An Electroporation Device with Microbead-Enhanced Electric Field for Bacterial Inactivation
Abstract
1. Introduction
Fundamentals
2. Materials and Methods
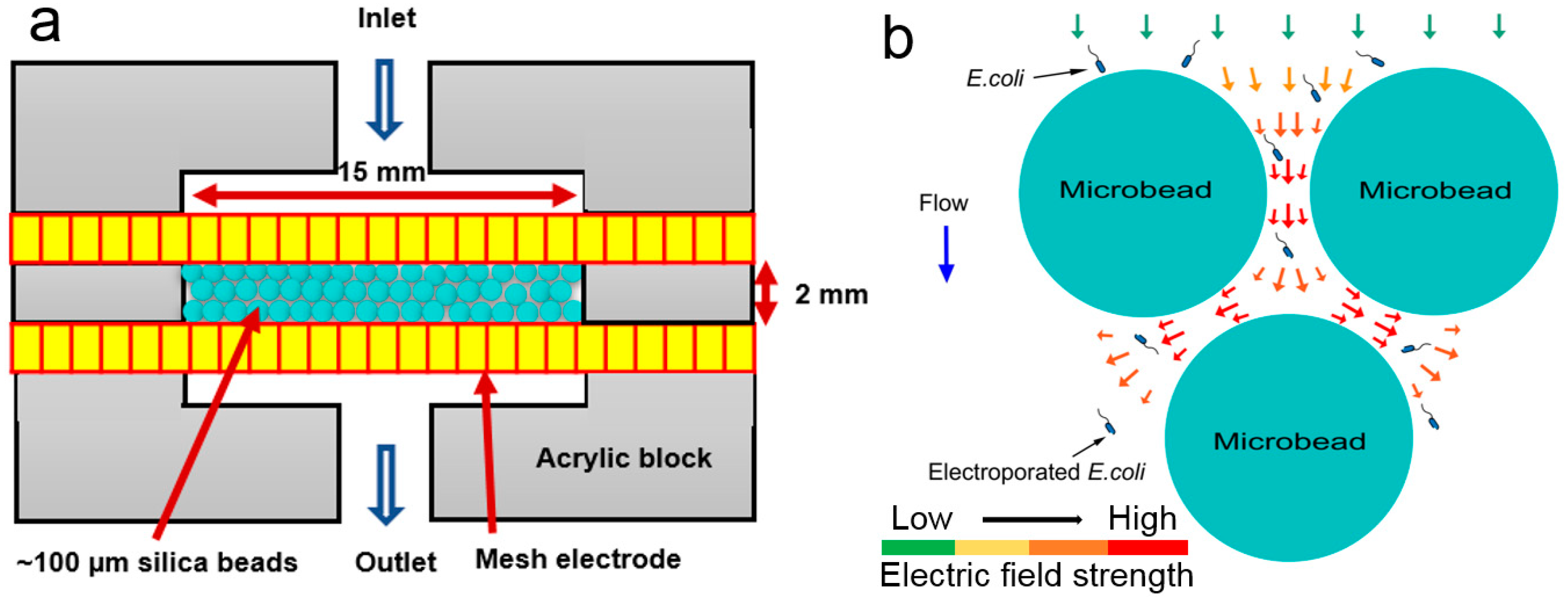
2.1. Device Design and Fabrication
2.2. Cell Preparation
2.3. Experimental Setup
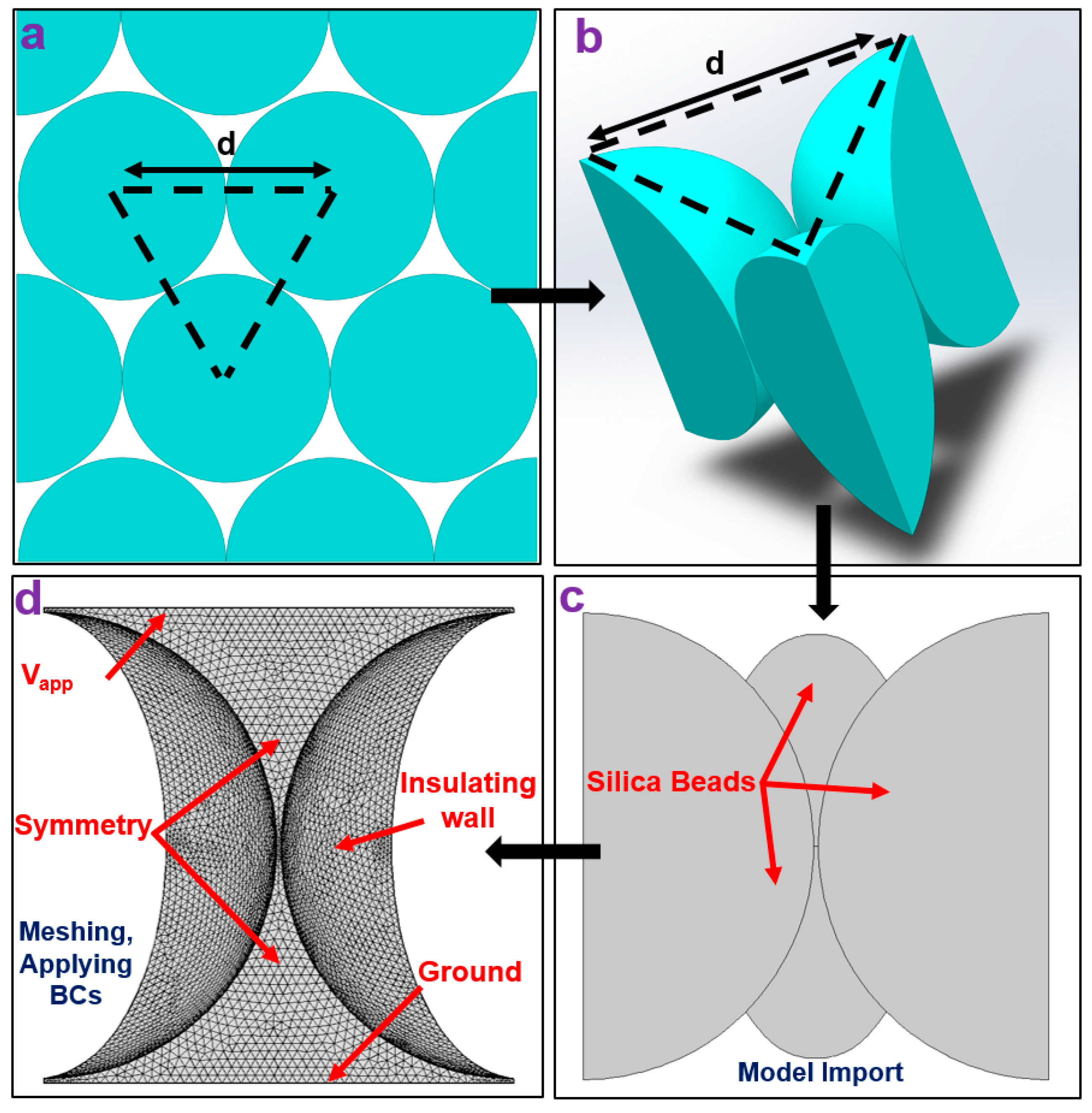
2.4. Numerical Simulation
3. Results and Discussion
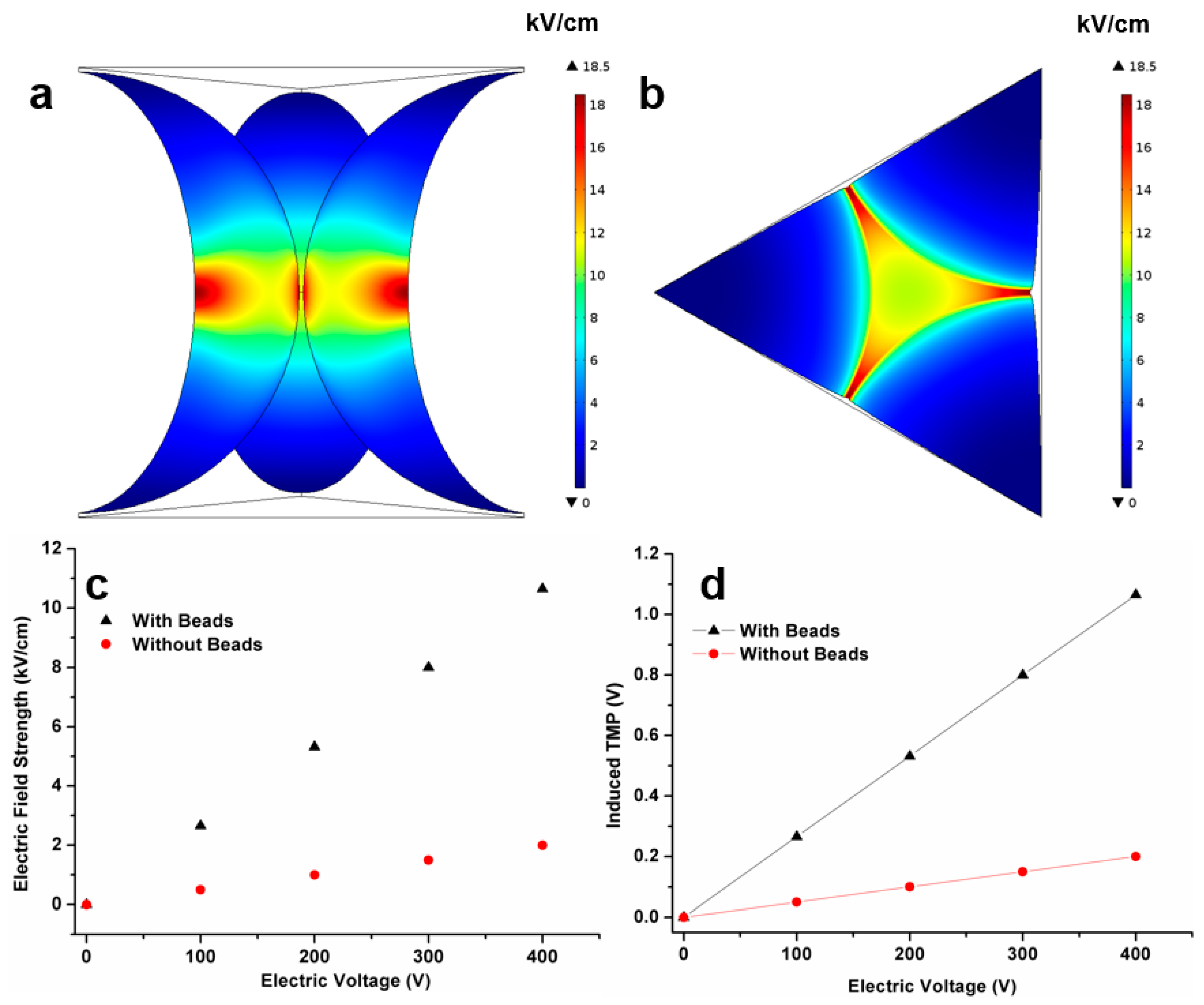
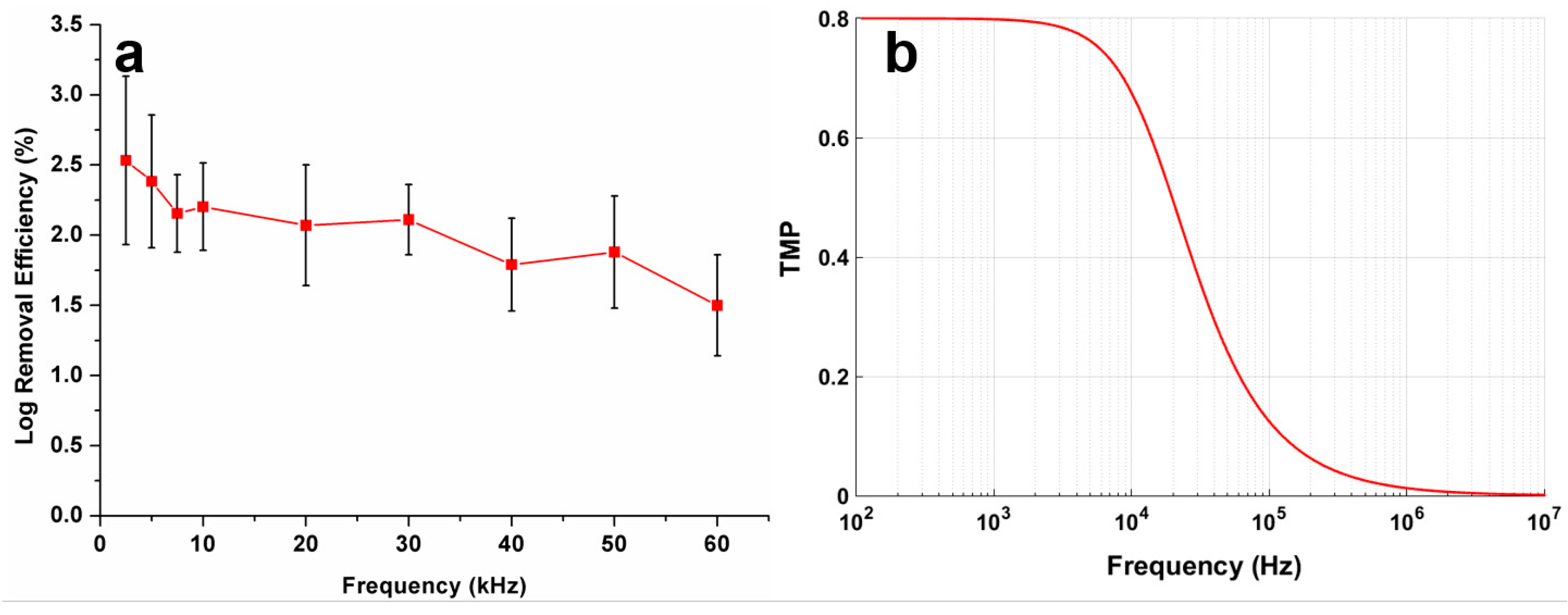
3.1. Numerical Simulation Results
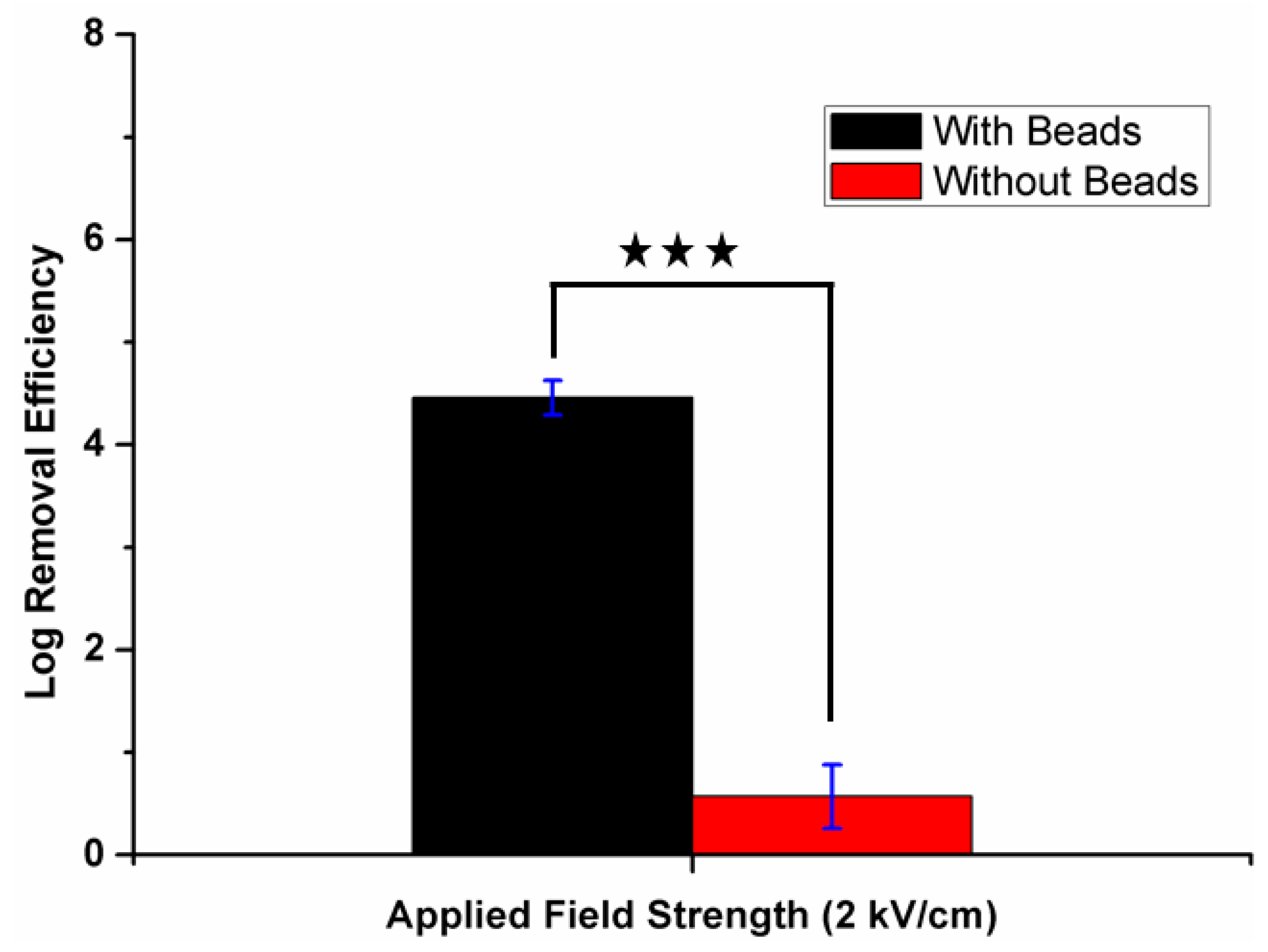
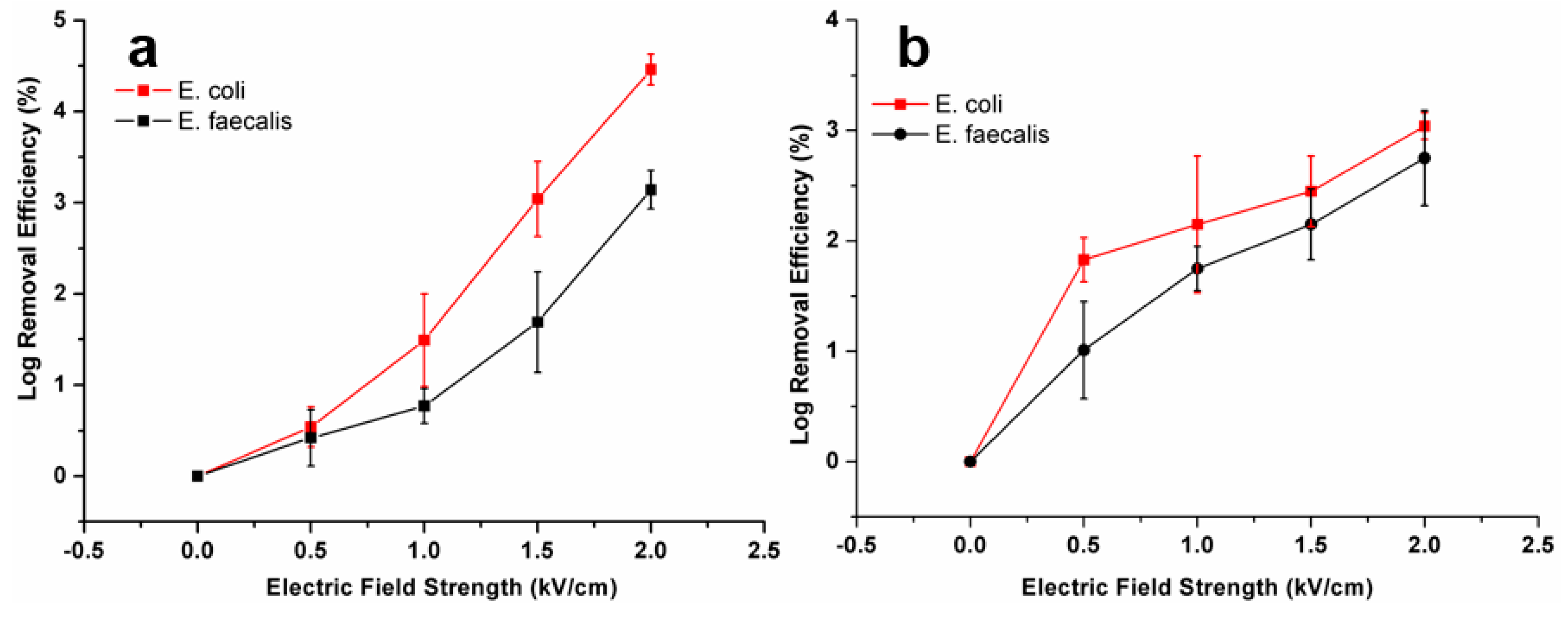
3.2. Inactivation Performance of the Device
3.3. Effect of Applied Electric Field
3.4. Effect of AC Frequency
4. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chang, D. Guide to Electroporation and Electrofusion; Academic Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Chu, G.; Hayakawa, H.; Berg, P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987, 15, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.A.; Neumann, E.; Sowers, A.E. Electroporation and Electrofusion in Cell Biology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Geng, T.; Lu, C. Microfluidic electroporation for cellular analysis and delivery. Lab Chip 2013, 13, 3803–3821. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.C.; Chizmadzhev, Y.A. Theory of electroporation: A review. Bioelectrochem. Bioenerg. 1996, 41, 135–160. [Google Scholar] [CrossRef]
- Chang, L.; Li, L.; Shi, J.; Sheng, Y.; Lu, W.; Gallego-Perez, D.; Lee, L.J. Micro-/nanoscale electroporation. Lab Chip 2016, 16, 4047–4062. [Google Scholar] [CrossRef] [PubMed]
- Santra, T.; Tseng, F. Recent trends on micro/nanofluidic single cell electroporation. Micromachines 2013, 4, 333–356. [Google Scholar] [CrossRef]
- Hsu, P.D.; Lander, E.S.; Zhang, F. Development and applications of crispr-cas9 for genome engineering. Cell 2014, 157, 1262–1278. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with crispr-cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Buganim, Y.; Faddah, D.A.; Jaenisch, R. Mechanisms and models of somatic cell reprogramming. Nat. Rev. Genet. 2013, 14, 427–439. [Google Scholar] [CrossRef]
- Golberg, A.; Fischer, J.; Rubinsky, B. The use of irreversible electroporation in food preservation. In Irreversible Electroporation; Springer: Berlin/Heidelberg, Germany, 2010; pp. 273–312. [Google Scholar]
- Griffiths, M.; Walkling-Ribeiro, M. Microbial decontamination of milk and dairy products. In Microbial Decontamination in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2012; pp. 190–238. [Google Scholar]
- Puértolas, E.; López, N.; Condón, S.; Raso, J.; Álvarez, I. Pulsed electric fields inactivation of wine spoilage yeast and bacteria. Int. J. Food Microbiol. 2009, 130, 49–55. [Google Scholar] [CrossRef]
- Mahnič-Kalamiza, S.; Vorobiev, E.; Miklavčič, D. Electroporation in food processing and biorefinery. J. Membr. Biol. 2014, 247, 1279–1304. [Google Scholar] [CrossRef]
- Huo, Z.-Y.; Xie, X.; Yu, T.; Lu, Y.; Feng, C.; Hu, H.-Y. Nanowire-modified three-dimensional electrode enabling low-voltage electroporation for water disinfection. Environ. Sci. Technol. 2016, 50, 7641–7649. [Google Scholar] [CrossRef] [PubMed]
- Huo, Z.-Y.; Zhou, J.-F.; Wu, Y.; Wu, Y.-H.; Liu, H.; Liu, N.; Hu, H.-Y.; Xie, X. A cu 3 p nanowire enabling high-efficiency, reliable, and energy-efficient low-voltage electroporation-inactivation of pathogens in water. J. Mater. Chem. A 2018, 6, 18813–18820. [Google Scholar] [CrossRef]
- Liu, C.; Xie, X.; Zhao, W.; Liu, N.; Maraccini, P.A.; Sassoubre, L.M.; Boehm, A.B.; Cui, Y. Conducting nanosponge electroporation for affordable and high-efficiency disinfection of bacteria and viruses in water. Nano Lett. 2013, 13, 4288–4293. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Huang, M.-Y. Electroporation microchips for in vitro gene transfection. J. Micromech. Microeng. 2001, 11, 542. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Li, M.; Fan, C.-S.; Wu, L.-W. A microchip for electroporation of primary endothelial cells. Sens. Actuators A Phys. 2003, 108, 12–19. [Google Scholar] [CrossRef]
- Lu, H.; Schmidt, M.A.; Jensen, K.F. A microfluidic electroporation device for cell lysis. Lab Chip 2005, 5, 23–29. [Google Scholar] [CrossRef]
- De la Rosa, C.; Tilley, P.A.; Fox, J.D.; Kaler, K.V. Microfluidic device for dielectrophoresis manipulation and electrodisruption of respiratory pathogen bordetella pertussis. IEEE Trans. Biomed. Eng. 2008, 55, 2426–2432. [Google Scholar] [CrossRef]
- Huang, H.; Wei, Z.; Huang, Y.; Zhao, D.; Zheng, L.; Cai, T.; Wu, M.; Wang, W.; Ding, X.; Zhou, Z. An efficient and high-throughput electroporation microchip applicable for sirna delivery. Lab Chip 2011, 11, 163–172. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.; Yu, C.; Xie, X. Rapid determination of the electroporation threshold for bacteria inactivation using a lab-on-a-chip platform. Environ. Int. 2019, 132, 105040. [Google Scholar] [CrossRef]
- Lu, K.-Y.; Wo, A.M.; Lo, Y.-J.; Chen, K.-C.; Lin, C.-M.; Yang, C.-R. Three dimensional electrode array for cell lysis via electroporation. Biosens. Bioelectron. 2006, 22, 568–574. [Google Scholar] [CrossRef]
- Huang, D.; Zhao, D.; Li, J.; Wu, Y.; Zhou, W.; Wang, W.; Liang, Z.; Li, Z. High cell viability microfluidic electroporation in a curved channel. Sens. Actuators B Chem. 2017, 250, 703–711. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Lu, C. Electroporation of mammalian cells in a microfluidic channel with geometric variation. Anal. Chem. 2006, 78, 5158–5164. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Kim, T.; Cho, Y.-H. Cell electroporation chip using multiple electric field zones in a single channel. Appl. Phys. Lett. 2012, 101, 223705. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, T.; Doh, I.; Cho, Y.-H. A cell electroporation characterization chip using a single tapered channel for continuous electric field variation. BioChip J. 2014, 8, 269–274. [Google Scholar] [CrossRef]
- Garcia, P.A.; Ge, Z.; Kelley, L.E.; Holcomb, S.J.; Buie, C.R. High efficiency hydrodynamic bacterial electrotransformation. Lab Chip 2017, 17, 490–500. [Google Scholar] [CrossRef]
- Garcia, P.A.; Ge, Z.; Moran, J.L.; Buie, C.R. Microfluidic screening of electric fields for electroporation. Sci. Rep. 2016, 6, 21238. [Google Scholar] [CrossRef]
- Church, C.; Zhu, J.; Huang, G.; Tzeng, T.-R.; Xuan, X. Integrated electrical concentration and lysis of cells in a microfluidic chip. Biomicrofluidics 2010, 4, 044101. [Google Scholar] [CrossRef]
- Gallo-Villanueva, R.C.; Jesús-Pérez, N.M.; Martínez-López, J.I.; Pacheco, A.; Lapizco-Encinas, B.H. Assessment of microalgae viability employing insulator-based dielectrophoresis. Microfluid. Nanofluid. 2011, 10, 1305–1315. [Google Scholar] [CrossRef]
- Gallo-Villanueva, R.C.; Perez-Gonzalez, V.H.; Cardenas-Benitez, B.; Jind, B.; Martinez-Chapa, S.O.; Lapizco-Encinas, B.H. Joule heating effects in optimized insulator-based dielectrophoretic devices: An interplay between post geometry and temperature rise. Electrophoresis 2019, 40, 1408–1416. [Google Scholar] [CrossRef]
- Sano, M.B.; Gallo-Villanueva, R.C.; Lapizco-Encinas, B.H.; Davalos, R.V. Simultaneous electrokinetic flow and dielectrophoretic trapping using perpendicular static and dynamic electric fields. Microfluid. Nanofluid. 2013, 15, 599–609. [Google Scholar] [CrossRef]
- Pudasaini, S.; Perera, A.; Das, D.; Ng, S.H.; Yang, C. Continuous flow microfluidic cell inactivation with use of insulating micropillars for multiple electroporation zones. Electrophoresis 2019, 40, 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Kissinga, H.D.; Mwombeki, F.; Said, K.; Katakweba, A.A.; Nonga, H.E.; Muhairwa, A.P. Antibiotic susceptibilities of indicator bacteria escherichia coli and enterococci spp. Isolated from ducks in morogoro municipality, tanzania. BMC Res. Notes 2018, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Worley-Morse, T.; Mann, M.; Khunjar, W.; Olabode, L.; Gonzalez, R. Evaluating the fate of bacterial indicators, viral indicators, and viruses in water resource recovery facilities. Water Environ. Res. 2019, 91, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Xu, B.; Sinton, D.; Li, D. Electroosmotic flow with joule heating effects. Lab Chip 2004, 4, 230–236. [Google Scholar] [CrossRef]
- Barz, D.P.; Ehrhard, P. Model and verification of electrokinetic flow and transport in a micro-electrophoresis device. Lab Chip 2005, 5, 949–958. [Google Scholar] [CrossRef]
- Tandon, V.; Bhagavatula, S.K.; Nelson, W.C.; Kirby, B.J. Zeta potential and electroosmotic mobility in microfluidic devices fabricated from hydrophobic polymers: 1. The origins of charge. Electrophoresis 2008, 29, 1092–1101. [Google Scholar] [CrossRef]
- Hyoung Kang, K.; Xuan, X.; Kang, Y.; Li, D. Effects of dc-dielectrophoretic force on particle trajectories in microchannels. J. Appl. Phys. 2006, 99, 064702. [Google Scholar] [CrossRef]
- Tsong, T.Y. Electroporation of cell membranes. Biophys. J. 1991, 60, 297–306. [Google Scholar] [CrossRef]
- Grahl, T.; Märkl, H. Killing of microorganisms by pulsed electric fields. Appl. Microbiol. Biotechnol. 1996, 45, 148–157. [Google Scholar] [CrossRef]
- Zimmermann, U.; Pilwat, G.; Riemann, F. Dielectric breakdown of cell membranes. Biophys. J. 1974, 14, 881–899. [Google Scholar] [CrossRef]
- Movahed, S.; Li, D. Microfluidics cell electroporation. Microfluid. Nanofluid. 2011, 10, 703–734. [Google Scholar] [CrossRef]
- Pucihar, G.; Kotnik, T.; Kandušer, M.; Miklavčič, D. The influence of medium conductivity on electropermeabilization and survival of cells in vitro. Bioelectrochemistry 2001, 54, 107–115. [Google Scholar] [CrossRef]
- Liu, C.; Xie, X.; Zhao, W.; Yao, J.; Kong, D.; Boehm, A.B.; Cui, Y. Static electricity powered copper oxide nanowire microbicidal electroporation for water disinfection. Nano Lett. 2014, 14, 5603–5608. [Google Scholar] [CrossRef] [PubMed]
- Keyser, M.; Műller, I.A.; Cilliers, F.P.; Nel, W.; Gouws, P.A. Ultraviolet radiation as a non-thermal treatment for the inactivation of microorganisms in fruit juice. Innov. Food Sci. Emerg. Technol. 2008, 9, 348–354. [Google Scholar] [CrossRef]
- Rhodes, M.J. Introduction to Particle Technology; John Wiley & Sons: Hobken, NJ, USA, 2008. [Google Scholar]
- Bai, M.; Zhang, Z.; Zhang, N.; Tian, Y.; Chen, C.; Meng, X. Treatment of 250 t/h ballast water in oceanic ships using·oh radicals based on strong electric-field discharge. Plasma Chem. Plasma Process. 2012, 32, 693–702. [Google Scholar] [CrossRef]
- Huo, Z.-Y.; Luo, Y.; Xie, X.; Feng, C.; Jiang, K.; Wang, J.; Hu, H.-Y. Carbon-nanotube sponges enabling highly efficient and reliable cell inactivation by low-voltage electroporation. Environ. Sci. Nano 2017, 4, 2010–2017. [Google Scholar] [CrossRef]
- Tsong, T.Y. Electroporation of cell membranes. In Electroporation and Electrofusion in Cell Biology; Springer: Berlin/Heidelberg, Germany, 1989; pp. 149–163. [Google Scholar]
- Mernier, G.; Piacentini, N.; Braschler, T.; Demierre, N.; Renaud, P. Continuous-flow electrical lysis device with integrated control by dielectrophoretic cell sorting. Lab Chip 2010, 10, 2077–2082. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pudasaini, S.; Perera, A.T.K.; Ahmed, S.S.U.; Chong, Y.B.; Ng, S.H.; Yang, C. An Electroporation Device with Microbead-Enhanced Electric Field for Bacterial Inactivation. Inventions 2020, 5, 2. https://doi.org/10.3390/inventions5010002
Pudasaini S, Perera ATK, Ahmed SSU, Chong YB, Ng SH, Yang C. An Electroporation Device with Microbead-Enhanced Electric Field for Bacterial Inactivation. Inventions. 2020; 5(1):2. https://doi.org/10.3390/inventions5010002
Chicago/Turabian StylePudasaini, Sanam, A. T. K. Perera, Syed. S. U. Ahmed, Yong Bing Chong, Sum Huan Ng, and Chun Yang. 2020. "An Electroporation Device with Microbead-Enhanced Electric Field for Bacterial Inactivation" Inventions 5, no. 1: 2. https://doi.org/10.3390/inventions5010002
APA StylePudasaini, S., Perera, A. T. K., Ahmed, S. S. U., Chong, Y. B., Ng, S. H., & Yang, C. (2020). An Electroporation Device with Microbead-Enhanced Electric Field for Bacterial Inactivation. Inventions, 5(1), 2. https://doi.org/10.3390/inventions5010002




