Siphon-Induced Droplet Break-Off for Enhanced Mixing on a Centrifugal Platform
Abstract
:1. Introduction
2. Materials and Methods
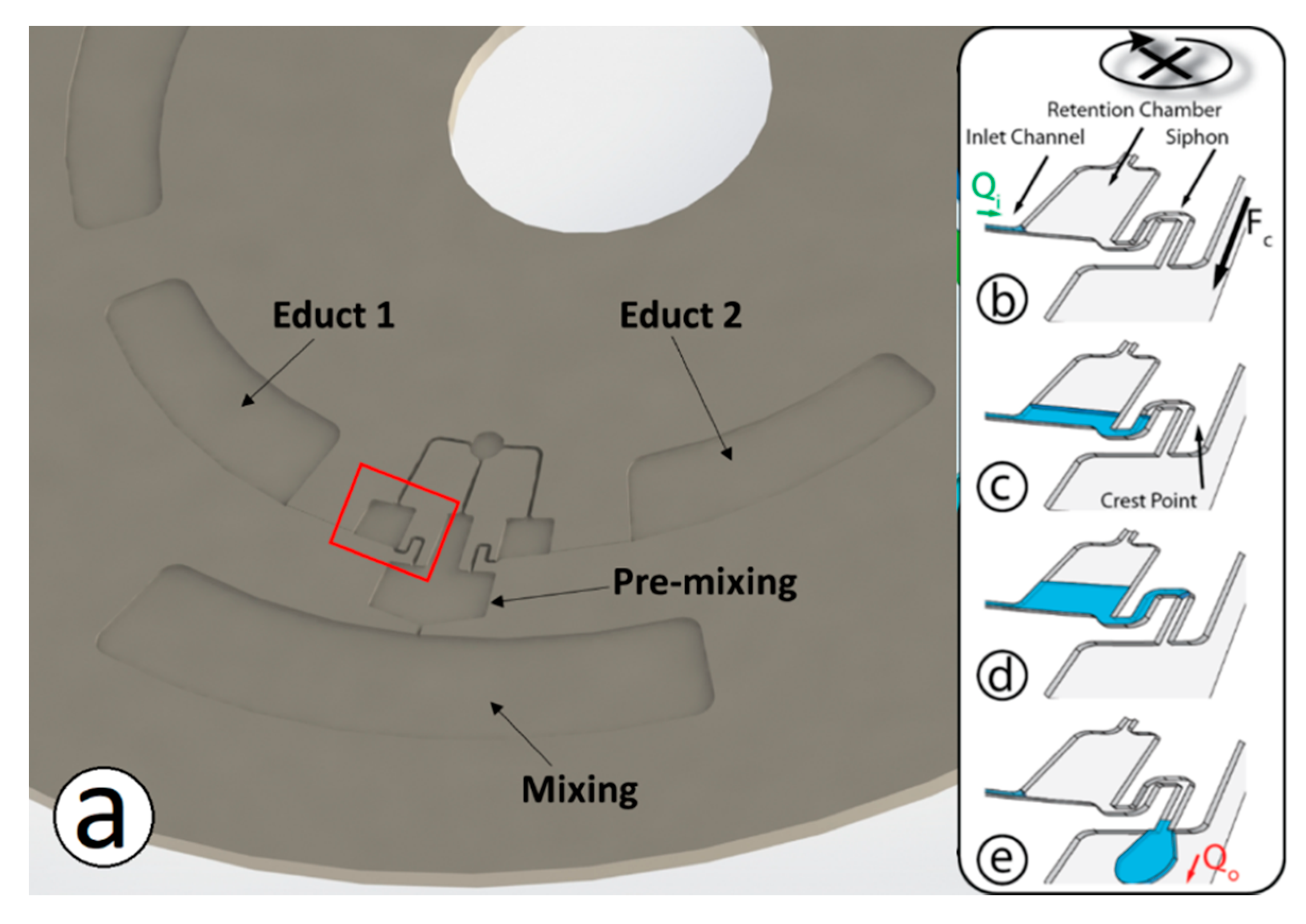
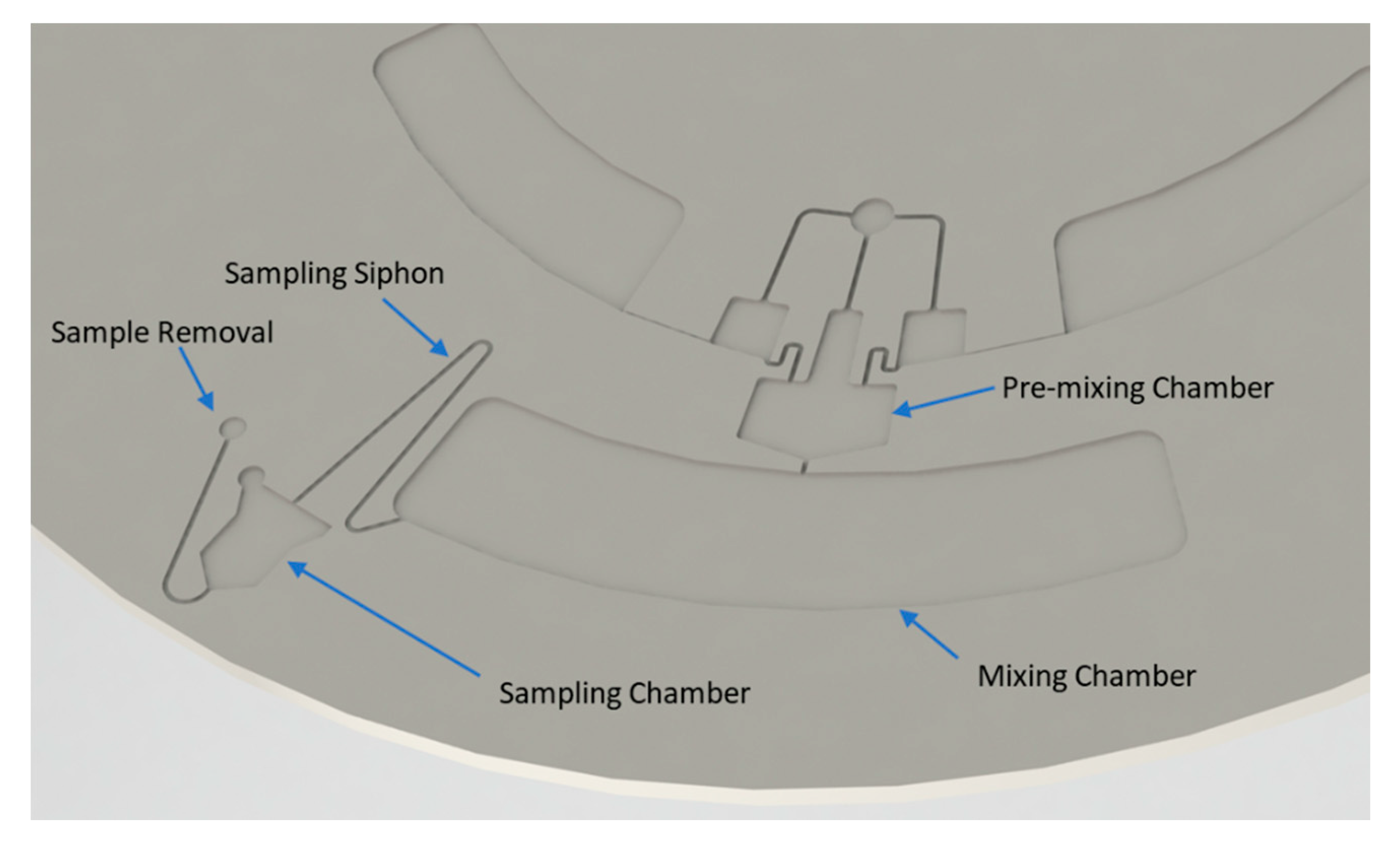
2.1. Method of Operation
- Discretization of continuous flow by siphon mechanism into droplets
- Merging of droplets in the intermediate chamber as enabled by the microfluidic shift register
- Mixing of regents upstream and during transfer through the capillary valve
- Mixing enhanced by droplet impact on liquid interface in the outer chamber
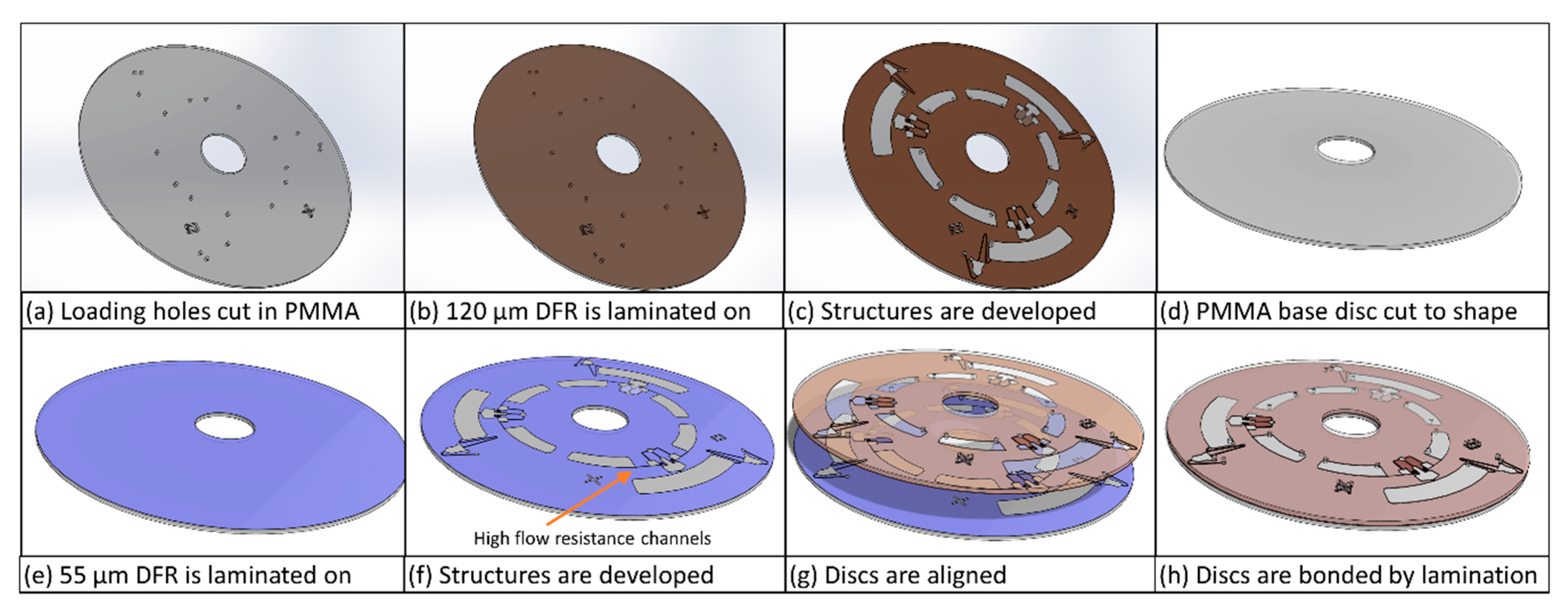
2.2. Materials and Fabrication
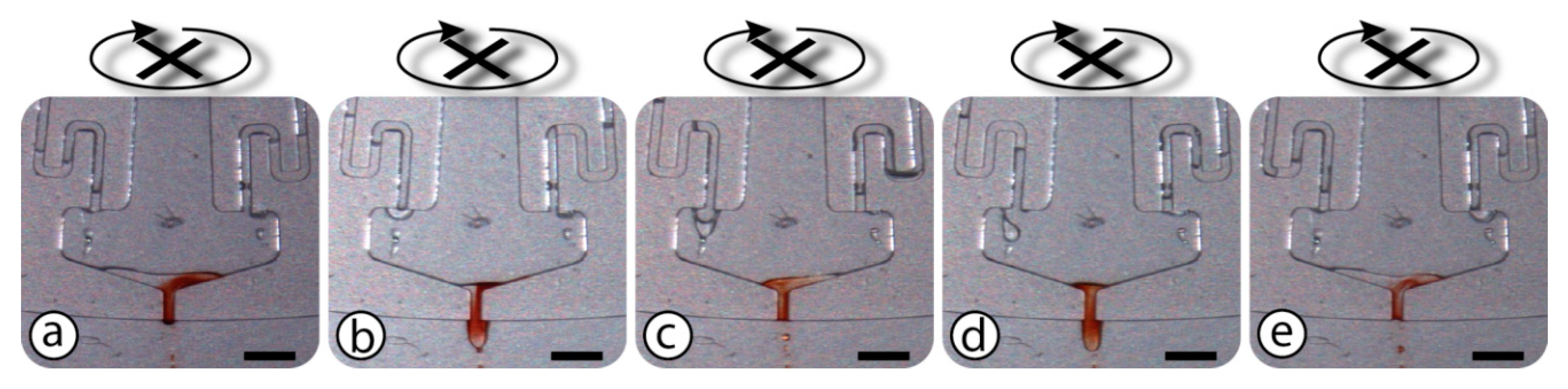
2.3. Measurement and Testing
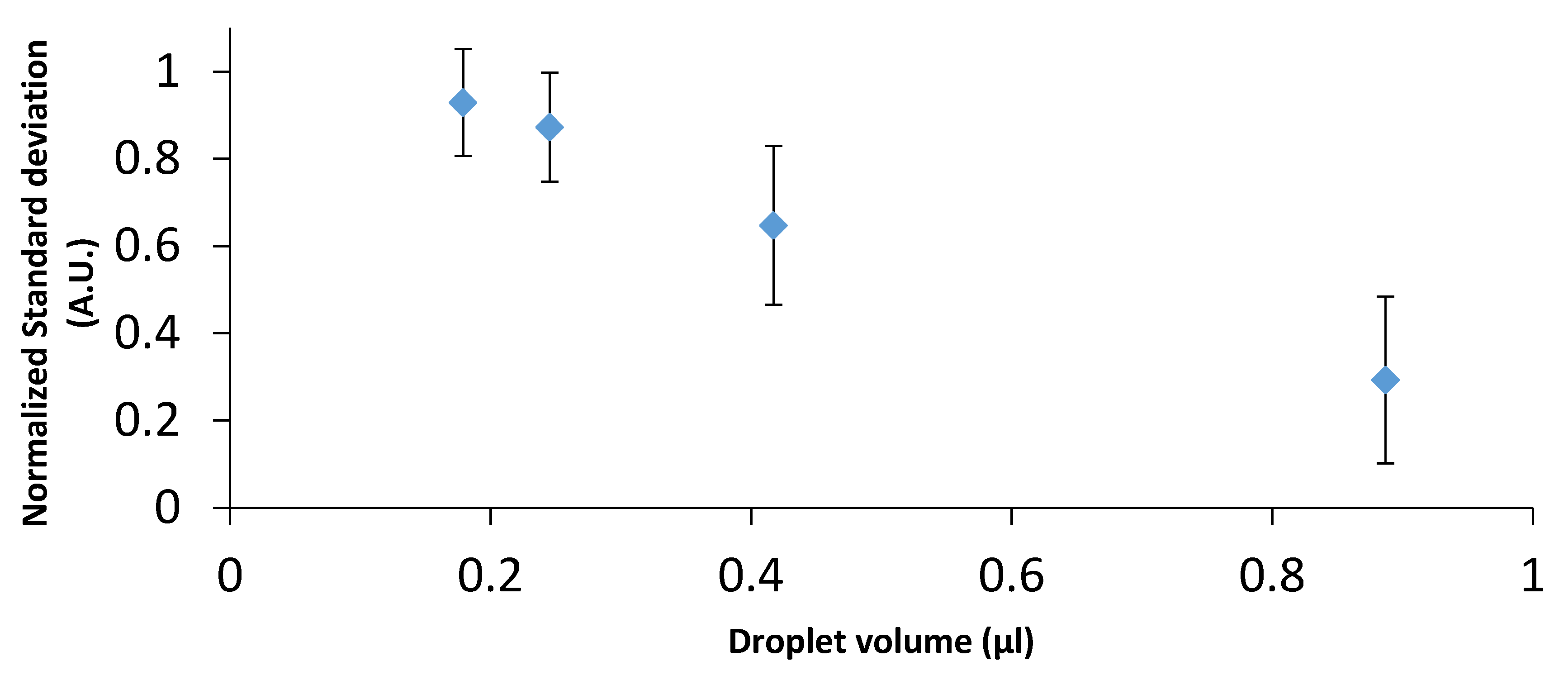
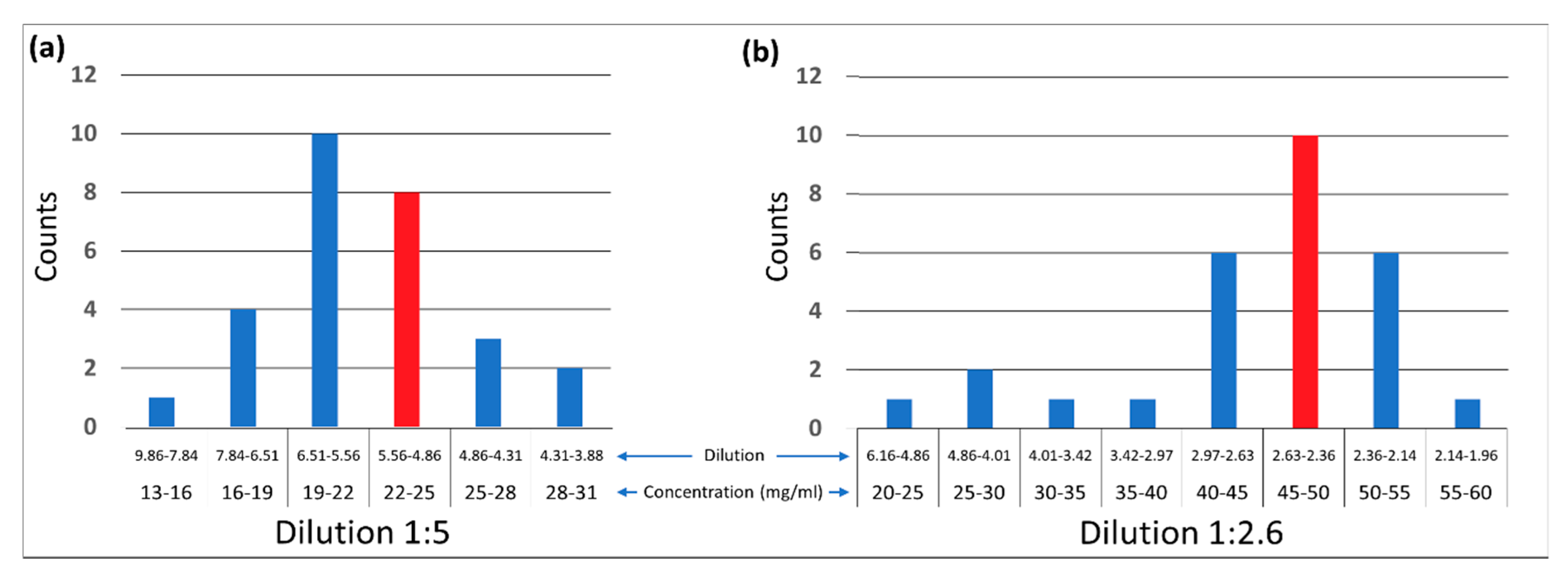
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dittrich, P.S.; Tachikawa, K.; Manz, A. Micro total analysis systems. Latest advancements and trends. Anal. Chem. 2006, 78, 3887–3908. [Google Scholar] [CrossRef] [PubMed]
- Craighead, H. Future lab-on-a-chip technologies for interrogating individual molecules. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 330–336. [Google Scholar]
- Demello, A.J. Control and detection of chemical reactions in microfluidic systems. Nature 2006, 442, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Haeberle, S.; Roth, G.; Von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. In Microfluidics Based Microsystems; Springer: Berlin/Heidelberg, Germany, 2010; pp. 305–376. [Google Scholar]
- Darwish, N.T.; Sekaran, S.D.; Khor, S.M. Point-of-care tests: A review of advances in the emerging diagnostic tools for dengue virus infection. Sens. Actuators B Chem. 2018, 255, 3316–3331. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Circulating tumor cells: Liquid biopsy of cancer. Clin. Chem. 2013, 59, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Avella-Oliver, M.; Puchades, R.; Wachsmann-Hogiu, S.; Maquieira, A. Label-free SERS analysis of proteins and exosomes with large-scale substrates from recordable compact disks. Sens. Actuators B Chem. 2017, 252, 657–662. [Google Scholar] [CrossRef]
- Burger, R.; Amato, L.; Boisen, A. Detection methods for centrifugal microfluidic platforms. Biosens. Bioelectron. 2016, 76, 54–67. [Google Scholar] [CrossRef]
- Barathur, R.; Bookout, J.; Sreevatsan, S.; Gordon, J.; Werner, M.; Thor, G.; Worthington, M. New disc-based technologies for diagnostic and research applications. Psychiatr. Genet. 2002, 12, 193–206. [Google Scholar] [CrossRef]
- Lange, S.A.; Roth, G.; Wittemann, S.; Lacoste, T.; Vetter, A.; Grässle, J.; Kopta, S.; Kolleck, M.; Breitinger, B.; Wick, M. Measuring biomolecular binding events with a compact disc player device. Angew. Chem. Int. Ed. 2006, 45, 270–273. [Google Scholar] [CrossRef]
- Arai, T.; Gopinath, S.C.B.; Mizuno, H.; Kumar, P.K.R.; Rockstuhl, C.; Awazu, K.; Tominaga, J. Toward biological diagnosis system based on digital versatile disc technology. Jpn. J. Appl. Phys. 2007, 46, 4003. [Google Scholar] [CrossRef]
- Morais, S.; Carrascosa, J.; Mira, D.; Puchades, R.; Maquieira, A. Microimmunoanalysis on standard compact discs to determine low abundant compounds. Anal. Chem. 2007, 79, 7628–7635. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, M.; Nolte, D.D. Combined fluorescent and interferometric detection of protein on a BioCD. Appl. Opt. 2008, 47, 2779–2789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schembri, C.T.; Burd, T.L.; Kopf-Sill, A.R.; Shea, L.R.; Braynin, B. Centrifugation and capillarity integrated into a multiple analyte whole blood analyser. J. Anal. Methods Chem. 1995, 17, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Madou, M.; Zoval, J.; Jia, G.; Kido, H.; Kim, J.; Kim, N. Lab on a CD. Annu. Rev. Biomed. Eng. 2006, 8, 601–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducrée, J.; Haeberle, S.; Lutz, S.; Pausch, S.; Von Stetten, F.; Zengerle, R. The centrifugal microfluidic Bio-Disk platform. J. Micromech. Microeng. 2007, 17, S103. [Google Scholar] [CrossRef]
- Gorkin, R.; Park, J.; Siegrist, J.; Amasia, M.; Lee, B.S.; Park, J.-M.; Kim, J.; Kim, H.; Madou, M.; Cho, Y.-K. Centrifugal microfluidics for biomedical applications. Lab Chip 2010, 10, 1758–1773. [Google Scholar] [CrossRef] [Green Version]
- Strohmeier, O.; Keller, M.; Schwemmer, F.; Zehnle, S.; Mark, D.; von Stetten, F.; Zengerle, R.; Paust, N. Centrifugal microfluidic platforms: Advanced unit operations and applications. Chem. Soc. Rev. 2015, 44, 6187–6229. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.; Mager, D.; Perebikovsky, A.; Shamloo, E.; Kinahan, D.; Mishra, R.; Torres Delgado, S.; Kido, H.; Saha, S.; Ducrée, J.; et al. CD-based microfluidics for primary care in extreme point-of-care settings. Micromachines 2016, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Kinahan, D.J.; Kearney, S.M.; Glynn, M.T.; Ducrée, J. Spira mirabilis enhanced whole blood processing in a lab-on-a-disk. Sens. Actuators A Phys. 2014, 215, 71–76. [Google Scholar] [CrossRef]
- Haeberle, S.; Brenner, T.; Zengerle, R.; Ducrée, J. Centrifugal extraction of plasma from whole blood on a rotating disk. Lab Chip 2006, 6, 776–781. [Google Scholar] [CrossRef]
- Kinahan, D.J.; Kearney, S.M.; Kilcawley, N.A.; Early, P.L.; Glynn, M.T.; Ducrée, J. Density-gradient mediated band extraction of leukocytes from whole blood using centrifugo-pneumatic siphon valving on centrifugal microfluidic discs. PLoS ONE 2016, 11, e0155545. [Google Scholar] [CrossRef] [PubMed]
- Burtis, C.A.; Mailen, J.C.; Johnson, W.F.; Scott, C.D.; Tiffany, T.O.; Anderson, N.G. Development of a miniature fast analyzer. Clin. Chem. 1972, 18, 753–761. [Google Scholar] [PubMed]
- Lai, S.; Wang, S.; Luo, J.; Lee, L.J.; Yang, S.-T.; Madou, M.J. Design of a compact disk-like microfluidic platform for enzyme-linked immunosorbent assay. Anal. Chem. 2004, 76, 1832–1837. [Google Scholar] [CrossRef] [PubMed]
- Honda, N.; Lindberg, U.; Andersson, P.; Hoffmann, S.; Takei, H. Simultaneous multiple immunoassays in a compact disc–shaped microfluidic device based on centrifugal force. Clin. Chem. 2005, 51, 1955–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inganäs, M.; Dérand, H.; Eckersten, A.; Ekstrand, G.; Honerud, A.-K.; Jesson, G.; Thorsén, G.; Söderman, T.; Andersson, P. Integrated microfluidic compact disc device with potential use in both centralized and point-of-care laboratory settings. Clin. Chem. 2005, 51, 1985–1987. [Google Scholar] [CrossRef] [PubMed]
- Delgado, S.M.T.; Kinahan, D.J.; Sandoval, F.S.; Julius, L.A.N.; Kilcawley, N.A.; Ducrée, J.; Mager, D. Fully automated chemiluminescence detection using an electrified-Lab-on-a-Disc (eLoaD) platform. Lab Chip 2016, 16, 4002–4011. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.Y.L.; Li, P.C.H.; Yu, H.-Z.; Ash, M.P.; Chou, W.L.J. Spiral microchannels on a CD for DNA hybridizations. Sens. Actuators B Chem. 2007, 128, 64–69. [Google Scholar] [CrossRef]
- Jia, G.; Ma, K.-S.; Kim, J.; Zoval, J.V.; Peytavi, R.; Bergeron, M.G.; Madou, M.J. Dynamic automated DNA hybridization on a CD (compact disc) fluidic platform. Sens. Actuators B Chem. 2006, 114, 173–181. [Google Scholar] [CrossRef]
- Cho, Y.-K.; Lee, J.-G.; Park, J.-M.; Lee, B.-S.; Lee, Y.; Ko, C. One-step pathogen specific DNA extraction from whole blood on a centrifugal microfluidic device. Lab Chip 2007, 7, 565–573. [Google Scholar] [CrossRef]
- Li, C.; Dong, X.; Qin, J.; Lin, B. Rapid nanoliter DNA hybridization based on reciprocating flow on a compact disk microfluidic device. Anal. Chim. Acta 2009, 640, 93–99. [Google Scholar] [CrossRef]
- Bañuls, M.-J.; García-Piñón, F.; Puchades, R.; Maquieira, Á. Chemical derivatization of compact disc polycarbonate surfaces for SNPs detection. Bioconjug. Chem. 2008, 19, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, J.; Gorkin, R.; Bastien, M.; Stewart, G.; Peytavi, R.; Kido, H.; Bergeron, M.; Madou, M. Validation of a centrifugal microfluidic sample lysis and homogenization platform for nucleic acid extraction with clinical samples. Lab Chip 2010, 10, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Kong, J.; Li, X.; Fang, X.; Chen, Q. Colorimetric LAMP microfluidic chip for detecting three allergens: Peanut, sesame and soybean. Sci. Rep. 2018, 8, 8682. [Google Scholar] [CrossRef] [PubMed]
- Hoehl, M.M.; Weißert, M.; Dannenberg, A.; Nesch, T.; Paust, N.; von Stetten, F.; Zengerle, R.; Slocum, A.H.; Steigert, J. Centrifugal LabTube platform for fully automated DNA purification and LAMP amplification based on an integrated, low-cost heating system. Biomed. Microdevices 2014, 16, 375–385. [Google Scholar] [CrossRef] [Green Version]
- Burger, R.; Kirby, D.; Glynn, M.; Nwankire, C.; O’Sullivan, M.; Siegrist, J.; Kinahan, D.; Aguirre, G.; Kijanka, G.; Gorkin, R.A. Centrifugal microfluidics for cell analysis. Curr. Opin. Chem. Biol. 2012, 16, 409–414. [Google Scholar] [CrossRef]
- Morijiri, T.; Sunahiro, S.; Senaha, M.; Yamada, M.; Seki, M. Sedimentation pinched-flow fractionation for size-and density-based particle sorting in microchannels. Microfluid. Nanofluidics 2011, 11, 105–110. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kang, J.Y.; Lee, I.-H.; Ryu, S.-S.; Kwak, S.-M.; Shin, K.-S.; Kim, C.; Jung, H.-I.; Kim, T.-S. Single-cell assay on CD-like lab chip using centrifugal massive single-cell trap. Sens. Actuators A Phys. 2008, 143, 64–69. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chen, K.-C.; Pan, Y.-C.; Lee, T.-P.; Hsiung, L.-C.; Lin, C.-M.; Chen, C.-Y.; Lin, C.-H.; Chiang, B.-L.; Wo, A.M. Separation and detection of rare cells in a microfluidic disk via negative selection. Lab Chip 2011, 11, 474–483. [Google Scholar] [CrossRef]
- Burger, R.; Reith, P.; Kijanka, G.; Akujobi, V.; Abgrall, P.; Ducrée, J. Array-based capture, distribution, counting and multiplexed assaying of beads on a centrifugal microfluidic platform. Lab Chip 2012, 12, 1289–1295. [Google Scholar] [CrossRef]
- Burger, R.; Kurzbuch, D.; Gorkin, R.; Kijanka, G.; Glynn, M.; McDonagh, C.; Ducrée, J. An integrated centrifugo-opto-microfluidic platform for arraying, analysis, identification and manipulation of individual cells. Lab Chip 2015, 15, 378–381. [Google Scholar] [CrossRef]
- Glynn, M.; Kirby, D.; Chung, D.; Kinahan, D.J.; Kijanka, G.; Ducrée, J. Centrifugo-magnetophoretic purification of CD4+ cells from whole blood toward future HIV/AIDS point-of-care applications. J. Lab. Autom. 2014, 19, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Xie, Y.; Yang, Y.; Cheng, M.M.-C.; Koh, C.-G.; Bai, Y.; Lee, L.J.; Juang, Y.-J. New valve and bonding designs for microfluidic biochips containing proteins. Anal. Chem. 2007, 79, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Huang, P.-C.; Lin, M.-G. Analysis and experiment of capillary valves for microfluidics on a rotating disk. Microfluid. Nanofluidics 2008, 4, 427–437. [Google Scholar] [CrossRef]
- Park, J.-M.; Cho, Y.-K.; Lee, B.-S.; Lee, J.-G.; Ko, C. Multifunctional microvalves control by optical illumination on nanoheaters and its application in centrifugal microfluidic devices. Lab Chip 2007, 7, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Delgado, S.M.T.; Kinahan, D.J.; Julius, L.A.N.; Mallette, A.; Ardila, D.S.; Mishra, R.; Miyazaki, C.M.; Korvink, J.G.; Ducrée, J.; Mager, D. Wirelessly powered and remotely controlled valve-array for highly multiplexed analytical assay automation on a centrifugal microfluidic platform. Biosens. Bioelectron. 2018, 109, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Kinahan, D.J.; Early, P.L.; Vembadi, A.; MacNamara, E.; Kilcawley, N.A.; Glennon, T.; Diamond, D.; Brabazon, D.; Ducrée, J. Xurography actuated valving for centrifugal flow control. Lab Chip 2016, 16, 3454–3459. [Google Scholar] [CrossRef]
- Steigert, J.; Brenner, T.; Grumann, M.; Riegger, L.; Lutz, S.; Zengerle, R.; Ducrée, J. Integrated siphon-based metering and sedimentation of whole blood on a hydrophilic lab-on-a-disk. Biomed. Microdevices 2007, 9, 675–679. [Google Scholar] [CrossRef]
- Mark, D.; Metz, T.; Haeberle, S.; Lutz, S.; Ducrée, J.; Zengerle, R.; von Stetten, F. Centrifugo-pneumatic valve for metering of highly wetting liquids on centrifugal microfluidic platforms. Lab Chip 2009, 9, 3599–3603. [Google Scholar] [CrossRef]
- Grumann, M.; Geipel, A.; Riegger, L.; Zengerle, R.; Ducrée, J. Batch-mode mixing on centrifugal microfluidic platforms. Lab Chip 2005, 5, 560–565. [Google Scholar] [CrossRef]
- Noroozi, Z.; Kido, H.; Micic, M.; Pan, H.; Bartolome, C.; Princevac, M.; Zoval, J.; Madou, M. Reciprocating flow-based centrifugal microfluidics mixer. Rev. Sci. Instrum. 2009, 80, 75102. [Google Scholar] [CrossRef]
- Zehnle, S.; Roth, G.; von Stetten, F.; Roland, Z.; Paust, N. Centrifugo-dynamic inward pumping of liquids on a centrifugal microfluidic platform. Lab Chip 2012, 12, 5142–5145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorkin, R., III; Clime, L.; Madou, M.; Kido, H. Pneumatic pumping in centrifugal microfluidic platforms. Microfluid. Nanofluidics 2010, 9, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, C.M.; Kinahan, D.J.; Mishra, R.; Mangwanya, F.; Kilcawley, N.; Ferreira, M.; Ducrée, J. Label-free, spatially multiplexed SPR detection of immunoassays on a highly integrated centrifugal Lab-on-a-Disc platform. Biosens. Bioelectron. 2018, 119, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Brenner, T.; Glatzel, T.; Zengerle, R.; Ducrée, J. Frequency-dependent transversal flow control in centrifugal microfluidics. Lab Chip 2005, 5, 146–150. [Google Scholar] [CrossRef]
- Kinahan, D.J.; Kearney, S.M.; Dimov, N.; Glynn, T.; Ducrée, J. Event-triggered logical flow control for comprehensive process integration of multi-step assays on centrifugal microfluidic platforms. Lab Chip 2014, 14, 2249–2258. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.-T.; Wu, Z. Micromixers—A review. J. Micromech. Microeng. 2004, 15, R1. [Google Scholar] [CrossRef]
- Hessel, V.; Löwe, H.; Schönfeld, F. Micromixers—A review on passive and active mixing principles. Chem. Eng. Sci. 2005, 60, 2479–2501. [Google Scholar] [CrossRef]
- Yang, Z.; Goto, H.; Matsumoto, M.; Maeda, R. Active micromixer for microfluidic systems using lead-zirconate-titanate (PZT)-generated ultrasonic vibration. Electrophor. Int. J. 2000, 21, 116–119. [Google Scholar] [CrossRef]
- Paik, P.; Pamula, V.K.; Fair, R.B. Rapid droplet mixers for digital microfluidic systems. Lab Chip 2003, 3, 253–259. [Google Scholar] [CrossRef]
- Glasgow, I.; Aubry, N. Enhancement of microfluidic mixing using time pulsing. Lab Chip 2003, 3, 114–120. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessoth, F.G.; deMello, A.J.; Manz, A. Microstructure for efficient continuous flow mixing. Anal. Commun. 1999, 36, 213–215. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, D.S.; Lee, S.S.; Kwon, T.H. A split and recombination micromixer fabricated in a PDMS three-dimensional structure. J. Micromech. Microeng. 2006, 16, 1067. [Google Scholar] [CrossRef]
- Schönfeld, F.; Hessel, V.; Hofmann, C. An optimised split-and-recombine micro-mixer with uniform ‘chaotic’mixing. Lab Chip 2004, 4, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Leung, W.W.-F. Numerical and experimental investigation on flow and mixing in batch-mode centrifugal microfluidics. Int. J. Heat Mass Transf. 2013, 60, 95–104. [Google Scholar] [CrossRef]
- Ducrée, J.; Haeberle, S.; Brenner, T.; Glatzel, T.; Zengerle, R. Patterning of flow and mixing in rotating radial microchannels. Microfluid. Nanofluidics 2006, 2, 97–105. [Google Scholar] [CrossRef]
- Ducree, J.; Brenner, T.; Haeberle, S.; Glatzel, T.; Zengerle, R. Multilamination of flows in planar networks of rotating microchannels. Microfluid. Nanofluidics 2006, 2, 78–84. [Google Scholar] [CrossRef]
- Kinahan, D.J.; Kearney, S.M.; Faneuil, O.P.; Glynn, M.T.; Dimov, N.; Ducrée, J. Paper imbibition for timing of multi-step liquid handling protocols on event-triggered centrifugal microfluidic lab-on-a-disc platforms. RSC Adv. 2015, 5, 1818–1826. [Google Scholar] [CrossRef] [Green Version]
- Haeberle, S.; Brenner, T.; Schlosser, H.; Zengerle, R.; Ducrée, J. Centrifugal micromixery. Chem. Eng. Technol. Ind. Chem. Equip.-Process Eng. 2005, 28, 613–616. [Google Scholar] [CrossRef]
- Kuo, J.-N.; Li, Y.-S. Centrifuge-based micromixer with three-dimensional square-wave microchannel for blood plasma mixing. Microsyst. Technol. 2017, 23, 2343–2354. [Google Scholar] [CrossRef]
- Burger, S.; Schulz, M.; von Stetten, F.; Zengerle, R.; Paust, N. Rigorous buoyancy driven bubble mixing for centrifugal microfluidics. Lab Chip 2016, 16, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Xiang, J.; Chen, H.; Wang, W. A rapid micromixer for centrifugal microfluidic platforms. Micromachines 2016, 7, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, R.; Reith, P.; Akujobi, V.; Ducrée, J. Rotationally controlled magneto-hydrodynamic particle handling for bead-based microfluidic assays. Microfluid. Nanofluidics 2012, 13, 675–681. [Google Scholar] [CrossRef]
- Kong, M.C.R.; Salin, E.D. Micromixing by pneumatic agitation on continually rotating centrifugal microfluidic platforms. Microfluid. Nanofluidics 2012, 13, 519–525. [Google Scholar] [CrossRef]
- Clime, L.; Brassard, D.; Geissler, M.; Veres, T. Active pneumatic control of centrifugal microfluidic flows for lab-on-a-chip applications. Lab Chip 2015, 15, 2400–2411. [Google Scholar] [CrossRef]
- Morelli, L.; Serioli, L.; Centorbi, F.A.; Jendresen, C.B.; Matteucci, M.; Ilchenko, O.; Demarchi, D.; Nielsen, A.T.; Zór, K.; Boisen, A. Injection molded lab-on-a-disc platform for screening of genetically modified E. coli using liquid–liquid extraction and surface enhanced Raman scattering. Lab Chip 2018, 18, 869–877. [Google Scholar] [CrossRef] [Green Version]
- Da Fonseca, J.G.; Reis, N.A.E.; Burger, R. Analytical Rotors and Methods for Analysis of Biological Fluids. U.S. Patent 8,440,147, 14 May 2013. [Google Scholar]
- Brennan, D.; Coughlan, H.; Clancy, E.; Dimov, N.; Barry, T.; Kinahan, D.; Ducrée, J.; Smith, T.J.; Galvin, P. Development of an on-disc isothermal in vitro amplification and detection of bacterial RNA. Sens. Actuators B Chem. 2017, 239, 235–242. [Google Scholar] [CrossRef] [Green Version]


| Description | Advantages | Disadvantages | References |
|---|---|---|---|
| Microchannels-based Liquid is split-and-recombined or flows through channels of specific orientations to induce secondary flows to increase the boundary area across which diffusion occurs. | Largely passive; Improves mixing speed. | Can occupy significant disc space; functions best with continuous flow | [68,69] |
| Euler Force (Shake-mode) Use of Euler force (disc acceleration and deceleration) to induce mixing. Can be combined with pneumatic chambers in enhance liquid displacement/reciprocating flows. | Fast mixing mechanism. Does not require additional support instrumentation. | Can occupy significand disc space. Requires a specific disc spin profile (i.e., acceleration and deceleration). | [52,70,71,72] |
| Mixing by bubbles. Provision of sources of pressurized air by on-rotor pneumatic pumps, via pneumatic slip-rings or through directing off-disc compressed air to generate air bubbles to enhance mixing. Use of chemical reactions to create bubbles. | Mechanism largely independent of disc spin-rate. Very rapid mixing mechanism. | Supporting instrumentation is complex and moves away from principal of Lab-on-a-Disc based solely on low-cost spindle motor. Can require specific chemical reagents stored on disc. | [73,76,77] |
| Wall deformation Mixing is enhanced by deforming chamber walls to induce liquid movement. | Mechanism largely independent of disc spin-rate. | Can require additional equipment such as magnet embedded in disc wall. Suitable only for discs made from soft material such as PDMS. | [74,75] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burger, R.; Kinahan, D.J.; Cayron, H.; Reis, N.; Fonseca, J.; Ducrée, J. Siphon-Induced Droplet Break-Off for Enhanced Mixing on a Centrifugal Platform. Inventions 2020, 5, 1. https://doi.org/10.3390/inventions5010001
Burger R, Kinahan DJ, Cayron H, Reis N, Fonseca J, Ducrée J. Siphon-Induced Droplet Break-Off for Enhanced Mixing on a Centrifugal Platform. Inventions. 2020; 5(1):1. https://doi.org/10.3390/inventions5010001
Chicago/Turabian StyleBurger, Robert, David J Kinahan, Hélène Cayron, Nuno Reis, João Fonseca, and Jens Ducrée. 2020. "Siphon-Induced Droplet Break-Off for Enhanced Mixing on a Centrifugal Platform" Inventions 5, no. 1: 1. https://doi.org/10.3390/inventions5010001







