A Microfluidic Cell Stretch Device to Investigate the Effects of Stretching Stress on Artery Smooth Muscle Cell Proliferation in Pulmonary Arterial Hypertension
Abstract
1. Introduction
2. Materials and Methods
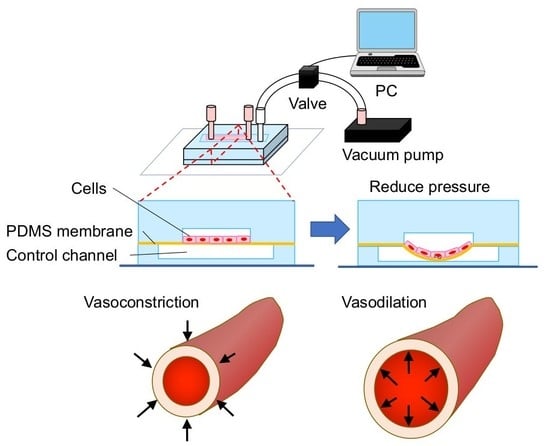
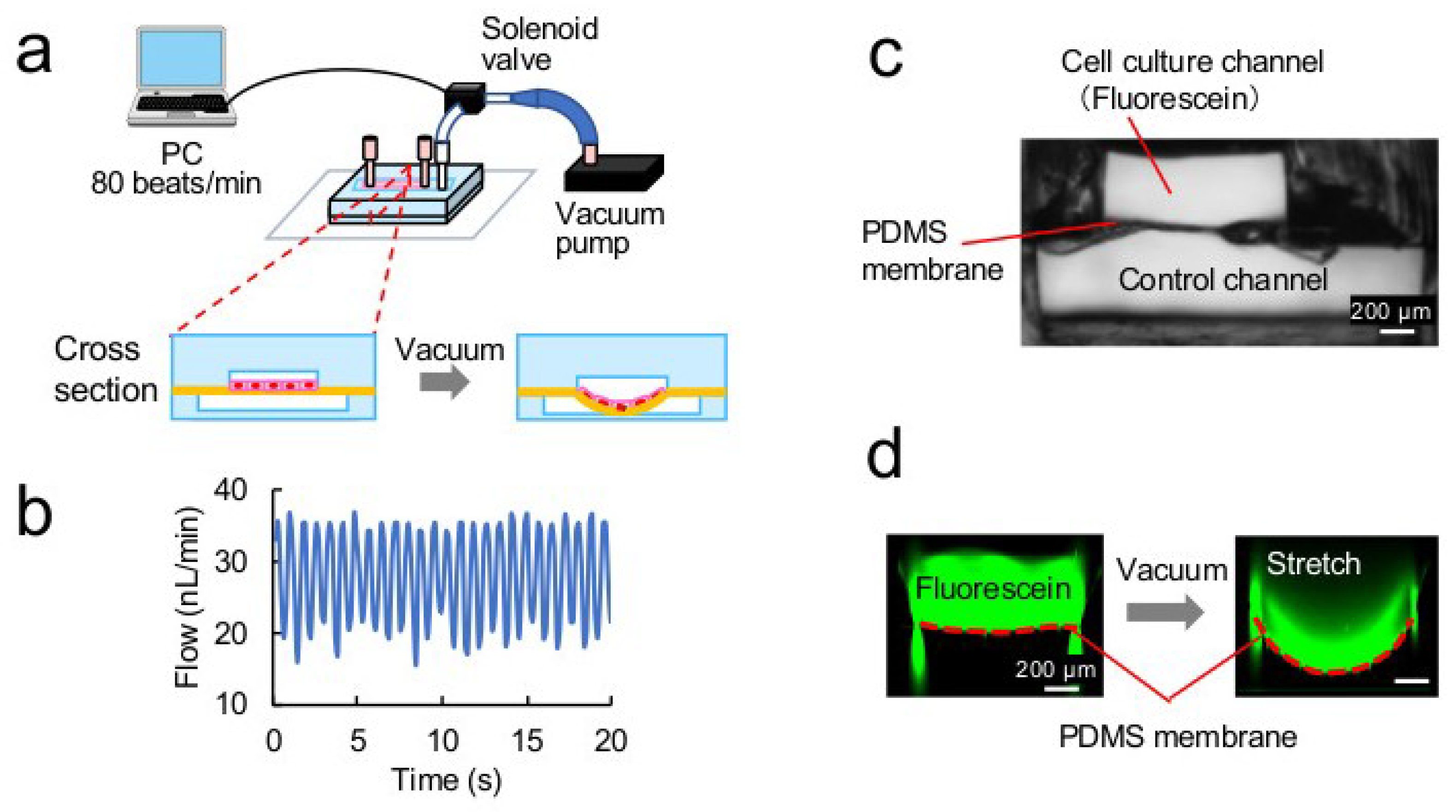
2.1. Fabrication of a Cell Stretch Device
2.2. Analysis of Stretching Properties of the PDMS Membrane
2.3. Microfluidic Cell Culture
2.4. Cell Staining
2.5. RNA Extraction, cDNA Synthesis, and Real-Time PCR
3. Results and Discussion
3.1. Microdevice
3.2. Stretching Properties of the PDMS Membrane
3.3. Stretch Culture
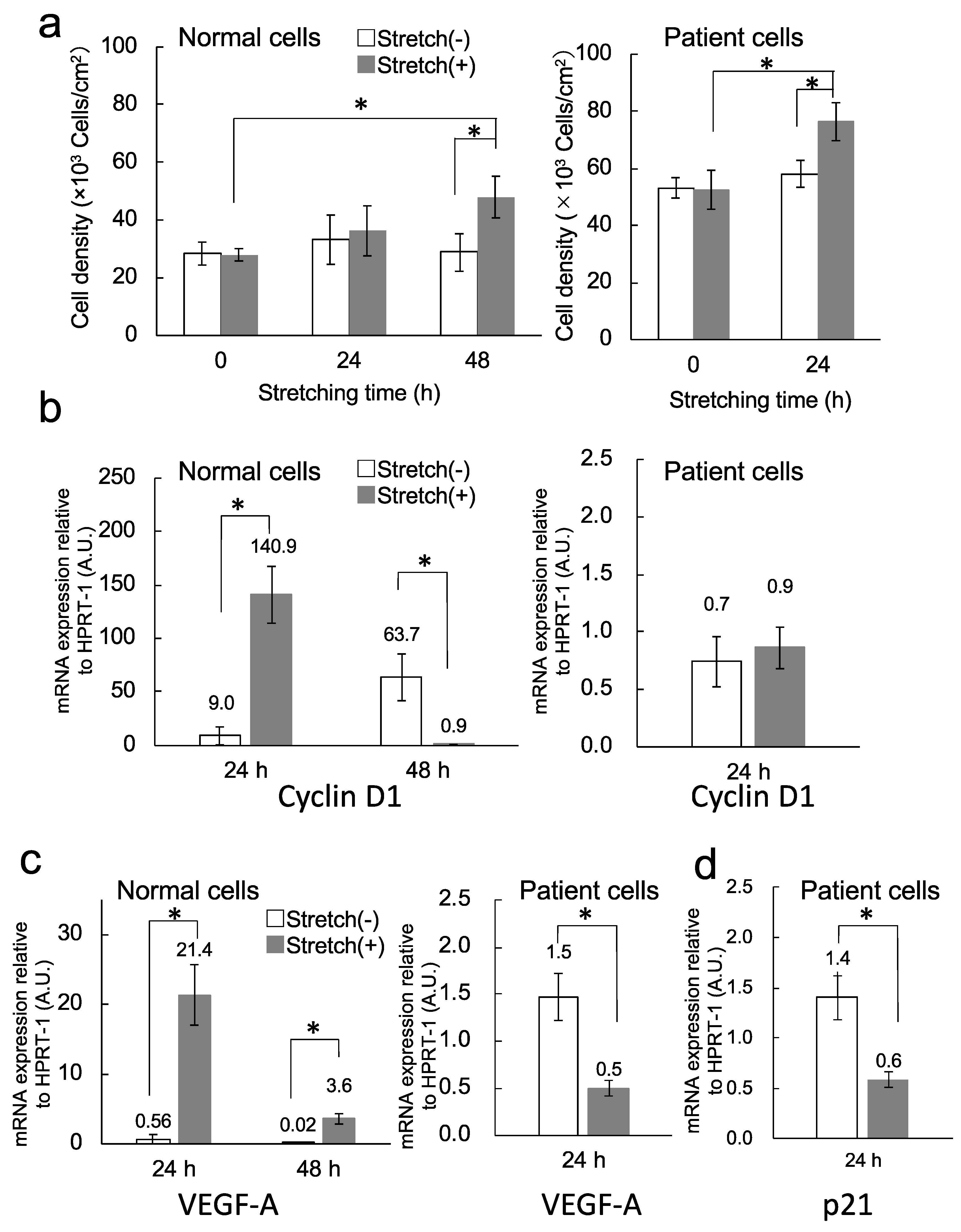
3.4. Cell Proliferation
3.5. Transcription Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vonk Noordegraaf, A.; Groeneveldt, J.A.; Bogaard, H.J. Pulmonary hypertension. Eur. Respir. Rev. 2016, 25, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Maarman, G.; Lecour, S.; Butrous, G.; Thienemann, F.; Sliwa, K. A comprehensive review: The evolution of animal models in pulmonary hypertension research; are we there yet? Pulm. Circ. 2013, 3, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Firth, A.L.; Yao, W.; Madani, M.M.; Kerr, K.M.; Auger, W.R.; Jamieson, S.W.; Thistlethwaite, P.A.; Yuan, J.X. Inhibition of mTOR attenuates store-operated Ca2+ entry in cells from endarterectomized tissues of patients with chronic thromboembolic pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L666–L676. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Firth, A.L.; Smith, K.A.; Maliakal, M.V.; Yuan, J.X. PDGF enhances store-operated Ca2+ entry by upregulating STIM1/Orai1 via activation of Akt/mTOR in human pulmonary arterial smooth muscle cells. Am. J. Physiol. Cell Physiol. 2012, 302, C405–C411. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Firth, A.L.; Ariyasu, S.; Yamadori, I.; Matsubara, H.; Song, S.; Fraidenburg, D.R.; Yuan, J.X. Thrombin-mediated activation of Akt signaling contributes to pulmonary vascular remodeling in pulmonary hypertension. Physiol. Rep. 2013, 1, e00190. [Google Scholar] [CrossRef]
- Sasaki, N.; Shinjo, M.; Hirakawa, S.; Nishinaka, M.; Tanaka, Y.; Mawatari, K.; Kitamori, T.; Sato, K. A palmtop-sized microfluidic cell culture system driven by a miniaturized infusion pump. Electrophoresis 2012, 33, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sasaki, N.; Svahn, H.A.; Sato, K. Microfluidics for nano-pathophysiology. Adv. Drug Deliv. Rev. 2014, 74, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Sasaki, N.; Ato, M.; Hirakawa, S.; Sato, K.; Sato, K. Microcirculation-on-a-chip: A microfluidic platform for assaying blood- and lymphatic-vessel permeability. PLoS ONE 2015, 10, e0137301. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sato, K. Recent Progress in the Development of Microfluidic Vascular Models. Anal. Sci. 2018, 34, 755–764. [Google Scholar] [CrossRef]
- Tazawa, H.; Sunaoshi, S.; Tokeshi, M.; Kitamori, T.; Ohtani-Kaneko, R. An easy-to-use polystyrene microchip-based cell culture system. Anal. Sci. 2016, 32, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Ohtani-Kaneko, R.; Sato, K.; Tsutiya, A.; Nakagawa, Y.; Hashizume, K.; Tazawa, H. Characterisation of human induced pluripotent stem cell-derived endothelial cells under shear stress using an easy-to-use microfluidic cell culture system. Biomed. Microdevices 2017, 19, 91. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, A.; Albers, H.J.; Passier, R.; Mummery, C.L.; van den Berg, A.; Orlova, V.V.; van der Meer, A.D. Advanced in vitro models of vascular biology: Human induced pluripotent stem cells and organ-on-chip technology. Adv. Drug Deliv. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.M.; Stroka, K.M. Vascular endothelial cell mechanosensing: New insights gained from biomimetic microfluidic models. Semin. Cell Dev. Biol. 2017, 71, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Niklason, L.E. Microfluidic artificial “vessels” for dynamic mechanical stimulation of mesenchymal stem cells. Integr. Biol. 2012, 4, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Jiang, B.; Wang, D.; Zhang, W.; Wang, Z.; Jiang, X. A microfluidic flow-stretch chip for investigating blood vessel biomechanics. Lab Chip 2012, 12, 3441–3450. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zheng, W.; Liu, W.; Zhang, W.; Long, Y.; Jiang, X. Investigation of tumor cell behaviors on a vascular microenvironment-mimicking microfluidic chip. Sci. Rep. 2015, 5, 17768. [Google Scholar] [CrossRef]
- Van Engeland, N.C.A.; Pollet, A.; den Toonder, J.M.J.; Bouten, C.V.C.; Stassen, O.; Sahlgren, C.M. A biomimetic microfluidic model to study signalling between endothelial and vascular smooth muscle cells under hemodynamic conditions. Lab Chip 2018, 18, 1607–1620. [Google Scholar] [CrossRef]
- Yasotharan, S.; Pinto, S.; Sled, J.G.; Bolz, S.S.; Gunther, A. Artery-on-a-chip platform for automated, multimodal assessment of cerebral blood vessel structure and function. Lab Chip 2015, 15, 2660–2669. [Google Scholar] [CrossRef]
- Sato, K.; Nakajima, M.; Tokuda, S.; Ogawa, A. Fluidic culture and analysis of pulmonary artery smooth muscle cells for the study of pulmonary hypertension. Anal. Sci. 2016, 32, 1217–1221. [Google Scholar] [CrossRef]
- Ogawa, A.; Satoh, T.; Tamura, Y.; Fukuda, K.; Matsubara, H. Survival of Japanese patients with idiopathic/heritable pulmonary arterial hypertension. Am. J. Cardiol. 2017, 119, 1479–1484. [Google Scholar] [CrossRef]
- Mata-Greenwood, E.; Grobe, A.; Kumar, S.; Noskina, Y.; Black, S.M. Cyclic stretch increases VEGF expression in pulmonary arterial smooth muscle cells via TGF-β1 and reactive oxygen species: A requirement for NADPH oxidase. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L288–L289. [Google Scholar] [CrossRef]
- Chang, H.; Shyu, K.G.; Wang, B.W.; Kuan, P. Regulation of hypoxia-inducible factor-1α by cyclical mechanical stretch in rat vascular smooth muscle cells. Clin. Sci. 2003, 105, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, Y.; Sato, K. Effects of microchannel shape and ultrasonic mixing on microfluidic padlock probe rolling circle amplification (RCA) reactions. Micromachines 2018, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sato, M.; Yokoyama, M.; Hirai, M.; Furuta, A. Influence of culture conditions on cell proliferation in a microfluidic channel. Anal. Sci. 2018. [Google Scholar] [CrossRef]
- Standley, P.R.; Cammarata, A.; Nolan, B.P.; Purgason, C.T.; Stanley, M.A. Cyclic stretch induces vascular smooth muscle cell alignment via NO signaling. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1907–H1914. [Google Scholar] [CrossRef]
- Bono, N.; Pezzoli, D.; Levesque, L.; Loy, C.; Candiani, G.; Fiore, G.B.; Mantovani, D. Unraveling the role of mechanical stimulation on smooth muscle cells: A comparative study between 2D and 3D models. Biotechnol. Bioeng. 2016, 113, 2254–2263. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Nakamura, K.; Matsubara, H.; Fujio, H.; Ikeda, T.; Kobayashi, K.; Miyazaki, I.; Asanuma, M.; Miyaji, K.; Miura, D.; et al. Prednisolone inhibits proliferation of cultured pulmonary artery smooth muscle cells of patients with idiopathic pulmonary arterial hypertension. Circulation 2005, 112, 1806–1812. [Google Scholar] [CrossRef]
- Fujio, H.; Nakamura, K.; Matsubara, H.; Kusano, K.F.; Miyaji, K.; Nagase, S.; Ikeda, T.; Ogawa, A.; Ohta-Ogo, K.; Miura, D.; et al. Carvedilol inhibits proliferation of cultured pulmonary artery smooth muscle cells of patients with idiopathic pulmonary arterial hypertension. J. Cardiovasc. Pharmacol. 2006, 47, 250–255. [Google Scholar] [CrossRef]
- Sherr, C.J. D-type cyclins. Trends Biochem. Sci. 1995, 20, 187–190. [Google Scholar] [CrossRef]
- Holmes, D.I.; Zachary, I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005, 6, 209. [Google Scholar] [CrossRef][Green Version]
- Quinn, T.P.; Schlueter, M.; Soifer, S.J.; Gutierrez, J.A. Cyclic mechanical stretch induces VEGF and FGF-2 expression in pulmonary vascular smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L897–L903. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Huang, X.; Xue, Z.; Cao, D.; Huang, K.; Chen, J.; Pan, Y.; Gao, Y. The role of p21 in apoptosis, proliferation, cell cycle arrest, and antioxidant activity in UVB-irradiated human HaCaT keratinocytes. Med. Sci. Monit. Basic Res. 2015, 21, 86–95. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, K.; Nitta, M.; Ogawa, A. A Microfluidic Cell Stretch Device to Investigate the Effects of Stretching Stress on Artery Smooth Muscle Cell Proliferation in Pulmonary Arterial Hypertension. Inventions 2019, 4, 1. https://doi.org/10.3390/inventions4010001
Sato K, Nitta M, Ogawa A. A Microfluidic Cell Stretch Device to Investigate the Effects of Stretching Stress on Artery Smooth Muscle Cell Proliferation in Pulmonary Arterial Hypertension. Inventions. 2019; 4(1):1. https://doi.org/10.3390/inventions4010001
Chicago/Turabian StyleSato, Kae, Manami Nitta, and Aiko Ogawa. 2019. "A Microfluidic Cell Stretch Device to Investigate the Effects of Stretching Stress on Artery Smooth Muscle Cell Proliferation in Pulmonary Arterial Hypertension" Inventions 4, no. 1: 1. https://doi.org/10.3390/inventions4010001
APA StyleSato, K., Nitta, M., & Ogawa, A. (2019). A Microfluidic Cell Stretch Device to Investigate the Effects of Stretching Stress on Artery Smooth Muscle Cell Proliferation in Pulmonary Arterial Hypertension. Inventions, 4(1), 1. https://doi.org/10.3390/inventions4010001