1. Introduction
Today, minimally invasive surgery is an established technique in various surgical procedures, and the development in this field continues. In laparoscopy, the original two-dimensional laparoscopic vision system (2D LVS) is being challenged by three-dimensional systems (3D LVS) that provide a stereoscopic perception [
1,
2,
3,
4,
5]. The advantages reported with 3D vision systems in surgery are shorter operative time, less blood loss, fewer perioperative complications, and shorter length of hospital stay, according to a large meta-analysis performed by Cheng et al. [
4]. In laparoscopic simulation studies, the 3D LVS is proven to be better in terms of shorter task durations, reduced error rates, and a better subjective experience [
3,
5,
6]. However, one major user complaint regarding the 3D LVSs is that it requires shutter glasses, and the system has also been associated with a bad image quality and experiences of headache, dizziness, eyestrain, nausea, and visual disturbance [
2,
3]. A new generation of glasses-less 3D LVSs has been invented, and the side effects mentioned above were not reported to be significantly higher than for the 2D LVSs in comparison studies [
1].
Several studies have indicated a better outcome of 3D LVSs without glasses (3D−) in comparison with the conventional 3D LVSs with glasses (3D+) [
1,
2,
5,
7,
8]. In a recent article by Liu et al. [
9], potential advantages of the 3D− systems were technically described, such as high brightness, a large viewing area, and a strong anti-interference capability, but the authors did not conduct any actual testing; instead, they expressed a need for further comparison studies.
A single-task study (running sutures on a rubber model) using a 3D LVS system (3D+) and a novel glasses-less system (3D−) was carried out by He et al. [
7]. They compared image quality, 3D effect, accessibility, and overall surgical performance, and found no significant difference (all
p > 0.5). However, the experiment was short, and the performance was only measured by operative time. Another study reported significantly less visual fatigue with a 3D− than with a 3D+ [
8]. However, a limitation of that study was that the tests only included watching videos with no connection to surgery.
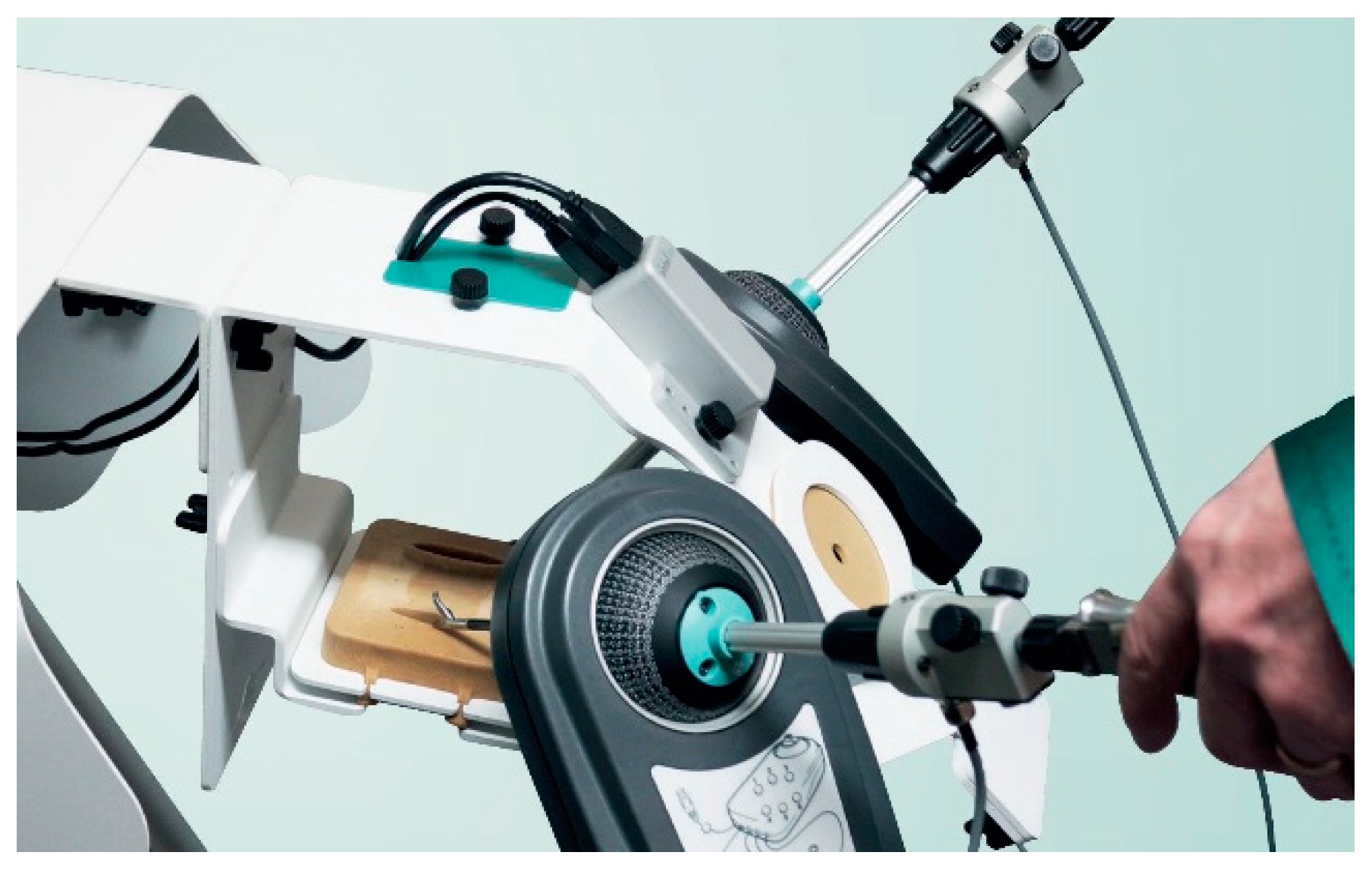
In consideration of the limitations from the previous studies, and with the remaining need to test the new 3D− invention, the purpose of this study is to compare a novel 3D− laparoscopic vision system with a conventional 3D+ system by objective and quantitative assessment of surgical performance (in a validated simulation box with authentic surgical instruments), as well as a questionnaire-based user experience assessment.
3. Results
In most of the outcomes and tasks, full data sets were obtained from all 18 participants. However, due to technical difficulty, we did not obtain a video recording during the first peg transfer task for three of the participants. Hence, they were excluded from the counting of errors in that task. These three participants all started with the 3D− system. We also had technical problems with another subject in the peg transfer task in the path length measurement; therefore, the subject was excluded from the calculations of path length as well. This was a participant that started with the 3D− system as well. Therefore, the peg transfer statistical comparisons were performed on 15 participants and the path length with 17.
3.1. Performance
The counted errors of the two reviewers were highly correlated; Spearman’s rank correlation coefficient was 0.883, and Cohen’s linearly weighted kappa was 0.642.
As shown in
Table 1 and
Table 2, there were no significant differences in performance between the two vision systems (3D+ and 3D−) for any of the performance tasks in terms of task duration, path length, or number of errors.
There was an overall improvement from the first to the second performance, which was expected since it was their first experience with a laparoscopic simulator. There was no significant difference in improvement between the participants who started with the 3D+ system and those who began with the 3D− system.
3.2. Questionnaires
The participants reported significantly higher spatial orientation when the 3D− monitor was used. However, direct comparisons of spatial orientation and sense of depth favored the 3D+ monitor (
Table 3). The question of the indirection comparison of the overall impression on each system was excluded from further analysis due to too much missing data. There were no significant differences between the systems in physical symptom ratings, i.e., headache and nausea (
Table 4), nor in eye symptoms (
Table 5).
3.3. Participant Comments
Two of the participants pointed out that it was nice not needing to use 3D glasses in the 3D− system, although two other participants stated that they got dizzy or got diplopia when moving their head using the 3D− system. One person reported bad peripheral depth vision with the 3D− system; conversely, one person also felt that their focus was bad in the upper left corner with the 3D+ system. Three participants thought that the 3D+ system had a darker image. One person commented that the 3D+ system required a longer time to focus, but another participant made the same comment about the 3D− system.
4. Discussion
The results in this study showed no significant differences in performance between the conventional 3D+ system and the novel 3D− system when the subjects performed four tasks in a laparoscopic simulator. In post-experience questionnaires, there was a statistically significant advantage for the 3D+ system regarding spatial orientation, sense of depth, and overall impression, although the actual difference was quite small. There was no significant difference in physical symptoms or in eye symptoms and all subjects reported very little discomfort in both systems. On the direct question regarding which system they preferred overall, ten participants answered 3D+ and eight answered 3D−.
These findings indicated no objective advantages of the 3D− system compared to the conventional 3D+ system. The subjective advantages of the conventional system were small in magnitude, and one can assume that the comfort was better without glasses, especially for surgeons with visual impairment who would also need their corrective lenses. The limitations of the 3D− system were that you must keep a certain distance between the monitor and eyes, and the surgeon must stay within a small range in front of the monitor and it is only suitable for one person to view at a time. In a robot console, this is immaterial since the surgeon is alone in the console, but in traditional laparoscopy, the assistant needs to have their own monitor since they cannot stand close enough to the surgeon to see a single display.
In this study, all participants were novices with only two who had used a laparoscopic 3D− system before. This means that their improvement from the first to the second performance should be comparable. We also had two surgeons who independently watched the videos of the tasks and counted the errors. While there were some differences in the counting, the two surgeons reached a good agreement with each other with a Spearman’s rank correlation coefficient of 0.883, and Cohen’s linearly weighted kappa of 0.642.
One limitation of this study was the short training time in which the participants learned to handle the simulation box. The learning effect during the experiment made it more difficult to compare the systems. However, the subjects were randomized into a balanced order. A remaining difficulty occurred in the peg transfer task, where three subjects who started with 3D− were excluded from the error counting due to technical reasons. This may have affected the results in favor of the 3D− system, although the difference was small, and the comparison would likely not have shown a significant difference even if all subjects were included in that task.
The number of participants (18 subjects) may be seen as another limitation. However, in comparing studies where paired comparisons were used, the power of statistics was higher than in unpaired comparisons. In other system comparison studies that we have conducted, significant differences, although small, were found with fewer subjects [
18,
19]. With more subjects included in this study, the power would naturally have been higher, but the differences may still be smaller than what may be of clinical interest. Hence, to show important differences between the systems, we estimated that 18 subjects would be enough.
It seems that 3D systems are superior to 2D systems when it comes to laparoscopy, in terms of performance time, error rate, and subjective assessments of cognitive workload, as reviewed by Fergo et al. and Sørensen et al. [
20,
21]. Both Fergo et al. and Sørensen et al. showed either an advantage of 3D systems or no significant differences in those aspects among screened and selected studies [
20,
21]. A recent comparison study of a 2D, a 3D+, and a 3D− system from Liu et al. concluded that the tested 3D systems showed an overall superiority over the tested 2D system in performance time and precision [
22]. The development of 3D diagnostic imaging may also facilitate support for surgeons during operation [
23].
However, whether it is a benefit to adopt a glasses-free 3D system is debatable. A study by He et al. indicated that the perceptions of benefits of glasses-free 3D systems could be conflicting among users with different backgrounds [
7]. In the current study, there were no significant differences between the systems in the objective performance data, which is in agreement (although with somewhat different parameters) with the findings of Liu et al. [
22]. Meanwhile, there were significant differences in the subjective comparisons (spatial orientation and sense of depth) in favor of the 3D+; however, the indicated advantages of the conventional 3D+ system were relatively small. The current results did not show a significant difference in terms of eye discomfort, but results from Liu et al. suggested that 3D+ system might increase eye fatigue [
22]. This may explain the bipolar division of opinions in regard to eyestrain in the study by He et al. [
7].