Optimization of Extraction Parameters of Anthocyanin Compounds and Antioxidant Properties from Red Grape (Băbească neagră) Peels
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Red Grape Skins Preparation
2.3. Extraction of Biologically Active Compounds
2.4. Determination of the Total Anthocyanins Content (TAC)
2.5. Total Phenolic Compounds Determination
2.6. Antioxidant Activity—DPPH Assay (AOA)
2.7. Experimental Design
2.8. Statistical Analysis
3. Results and Discussion
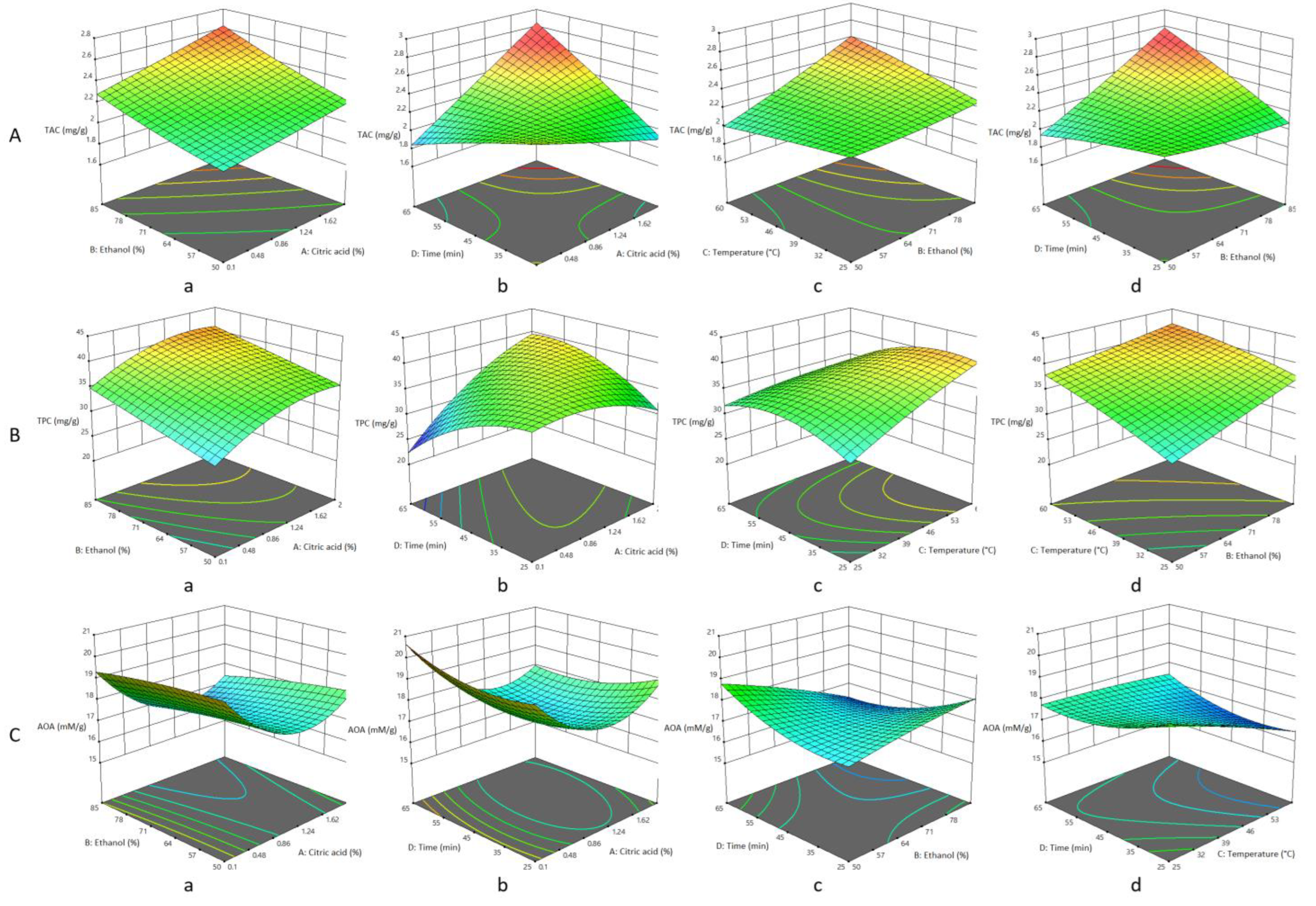
3.1. Effect of the Extraction Parameters on TAC
3.2. Effect of the Extraction Parameters on TPC
3.3. Effect of Extraction Parameters on AOA
3.4. Extraction Parameter Optimization and Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Djilas, S.M.; Canadanović-Brunet, J.; Ćetković, G. By-Products of Fruits Processing as a Source of Phytochemicals. Chem. Ind. Chem. Eng. Q. 2009, 15, 191–202. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Balbinoti, T.C.V.; Stafussa, A.P.; Haminiuk, C.W.I.; Maciel, G.M.; Sassaki, G.L.; de Matos Jorge, L.M.; Jorge, R.M.M. Addition of Grape Pomace in the Hydration Step of Parboiling Increases the Antioxidant Properties of Rice. Int. J. Food Sci. Technol. 2020, 55, 2370–2380. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Cifuentes-Gomez, T.; Escudero-Gilete, M.L.; Heredia, F.J.; Spencer, J.P.E. Assessment of White Grape Pomace from Winemaking as Source of Bioactive Compounds, and Its Antiproliferative Activity. Food Chem. 2015, 183, 78–82. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis Vinifera (Grape) and Its Bioactive Constituents: An Update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape Bioactive Molecules, and the Potential Health Benefits in Reducing the Risk of Heart Diseases. Food Chem. X 2021, 12, 100149. [Google Scholar] [CrossRef]
- Rebello, L.P.G.; Lago-Vanzela, E.S.; Barcia, M.T.; Ramos, A.M.; Stringheta, P.C.; Da-Silva, R.; Castillo-Muñoz, N.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Phenolic Composition of the Berry Parts of Hybrid Grape Cultivar BRS Violeta (BRS Rubea×IAC 1398-21) Using HPLC–DAD–ESI-MS/MS. Food Res. Int. 2013, 54, 354–366. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Zhang, M.; Sun, B. Novel Approach for Extraction of Grape Skin Antioxidants by Accelerated Solvent Extraction: Box–Behnken Design Optimization. J. Food Sci. Technol. 2019, 56, 4879–4890. [Google Scholar] [CrossRef]
- Brazinha, C.; Cadima, M.; Crespo, J.G. Optimization of Extraction of Bioactive Compounds from Different Types of Grape Pomace Produced at Wineries and Distilleries. J. Food Sci. 2014, 79, E1142–E1149. [Google Scholar] [CrossRef]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic Compounds Recovery from Grape Skin Using Conventional and Non-Conventional Extraction Methods. Ind. Crops Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; de Souza Schmidt Gonçalves, A.E.; Genovese, M.I.; Fett, R. Phenolic Compounds and Antioxidant Activity of Seed and Skin Extracts of Red Grape (Vitis Vinifera and Vitis Labrusca) Pomace from Brazilian Winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Radha; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; et al. Onion (Allium cepa L.) Peels: A Review on Bioactive Compounds and Biomedical Activities. Biomed. Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated Anthocyanins from Edible Sources and Their Applications in Food Systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Walker, A.R.; Barril, C. Grape Berry Flavonoids: A Review of Their Biochemical Responses to High and Extreme High Temperatures. J. Exp. Bot. 2019, 70, 397–423. [Google Scholar] [CrossRef]
- Putta, S.; Yarla, N.S.; Peluso, I.; Tiwari, D.K.; Reddy, G.V.; Giri, P.V.; Kumar, N.; Malla, R.; Rachel, V.; Bramhachari, P.V.; et al. Anthocyanins: Multi-Target Agents for Prevention and Therapy of Chronic Diseases. Curr. Pharm. Des. 2017, 23, 6321–6346. [Google Scholar] [CrossRef]
- Ren, K.-W.; Li, Y.-H.; Wu, G.; Ren, J.-Z.; Lu, H.-B.; Li, Z.-M.; Han, X.-W. Quercetin Nanoparticles Display Antitumor Activity via Proliferation Inhibition and Apoptosis Induction in Liver Cancer Cells. Int. J. Oncol. 2017, 50, 1299–1311. [Google Scholar] [CrossRef]
- Rasines-Perea, Z.; Teissedre, P.-L. Grape Polyphenols’ Effects in Human Cardiovascular Diseases and Diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef]
- Costa, E.; Cosme, F.; Jordão, A.M.; Mendes-Faia, A. Anthocyanin Profile and Antioxidant Activity from 24 Grape Varieties Cultivated in Two Portuguese Wine Regions. OENO One 2014, 48, 51–62. [Google Scholar] [CrossRef]
- Samota, M.K.; Sharma, M.; Kaur, K.; Sarita; Yadav, D.K.; Pandey, A.K.; Tak, Y.; Rawat, M.; Thakur, J.; Rani, H. Onion Anthocyanins: Extraction, Stability, Bioavailability, Dietary Effect, and Health Implications. Front. Nutr. 2022, 9, 917617. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Sun, B.; de Sá, M.; Leandro, C.; Caldeira, I.; Duarte, F.L.; Spranger, I. Reactivity of Polymeric Proanthocyanidins toward Salivary Proteins and Their Contribution to Young Red Wine Astringency. J. Agric. Food Chem. 2013, 61, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Kyraleou, M.; Kallithraka, S.; Theodorou, N.; Teissedre, P.-L.; Kotseridis, Y.; Koundouras, S. Changes in Tannin Composition of Syrah Grape Skins and Seeds during Fruit Ripening under Contrasting Water Conditions. Molecules 2017, 22, 1453. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized Liquid Extraction of Bioactive Compounds from Blackberry (Rubus Fruticosus L.) Residues: A Comparison with Conventional Methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Martiny, T.R.; Raghavan, V.; de Moraes, C.C.; da Rosa, G.S.; Dotto, G.L. Optimization of Green Extraction for the Recovery of Bioactive Compounds from Brazilian Olive Crops and Evaluation of Its Potential as a Natural Preservative. J. Environ. Chem. Eng. 2021, 9, 105130. [Google Scholar] [CrossRef]
- da Silva Meirelles, L.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms Involved in the Therapeutic Properties of Mesenchymal Stem Cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef]
- Mei, Z.; Zhang, R.; Zhao, Z.; Zheng, G.; Xu, X.; Yang, D. Extraction Process and Method Validation for Bioactive Compounds from Citrus Reticulata Cv. Chachiensis: Application of Response Surface Methodology and HPLC–DAD. Acta Chromatogr. 2020, 33, 270–280. [Google Scholar] [CrossRef]
- Weremfo, A.; Abassah-Oppong, S.; Adulley, F.; Dabie, K.; Seidu-Larry, S. Response Surface Methodology as a Tool to Optimize the Extraction of Bioactive Compounds from Plant Sources. J. Sci. Food Agric. 2022, 103, 26–36. [Google Scholar] [CrossRef]
- Turturică, M.; Stănciuc, N.; Bahrim, G.; Râpeanu, G. Effect of Thermal Treatment on Phenolic Compounds from Plum (Prunus Domestica) Extracts—A Kinetic Study. J. Food Eng. 2016, 171, 200–207. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Castro-Vargas, H.I.; Rodríguez-Varela, L.I.; Ferreira, S.R.S.; Parada-Alfonso, F. Extraction of Phenolic Fraction from Guava Seeds (Psidium guajava L.) Using Supercritical Carbon Dioxide and Co-Solvents. J. Supercrit. Fluids 2010, 51, 319–324. [Google Scholar] [CrossRef]
- Anderson, M.J.; Whitcomb, P.J. RSM Simplified: Optimizing Processes Using Response Surface Methods for Design of Experiments, 2nd ed.; Productivity Press: London, UK, 2016. [Google Scholar]
- Khazaei, K.M.; Jafari, S.M.; Ghorbani, M.; Kakhki, A.H.; Sarfarazi, M. Optimization of Anthocyanin Extraction from Saffron Petals with Response Surface Methodology. Food Anal. Methods 2016, 9, 1993–2001. [Google Scholar] [CrossRef]
- de Andrade, R.B.; Machado, B.A.S.; de Abreu Barreto, G.; Nascimento, R.Q.; Corrêa, L.C.; Leal, I.L.; Tavares, P.P.L.G.; Ferreira, E.d.S.; Umsza-Guez, M.A. Syrah Grape Skin Residues Has Potential as Source of Antioxidant and Anti-Microbial Bioactive Compounds. Biology 2021, 10, 1262. [Google Scholar] [CrossRef]
- Yammine, S. Extraction of High-Value Added Compounds by Subcritical Water and Fractionation by Membrane Processes: Valorization of Vine and Wine by-Products by Eco-Innovative Processes. Ph.D. Thesis, Université de Bordeaux, Bordeaux, France, 2016. [Google Scholar]
- Arozarena, I.; Casp, A.; Marín, R.; Navarro, M. Multivariate Differentiation of Spanish Red Wines According to Region and Variety. J. Sci. Food Agric. 2000, 80, 1909–1917. [Google Scholar] [CrossRef]
- Katalinić, V.; Možina, S.S.; Skroza, D.; Generalić, I.; Abramovič, H.; Miloš, M.; Ljubenkov, I.; Piskernik, S.; Pezo, I.; Terpinc, P.; et al. Polyphenolic Profile, Antioxidant Properties and Antimicrobial Activity of Grape Skin Extracts of 14 Vitis Vinifera Varieties Grown in Dalmatia (Croatia). Food Chem. 2010, 119, 715–723. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic Compounds and Antioxidant Activity from Red Grape Marc Extracts. Bioresour. Technol. 2003, 87, 41–44. [Google Scholar] [CrossRef]
- Poudel, P.R.; Tamura, H.; Kataoka, I.; Mochioka, R. Phenolic Compounds and Antioxidant Activities of Skins and Seeds of Five Wild Grapes and Two Hybrids Native to Japan. J. Food Compos. Anal. 2008, 21, 622–625. [Google Scholar] [CrossRef]
- Tournour, H.H.; Segundo, M.A.; Magalhães, L.M.; Barreiros, L.; Queiroz, J.; Cunha, L.M. Valorization of Grape Pomace: Extraction of Bioactive Phenolics with Antioxidant Properties. Ind. Crops Prod. 2015, 74, 397–406. [Google Scholar] [CrossRef]
- Constantin, O.; Skrt, M.; Ulrih, N.; Rapeanu, G. Anthocyanins Profile, Total Phenolics and Antioxidant Activity of Two Romanian Red Grape Varieties: Feteasca Neagra and Babeasca Neagra (Vitis Vinifera). Chem. Pap. 2015, 69, 1573–1581. [Google Scholar] [CrossRef]
- Kupe, M.; Karatas, N.; Unal, M.S.; Ercisli, S.; Baron, M.; Sochor, J. Phenolic Composition and Antioxidant Activity of Peel, Pulp and Seed Extracts of Different Clones of the Turkish Grape Cultivar ‘Karaerik. ’ Plants 2021, 10, 2154. [Google Scholar] [CrossRef] [PubMed]
- De, B.; Bhandari, K.; Katakam, P.; Goswami, T.K. Development of a Standardized Combined Plant Extract Containing Nutraceutical Formulation Ameliorating Metabolic Syndrome Components. SN Appl. Sci. 2019, 1, 1484. [Google Scholar] [CrossRef]


| Code | Independent Variables | Units | Minimum | Maximum | Coded Low | Coded High |
|---|---|---|---|---|---|---|
| A | Citric acid | % | 0.0100 | 2.64 | −1 = 0.10 | +1 = 2.00 |
| B | Ethanol | % | 38.06 | 96.93 | −1 = 50.00 | +1 = 85.00 |
| C | Temperature | °C | 13.06 | 71.90 | −1 = 25.00 | +1 = 60.00 |
| D | Time | min | 11.36 | 78.63 | −1 = 25.00 | +1 = 65.00 |
| Run | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Response 1 (R1) | Response 2 (R2) | Response 3 (R3) |
|---|---|---|---|---|---|---|---|
| A: Citric Acid % | B: Ethanol % | C: Temperature °C | D: Time min | TAC mg/g | TPC mg/g | AOA mM/g | |
| 1 | 0.1 | 85 | 25 | 65 | 2.09 ± 0.08 | 29.01 ± 1.70 | 19.76 ± 0.25 |
| 2 | 1 | 67 | 13.06 | 45 | 2.15 ± 0.11 | 29.97 ± 1.55 | 18.24 ± 0.13 |
| 3 | 1 | 67 | 71.9 | 45 | 2.29 ± 0.02 | 40.97 ± 1.70 | 15.95 ± 0.39 |
| 4 | 1 | 67 | 42 | 45 | 2.24 ± 0.02 | 36.94 ± 1.67 | 17.26 ± 0.14 |
| 5 | 1 | 67 | 42 | 45 | 2.25 ± 0.18 | 37.04 ± 1.49 | 17.25 ± 0.16 |
| 6 | 0.1 | 50 | 25 | 25 | 2.45 ± 0.03 | 29.93 ± 0.38 | 19.65 ± 0.66 |
| 7 | 2 | 50 | 25 | 65 | 2.64 ± 0.18 | 30.12 ± 1.71 | 19.05 ± 0.89 |
| 8 | 0.1 | 85 | 60 | 65 | 2.4 ± 0.39 | 31.09 ± 0.56 | 18.91 ± 0.50 |
| 9 | 2 | 50 | 60 | 65 | 2.17 ± 0.22 | 31.92 ± 0.89 | 19.31 ± 0.24 |
| 10 | 0.01 | 67 | 42 | 45 | 2.1 ± 0.09 | 30.86 ± 1.23 | 20.16 ± 0.64 |
| 11 | 0.1 | 50 | 60 | 25 | 2.53 ± 0.29 | 43.97 ± 0.94 | 18.38 ± 0.20 |
| 12 | 1 | 67 | 42 | 11.36 | 2.05 ± 0.10 | 31.41 ± 1.39 | 18.77 ± 0.29 |
| 13 | 2.64 | 67 | 42 | 45 | 2.49 ± 0.15 | 35.88 ± 0.98 | 20.49 ± 0.55 |
| 14 | 2 | 85 | 25 | 25 | 1.71 ± 0.08 | 24.67 ± 1.11 | 20.98 ± 0.40 |
| 15 | 1 | 67 | 42 | 78.63 | 2.32 ± 0.11 | 26.45 ± 1.92 | 18.72 ± 0.18 |
| 16 | 2 | 85 | 60 | 25 | 2.07 ± 0.07 | 31.1 ± 0.56 | 17.47 ± 0.50 |
| 17 | 1 | 67 | 42 | 45 | 2.24 ± 0.02 | 37.12 ± 1.94 | 17.26 ± 0.27 |
| 18 | 1 | 67 | 42 | 45 | 2.25 ± 0.00 | 37.01 ± 1.15 | 17.26 ± 0.13 |
| 19 | 1 | 96.93 | 42 | 45 | 2.59 ± 0.10 | 42.07 ± 1.66 | 16.35 ± 1.25 |
| 20 | 1 | 38.06 | 42 | 45 | 2.03 ± 0.16 | 32.25 ± 1.44 | 17.61 ± 0.80 |
| 21 | 1 | 67 | 42 | 45 | 2.25 ± 0.11 | 37.14 ± 1.96 | 17.35 ± 0.21 |
| TAC | TPC | AOA | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | SS | df | MS | F-Value | p-Value | SS | df | MSquare | F-Value | p-Value | SS | df | MS | F-Value | p-Value |
| Model a | 0.9492 | 14 | 0.0678 | 1003.34 | <0.0001 | 518.33 | 13 | 39.87 | 2495.65 | <0.0001 | 37.31 | 14 | 2.67 | 1241.30 | <0.0001 |
| A-Citric acid | 0.0547 | 1 | 0.0547 | 809.95 | <0.0001 | 29.58 | 1 | 29.58 | 1851.33 | <0.0001 | 2.06 | 1 | 2.06 | 960.80 | <0.0001 |
| B-Ethanol | 0.1661 | 1 | 0.1661 | 2457.72 | <0.0001 | 46.57 | 1 | 46.57 | 2915.05 | <0.0001 | 0.8648 | 1 | 0.8648 | 402.78 | <0.0001 |
| C-Temperature | 0.0197 | 1 | 0.0197 | 290.88 | <0.0001 | 131.49 | 1 | 131.49 | 8230.00 | <0.0001 | 6.37 | 1 | 6.37 | 2967.68 | <0.0001 |
| D-Time | 0.0606 | 1 | 0.0606 | 896.65 | <0.0001 | 6.78 | 1 | 6.78 | 424.19 | <0.0001 | 0.0137 | 1 | 0.0137 | 6.40 | 0.0447 |
| AB | 0.0044 | 1 | 0.0044 | 65.51 | 0.0002 | 0.0853 | 1 | 0.0853 | 5.34 | 0.0541 | 0.0459 | 1 | 0.0459 | 21.39 | 0.0036 |
| AC | 0.0314 | 1 | 0.0314 | 465.24 | <0.0001 | 7.84 | 1 | 7.84 | 490.84 | <0.0001 | 0.1584 | 1 | 0.1584 | 73.76 | 0.0001 |
| AD | 0.4312 | 1 | 0.4312 | 6380.64 | <0.0001 | 95.57 | 1 | 95.57 | 5982.19 | <0.0001 | 0.7707 | 1 | 0.7707 | 358.95 | <0.0001 |
| BC | 0.1403 | 1 | 0.1403 | 2076.49 | <0.0001 | 6.74 | 1 | 6.74 | 422.14 | <0.0001 | 1.40 | 1 | 1.40 | 652.50 | <0.0001 |
| BD | 0.1302 | 1 | 0.1302 | 1926.32 | <0.0001 | 57.93 | 1 | 57.93 | 3626.06 | <0.0001 | 1.56 | 1 | 1.56 | 728.21 | <0.0001 |
| CD | 0.0450 | 1 | 0.0450 | 665.91 | <0.0001 | 34.40 | 1 | 34.40 | 2153.40 | <0.0001 | 2.19 | 1 | 2.19 | 1022.08 | <0.0001 |
| A² | 0.0001 | 1 | 0.0001 | 1.57 | 0.2568 | 44.28 | 1 | 44.28 | 2771.33 | <0.0001 | 20.60 | 1 | 20.60 | 9596.00 | <0.0001 |
| B² | 0.0076 | 1 | 0.0076 | 112.10 | <0.0001 | - | - | - | - | - | 0.1771 | 1 | 0.1771 | 82.50 | <0.0001 |
| C² | 0.0010 | 1 | 0.0010 | 14.16 | 0.0094 | 5.49 | 1 | 5.49 | 343.61 | <0.0001 | 0.0642 | 1 | 0.0642 | 29.90 | 0.0016 |
| D² | 0.0060 | 1 | 0.0060 | 88.49 | <0.0001 | 125.06 | 1 | 125.06 | 7827.78 | <0.0001 | 3.91 | 1 | 3.91 | 1820.54 | <0.0001 |
| Residual | 0.0004 | 6 | 0.0001 | 0.1118 | 7 | 0.0160 | 0.0129 | 6 | 0.0021 | ||||||
| Lack of Fit b | 0.0003 | 2 | 0.0001 | 4.76 | 0.0876 | 0.085 | 3 | 0.0283 | 4.23 | 0.0986 | 0.0060 | 2 | 0.0030 | 1.72 | 0.2885 |
| Pure Error | 0.0001 | 4 | 0.000 | 0.0268 | 4 | 0.0067 | 0.0069 | 4 | 0.0017 | ||||||
| Cor Total | 0.9496 | 20 | 518.44 | 20 | 37.33 | 20 | |||||||||
| Dependent Variable | Predicted Value | 95% Confidence Intervals | Experimental Value |
|---|---|---|---|
| TAC (mg C3G/g DW) | 2.25 | 2.23–2.27 | 2.26 |
| TPC (mg GAE/g DW) | 37.41 | 37.09–37.73 | 37.22 |
| AOA (mM TE/g DW) | 17.20 | 17.08–17.32 | 17.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serea, D.; Constantin, O.E.; Horincar, G.; Stănciuc, N.; Aprodu, I.; Bahrim, G.E.; Râpeanu, G. Optimization of Extraction Parameters of Anthocyanin Compounds and Antioxidant Properties from Red Grape (Băbească neagră) Peels. Inventions 2023, 8, 59. https://doi.org/10.3390/inventions8020059
Serea D, Constantin OE, Horincar G, Stănciuc N, Aprodu I, Bahrim GE, Râpeanu G. Optimization of Extraction Parameters of Anthocyanin Compounds and Antioxidant Properties from Red Grape (Băbească neagră) Peels. Inventions. 2023; 8(2):59. https://doi.org/10.3390/inventions8020059
Chicago/Turabian StyleSerea, Daniela, Oana Emilia Constantin, Georgiana Horincar, Nicoleta Stănciuc, Iuliana Aprodu, Gabriela Elena Bahrim, and Gabriela Râpeanu. 2023. "Optimization of Extraction Parameters of Anthocyanin Compounds and Antioxidant Properties from Red Grape (Băbească neagră) Peels" Inventions 8, no. 2: 59. https://doi.org/10.3390/inventions8020059
APA StyleSerea, D., Constantin, O. E., Horincar, G., Stănciuc, N., Aprodu, I., Bahrim, G. E., & Râpeanu, G. (2023). Optimization of Extraction Parameters of Anthocyanin Compounds and Antioxidant Properties from Red Grape (Băbească neagră) Peels. Inventions, 8(2), 59. https://doi.org/10.3390/inventions8020059








