Effects of Electrical Pulse and 6-DMAP on Cleavage of Golden Hamster Oocytes—Morphological and Phisiological Observations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Animals
2.2. Collection of Oocytes
2.3. Culture Media
2.4. Activation and Cleavage
2.5. Assessment of Oocyte Cleavage
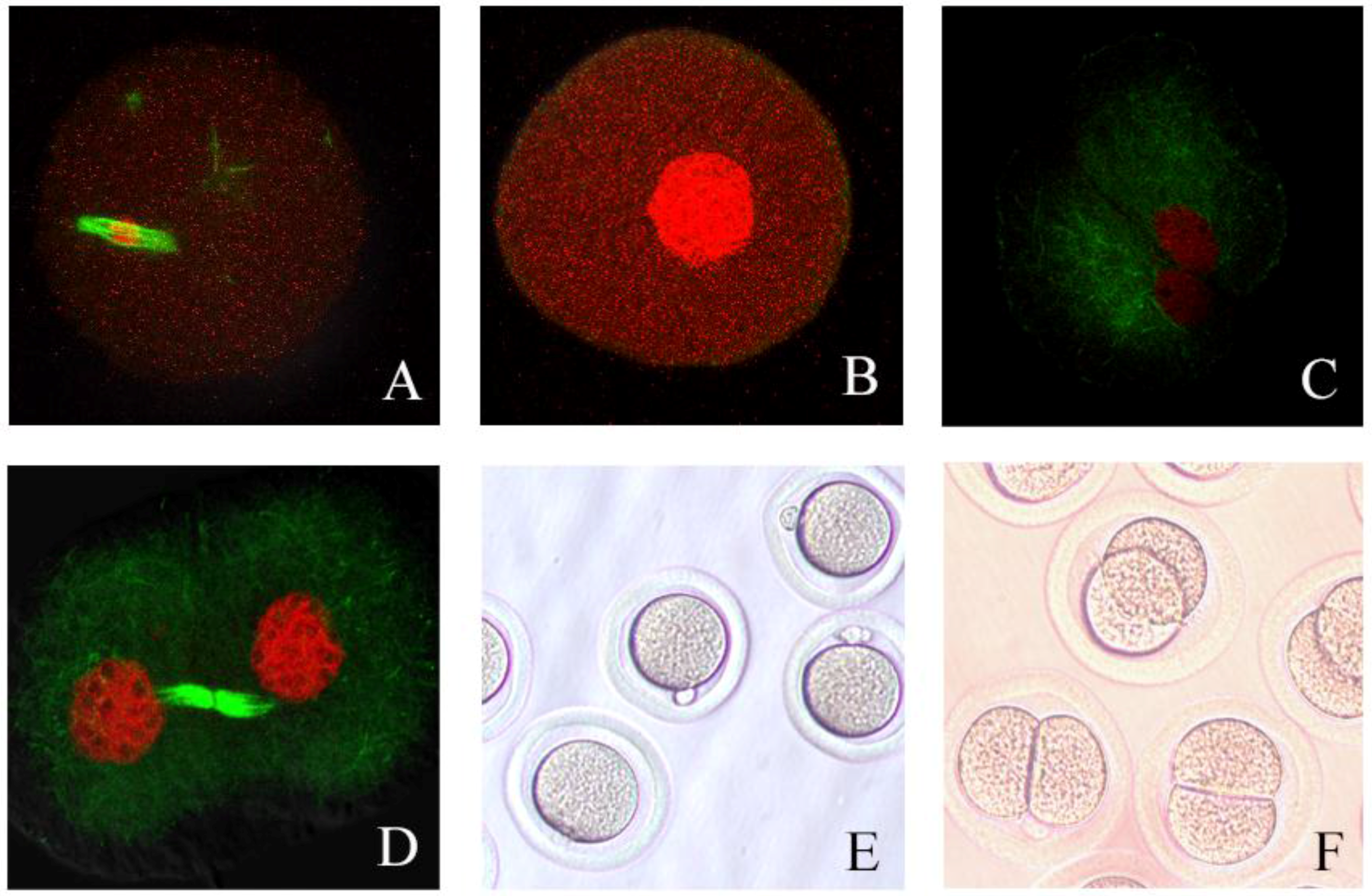
2.6. Staining and Observation of the Oocyte Cleavage Process
2.7. Data Analysis
3. Results
3.1. Cleavage of Oocytes at 15 h Post-hCG after Electrical Stimulus
3.2. Cleavage of Oocytes at 13.5–19 h Post-hCG after Electrical Stimulus
3.3. Cleavage of Oocytes at 15 h Post-hCG after 6-Dimethylaminopurine (6-DMAP) Treatment
3.4. Cleavage of Oocytes at 13.5–19 h Post-hCG after 6-DMAP Treatment
3.5. Cleavage of Oocytes after Combined Treatment of Electrical Pulse and 6-DMAP
3.6. Process of Hamster Oocyte Cleavage
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, L.; Jiang, H.; Su, L.; Tang, B.; Li, D.; Li, Z. Effects of colchicine or demecolcine on cytoplasmic protrusions and assisted enucleation of golden hamster oocytes. Cell Biol. Int. 2009, 33, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, D.; Li, Z. Changes in the reciprocal position of the first polar body and oocyte chromosome set in golden hamsters. Biosci. Rep. 2009, 29, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Ware, C.B.; Barnes, F.L.; Maiki-Laurila, M.; First, N.L. Age dependence of bovine oocyte activation. Gamete Res. 1989, 22, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Marcus, G.J. Activation of cumulus-free mouse oocytes. Mol. Reprod. Dev. 1990, 26, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.C.; Ma, S.F.; Wang, Z.Y.; Luo, M.J.; Chang, Z.L.; Tan, J.H. Effects of post-treatment with 6-dimethylaminopurine (6-DMAP) on ethanol activation of mouse oocytes at different ages. J. Exp. Zool. A Comp. Exp. Biol. 2004, 301, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Presicce, G.A.; Yang, X. Parthenogenetic development of bovine oocytes matured in vitro for 24 h and activated by ethanol and cycloheximide. Mol. Reprod. Dev. 1994, 38, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Bodart, J.F.; Bechard, D.; Bertout, M.; Gannon, J.; Rousseau, A.; Vilain, J.P.; Flament, S. Activation of xenopus eggs by the kinase inhibitor 6-DMAP suggests a differential regulation of cyclin B and p39mos proteolysis. Exp. Cell Res. 1999, 253, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Escriba, M.J.; Garcia-Ximenez, F. Use of a variable electrical pulsing sequence in rabbit oocyte activation. Reprod. Nutr. Dev. 2000, 40, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, C.; Guan, J.; Wang, L.; Li, Z. Changes of spontaneous parthenogenetic activation and development potential of golden hamster oocytes during the aging process. Acta Histochem. 2015, 117, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.S.; Yue, K.Z.; Zhou, J.B.; Chen, Q.X.; Tan, J.H. In vitro spontaneous parthenogenetic activation of golden hamster oocytes. Theriogenology 2002, 57, 845–851. [Google Scholar] [CrossRef]
- Gwatkin, R.B.; Williams, D.T.; Hartmann, J.F.; Kniazuk, M. The zona reaction of hamster and mouse eggs: Production in vitro by a trypsin-like protease from cortical granules. J. Reprod. Fertil. 1973, 32, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.J.; Park, C.S. Parthenogenetic development of porcine oocytes treated by ethanol, cycloheximide, cytochalasin B and 6-dimethylaminopurine. Anim. Reprod. Sci. 2005, 86, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, Q.; Rezaei Sabet, M.; Zhang, Y.; Ritchie, T.C.; Engelhardt, J.F. Conditions for in vitro maturation and artificial activation of ferret oocytes. Biol. Reprod. 2002, 66, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Grupen, C.G.; Mau, J.C.; McIlfatrick, S.M.; Maddocks, S.; Nottle, M.B. Effect of 6-dimethylaminopurine on electrically activated in vitro matured porcine oocytes. Mol. Reprod. Dev. 2002, 62, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Gertsenstein, M.; Vintersten, K.; Behringer, R. Manipulating the Mouse Embryo: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2003. [Google Scholar]
- Yamauchi, Y.; Yanagimachi, R.; Horiuchi, T. Full-term development of golden hamster oocytes following intracytoplasmic sperm head injection. Biol. Reprod. 2002, 67, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, U.; Vienken, J. Electric field-induced cell-to-cell fusion. J. Membr. Biol. 1982, 67, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Swann, K.; Ozil, J.P. Dynamics of the calcium signal that triggers mammalian egg activation. Int. Rev. Cytol. 1994, 152, 183–222. [Google Scholar] [PubMed]
- Cheng, W.M.; Sun, X.L.; An, L.; Zhu, S.E.; Li, X.H.; Li, Y.; Tian, J.H. Effect of different parthenogenetic activation methods on the developmental competence of in vitro matured porcine oocytes. Anim. Biotechnol. 2007, 18, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.C.; Yamazaki, W.; Ferreira, C.R.; Perecin, F.; Saraiva, N.Z.; Leal, C.L.; Garcia, J.M. Parthenogenetic activation of bovine oocytes using single and combined strontium, ionomycin and 6-dimethylaminopurine treatments. Zygote 2007, 15, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.A. Chemical activation of in vitro matured dromedary camel (Camelus dromedarius) oocytes: Optimization of protocols. Theriogenology 2008, 69, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.; Yanagimachi, R. Round spermatid nuclei injected into hamster oocytes from pronuclei and participate in syngamy. Biol. Reprod. 1993, 48, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T. Parthenogenetic activation of cattle follicular oocytes in vitro with ethanol. Gamete Res. 1987, 16, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Hagen, D.R.; Prather, R.S.; First, N.L. Response of porcine oocytes to electrical and chemical activation during maturation in vitro. Mol. Reprod. Dev. 1991, 28, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R.; Chang, M.C. Fertilizable life of golden hamster ova and their morphological changes at the time of losing fertilizability. J. Exp. Zool. 1961, 148, 185–203. [Google Scholar] [CrossRef] [PubMed]
- Moses, R.M.; Kline, D.; Masui, Y. Maintenance of metaphase in colcemid-treated mouse eggs by distinct calcium- and 6-dimethylaminopurine (6-DMAP)-sensitive mechanisms. Dev. Biol. 1995, 167, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Ledda, S.; Loi, P.; Bogliolo, L.; Moor, R.M.; Fulka, J., Jr. The effect of 6-dimethylaminopurine (6-DMAP) on DNA synthesis in activated mammalian oocytes. Zygote 1996, 4, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Fan, B.Q.; Wang, H.H.; Cheng, Y.F.; Wang, G.J. [effect of 6-dimethylaminopurine on the resumption of meiosis and parthenogenetic activation in mouse oocytes]. Shi Yan Sheng Wu Xue Bao 1997, 30, 403–406. [Google Scholar] [PubMed]
- Moses, R.M.; Masui, Y. Enhancement of mouse egg activation by the kinase inhibitor, 6-dimethylaminopurine (6-DMAP). J. Exp. Zool. 1994, 270, 211–218. [Google Scholar] [CrossRef] [PubMed]
| Electrical Pulse (V/mm) | No. of Oocytes Examined | No. of Oocytes Cleaved | % of Cleavage (mean ± SE) |
|---|---|---|---|
| 10 | 32 | 0 | 0.00 ± 0.00 a |
| 30 | 38 | 12 | 31.00 ± 7.57 b |
| 50 | 27 | 11 | 41.33 ± 8.25 b |
| 100 | 33 | 24 | 75.00 ± 7.00 c |
| 200 | 30 | 20 | 64.00 ± 14.18 c |
| 300 | 60 | 48 | 81.00 ± 9.71 c,d |
| 400 | 28 | 27 | 96.33 ± 3.67 d |
| 500 | 45 | 45 | 100.00 ± 0.00 d |
| 550 | 27 | 20 | 74.33 ± 5.46 c |
| 600 | 24 | 0 * | 0.00 ± 0.00 a |
| Electrical Pulse (V/mm) | Hours Post-hCG | No. of Oocytes Examined | No. of Oocytes Cleaved | % of Cleavage (mean ± SE) |
|---|---|---|---|---|
| 50 | 13.5 | 36 | 6 | 12.67 ± 12.67 a,b |
| 15 | 27 | 11 | 41.33 ± 8.25 a | |
| 17 | 27 | 4 | 14.00 ± 7.37 a,b | |
| 19 | 21 | 2 | 7.33 ± 7.33 b | |
| 100 | 13.5 | 33 | 3 | 7.33 ± 3.84 a,c |
| 15 | 33 | 24 | 75.00 ± 7.00 b | |
| 17 | 29 | 3 | 60.33 ± 11.69 b | |
| 19 | 29 | 18 | 13.67 ± 9.94 c | |
| 300 | 13.5 | 31 | 7 | 21.67 ± 11.28 a |
| 15 | 60 | 48 | 81.00 ± 9.71 b | |
| 17 | 28 | 16 | 63.33 ± 9.28 b,c | |
| 19 | 20 | 8 | 39.00 ± 3.22 a,c | |
| 500 | 13.5 | 33 | 0 * | 0.00 ± 0.00 a,* |
| 15 | 45 | 45 | 100.00 ± 0.00 b | |
| 17 | 22 | 9 | 41.00 ± 14.29 c | |
| 19 | 38 | 14 | 31.67 ± 9.28 c |
| Duration of 6-DMAP (h) | No. of Oocytes Examined | No. of Cleavage | % of Cleavage (mean ± SE) |
|---|---|---|---|
| 0.5 | 39 | 8 | 19.44 ± 4.24 a |
| 1 | 40 | 12 | 34.33 ± 11.37 a,b |
| 2 | 51 | 23 | 45.21 ± 10.65 a,b |
| 4 | 50 | 23 | 46.82 ± 5.40 b |
| 6 | 50 | 18 | 35.89 ± 13.33 a,b |
| 8 | 39 | 9 | 24.23 ± 2.25 a,b |
| Hours Post-hCG | Duration of 6-DMAP (h) | No. of Oocytes Examined | No. of Oocytes Cleaved | % of Cleavage (mean ± SE) |
|---|---|---|---|---|
| 13.5 | 2 | 43 | 18 | 47.12 ± 15.44 a |
| 4 | 43 | 14 | 39.23 ± 17.12 a | |
| 6 | 41 | 19 | 46.21 ± 9.04 a | |
| 15 | 2 | 50 | 23 | 45.21 ± 10.65 a |
| 4 | 51 | 23 | 46.82 ± 5.40 a | |
| 6 | 51 | 18 | 35.89 ± 13.33 a | |
| 17 | 2 | 32 | 5 | 13.89 ± 7.35 a |
| 4 | 32 | 1 | 4.17 ± 4.17 a | |
| 6 | 32 | 0 | 0.00 ± 0.00 a | |
| 19 | 2 | 34 | 6 | 16.27 ± 12.87 a |
| 4 | 34 | 1 | 2.78 ± 2.78 a | |
| 6 | 34 | 0 | 0.00 ± 0.00 a |
| Duration of 6-DMAP (h) | Electrical Stimulus | No. of Oocytes Examined | No. of Oocytes Cleaved | % of Cleavage (mean ± SE) |
|---|---|---|---|---|
| 0 | + | 60 | 48 | 81.00 ± 9.71 a |
| 2 | + | 54 | 52 | 96.49 ± 3.51 a |
| 2 | − | 51 | 23 | 45.21 ± 10.65 b |
| 4 | + | 60 | 54 | 90.44 ± 1.55 a |
| 4 | − | 50 | 23 | 46.82 ± 5.40 b |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Jiang, H.; Li, Z. Effects of Electrical Pulse and 6-DMAP on Cleavage of Golden Hamster Oocytes—Morphological and Phisiological Observations. J. Funct. Morphol. Kinesiol. 2016, 1, 240-248. https://doi.org/10.3390/jfmk1020240
Wang L, Jiang H, Li Z. Effects of Electrical Pulse and 6-DMAP on Cleavage of Golden Hamster Oocytes—Morphological and Phisiological Observations. Journal of Functional Morphology and Kinesiology. 2016; 1(2):240-248. https://doi.org/10.3390/jfmk1020240
Chicago/Turabian StyleWang, Lingyan, Han Jiang, and Ziyi Li. 2016. "Effects of Electrical Pulse and 6-DMAP on Cleavage of Golden Hamster Oocytes—Morphological and Phisiological Observations" Journal of Functional Morphology and Kinesiology 1, no. 2: 240-248. https://doi.org/10.3390/jfmk1020240
APA StyleWang, L., Jiang, H., & Li, Z. (2016). Effects of Electrical Pulse and 6-DMAP on Cleavage of Golden Hamster Oocytes—Morphological and Phisiological Observations. Journal of Functional Morphology and Kinesiology, 1(2), 240-248. https://doi.org/10.3390/jfmk1020240