Development of Membrane Electrode Assembly with Double-Catalytic Layer for Micro Direct Methanol Fuel Cell
Abstract
1. Introduction
2. Materials and Methods
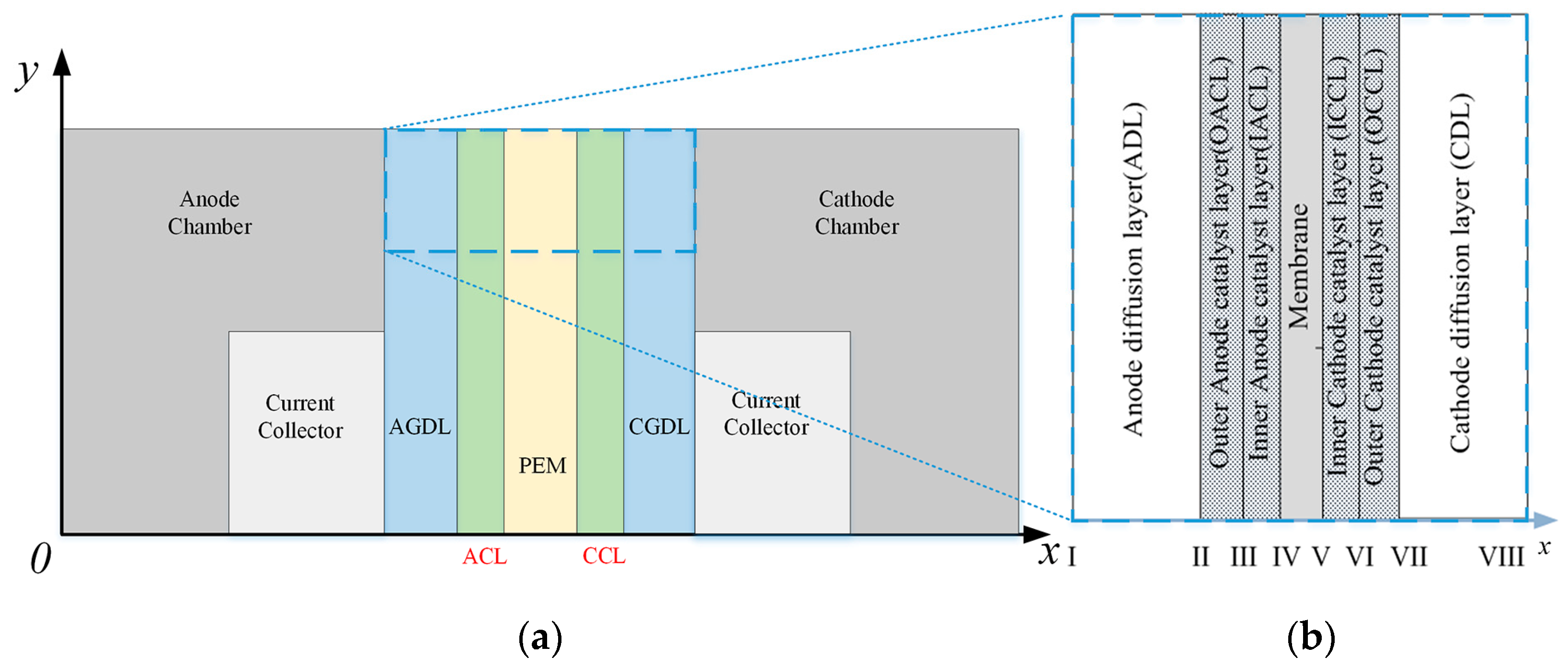
2.1. Mathematical
2.2. Experimental
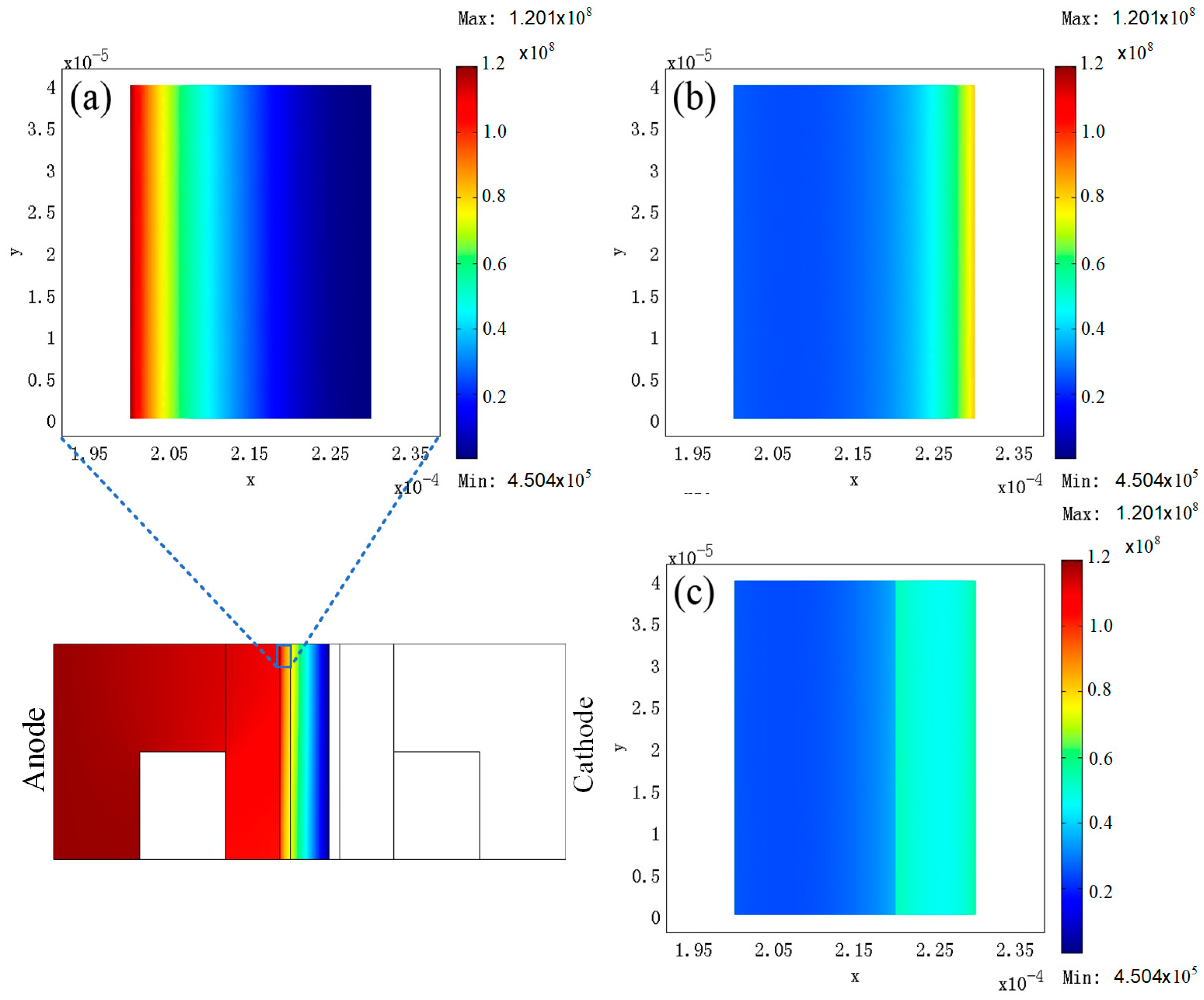
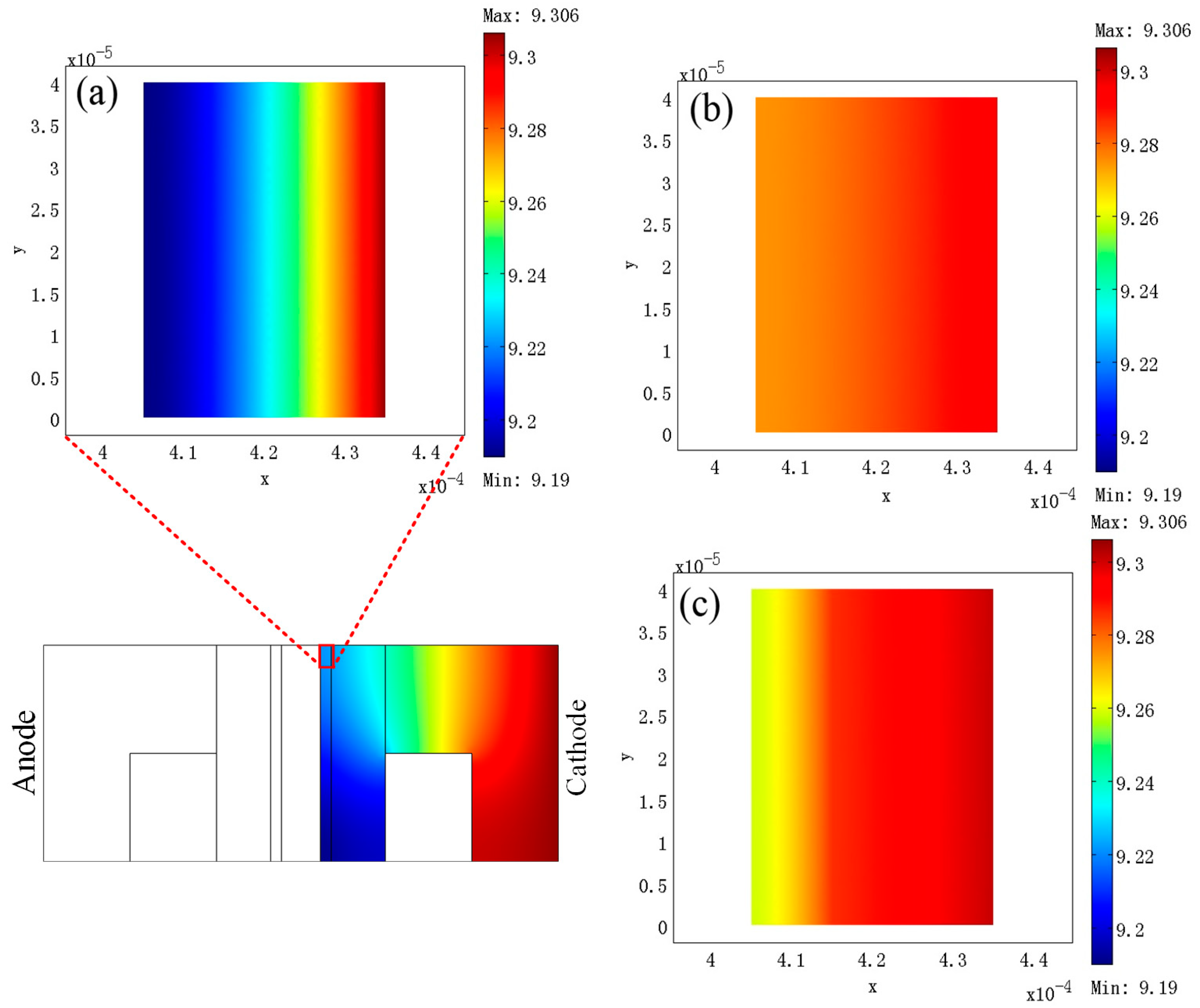
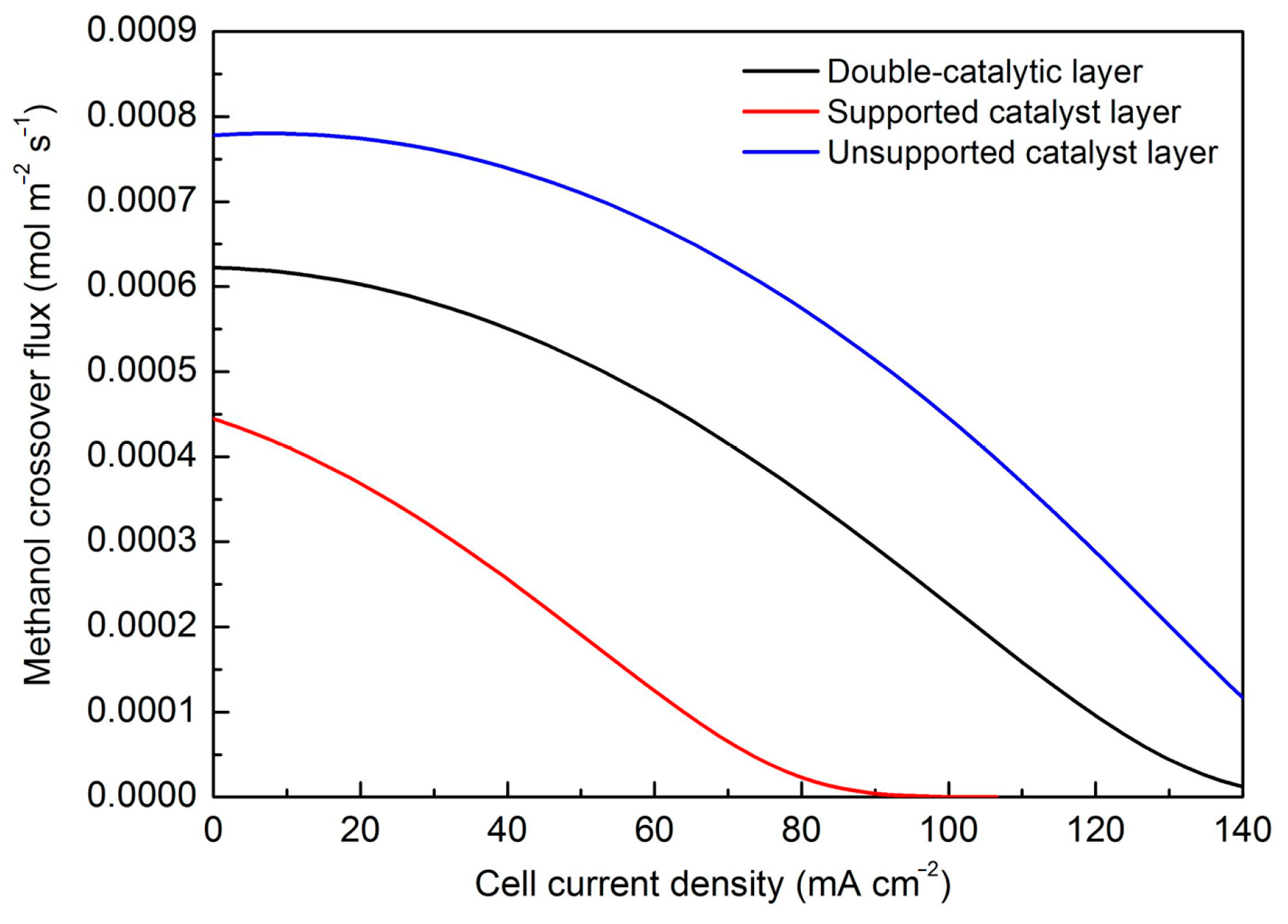
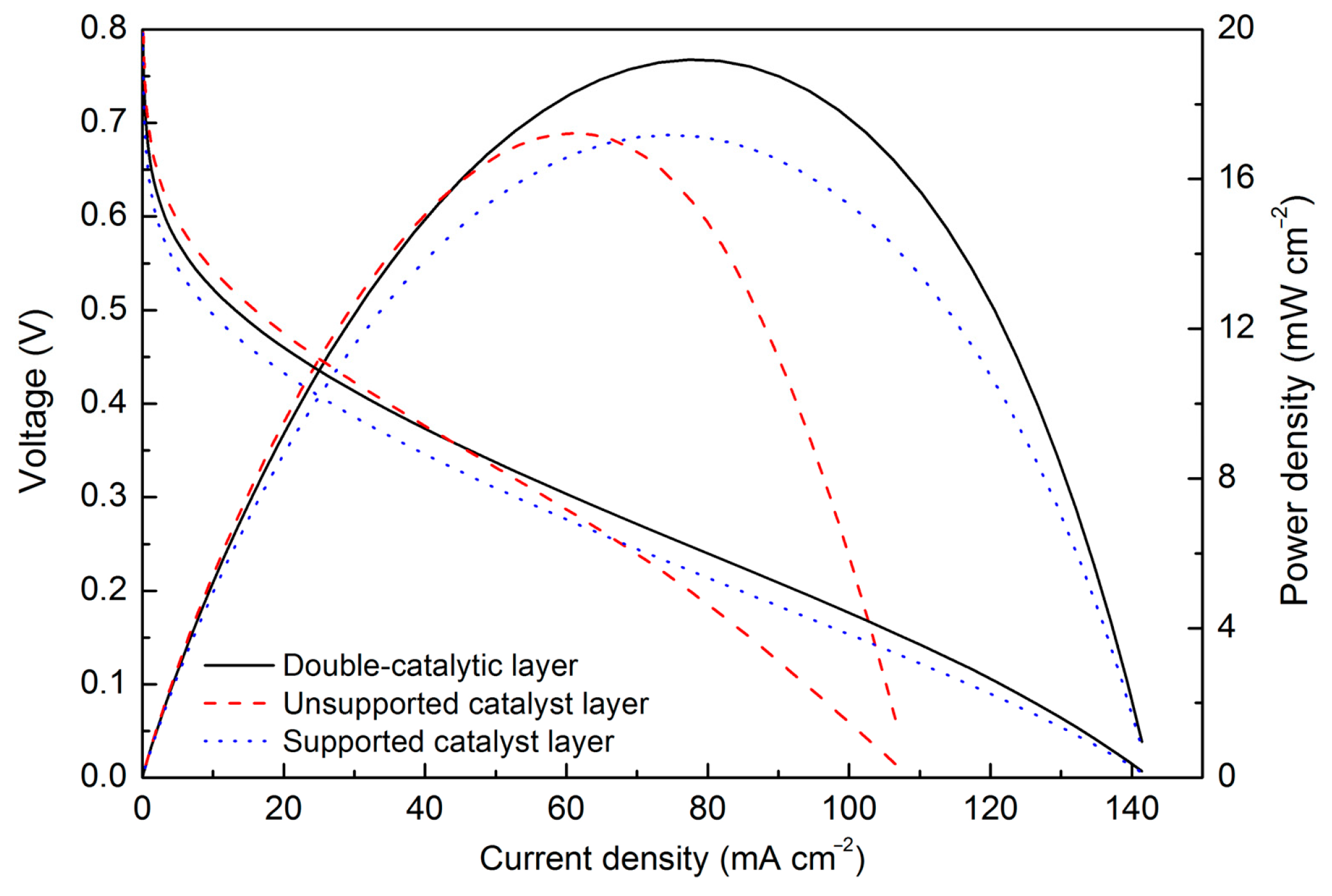
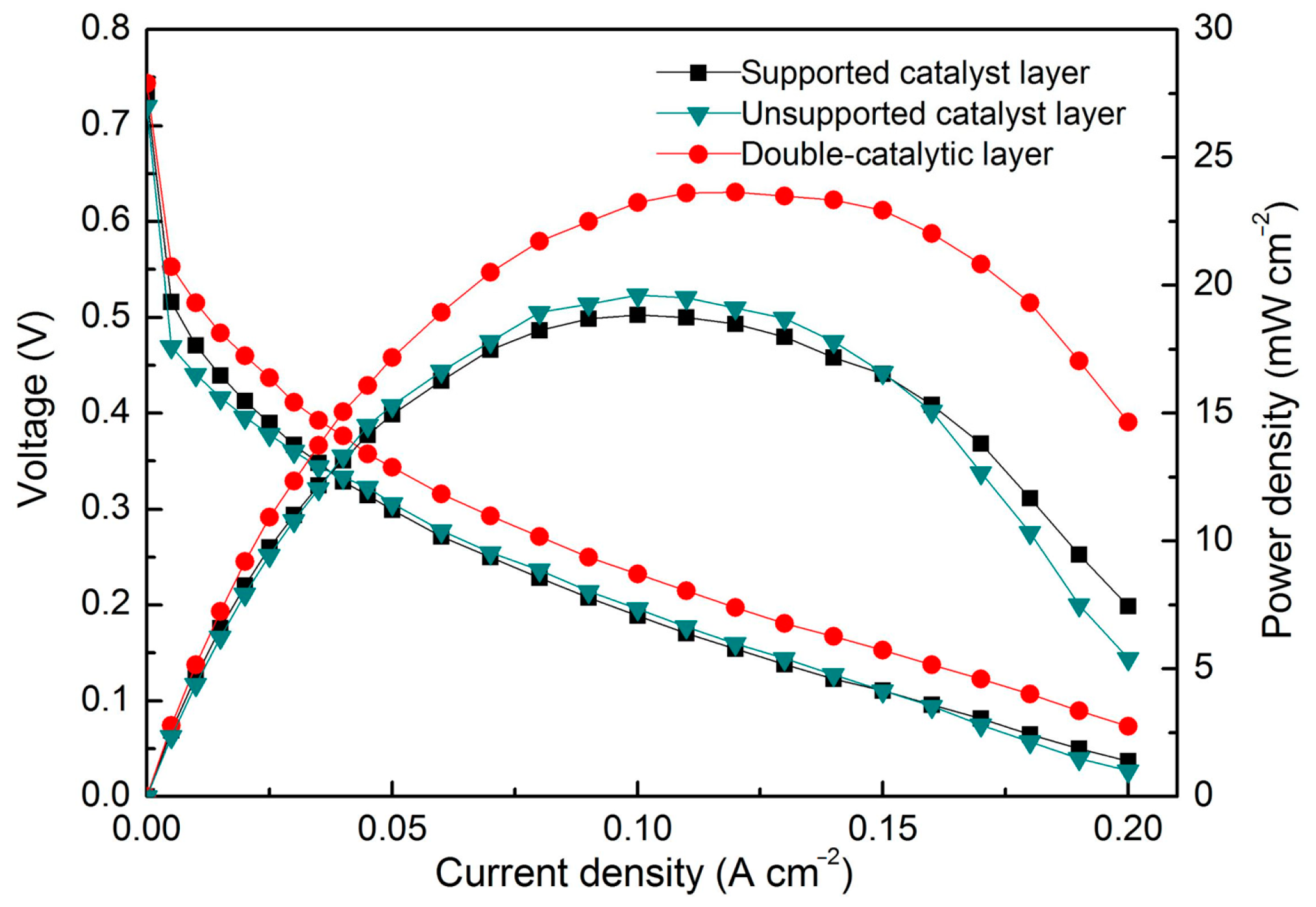
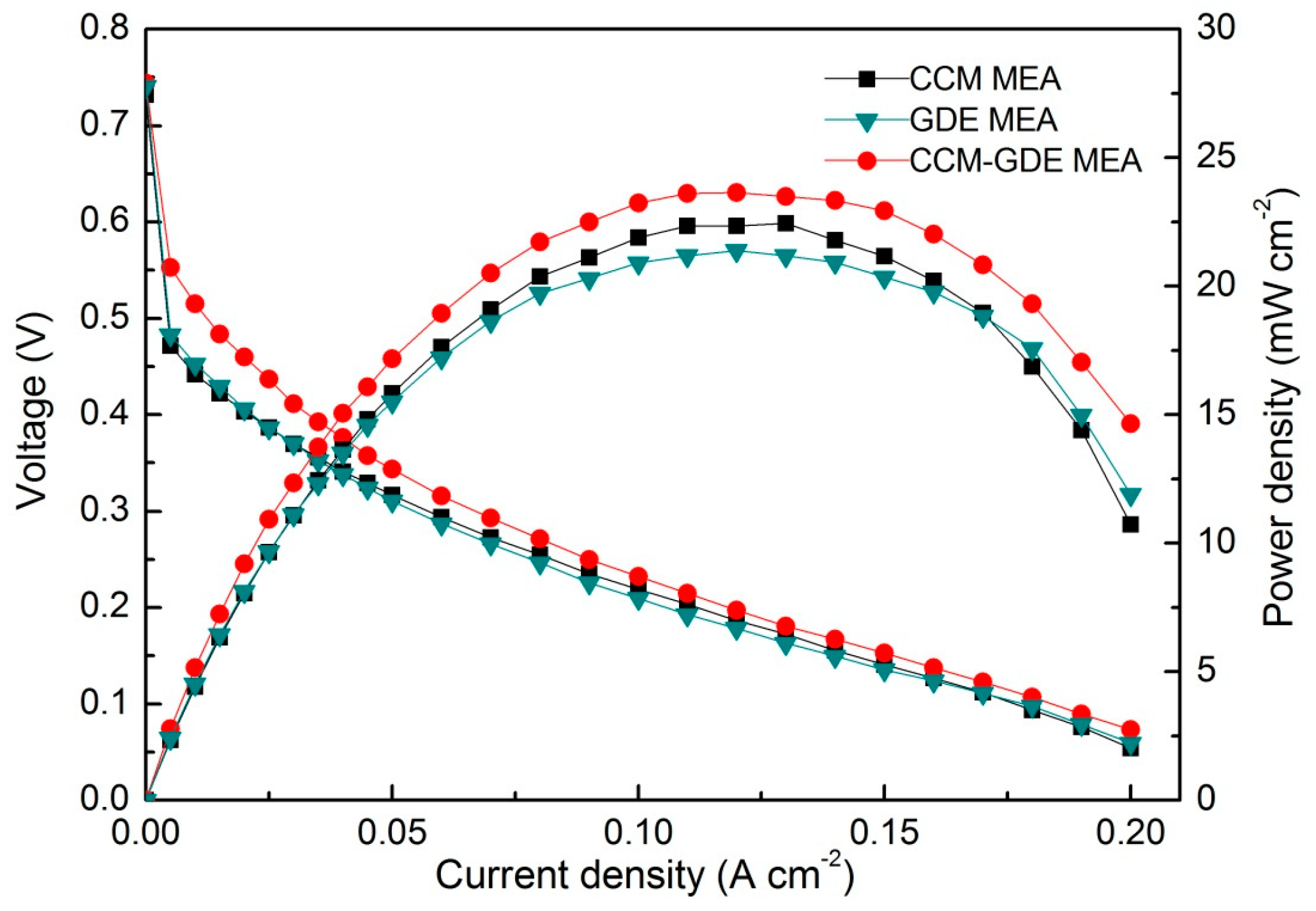
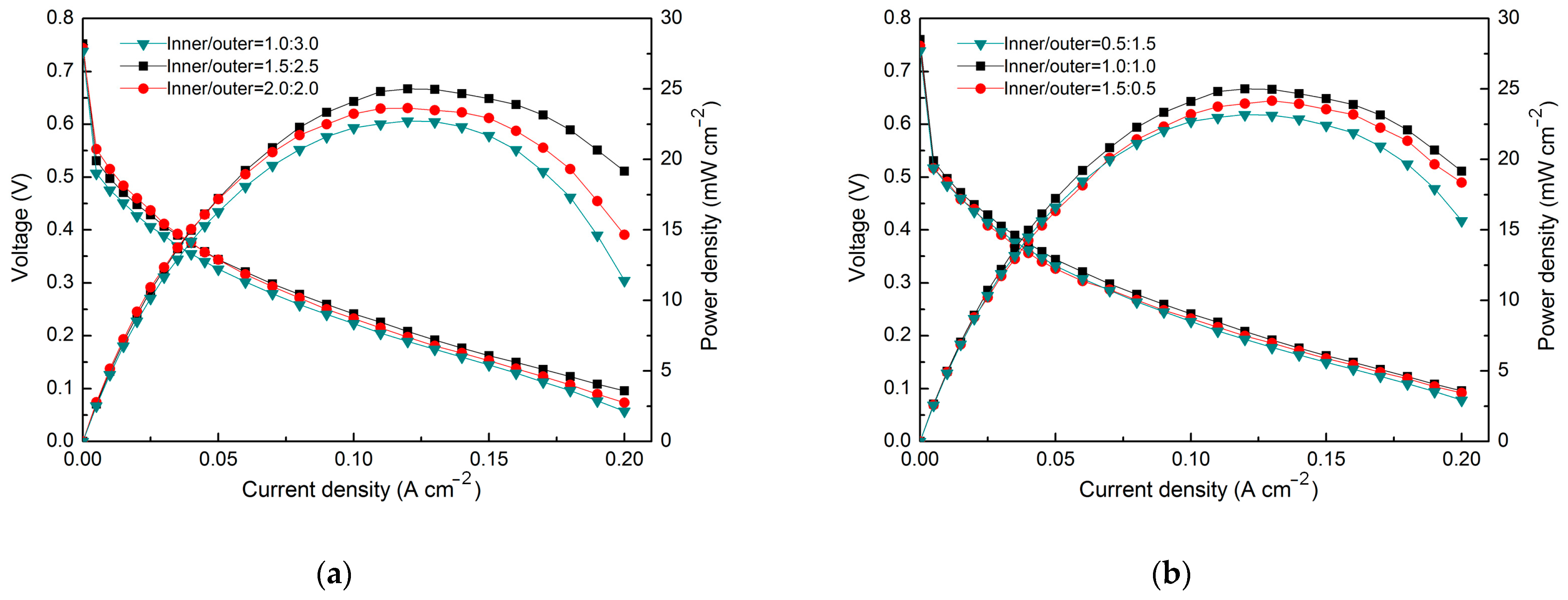
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.R.; Li, H.W.; Zhao, Y.; Ji, D.; Guo, P.; Li, G.X.; Zhao, X.H. Insights on the Roles of Nitrogen Configuration in Enhancing the Performance of Electrocatalytic Methanol Oxidation over Pt Nanoparticles. Small 2023, 19, e2303065. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.L.; Xu, J.F.; Zhao, J.N.; Sun, S.S.; Tang, W.B.; Huang, Q.H.; Yu, N.F.; Wu, Y.P. Incorporation of ultralow manganese dioxide for improving the durability of sulfonated poly (ether ether ketone) membranes in passive direct methanol fuel cell. Polymer 2023, 283, 126204. [Google Scholar] [CrossRef]
- Guo, S.Q.; Yu, S.Y.; Chen, F.; Wang, L.; Guo, M.; Ren, T.L.; Zhang, C.; Li, C.J. Direct methanol fuel cell with enhanced oxygen reduction performance enabled by CoFe alloys embedded into N-doped carbon nanofiber and bamboo-like carbon nanotube. J. Colloid Interface Sci. 2023, 652, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.S.; Zhang, Y.J.; Zhang, P.; Zhang, X.T.; Hu, T.P. In situ growth of Ni/Ni3S2-MoO2 nanocrystals on carbon cloth for the enhanced electrocatalytic oxidation of methanol. Appl. Surf. Sci. 2023, 640, 158348. [Google Scholar] [CrossRef]
- Zuo, Y.H.; Sheng, W.C.; Tao, W.Q.; Li, Z. Direct methanol fuel cells system-A review of dual-role electrocatalysts for oxygen reduction and methanol oxidation. J. Mater. Sci. Technol. 2022, 114, 29–41. [Google Scholar] [CrossRef]
- Bonham, D.; Choi, J.Y.; Kishimoto, T.; Ye, S.Y. Integrating PGM-Free Catalysts into Catalyst Layers and Proton Exchange Membrane Fuel Cell Devices. Adv. Mater. 2019, 31, e1804846. [Google Scholar] [CrossRef]
- Yang, L.J.; Shui, J.L.; Du, L.; Shao, Y.Y.; Liu, J.; Dai, L.M.; Hu, Z. Carbon-Based Metal-Free ORR Electrocatalysts for Fuel Cells: Past, Present, and Future. Adv. Mater. 2019, 31, 1804799. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Chen, H.M.; Wei, Z.D. Recent Progress in Precious Metal-Free Carbon-Based Materials towards the Oxygen Reduction Reaction: Activity, Stability, and Anti-Poisoning. Chem. Eur. J. 2020, 26, 3973–3990. [Google Scholar] [CrossRef]
- de Sá, M.H.; Moreira, C.S.; Pinto, A.M.F.R.; Oliveira, V.B. Recent Advances in the Development of Nanocatalysts for Direct Methanol Fuel Cells. Energies 2022, 15, 6335. [Google Scholar] [CrossRef]
- Kotp, A.A.; Abdelwahab, A.; Farghali, A.A.; El Rouby, W.M.A.; Allah, A.E. Evaluating the electrocatalytic activity of flower-like Co-MOF/CNT nanocomposites for methanol oxidation in basic electrolytes. RSC Adv. 2023, 13, 27934–27945. [Google Scholar] [CrossRef]
- Lou, W.H.; Ali, A.; Shen, P.K. Recent development of Au arched Pt nanomaterials as promising electrocatalysts for methanol oxidation reaction. Nano Res. 2022, 15, 18–37. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Kim, H.T.; Krewer, U.; Kweon, H.J. The effect of the anode loading and method of MEA fabrication on DMFC performance. Fuel Cells 2007, 7, 238–245. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Kim, H.T.; Kweon, H.J. Cathode structure optimization for air-breathing DMFC by application of pore-forming agents. J. Power Sources 2007, 171, 433–440. [Google Scholar] [CrossRef]
- Wang, G.X.; Sun, G.Q.; Wang, Q.; Wang, S.; Guo, J.S.; Gao, Y.; Xin, Q. Improving the DMFC performance with Ketien Black EC 300J as the additive in the cathode catalyst layer. J. Power Sources 2008, 180, 176–180. [Google Scholar] [CrossRef]
- Wang, G.X.; Sun, G.Q.; Wang, Q.; Wang, S.L.; Sun, H.; Xin, Q. Effect of carbon black additive in Pt black cathode catalyst layer on direct methanol fuel cell performance. Int. J. Hydrogen Energy 2010, 35, 11245–11253. [Google Scholar] [CrossRef]
- Roul, B.; Gorle, D.B.; Raj, G.; Kumar, K.; Kumari, M.; Nanda, K.K.; Krupanidhi, S.B. Solid-state synthesis of Pt/C cathode catalysts for direct methanol fuel cells. J. Mater. Chem. C 2023, 11, 11072–11081. [Google Scholar] [CrossRef]
- Singh, H.; Zhuang, S.Q.; Ingis, B.; Nunna, B.B.; Lee, E.S. Carbon-based catalysts for oxygen reduction reaction: A review on degradation mechanisms. Carbon 2019, 151, 160–174. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, G.; Singh, P.P.; Kaushal, S. Supported bimetallic nanoparticles as anode catalysts for direct methanol fuel cells: A review. Int. J. Hydrogen Energy 2021, 46, 15820–15849. [Google Scholar] [CrossRef]
- Yuan, W.J.; Hou, C.J.; Wu, J.F.; Zhang, Y.F.; Zhang, X.L. A direct methanol fuel cell with outstanding performance via capillary distillation. Chem. Eng. J. 2023, 470, 143663. [Google Scholar] [CrossRef]
- Alias, M.S.; Kamarudin, S.K.; Zainoodin, A.M.; Masdar, M.S. Active direct methanol fuel cell: An overview. Int. J. Hydrogen Energy 2020, 45, 19620–19641. [Google Scholar] [CrossRef]
- Moura, A.S.; Fajín, J.L.C.; Mandado, M.; Cordeiro, M.N.D.S. Ruthenium-Platinum Catalysts and Direct Methanol Fuel Cells (DMFC): A Review of Theoretical and Experimental Breakthroughs. Catalysts 2017, 7, 47. [Google Scholar] [CrossRef]
- Zhou, W.J.; Zhou, B.; Li, W.Z.; Zhou, Z.H.; Song, S.Q.; Sun, G.Q.; Xin, Q.; Douvartzides, S.; Goula, A.; Tsiakaras, P. Performance comparison of low-temperature direct alcohol fuel cells with different anode catalysts. J. Power Sources 2004, 126, 16–22. [Google Scholar] [CrossRef]
- Tsai, M.C.; Yeh, T.K.; Chen, C.Y.; Tsai, C.H. A catalytic gas diffusion layer for improving the efficiency of a direct methanol fuel cell. Electrochem. Commun. 2007, 9, 2299–2303. [Google Scholar] [CrossRef]
- Chang, Z.X.; Guan, L.; Zhang, J.J.; Zhang, W.Q.; Ma, Q.; Shah, A.; Xing, L.; Su, H.N.; Xu, Q. Construction of gradient catalyst layer anode by incorporating covalent organic framework to improve performance of direct methanol fuel cells. Int. J. Hydrogen Energy 2022, 47, 37013–37024. [Google Scholar] [CrossRef]
- Li, X.; Yao, K.X.; Zhao, F.L.; Yang, X.T.; Li, J.W.; Li, Y.F.; Yuan, Q. Interface-rich Au-doped PdBi alloy nanochains as multifunctional oxygen reduction catalysts boost the power density and durability of a direct methanol fuel cell device. Nano Res. 2022, 15, 6036–6044. [Google Scholar] [CrossRef]
- Wan, N.F. Durability study of direct methanol fuel cell under accelerated stress test. J. Power Sources 2023, 556, 232470. [Google Scholar] [CrossRef]
- Tang, H.L.; Wang, S.L.; Pan, M.; Jiang, S.P.; Ruan, Y.Z. Performance of direct methanol fuel cells prepared by hot-pressed MEA and catalyst-coated membrane (CCM). Electrochim. Acta 2007, 52, 3714–3718. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, T.S.; Yang, W.W. Modeling of water transport through the membrane electrode assembly for direct methanol fuel cells. J. Power Sources 2008, 178, 291–308. [Google Scholar] [CrossRef]
- Yang, W.W.; Zhao, T.S.; He, Y.L. Modelling of coupled electron and mass transport in anisotropic proton-exchange membrane fuel cell electrodes. J. Power Sources 2008, 185, 765–775. [Google Scholar] [CrossRef]
- Yang, W.W.; Zhao, T.S. A transient two-phase mass transport model for liquid feed direct methanol fuel cells. J. Power Sources 2008, 185, 1131–1140. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, J.M.; Zhang, Y.F.; Liu, J.F.; Chen, J.Y.; Li, M.X.; Wang, W.Q.; Liu, X.W. Overcoming undesired fuel crossover: Goals of methanol-resistant modification of polymer electrolyte membranes. Renew. Sustain. Energy Rev. 2021, 138, 110660. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, Z.; Zhang, W.; Wang, Y.X. Nanosized Mo-doped CeO enhances the electrocatalytic properties of the Pt anode catalyst in direct methanol fuel cells. J. Mater. Chem. A 2017, 5, 1481–1487. [Google Scholar] [CrossRef]
- Lu, G.B.; Ning, F.D.; Wei, J.; Li, Y.B.; Bai, C.; Shen, Y.B.; Li, Y.L.; Zhou, X.C. All-solid-state passive direct methanol fuel cells with great orientation stability and high energy density based on solid methanol fuels. J. Power Sources 2020, 450, 227669. [Google Scholar] [CrossRef]
- Wu, H.J.; Yuan, T.; Huang, Q.H.; Zhang, H.F.; Zou, Z.Q.; Zheng, J.W.; Yang, H. Polypyrrole nanowire networks as anodic micro-porous layer for passive direct methanol fuel cells. Electrochim. Acta 2014, 141, 1–5. [Google Scholar] [CrossRef]


| Parameters | Symbols | Value | Unit | Ref. | |
|---|---|---|---|---|---|
| Thickness Porosity Permeability | ADL | δADL, εADL, KADL | 2 × 10−4, 0.7, 1 × 10−12 | m, -, m2 | [28] |
| OACL | δOACL, εOACL, KOACL | 2 × 10−5, 0.3, 2 × 10−14 | m, -, m2 | [28] | |
| IACL | δIACL, εIACL, KIACL | 1 × 10−5, 0.1, 2 × 10−14 | m, -, m2 | [28] | |
| MEM | δMEM, -, KMEM | 1.8 × 10−4, -, 2 × 10−18 | m, -, m2 | [28] | |
| ICCL | δICCL, εICCL, KICCL | 0.1 × 10−4, 0.1, 2 × 10−14 | m, -, m2 | [28] | |
| OCCL | δOCCL, εOCCL, KOCCL | 0.2 × 10−4, 0.3, 2 × 10−14 | m, -, m2 | [28] | |
| CDL | δCDL, εCDL, KCDL | 2 × 10−4, 0.7, 1 × 10−12 | m, -, m2 | [28] | |
| Diffusivities | Dm | 1.58 × 10−9e0.02623(T−298) | m2 s−1 | [28] | |
| 1.78 × 10−5(T/273)1.823 | m2 s−1 | [28] | |||
| Dm,mem | 4.9 × 10−10e[2463(1/333−1/T)] | m2 s−1 | [28] | ||
| Operating temperature | T | 298 | K | - | |
| Anode inlet pressure | 1.01 × 105 | Pa | - | ||
| Cathode inlet pressure | 1.01 × 105 | Pa | - | ||
| Inlet methanol concentration | Cm,in | 2.0 | M | - | |
| Inlet oxygen concentration | 9.35 × 10−3 | M | - | ||
| Viscosity of gas phase | μg | 2.03 × 10−5 | kg m−1 s−1 | [28] | |
| Viscosity of liquid phase | μl | 4.05 × 10−4 | kg m−1 s−1 | [28] | |
| Electro-osmotic drag coefficients of water and methanol | 2.9e[1029(1/333−1/T)] | - | [29] | ||
| - | [28] | ||||
| Proton conductivity in membrane and catalyst layers | κm κc | 7.3e[1268(1/298−1/T)] 0.1416 | S m−1 S m−1 | [28] [30] | |
| Thermodynamic potential | E0 | 1.21 | V | [28] | |
| Transfer coefficient of anode and cathode | αa αc | 0.5 1.0 | - - | [28] [28] | |
| Anode exchange current density in OACL and IACL | 1.0 × 105 1.5 × 105 | A m−3 A m−3 | [28] - | ||
| Cathode exchange current density in ICCL and OCCL | 6.97 × 102 1.05 × 103 | A m−3 A m−3 | [28] - | ||
| Anode reference concentration | 0.1 | M | [28] | ||
| cathode reference concentration | 3.65 × 10−2 | M | [28] | ||
| Surface tension | σ | 0.0644 | N m−1 | [28] | |
| Contact resistance | Rcontact | 8 × 10−5 | Ω m2 | [28] | |
| Ref. | Catalyst Layer Design | Temperature (°C) | Power Density (mW cm−2) | Total Noble Metal Loadings (mg cm−2) |
|---|---|---|---|---|
| This work | Double-catalytic layer | 25 | 25.1 | 2.4 |
| Non-supported Pt | 19.6 | |||
| Pt/C | 18.8 | |||
| [16] | Pt/C | 60 | 82.28 | 3.2 |
| [19] | Pt/C | 25 | 22.3 | 2.4 |
| [32] | Pt-CeMoO/C | 60 | 69.4 | 3.2 |
| [33] | Pt/C | 25 | 17.2 | 3 |
| [34] | Pt/Polypyrrole nanowire | 25 | 34.3 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Jiang, Y. Development of Membrane Electrode Assembly with Double-Catalytic Layer for Micro Direct Methanol Fuel Cell. Inventions 2024, 9, 19. https://doi.org/10.3390/inventions9010019
Zhang S, Jiang Y. Development of Membrane Electrode Assembly with Double-Catalytic Layer for Micro Direct Methanol Fuel Cell. Inventions. 2024; 9(1):19. https://doi.org/10.3390/inventions9010019
Chicago/Turabian StyleZhang, Shubin, and Yanfeng Jiang. 2024. "Development of Membrane Electrode Assembly with Double-Catalytic Layer for Micro Direct Methanol Fuel Cell" Inventions 9, no. 1: 19. https://doi.org/10.3390/inventions9010019
APA StyleZhang, S., & Jiang, Y. (2024). Development of Membrane Electrode Assembly with Double-Catalytic Layer for Micro Direct Methanol Fuel Cell. Inventions, 9(1), 19. https://doi.org/10.3390/inventions9010019







