The Role of Compensatory Adaptations and Individual Variability in Exercise Prescription
Abstract
:1. Introduction
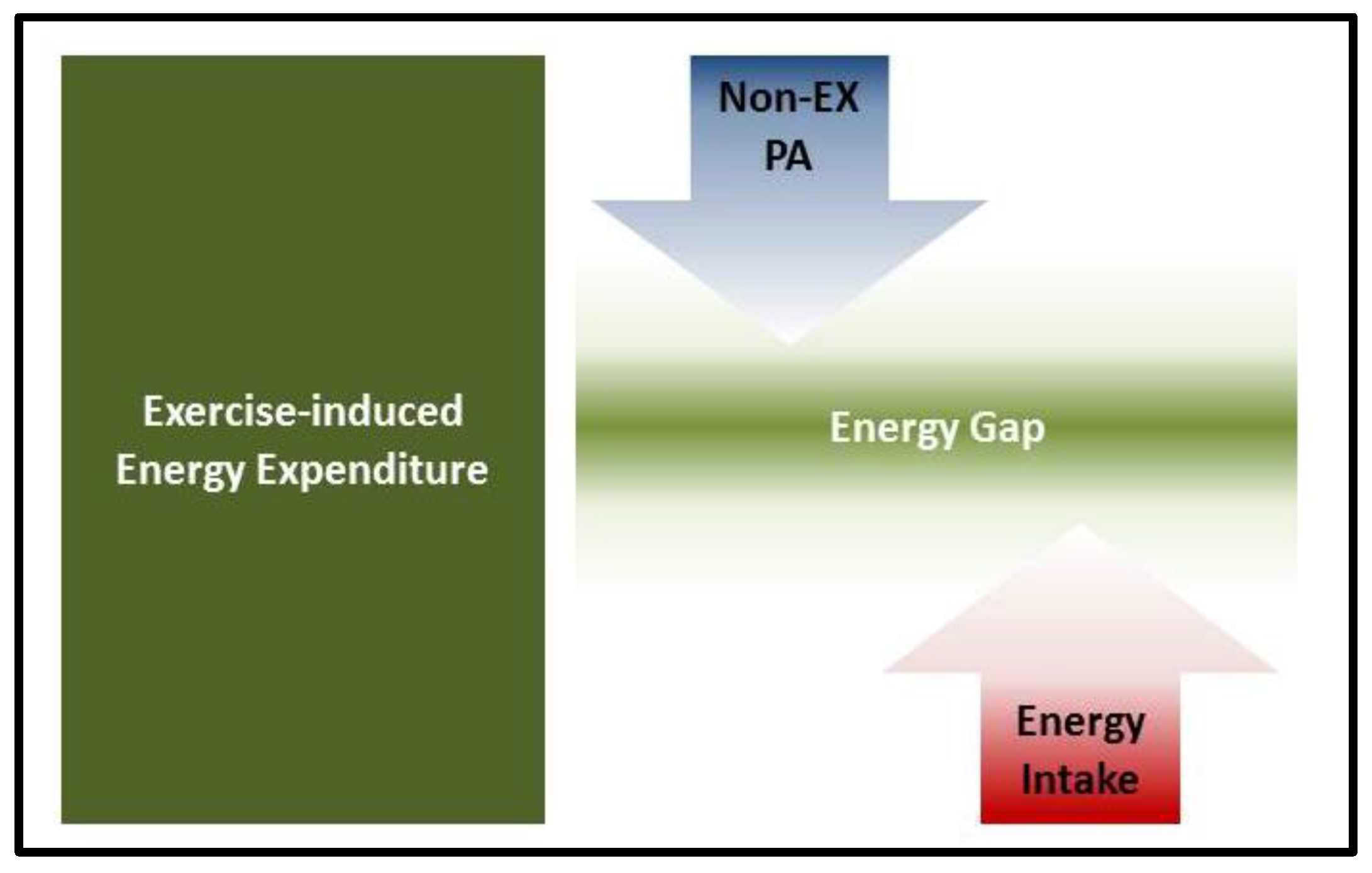
2. Compensatory Adaptations in Response to Exercise Interventions
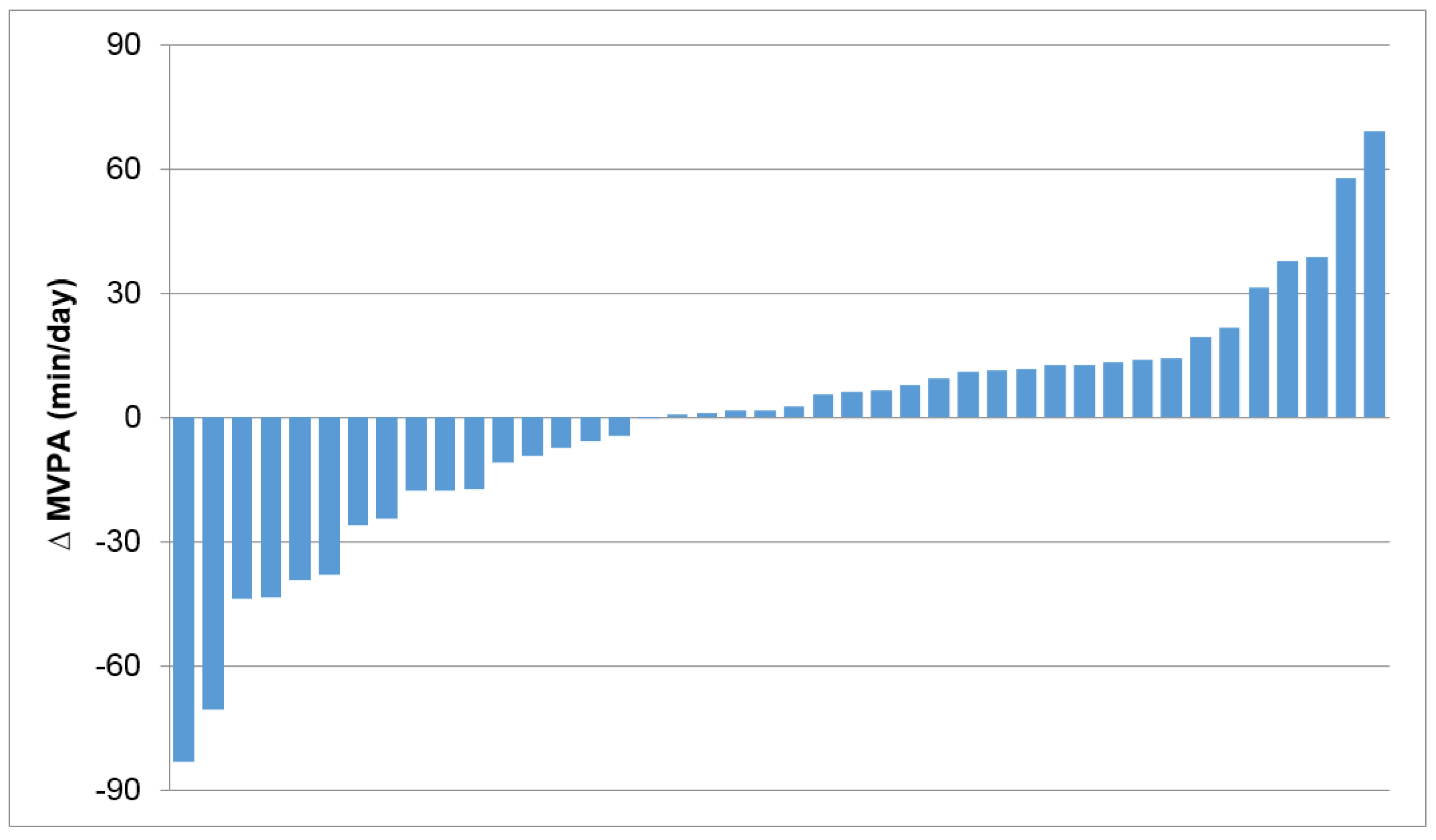
3. Individual Variability in Compensatory Adaptations in Response to Exercise
4. Summary and Conclusions
Conflicts of Interest
Abbreviations
| CVD | Cardio-Vascular Disease |
| EPOC | Excess Post-exercise Oxygen Consumption |
| PA | Physical Activity |
| RMR | Resting Metabolic Rate |
| TDEE | Total Daily Energy Expenditure |
References
- O’Donovan, G.; Blazevich, A.J.; Boreham, C.; Cooper, A.R.; Crank, H.; Ekelund, U.; Fox, K.R.; Gately, P.; Giles-Corti, B.; Gill, J.M.; et al. The ABC of physical activity for health: A consensus statement from the British Association of Sport and Exercise Sciences. J. Sports Sci. 2010, 28, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Brønnum-Hansen, H.; Juel, K.; Davidsen, M.; Sørensen, J. Impact of selected risk factors on expected lifetime without long-standing, limiting illness in Denmark. Prev. Med. 2007, 45, 49–53. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Observatory Data Repository, 2011. Available online: http://apps.who.int/gho/data/node.main.A893?lang=en (accessed on 30 March 2016).
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Masse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Global Health Risks; WHO Press: Geneva, Switzerland, 2009. [Google Scholar]
- World Health Organization. Global Recommendations on Physical Activity for Health; WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Church, T.S.; Thomas, D.M.; Tudor-Locke, C.; Katzmarzyk, P.T.; Earnest, C.P.; Rodarte, R.Q.; Martin, C.K.; Blair, S.N.; Bouchard, C. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS ONE 2011, 6, e19657. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Shook, R.P.; Thomas, D.M.; Church, T.S.; Katzmarzyk, P.T.; Hébert, J.R.; McIver, K.L.; Hand, G.A.; Lavie, C.J.; Blair, S.N. 45-year trends in women’s use of time and household management energy expenditure. PLoS ONE 2013, 8, e56620. [Google Scholar] [CrossRef] [PubMed]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- Miller, W.C.; Koceja, D.M.; Hamilton, E.J. A meta-analysis of the past 25 years of weight loss research using diet, exercise or diet plus exercise intervention. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, E.J.; Kaiser, K.A.; Dawson, J.A.; Alcorn, A.S.; Keating, K.D.; Allison, D.B. Predicting adult weight change in the real world: A systematic review and meta-analysis accounting for compensatory changes in energy intake or expenditure. Int. J. Obes. (Lond.) 2014, 39, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Melanson, E.L.; Keadle, S.K.; Donnelly, J.E.; Braun, B.; King, N.A. Resistance to exercise-induced weight loss: Compensatory behavioral adaptations. Med. Sci. Sports Exerc. 2013, 45, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.M.; Bouchard, C.; Church, T.; Slentz, C.; Kraus, W.E.; Redman, L.M.; Martin, C.K.; Silva, A.M.; Vossen, M.; Westerterp, K.; et al. Why do individuals not lose more weight from an exercise intervention at a defined dose? An energy balance analysis. Obes. Rev. 2012, 13, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Pontzer, H.; Durazo-Arvizu, R.; Dugas, L.R.; Plange-Rhule, J.; Bovet, P.; Forrester, T.E.; Lambert, E.V.; Cooper, R.S.; Schoeller, D.A.; Luke, A. Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans. Curr. Biol. 2016, 26, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Drenowatz, C. Reciprocal compensation to changes in dietary intake and energy expenditure within the concept of energy balance. Adv. Nutr. 2015, 6, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Rankinen, T. Individual differences in response to regular physical activity. Med. Sci. Sports Exerc. 2001, 33, S446–S453. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Blair, S.N.; Church, T.S.; Earnest, C.P.; Hagberg, J.M.; Häkkinen, K.; Jenkins, N.T.; Karavirta, L.; Kraus, W.E.; Leon, A.S.; et al. Adverse metabolic response to regular exercise: Is it a rare or common occurrence? PLoS ONE 2012, 7, e37887. [Google Scholar] [CrossRef] [PubMed]
- King, N.; Caudwell, P.; Hopkins, M.; Byrne, N.; Colley, R.; Hills, A.; Stubbs, J.; Blundell, J. Metabolic and behavioral compensatory responses to exercise interventions: Barriers to weight loss. Obesity 2007, 15, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- King, N.A.; Horner, K.; Hills, A.P.; Byrne, N.M.; Wood, R.E.; Bryant, E.; Caudwell, P.; Finlayson, G.; Gibbons, C.; Hopkins, M.; et al. Exercise, appetite and weight management: Understanding the compensatory responses in eating behaviour and how they contribute to variability in exercise-induced weight loss. Br. J. Sports Med. 2012, 46, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Holloszy, J.O. Exercise: It’s the real thing! Nutr. Rev. 2009, 67, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L.H.; Wing, R.R. Aerobic exercise and weight. Addict. Behav. 1980, 5, 371–388. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Smith, B.K. Is exercise effective for weight loss with ad libitum diet? Energy balance, compensation, and gender differences. Exerc. Sport Sci. Rev. 2005, 33, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Colley, R.C.; Hills, A.P.; King, N.A.; Byrne, N.M. Exercise-induced energy expenditure: Implications for exercise prescription and obesity. Patient Educ. Couns. 2010, 79, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Schutz, Y.; Nguyen, D.M.; Byrne, N.M.; Hills, A.P. Effectiveness of three different walking prescription durations on total physical activity in normal- and over-weight women. Obes. Facts 2014, 7, 264–273. [Google Scholar] [PubMed]
- Li, J.; O’Connor, L.E.; Zhou, J.; Campbell, W.W. Exercise patterns, ingestive behaviors, and energy balance. Physiol. Behav. 2014, 134, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Schobersberger, W. Evidence for resistance training as a treatment therapy in obesity. J. Obes. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.R.; Wetzstein, C.J.; Fields, D.A.; Brown, A.; Bamman, M.M. Resistance training increases total energy expenditure and free-living physical activity in older adults. J. Appl. Physiol. 2000, 89, 977–984. [Google Scholar] [PubMed]
- Drenowatz, C.; Grieve, G.L.; DeMello, M.M. Change in energy expenditure and physical activity in response to aerobic and resistance exercise programs. Springerplus 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Herrmann, S.D.; Lambourne, K.; Szabo, A.N.; Honas, J.J.; Washburn, R.A. Does increased exercise or physical activity alter ad-libitum daily energy intake or macronutrient composition in healthy adults? A systematic review. PLoS ONE 2014, 9, e83498. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Caudwell, P.; Gibbons, C.; Hopkins, M.; Naslund, E.; King, N.; Finlayson, G. Role of resting metabolic rate and energy expenditure in hunger and appetite control: A new formulation. Dis. Model. Mech. 2012, 5, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.; King, N.A.; Blundell, J.E. Acute and long-term effects of exercise on appetite control: Is there any benefit for weight control? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, R.J.; Sepp, A.; Hughes, D.A.; Johnstone, A.M.; King, N.; Horgan, G.; Blundell, J.E. The effect of graded levels of exercise on energy intake and balance in free-living women. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 866–869. [Google Scholar] [PubMed]
- Stubbs, R.J.; Sepp, A.; Hughes, D.A.; Johnstone, A.M.; Horgan, G.W.; King, N.; Blundell, J. The effect of graded levels of exercise on energy intake and balance in free-living men, consuming their normal diet. Eur. J. Clin. Nutr. 2002, 56, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Church, T.S.; Martin, C.K.; Thompson, A.M.; Earnest, C.P.; Mikus, C.R.; Blair, S.N. Changes in weight, waist circumference and compensatory responses with different doses of exercise among sedentary, overweight postmenopausal women. PLoS ONE 2009, 4, e4515. [Google Scholar] [CrossRef] [PubMed]
- Pomerleau, M.; Imbeault, P.; Parker, T.; Doucet, E. Effects of exercise intensity on food intake and appetite in women. Am. J. Clin. Nutr. 2004, 80, 1230–1236. [Google Scholar] [PubMed]
- Pannacciulli, N.; Salbe, A.D.; Ortega, E.; Venti, C.A.; Bogardus, C.; Krakoff, J. The 24-h carbohydrate oxidation rate in a human respiratory chamber predicts ad libitum food intake. Am. J. Clin. Nutr. 2007, 86, 625–632. [Google Scholar] [PubMed]
- Badman, M.K.; Flier, J.S. The gut and energy balance: Visceral allies in the obesity wars. Science 2005, 307, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- King, N.A.; Hopkins, M.; Caudwell, P.; Stubbs, R.J.; Blundell, J.E. Individual variability following 12 weeks of supervised exercise: Identification and characterization of compensation for exercise-induced weight loss. Int. J. Obes. (Lond.) 2008, 32, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, G.; Bryant, E.; Blundell, J.E.; King, N.A. Acute compensatory eating following exercise is associated with implicit hedonic wanting for food. Physiol. Behav. 2009, 97, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.H.; Oliver, A.J. Acute effects of brisk walking on urges to eat chocolate, affect, and responses to a stressor and chocolate cue. An experimental study. Appetite 2009, 52, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Schneider, K.L.; Spring, B.; Pagoto, S.L. Exercise and energy intake in overweight, sedentary individuals. Eat. Behav. 2009, 10, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Pontzer, H. Constrained total energy expenditure and the evolutionary biology of energy balance. Exerc. Sport Sci. Rev. 2015, 43, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R.; Meijer, G.A.; Janssen, E.M.; Saris, W.H.; Ten Hoor, F. Long-term effect of physical activity on energy balance and body composition. Br. J. Nutr. 1992, 68, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.; Gibbons, C.; Caudwell, P.; Hellström, P.M.; Näslund, E.; King, N.A.; Finlayson, G.; Blundell, J.E. The adaptive metabolic response to exercise-induced weight loss influences both energy expenditure and energy intake. Eur. J. Clin. Nutr. 2014, 68, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Doucet, E.; Imbeault, P.; St-Pierre, S.; Alméras, N.; Mauriège, P.; Després, J.P.; Bouchard, C.; Tremblay, A. Greater than predicted decrease in energy expenditure during exercise after body weight loss in obese men. Clin. Sci. (Lond.) 2003, 105, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Selman, C. Physical activity and resting metabolic rate. Proc. Nutr. Soc. 2003, 62, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Wilmore, J.H.; Stanforth, P.R.; Hudspeth, L.A.; Gagnon, J.; Daw, E.W.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Bouchard, C. Alterations in resting metabolic rate as a consequence of 20 wk of endurance training: The heritage family study. Am. J. Clin. Nutr. 1998, 68, 66–71. [Google Scholar] [PubMed]
- Manthou, E.; Gill, J.M.; Wright, A.; Malkova, D. Behavioural compensatory adjustments to exercise training in overweight women. Med. Sci. Sports Exerc. 2010, 42, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R. Impacts of vigorous and non-vigorous activity on daily energy expenditure. Proc. Nutr. Soc. 2003, 62, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Tremblay, A.; Nadeau, A.; Dussault, J.; Després, J.P.; Theriault, G.; Lupien, P.J.; Serresse, O.; Boulay, M.R.; Fournier, G. Long-term exercise training with constant energy intake. 1: Effect on body composition and selected metabolic variables. Int. J. Obes. 1990, 14, 57–73. [Google Scholar] [PubMed]
- Sawyer, B.J.; Bhammar, D.M.; Angadi, S.S.; Ryan, D.M.; Ryder, J.R.; Sussman, E.J.; Bertmann, F.M.; Gaesser, G.A. Predictors of fat mass changes in response to aerobic exercise training in women. J. Strength Cond. Res. 2015, 29, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.A.; Eberhardt, N.L.; Jensen, M.D. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 1999, 283, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Caudwell, P.; Gibbons, C.; Hopkins, M.; King, N.; Finlayson, G.; Blundell, J. No sex difference in body fat in response to supervised and measured exercise. Med. Sci. Sports Exerc. 2013, 45, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Hill, J.O.; Jacobsen, D.J.; Potteiger, J.; Sullivan, D.K.; Johnson, S.L.; Heelan, K.; Hise, M.; Fennessey, P.V.; Sonko, B.; et al. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: The midwest exercise trial. Arch. Intern. Med. 2003, 163, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.E.; Honas, J.J.; Smith, B.K.; Mayo, M.S.; Gibson, C.A.; Sullivan, D.K.; Lee, J.; Herrmann, S.D.; Lambourne, K.; Washburn, R.A. Aerobic exercise alone results in clinically significant weight loss for men and women: Midwest exercise trial 2. Obesity (Silver Spring) 2013, 21, E219–E228. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C.; Tremblay, A.; Després, J.P.; Thériault, G.; Nadeau, A.; Lupien, P.J.; Moorjani, S.; Prudhomme, D.; Fournier, G. The response to exercise with constant energy intake in identical twins. Obes. Res. 1994, 2, 400–410. [Google Scholar] [CrossRef] [PubMed]
- King, N.A.; Caudwell, P.P.; Hopkins, M.; Stubbs, J.R.; Naslund, E.; Blundell, J.E. Dual-process action of exercise on appetite control: Increase in orexigenic drive but improvement in meal-induced satiety. Am. J. Clin. Nutr. 2009, 90, 921–927. [Google Scholar] [CrossRef] [PubMed]
- King, N.A.; Hopkins, M.; Caudwell, P.; Stubbs, R.J.; Blundell, J.E. Beneficial effects of exercise: Shifting the focus from body weight to other markers of health. Br. J. Sports Med. 2009, 43, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.; Blundell, J.E.; King, N.A. Individual variability in compensatory eating following acute exercise in overweight and obese women. Br. J. Sports Med. 2014, 48, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Oliveros, E.; Somers, V.K.; Sochor, O.; Goel, K.; Lopez-Jimenez, F. The concept of normal weight obesity. Prog. Cardiovasc. Dis. 2014, 56, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Fogelholm, M. Physical activity, fitness and fatness: Relations to mortality, morbidity and disease risk factors. A systematic review. Obes. Rev. 2010, 11, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C. Individual differences in the response to regular exercise. Int. J. Obes. Relat. Metab. Disord. 1995, 19, S5–S8. [Google Scholar] [PubMed]
- Bouchard, C.; An, P.; Rice, T.; Skinner, J.S.; Wilmore, J.H.; Gagnon, J.; Pérusse, L.; Leon, A.S.; Rao, D.C. Familial aggregation of VO(2max) response to exercise training: Results from the heritage family study. J. Appl. Physiol. 1999, 87, 1003–1008. [Google Scholar] [PubMed]
- Leon, A.S.; Gaskill, S.E.; Rice, T.; Bergeron, J.; Gagnon, J.; Rao, D.C.; Skinner, J.S.; Wilmore, J.H.; Bouchard, C. Variability in the response of HDL cholesterol to exercise training in the HERITAGE family study. Int. J. Sports Med. 2002, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.; Bredin, S.S. Reflections on physical activity and health: What should we recommend? Can. J. Cardiol. 2016, 32, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Gibbons, C.; Caudwell, P.; Finlayson, G.; Hopkins, M. Appetite control and energy balance: Impact of exercise. Obes. Rev. 2015, 16, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Dilnot, A. The Tiger that Isn’t: Seeing through a World of Numbers; Profile Books: London, UK, 2008. [Google Scholar]
- Wilkinson, G.R. Drug metabolism and variability among patients in drug response. N. Engl. J. Med. 2005, 352, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Kozey-Keadle, S.; Staudenmayer, J.; Libertine, A.; Mavilia, M.; Lyden, K.; Braun, B.; Freedson, P. Changes in sedentary time and physical activity in response to an exercise training and/or lifestyle intervention. J. Phys. Act. Health 2014, 11, 1324–1333. [Google Scholar] [CrossRef] [PubMed]
| Study | Population | Duration | Intervention | Avg. Weight Change | Range of Weight Change |
|---|---|---|---|---|---|
| Bouchard et al. [50] | 5 healthy young men | 84 days | cycling for 1000 kcal/day | −8.0 kg | −3 to −12 kg |
| Bouchard et al. [51] | 14 young men (7 twin pairs) | 93 days | 9 of 10 days cycling for 1000 kcal/day | −5.0 kg | −8 to −1 kg |
| Caudwell et al. [52] | 107 overweight/obese adults | 12 weeks | aerobic exercise at 2500 kcal/week | −2.5 kg | −15 to +4 kg |
| Donelly et al. [22,53] | 74 overweight/obese adults | 16 months | 5 days/week aerobic exercise for 45 min | ♂: −5.2 kg ♀: +0.6 kg | −14 to +12 kg |
| Donnelly et al. [54] | 92 overweight/obese adults | 10 months | 5 days/week treadmill for 400 or 600 kcal/session | −3.0 kg | −22% to +5% |
| Hopkins et al. [44] | 30 overweight/obese women | 12 weeks | aerobic exercise at 2500 kcal/week | −1.3 kg | −8 to +4 kg |
| King et al. [38] | 35 overweight/obese adults | 12 weeks | aerobic exercise at 2500 kcal/week | −3.7 kg | −15 to +2 kg |
| King et al. [55,56] | 58 overweight/obese adults | 12 weeks | aerobic exercise at 2500 kcal/week | −3.2 kg | −14 to +3 kg |
| Manthou et al. [48] | 34 overweight/obese women | 8 weeks | Aerobic exercise for 150 min/week | 0.0 kg † | −3 to +3 kg † |
| Sawyer et al. [57] | 81 sedentarywomen | 12 weeks | 3 days/week walking for 30 min/session | 0.0 kg | −12 to +5 kg |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drenowatz, C. The Role of Compensatory Adaptations and Individual Variability in Exercise Prescription. J. Funct. Morphol. Kinesiol. 2016, 1, 230-239. https://doi.org/10.3390/jfmk1020230
Drenowatz C. The Role of Compensatory Adaptations and Individual Variability in Exercise Prescription. Journal of Functional Morphology and Kinesiology. 2016; 1(2):230-239. https://doi.org/10.3390/jfmk1020230
Chicago/Turabian StyleDrenowatz, Clemens. 2016. "The Role of Compensatory Adaptations and Individual Variability in Exercise Prescription" Journal of Functional Morphology and Kinesiology 1, no. 2: 230-239. https://doi.org/10.3390/jfmk1020230
APA StyleDrenowatz, C. (2016). The Role of Compensatory Adaptations and Individual Variability in Exercise Prescription. Journal of Functional Morphology and Kinesiology, 1(2), 230-239. https://doi.org/10.3390/jfmk1020230






