The Development of a Highly Sensitive Fiber-Optic Oxygen Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Two Fiber-Optic Oxygen Sensors
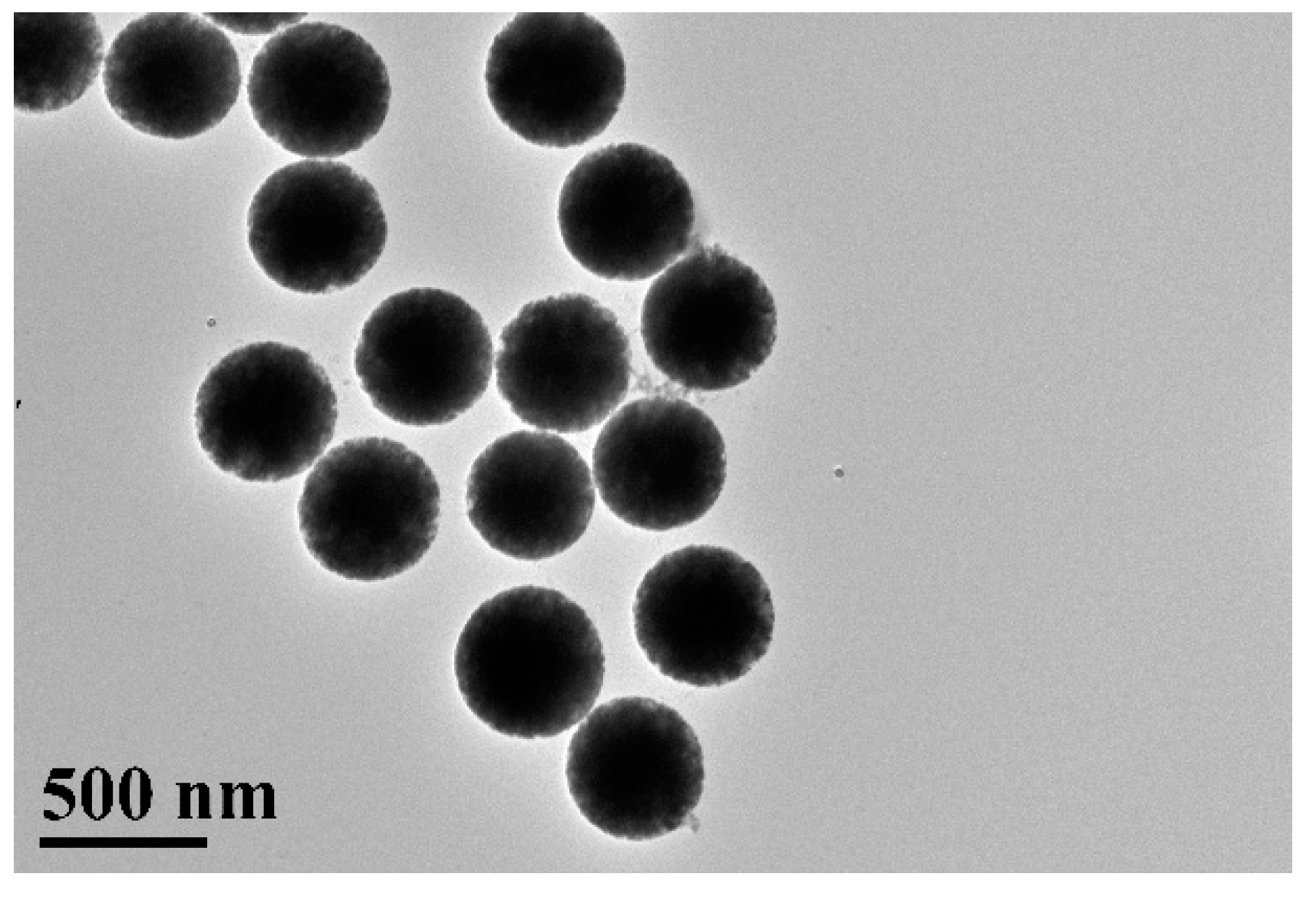
2.2. Synthesis of Solid Monodispersive SiO2 Spheres
2.3. Synthesis of Porous Silica Nanoparticles
2.4. The Sol-Gel Mixing and Dip-Coating Processes
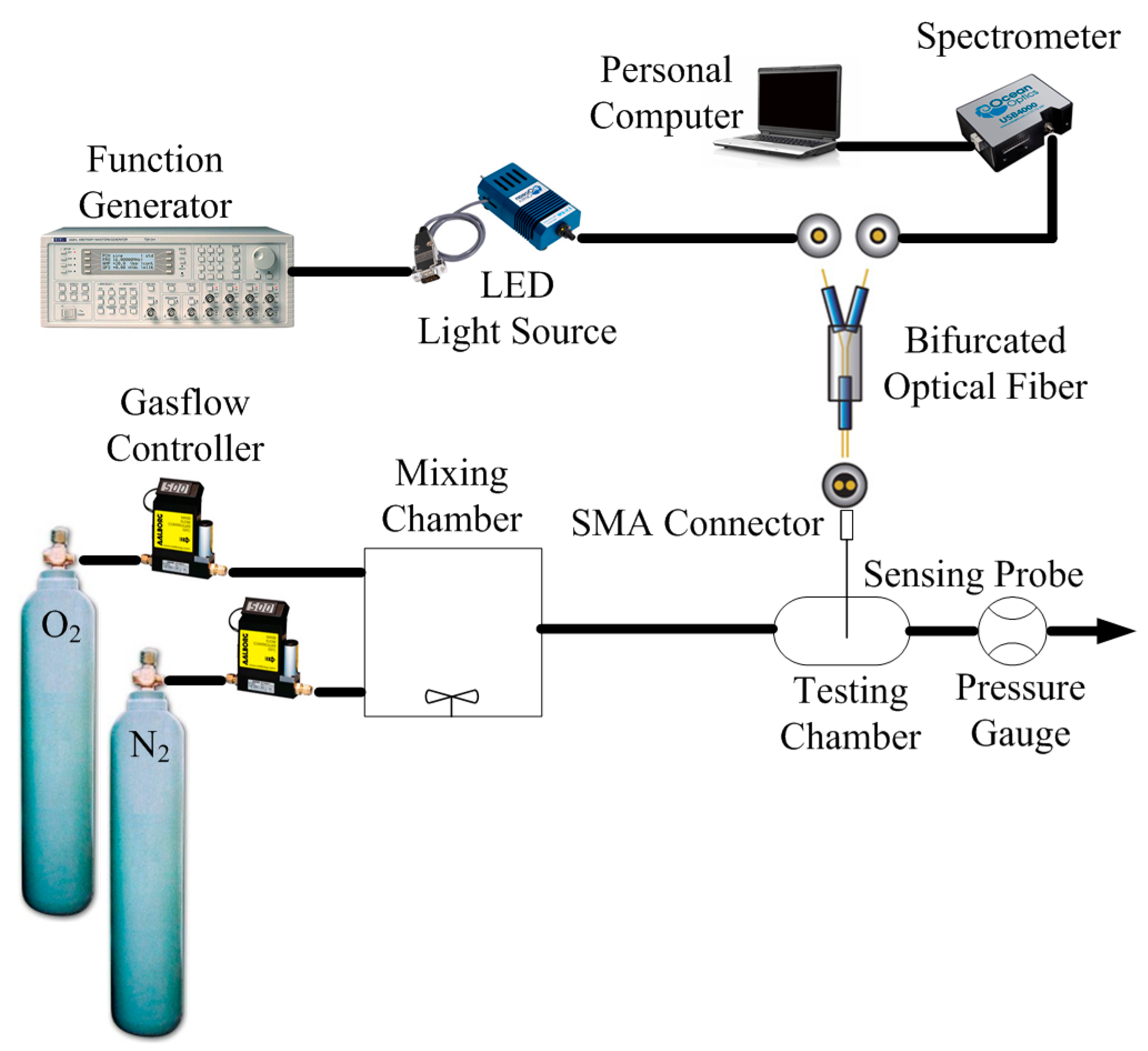
2.5. Instrumentation
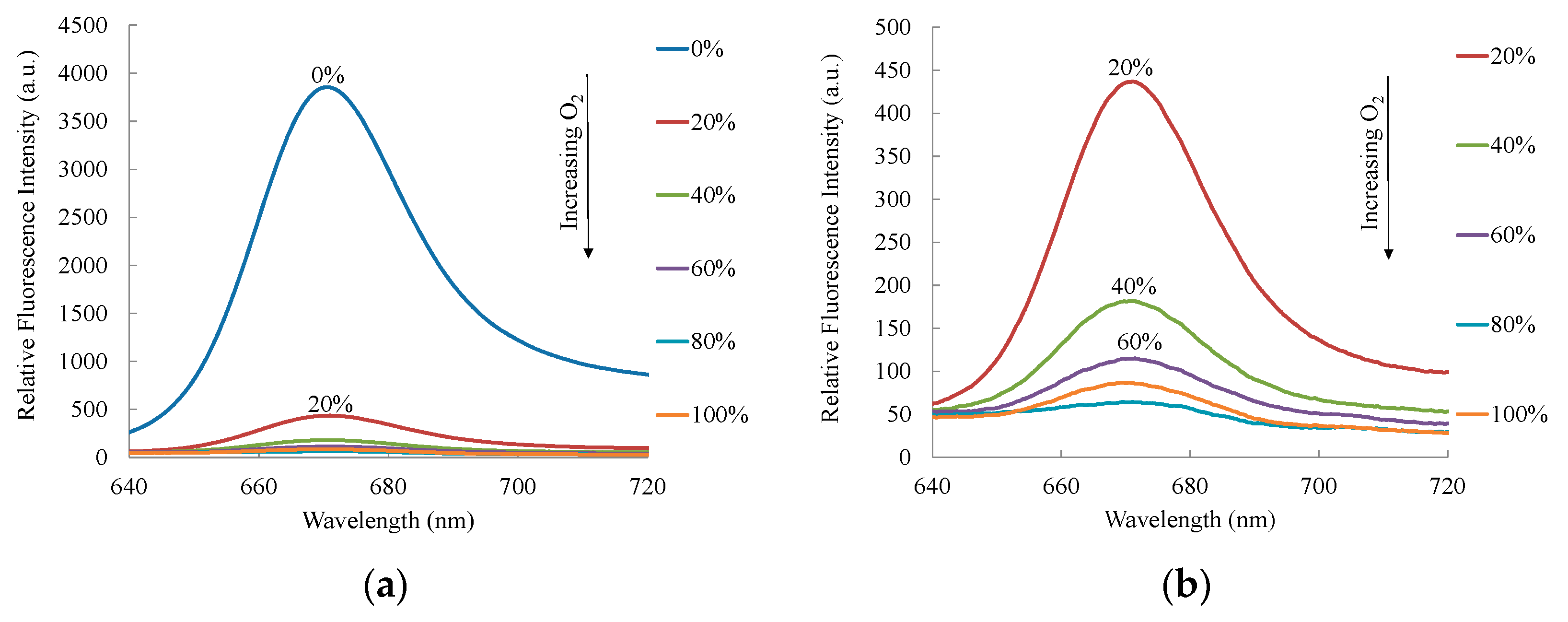
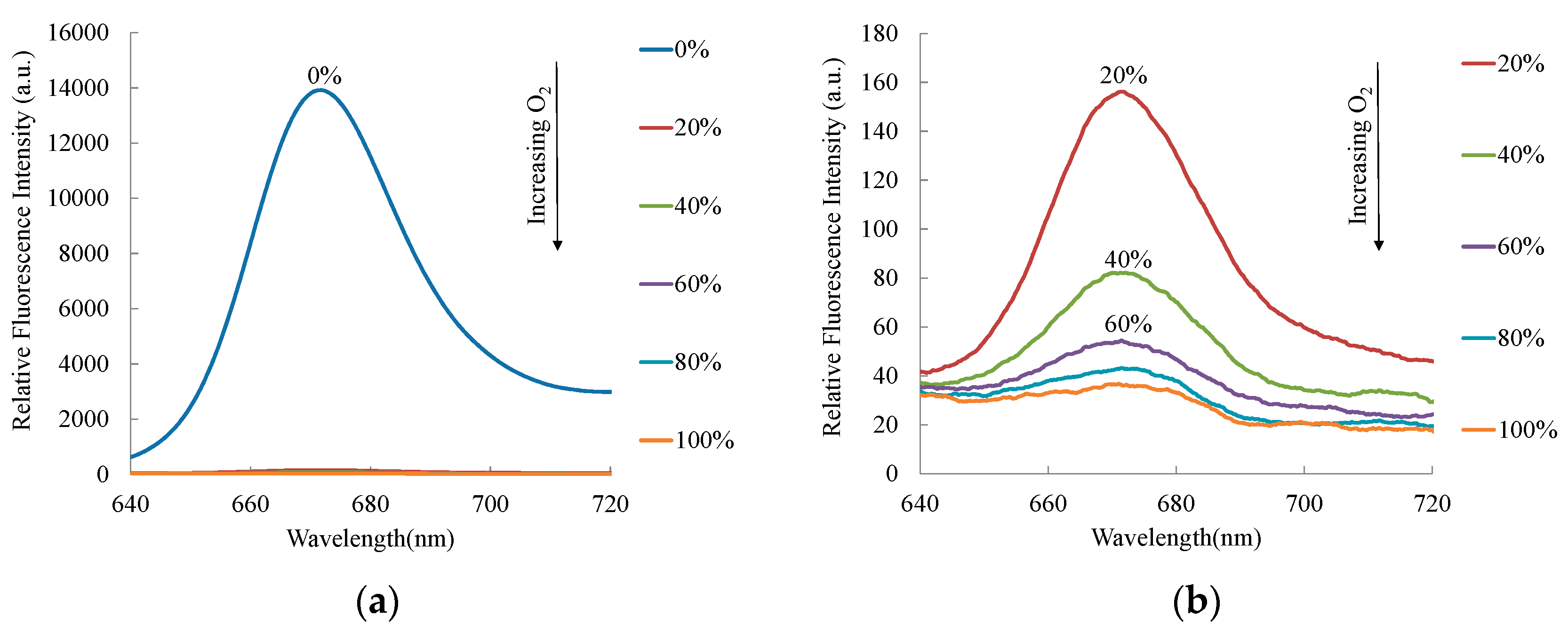
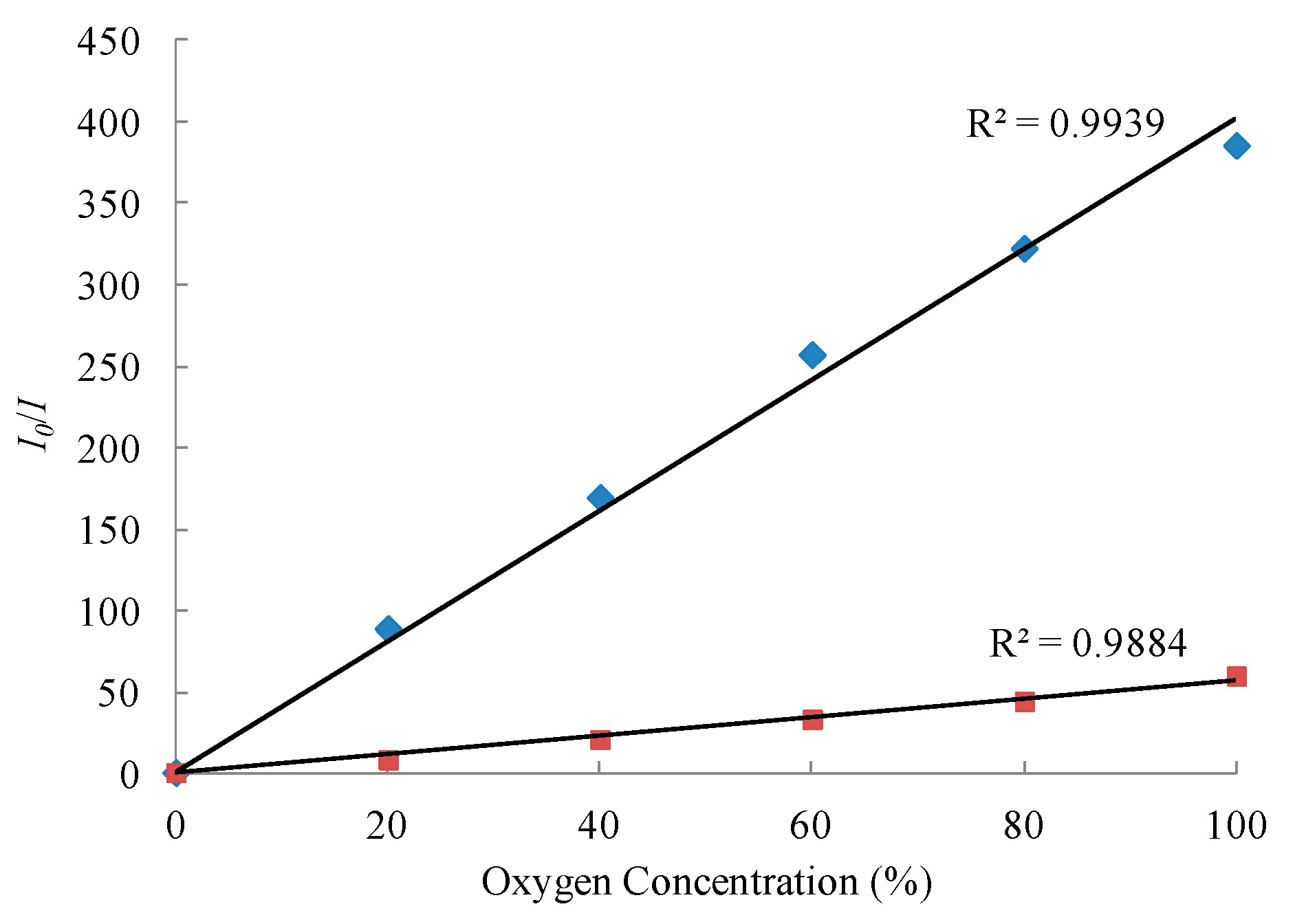
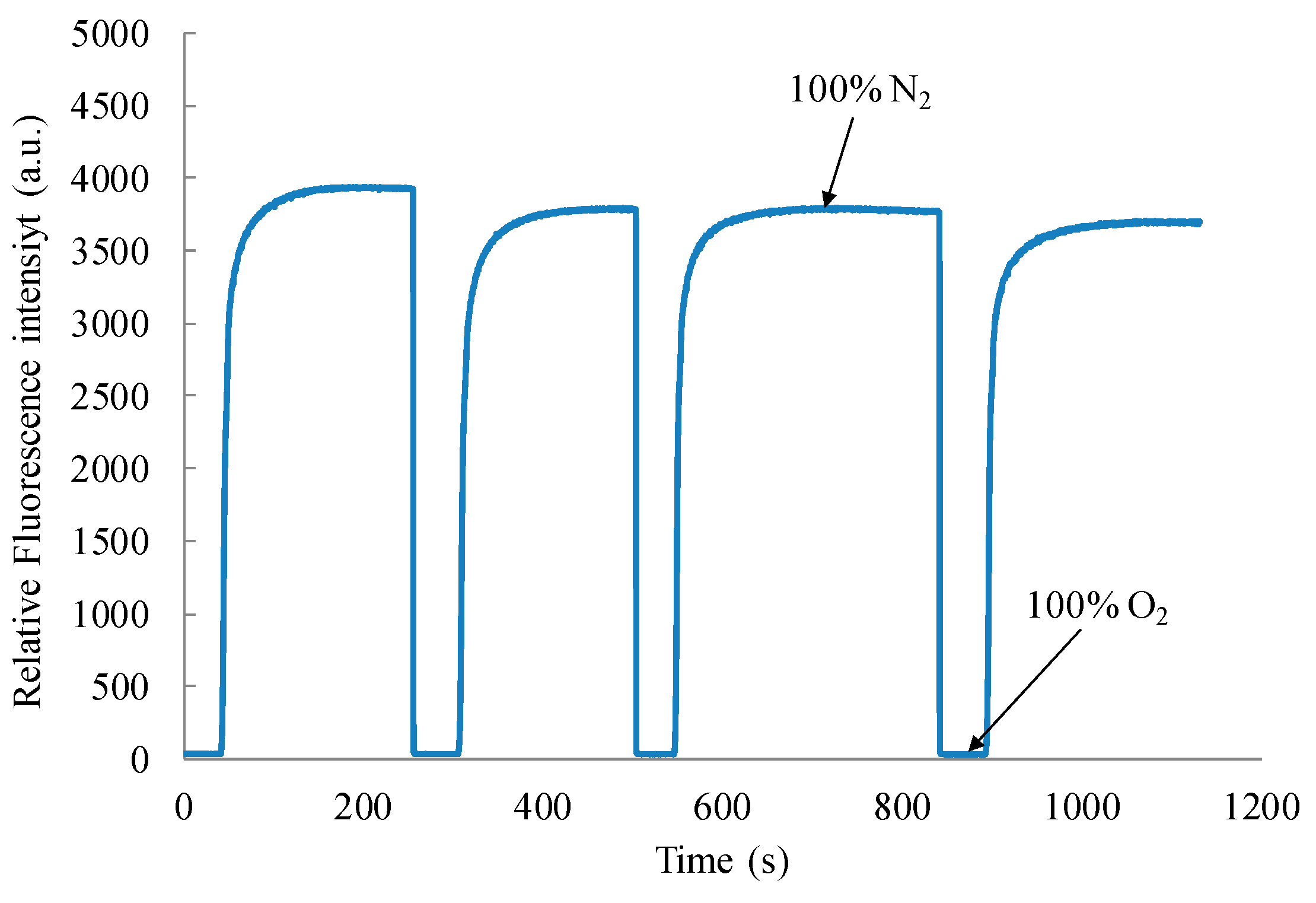
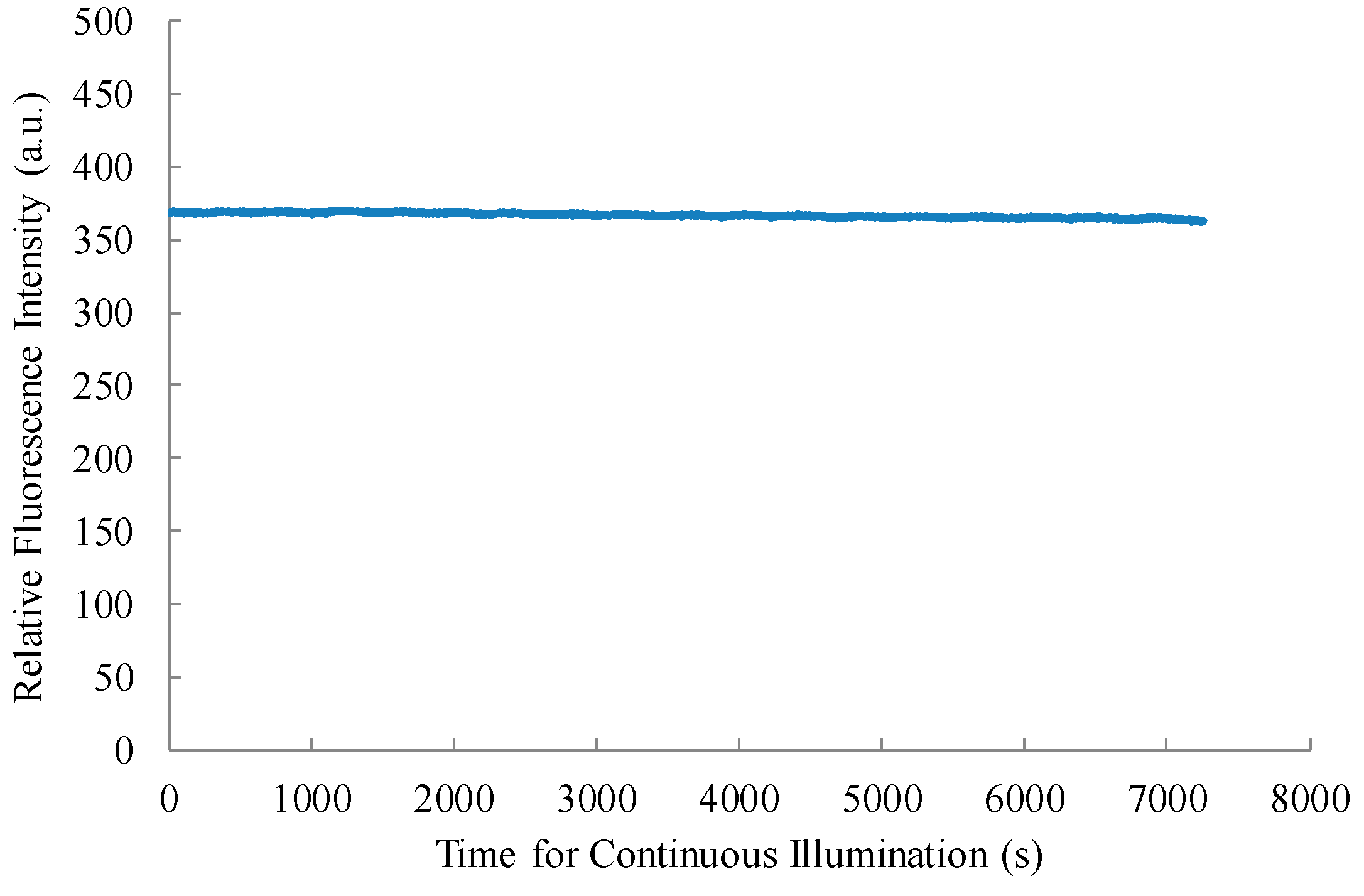
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Clark, L.C., Jr. Monitor and control of blood and tissue oxygen tensions. Trans. Am. Soc. Artif. Intern. Organs 1956, 2, 41–48. [Google Scholar]
- Papkovsky, D.B. New oxygen sensors and their application to biosensing. Sens. Actuators B Chem. 1995, 29, 213–218. [Google Scholar] [CrossRef]
- Demas, J.N.; Degraff, B.A.; Coleman, P.B. Oxygen sensors based on luminescence quenching. Anal. Chem. 1999, 71, 793A–800A. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, K.; Sakai, S.; Hase, K.; Minamitani, H. Development of catheter type optical oxygen sensor and applications to bioinstrumentation. Biosens. Bioelectron. 2003, 18, 1439–1445. [Google Scholar] [CrossRef]
- Vander Donckt, E.; Camerman, B.; Herne, R.; Vandeloise, R. Fiber-optic oxygen sensor based on luminescence quenching of a Pt(II) complex embedded in polymer matrices. Sens. Actuators B Chem. 1996, 32, 121–127. [Google Scholar] [CrossRef]
- Lee, S.K.; Okura, I. Photostable optical oxygen sensing material: Platinum tetrakis (pentafluorophenyl) porphyrin immobilized in polystyrene. Anal. Comm. 1997, 34, 185–188. [Google Scholar] [CrossRef]
- Zhang, H.R.; Lei, B.F.; Liu, Y.L.; Liu, X.T.; Zhang, M.T.; Dong, H.W.; Xiao, Y.; Zhang, J.Y. Sol-gel-derived highly sensitive optical oxygen sensing materials using Ru(II) complex via covalent grafting strategy. J. Nanosci. Nanotechnol. 2014, 14, 4615–4621. [Google Scholar] [CrossRef] [PubMed]
- Mill, A.; Graham, A.; O’Rourke, C. A novel, titania sol-gel derived film for luminescence-based oxygen sensing. Sens. Actuators B Chem. 2014, 190, 907–912. [Google Scholar] [CrossRef]
- Farooq, A.; AI-jowder, R.; Narayanaswamy, R.; Azzawi, M.; Roche, P.J.R.; Whitrhead, D.E. Gas detection using quenching fluorescence of dye-immobilised silica nanoparticles. Sens. Actuators B Chem. 2013, 183, 230–238. [Google Scholar] [CrossRef]
- Chu, C.S.; Lin, C.A. Optical fiber sensor for dual sensing of temperature and oxygen based on PtTFPP/CF embedded in sol-gel matrix. Sens. Actuators B Chem. 2014, 195, 259–265. [Google Scholar] [CrossRef]
- Chu, C.S.; Lin, T.H. Ratiometric optical sensor for dual sensing of temperature and oxygen. Sens. Actuators B Chem. 2015, 210, 302–307. [Google Scholar] [CrossRef]
- Chu, C.S.; Lin, K.Z.; Tang, Y.H. A new optical sensor for sensing oxygen based on phase shift detection. Sens. Actuators B Chem. 2016, 223, 606–612. [Google Scholar] [CrossRef]
- McDonagh, C.; MacCraith, B.D.; McEcoy, A.K. Tailoring of sol-gel films for optical sensing of oxygen in gas and aqueous phase. Anal. Chem. 1998, 70, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Lehner, P.; Klimant, I. Novel optical trace oxygen sensors based on platinum(II) and palladium(II) complexes with 5,10,15,20-meso-tetrakis-(2,3,4,5,6-pentafluorphenyl)-porphyrin covalently immobilized on silica-gel particles. Anal. Chim. Acta 2011, 690, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Borisov, S.M.; Vasylevska, A.S.; Krause, C.; Wolfbeis, O.S. Composite luminescent material for dual sensing of oxygen and temperature. Adv. Mater. 2006, 16, 1536–1542. [Google Scholar] [CrossRef]
- Chu, C.S.; Lo, Y.L. Ratiometric fiber-optic oxygen sensors based on sol-gel matrix doped with metalloporphyrin and 7-amino-4-trifluoromethyl coumarin. Sens. Actuators B Chem. 2008, 134, 711–717. [Google Scholar] [CrossRef]
- Badocco, D.; Mondin, A.; Pastore, P. Rationalization of the behavior of a bi-label oxygen optical sensor. Sens. Actuators B Chem. 2011, 158, 54–61. [Google Scholar] [CrossRef]
- Chu, C.S. Optical fiber oxygen sensor based on Pd(II) complex embedded in sol-gel matrix. J. Lumin. 2013, 135, 5–9. [Google Scholar] [CrossRef]
- Xue, R.P.; Ge, C.; Richardson, K.; Palmer, A.; Viapiano, M.; lannutti, J.L. Microscal sensing of oxygen via encapsulated of porphyrin nanofibers: Effect of indicator and polymer “core” permeability. ACS Appl. Mater. Interfaces 2015, 7, 8606–8614. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Chuang, C.Y. Highly sensitive fiber-optic oxygen sensor based on palladium tetrakis (4-carboxyphenyl)porphyrin doped in ormosil. J. Lumin. 2014, 154, 475–478. [Google Scholar] [CrossRef]
- Yin, Y.D.; Lu, Y.; Sun, Y.G.; Xia, Y.N. Silver nanowires can be directly coated with amorphous silica to generate well-controlled coaxial nanocables of silver/silica. Nano Lett. 2002, 2, 427–430. [Google Scholar] [CrossRef]
- Zhang, T.R.; Ge, J.P.; Hu, Y.X.; Zhang, Q.; Aloni, S.; Yin, Y.D. Formation of hollow silica colloids through a spontaneous dissolution-regrowth process. Angew. Chem. Int. Ed. 2008, 47, 5806–5811. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, W.J.; Tan, W.H. Bioconjugated silica nanoparticles: Development and applications. Nano Res. 2008, 1, 99–115. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Q.; Ge, J.; Goebl, J.; Sun, M.; Yan, Y.; Liu, Y.S.; Chang, C.; Guo, J.; Yin, Y. A self templated route to hollow silica microspheres. J. Phys. Chem. C 2009, 113, 3168–3175. [Google Scholar]
- Chu, C.S. Optical oxygen sensing properties of Ru(II) complex and porous silica nanoparticles embedded in sol-gel matrix. Appl. Opt. 2011, 50, E145–E151. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Chu, C.S.; Lo, Y.L. Highly sensitive and linear optical fiber carbon dioxide sensor based on sol-gel matrix doped with silica particles and HPTS. Sens. Actuators B Chem. 2009, 143, 205–210. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, T.R.; Ge, J.P.; Yin, Y.D. Permeable silica shell through surface-protected etching. Nano Lett. 2008, 8, 2867–2871. [Google Scholar] [CrossRef] [PubMed]
- Han, B.H.; Manners, I.; Winnik, M.A. Oxygen sensors based on mesoporous silica particles on layer-by-layer self-assembled films. Chem. Mater. 2005, 17, 3160–3171. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kluwer Academic/Plenum Press: New York, NY, USA, 1999. [Google Scholar]

| Oxygen Sensitive Dye | Support Matrix | Sensitivity | Reference |
|---|---|---|---|
| PdTFPP | silica-gel beads in silicone | IN2/I100Pa,pO2~8 | [14] |
| PdTFPP | polyurethane hydrogel | IN2/I25%O2~8 | [15] |
| PdTFPP | TEOS/Octyl-triEOS | IN2/I100%O2~72 | [16] |
| PdTFPP | PVC | IN2/I25%O2~7 | [17] |
| PdTFPP | n-propyl-TriMOS/TEOS/Octyl-triEOS | IN2/I100%O2~263 | [18] |
| PdTFPP | PEC-PCL | IN2/I100%O2~80.6 | [19] |
| PdTFPP | PSU-PCL | IN2/I100%O2~106.7 | [19] |
| PdTFPP and porous silica nanoparticles | TEOS/Octyl-triEOS | IN2/I100%O2~386 | present study |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, C.-S.; Syu, J.-J. The Development of a Highly Sensitive Fiber-Optic Oxygen Sensor. Inventions 2016, 1, 9. https://doi.org/10.3390/inventions1020009
Chu C-S, Syu J-J. The Development of a Highly Sensitive Fiber-Optic Oxygen Sensor. Inventions. 2016; 1(2):9. https://doi.org/10.3390/inventions1020009
Chicago/Turabian StyleChu, Cheng-Shane, and Jhih-Jheng Syu. 2016. "The Development of a Highly Sensitive Fiber-Optic Oxygen Sensor" Inventions 1, no. 2: 9. https://doi.org/10.3390/inventions1020009
APA StyleChu, C.-S., & Syu, J.-J. (2016). The Development of a Highly Sensitive Fiber-Optic Oxygen Sensor. Inventions, 1(2), 9. https://doi.org/10.3390/inventions1020009






