Chemical-Free Extraction of Functional Mitochondria Using a Microfluidic Device
Abstract
1. Introduction
2. Materials and Methods
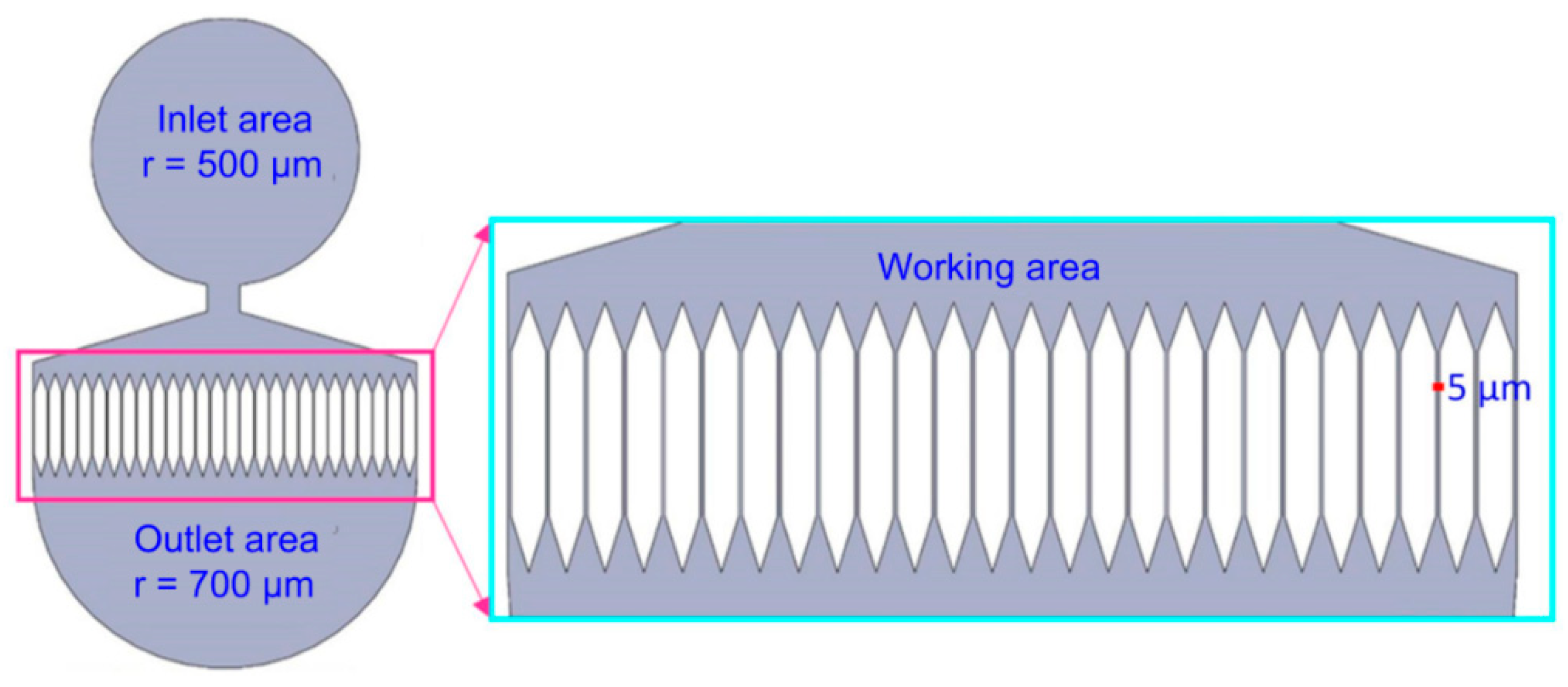
2.1. Design of the Microfluidic Mitochondria Extraction Device
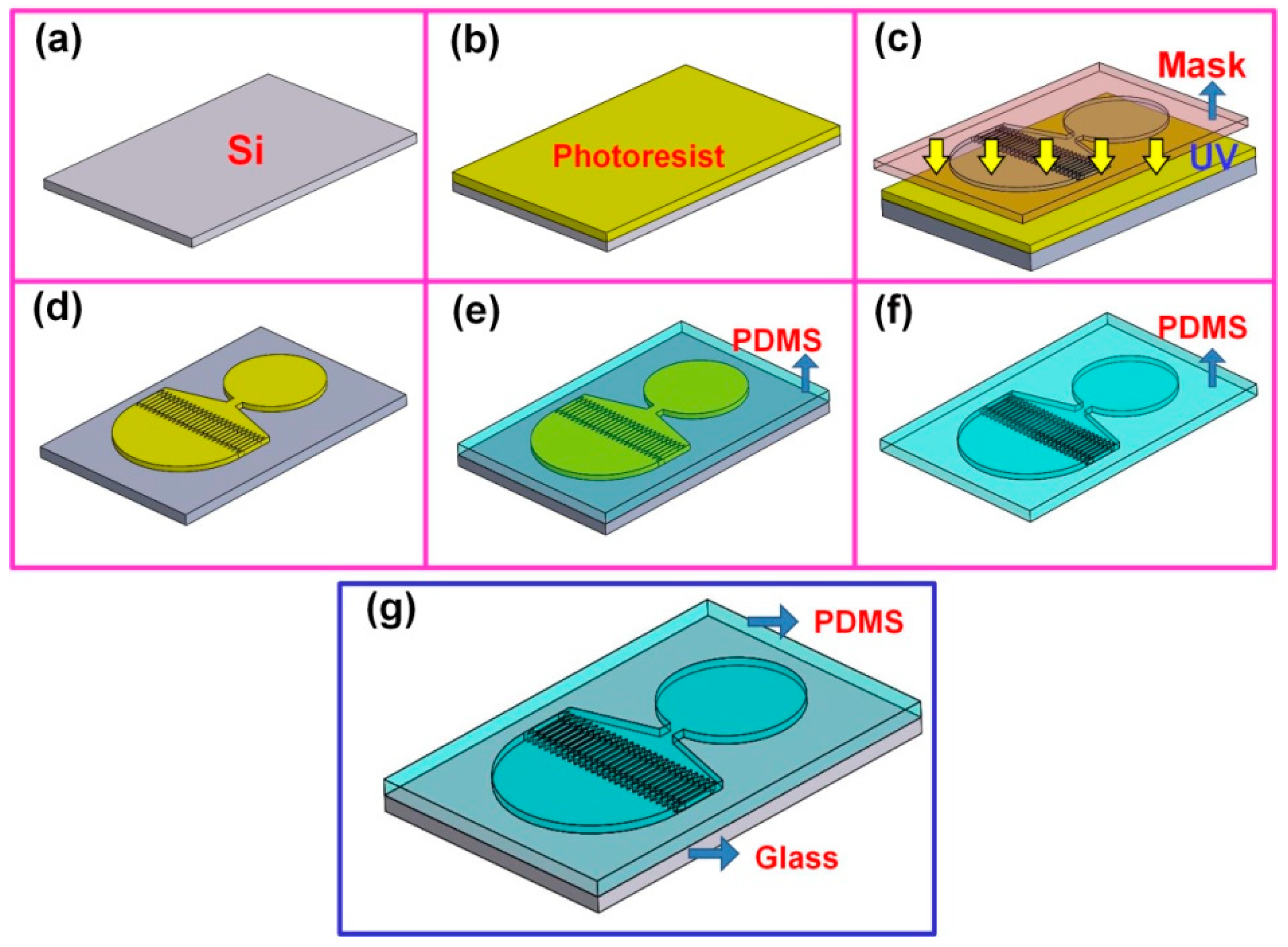
2.2. Fabrication of the Microfluidic Device
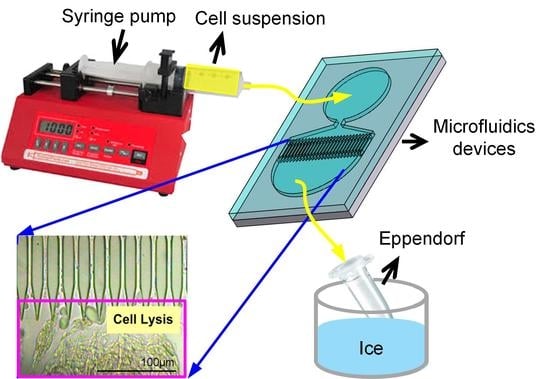
2.3. Cell Culture and Mitochondria Extraction
2.4. Protein Assay
2.5. SDS-PAGE and Western Blot
2.6. Flow Cytometry
2.7. Examination of Mitochondrial Morphology
3. Results
3.1. Device Structure Selection
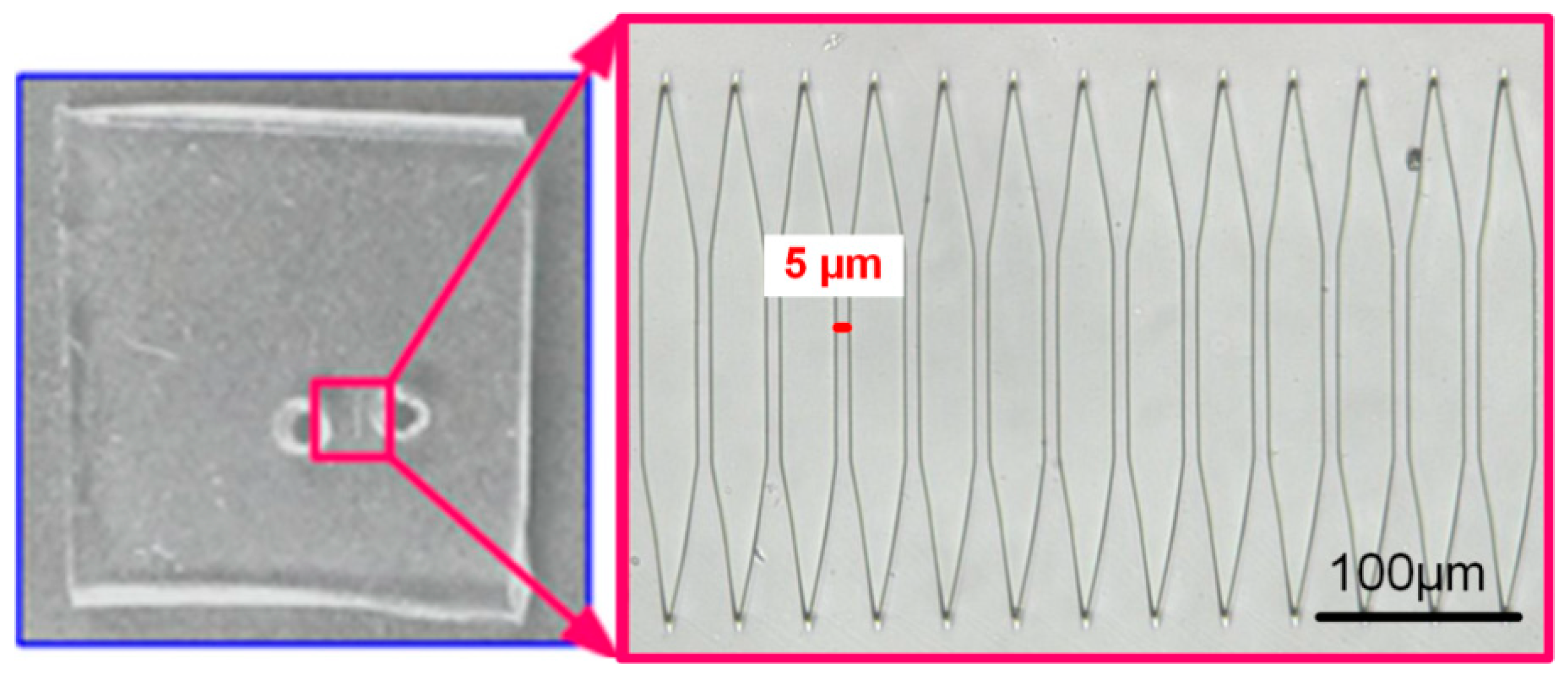
3.2. Chip-Based Microfluidic Device
3.3. Pressure Drop and Shear Stress
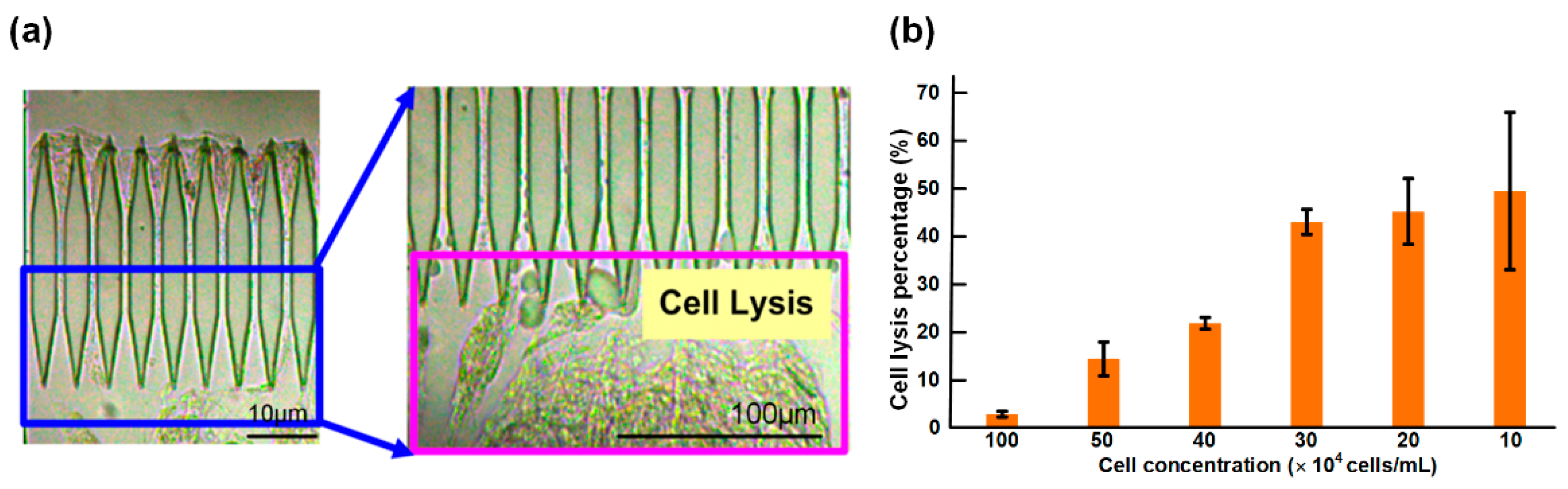
3.4. Working Concentration of the Cell Suspension
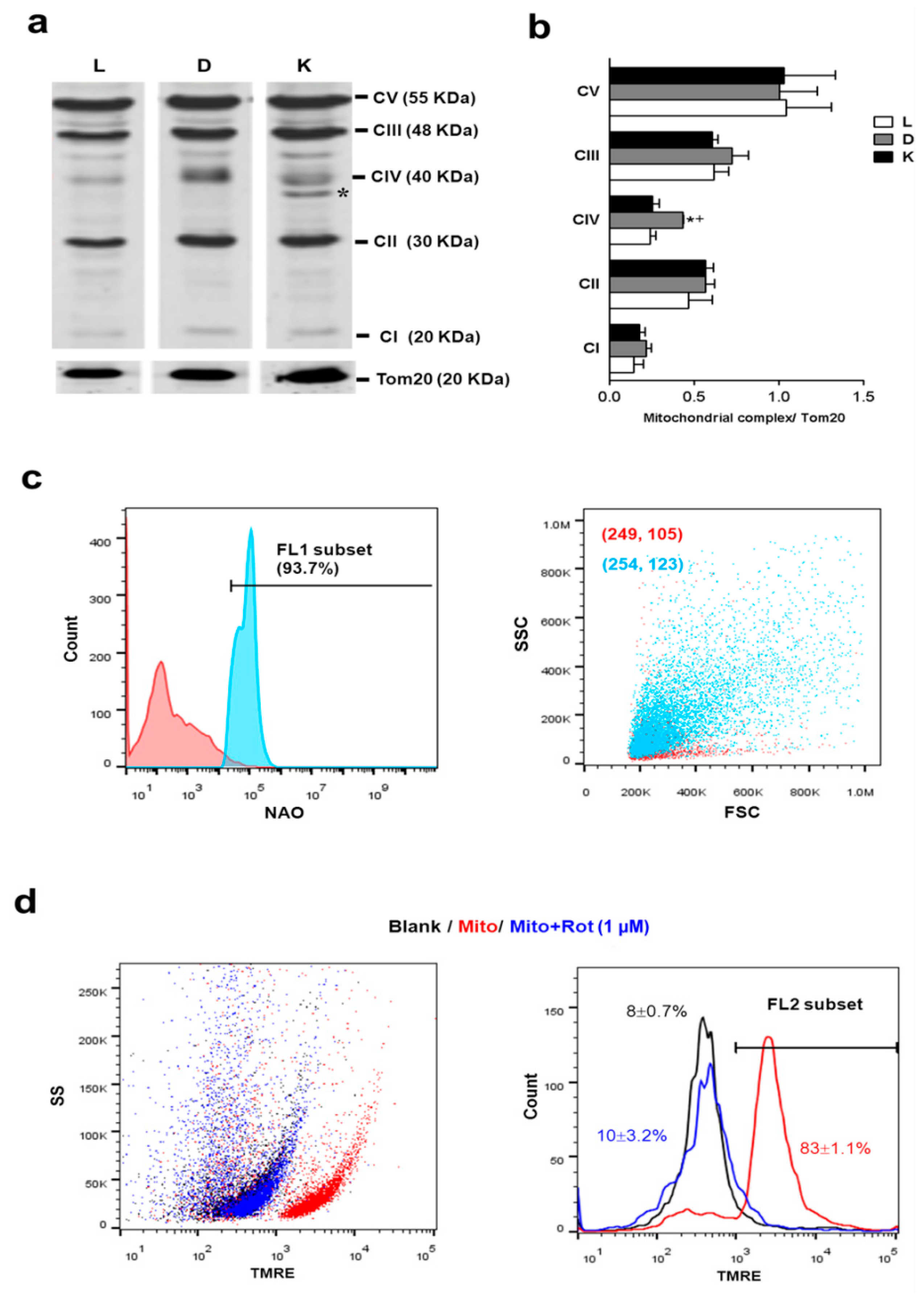
3.5. Function of Device-Extracted Mitochondria
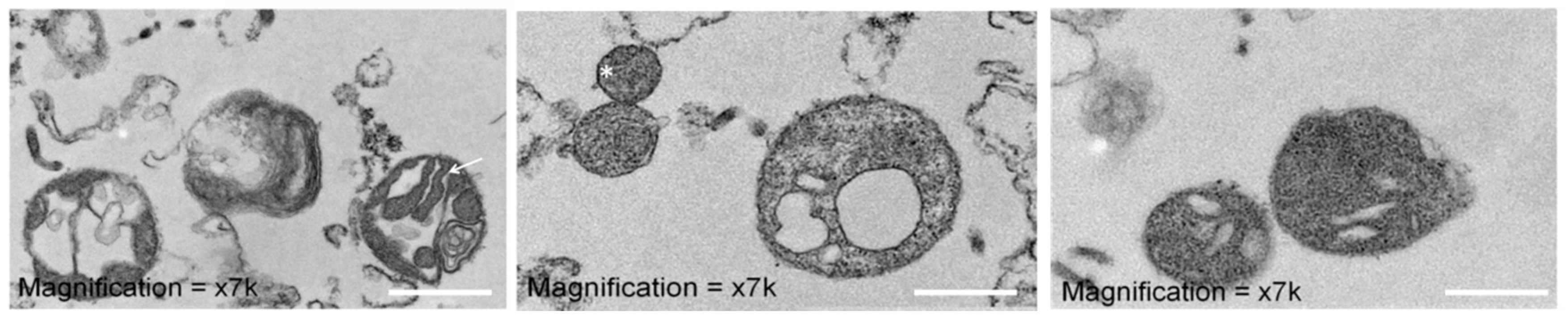
3.6. Mitochondrial Ultrastructure of Device-Extracted Mitochondria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Desler, C.; Rasmussen, L.J. Mitochondria in biology and medicine. Mitochondrion 2012, 12, 472–476. [Google Scholar] [CrossRef] [PubMed]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More than just a powerhouse. Curr. Biol. 2006, 16, R551–560. [Google Scholar] [CrossRef] [PubMed]
- Voet, D.; Voet, J.G.; Pratt, C.W. Fundamentals of Biochemistry, 2nd ed.; John Wiley and Sons, Inc.: New York, NY, USA, 2006. [Google Scholar]
- Richter, C.; Park, J.; Ames, B.N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc. Natl. Acad. Sci. USA 1988, 85, 6465–6467. [Google Scholar] [CrossRef] [PubMed]
- Pourahmad, J.; Hosseini, M.J. Toxicity of Arsenic (III) on Isolated Liver Mitochondria: A New Mechanistic Approach. Iran. J. Pharm. Res. 2012, 11, 703–704. [Google Scholar] [PubMed]
- Giang, A.H.; Raymond, T.; Brookes, P.; de Mesy Bentley, K.; Schwarz, E.; O’Keefe, R.; Eliseev, R. Mitochondrial Dysfunction and permeability transition in osteosarcoma cells showing the warburg effect. J. Biol. Chem. 2013, 288, 33303–33311. [Google Scholar] [CrossRef] [PubMed]
- Knott, A.B.; Perkins, G.; Schwarzenbacher, R.; Bossy-Wetzel, E. Mitochondrial fragmentation in neurodegeneration. Nat. Rev. Neurosci. 2008, 9, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Spees, J.L.; Olson, S.D.; Whitney, M.J.; Prockop, D.J. Mitochondrial transfer between cells can rescue aerobic respiration. Cell Biol. 2006, 103, 1283–1288. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.L.; Jiang, X.P.; Head, J.F. Mitochondria organelle transplantation: Introduction of normal epithelial mitochondria into human cancer cells inhibits proliferation and increases drug sensitivity. Breast Cancer Res. Treat. 2012, 136, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Masuzawa, A.; Black, K.M.; Pacak, C.A.; Ericsson, M.; Barnett, R.J.; Drumm, C.; Seth, P.; Bloch, D.B.; Levitsky, S.; Cowan, D.B.; et al. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H966–H982. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Kwon, K.W.; Park, M.C.; Lee, S.H.; Kim, S.M.; Suh, K.Y. Soft Lithography for Microfluidics: A Review. Biochip J. 2008, 2, 1–11. [Google Scholar]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Kido, H.; Micic, M.; Smith, D.; Zoval, J.; Norton, J.; Madou, M. A novel compact disk-like centrifugal microfluidics system for cell lysis and sample homogenization. Colloids Surf. B Biointerfaces 2007, 58, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.Y.; Wo, A.M.; Lo, Y.J.; Chen, K.C.; Lin, C.M.; Yang, C.R. Three dimensional electrode array for cell lysis via electroporation. Biosens. Bioelectron. 2006, 22, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.K.; Ra, G.S.; Joo, G.S.; Kim, Y.S. Electrochemical cell lysis on a miniaturized flow-through device. Curr. Appl. Phys. 2009, 9, e301–e303. [Google Scholar] [CrossRef]
- Ikeda, N.; Tanaka, N.; Yanagida, Y.; Hatsuzawa, T. On-chip single-cell lysis for extracting intracellular material. Jpn. J. Appl. Phys. 2007, 46, 6410. [Google Scholar] [CrossRef]
- Liu, C. Recent Developments in Polymer MEMS. Adv. Mater. 2007, 19, 3783–3790. [Google Scholar] [CrossRef]
- Nisar, A.; Afzulpurkar, N.; Mahaisavariya, B.; Tuantranont, A. MEMS-based, micropumps in drug delivery and biomedical applications. Sens. Actuators B Chem. 2008, 130, 917–942. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, G.Y.; Lin, Y.C.; Wang, G.J. A lab-on-a-chip capillary network for red blood cell hydrodynamics. Microfluid. Nnaofluid. 2010, 9, 585–591. [Google Scholar] [CrossRef]
- Li, Y.; Park, J.S.; Deng, J.H.; Bai, Y. Cytochrome c oxidase subunit IV is essential for assembly and respiratory function of the enzyme complex. J. Bioenerg. Biomembr. 2006, 38, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Terada, K.; Kanazawa, M.; Yano, M.; Hanson, B.; Hoogenraad, N.; Mori, M. Participation of the import receptor Tom20 in protein import into mammalian mitochondria: Analyses in vitro and in cultured cells. FEBS Lett. 1997, 403, 309–312. [Google Scholar] [CrossRef]
- Ramirez-Aguilar, S.J.; Keuthe, M.; Rocha, M.; Fedyaev, V.V.; Kramp, K.; Gupta, K.J.; Rasmusson, A.G.; Schulze, W.X.; van Dongen, J.T. The composition of plant mitochondrial supercomplexes changes with oxygen availability. J. Biol. Chem. 2011, 286, 43045–43053. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C.; Liu, K.H.; Chuang, C.S.; Su, H.L.; Wei, Y.H.; Kuo, S.J.; Liu, C.S. Treatment of human cells derived from MERRF syndrome by peptide-mediated mitochondrial delivery. Cytotherapy 2013, 15, 1580–1596. [Google Scholar] [CrossRef] [PubMed]
- Corcelli, A.; Saponetti, M.S.; Zaccagnino, P.; Lopalco, P.; Mastrodonato, M.; Liquori, G.E.; Lorusso, M. Mitochondria isolated in nearly isotonic KCl buffer: Focus on cardiolipin and organelle morphology. Biochim. Biophys. Acta 2010, 1798, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Gross, V.S.; Greenberg, H.K.; Baranov, S.V.; Carlson, G.M.; Stavrovskaya, I.G.; Lazarev, A.V.; Kristal, B.S. Isolation of functional mitochondria from rat kidney and skeletal muscle without manual homogenization. Anal. Biochem. 2011, 418, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Hornig-Do, H.T.; Günther, G.; Bust, M.; Lehnartz, P.; Bosio, A.; Wiesnera, R.J. Isolation of functional pure mitochondria by superparamagnetic microbeads. Anal. Biochem. 2009, 389, 1–5. [Google Scholar] [CrossRef] [PubMed]
| n | m | ΔP (mPa) | σ (mPa) |
|---|---|---|---|
| 30 | 0 | 6.73 × 10−5 | 1.722 × 10−6 |
| 30 | 5 | 8.40 × 10−5 | 2.15 × 10−6 |
| 30 | 10 | 10.49 × 10−5 | 2.69 × 10−6 |
| 30 | 15 | 13.99 × 10−5 | 3.58 × 10−6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, Y.-H.; Li, C.-W.; Chang, J.-C.; Chen, S.-T.; Liu, C.-S.; Wang, G.-J. Chemical-Free Extraction of Functional Mitochondria Using a Microfluidic Device. Inventions 2018, 3, 68. https://doi.org/10.3390/inventions3040068
Hsiao Y-H, Li C-W, Chang J-C, Chen S-T, Liu C-S, Wang G-J. Chemical-Free Extraction of Functional Mitochondria Using a Microfluidic Device. Inventions. 2018; 3(4):68. https://doi.org/10.3390/inventions3040068
Chicago/Turabian StyleHsiao, Yu-Han, Ching-Wen Li, Jui-Chih Chang, Sung-Tzu Chen, Chin-San Liu, and Gou-Jen Wang. 2018. "Chemical-Free Extraction of Functional Mitochondria Using a Microfluidic Device" Inventions 3, no. 4: 68. https://doi.org/10.3390/inventions3040068
APA StyleHsiao, Y.-H., Li, C.-W., Chang, J.-C., Chen, S.-T., Liu, C.-S., & Wang, G.-J. (2018). Chemical-Free Extraction of Functional Mitochondria Using a Microfluidic Device. Inventions, 3(4), 68. https://doi.org/10.3390/inventions3040068