Abstract
This review synthesizes the multifaceted role of vitamin A in fish well-being, encompassing immune function, antioxidant capacity and growth regulation, providing a comprehensive understanding of its significance in fish diets and implications for aquaculture. Key objectives include exploring the role of vitamin A in fish immunology, growth impact, oxidative stress status, and future directions. Vitamin A is critical for immune cell development, enhancing both innate and adaptive immune responses. It maintains mucosal integrity and modulates phagocytic activity, crucial for pathogen defense. Growth regulation is influenced by vitamin A through cellular differentiation and morphogenesis, essential for tissue and skeletal development. Studies indicate that vitamin A supplementation improves growth metrics and body composition across various fish species. Vitamin A also combats oxidative stress by scavenging free radicals, enhancing antioxidant enzyme activity, and inducing autophagy, thus protecting cellular components from oxidative damage. Interactions with other vitamins like E, D, and C highlight the importance of balanced nutrition in aquaculture. Integrative management practices, including nutritionally balanced diets, optimal water quality, stress reduction, and environmental enrichment, are recommended to maximize fish health and productivity through adequate vitamin A utilization.
Key Contribution:
The balance between reactive oxygen species production and antioxidant defenses in immune cells is essential for maintaining cellular health, highlighting the crucial interplay between immunity and oxidative capacity in supporting the achievement of genetic growth potential.
1. Introduction
In aquatic ecosystems, fish primarily acquire vitamin A through dietary intake due to their inability to synthesize it de novo [1,2]. Vitamin A, an essential micronutrient, plays a pivotal role in fish physiology by influencing crucial processes essential for their health, growth, and survival [3,4].
Vitamin A exists in multiple forms, including retinol, retinal, and all-trans-retinoic acid (ATRA), each serving distinct biological functions [5,6] (Table 1). The diversity in chemical forms allows vitamin A to fulfill its multiple roles in vision, cellular function, and systemic health. ATRA, the most biologically active form, plays a critical role in regulating gene expression, cellular differentiation, and immune response [7].

Table 1.
Roles of various vitamin A forms in fish.
The growth and development of fish are complex processes influenced by genetic, nutritional, and environmental factors. Vitamin A and its active forms are crucial regulators of growth, influencing cell proliferation, differentiation, apoptosis, skeletal development, vision, and reproductive health [23,24] (Table 2).

Table 2.
Signs of vitamin A deficiency and excess in fish.
Likewise, vitamin A plays a key role in enhancing immune strength in fish by supporting the integrity of mucosal barriers, which serve as the first line of defense against infections [15]. Additionally, the active forms of vitamin A are essential for the proper functioning of immune cells, such as T-cells and macrophages, which are vital for combating pathogens [4,37].
Fish inhabit diverse and challenging environments characterized by variables such as water temperature, salinity, and pollution. These factors can impact the antioxidative capacity of fish tissues and affect the availability and metabolism of vitamin A and other micronutrients [16,38]. Stressors like hypoxia, pathogen exposure, and poor water quality can increase oxidative stress and elevate the requirement for vitamin A, which serves crucial antioxidant functions in the body [16,39]. Understanding these interactions is important for enhancing fish resilience and well-being in both aquaculture and natural habitats.
Aquafarming, vital for global food security, provides a significant portion of the world’s fish supply [40]. Optimizing the health and growth of farmed fish is imperative for sustainable aquaculture operations. Vitamin A supplementation, typically in the form of retinyl acetate, is widely practiced in fish diets to promote health and growth [2]. However, the specific vitamin A requirements vary widely among fish species and developmental stages [2,41].
This review explains the many ways vitamin A supports fish health, including boosting the immune system, protecting against oxidative stress, and helping with growth. It provides a clear understanding of why vitamin A is important in fish diets and what it means for aquaculture. Upcoming sections will explore innovative perspectives on vitamin A mechanisms in fish, enriched by the latest research and practical insights from aquaculture. This integrated approach underscores the critical necessity of adequate vitamin A to enhance fish health and productivity across diverse aquatic environments, making it an essential consideration for aquaculture professionals, nutritionists, and researchers. The specific objectives of this synthesis are as follows:
- Role of vitamin A in fish immunology.
- Vitamin A status and oxidative stress.
- Impact of vitamin A on fish growth.
- Integrative discussion.
- Future directions.
To identify and evaluate relevant studies for this manuscript, a comprehensive search was conducted using databases such as PubMed and Scopus, with specific keywords related to vitamin A’s role in fish immunology, oxidative stress, and growth. Studies were selected based on predefined inclusion and exclusion criteria, and their quality was assessed using standardized tools, ensuring a robust synthesis of data to address the manuscript’s objectives effectively.
2. Role of Vitamin A in Fish Immunology
2.1. Overview of Fish Immune System
The immune system of fish, like that of other vertebrates, has both innate and adaptive parts that work together to fight off infections [42]. The innate immune system serves as the initial defense line, characterized by its rapid response to a broad spectrum of pathogens [43]. Key elements include physical barriers like skin and mucous membranes, cellular defenses such as macrophages and neutrophils, and humoral factors like lysozymes, complement proteins, and antimicrobial peptides [44].
In contrast, the adaptive immune system, while slower, provides highly specific and enduring protection against pathogens [45]. It involves lymphocytes, notably T and B cells, and the production of antibodies [46]. T cells are pivotal in cell-mediated immunity, while B cells generate antibodies that neutralize pathogens [47] (Figure 1). Moreover, the adaptive immune system retains immunological memory, enabling a swifter and more potent response upon subsequent encounters with the same pathogen [48].
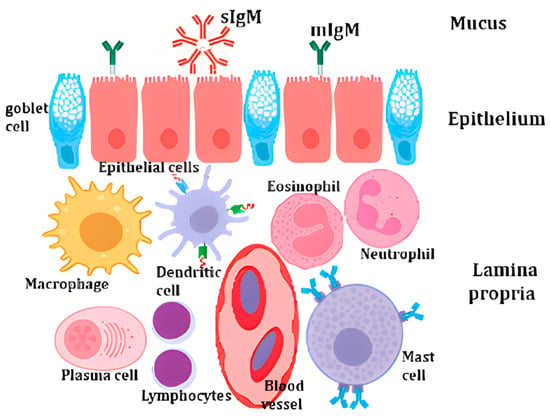
Figure 1.
Schematic representation of the fish mucosal immunity [43].
Fish, being ectothermic organisms, experience immune system dynamics influenced by environmental factors such as water temperature and quality [49]. Despite these differences, the basic principles of immune response in fish are similar to those in higher vertebrates, including humans [50,51].
2.2. Vitamin A and Immune Function
Vitamin A is essential for maintaining and modulating the immune system in fish and other species. Its critical role was first recognized as early as 1928 by Green and Mellanby, who referred to it as “the anti-infective vitamin” [52,53]. Vitamin A is essential for the development and function of immune cells, influencing both their differentiation and proliferation [54]. Particularly noteworthy is its active form, ATRA, which plays a key role in immune response regulation [55]. ATRA affects T and B lymphocytes and enhances blood serum bactericidal activity [14,25,56].
Additionally, vitamin A helps keep mucosal surfaces healthy, acting as important barriers that prevent pathogens from entering the body [57,58]. By supporting the health and function of epithelial tissues, vitamin A helps prevent infections. Additionally, it modulates the activity of phagocytic cells such as macrophages and neutrophils, boosting their ability to engulf and eliminate pathogens [15,59].
In fish, deficiency in vitamin A can impair immune responses and heighten susceptibility to infections [25,60]. Conversely, adequate levels of vitamin A are essential for supporting a robust immune system, thereby promoting overall health and resilience against diseases [2,61].
2.3. Vitamin A Modulation of Immune Responses
Vitamin A exerts its effects on the immune system through a variety of cellular and molecular mechanisms. One of the primary ways vitamin A influences immune function is by regulating gene expression [7,25]. ATRA, the most active metabolite of vitamin A, binds to retinoic acid receptors (RARs) and retinoid X receptors (RXRs) in the nucleus of immune cells [18,62,63]. These receptor complexes then bind to specific DNA sequences, called retinoic acid response elements (RAREs), leading to the transcription of genes involved in immune responses [64,65,66].
At the cellular level, vitamin A affects the differentiation, proliferation, and function of various immune cells. For instance, ATRA promotes the differentiation of naive T cells into regulatory T cells (Tregs), which play a crucial role in maintaining immune tolerance and preventing autoimmunity [37,67,68] (Figure 2). It also enhances the differentiation of B cells into plasma cells, which are responsible for antibody production [43,69,70].
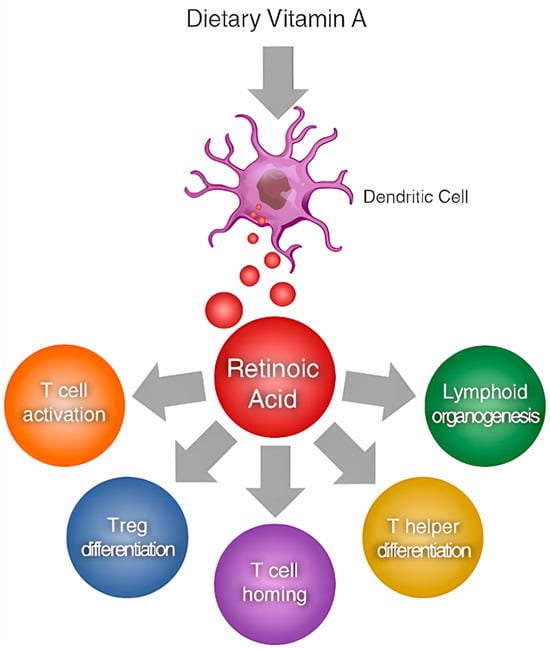
Figure 2.
ATRA (all-trans-retinoic acid) as a modulator of T cell immunity [71].
Vitamin A modulates the function of antigen-presenting cells (APCs), such as dendritic cells and macrophages, by enhancing their ability to process and present antigens to T cells [44,72,73,74,75]. This helps start the body’s adaptive immune response. Additionally, vitamin A influences the production of cytokines, which are signaling molecules that mediate and regulate immunity, inflammation, and hematopoiesis [15,25,76]. Through these mechanisms, vitamin A ensures a balanced and effective immune response, protecting fish from infections and contributing to their overall health and well-being.
2.4. Summary of Key Studies and Findings
Many studies have shown that vitamin A plays a key role in fish immune systems. For example, research on various species like Nile tilapia and Atlantic salmon demonstrates that dietary supplementation with vitamin A can bolster immune responses and enhance resistance to diseases.
Guimaraes et al. [61] evaluated the effect of various levels of dietary vitamin A (0, 2500, 5000, 10,000, and 20,000 IU/kg diet) on the immune response and resistance of Nile tilapia to Streptococcus iniae challenge. Fish fed a diet without vitamin A showed significantly lower weight gain and feed efficiency, along with signs of vitamin A deficiency such as hemorrhages and lethargy. Vitamin A supplementation significantly enhanced immune responses compared to the control group (0 IU/kg), increasing serum protein levels by up to 12%, lysozyme activity by up to 59%, and superoxide anion production by up to 233%.
Hernandez et al. [77] investigated the effects of dietary vitamin A deficiency and excess on immune responses in juvenile Japanese flounder. Juvenile flounder were fed diets containing 0, 10,000, and 25,000 IU vitamin A/kg feed for 120 days. Serum antibacterial activity was significantly elevated—by up to 57 percentage points—in groups fed with 10,000 and 25,000 IU of vitamin A per kg, compared to the group that received no vitamin A. The results indicate that dietary vitamin A supplementation significantly improved antibacterial activity in juvenile Japanese flounder.
Cuesta et al. [78] examined the effects of dietary retinyl acetate on the innate immune system of gilthead seabream. Serum lysozyme activity and myeloperoxidase (MPO) content were unaffected by dietary vitamin A. However, dietary supplements of 150 and 300 mg retinyl acetate per kg diet enhanced respiratory burst activity in head-kidney leucocytes after 1 or 2 weeks. Leucocyte MPO content increased with the highest vitamin A dose over 2 or 4 weeks, and with 150 or 50 mg supplements over 4 or 6 weeks. These findings underscore the significant impact of retinyl acetate on enhancing certain immune responses in gilthead seabream.
Zhang et al. [25] investigated the impact of dietary vitamin A on immune function in young grass carp. Over 10 weeks, fish were fed varying levels of vitamin A as retinyl acetate (0.206, 0.413, 0.619, 0.963, and 1.307 mg/kg diet) followed by a 14-day challenge with Aeromonas hydrophila. Vitamin A deficiency significantly impaired growth, increased enteritis morbidity, decreased intestinal innate humoral immune response (e.g., lysozyme activity in the proximal intestine decreased by up to 62% compared to adequate vitamin A levels), and worsened intestinal inflammation. The deficiency also disrupted inflammatory cytokines in the intestines, potentially linked to p38MAPK and NF-κB canonical signaling pathways. The optimum dietary vitamin A levels required for optimal young grass carp growth, enteritis resistance, and immune function were estimated to be 0.664, 0.707, and 0.722 mg/kg, respectively, indicating higher requirements for optimal immunity.
Hassan et al. [4] studied the vitamin A requirement in fingerling common carp, Cyprinus carpio var. communis, over a 10-week period. Fish were fed diets with six graded levels of vitamin A (0, 0.03, 0.07, 0.11, 0.15, and 0.19 g as retinyl acetate/kg diet), with optimal results observed at 0.11 g/kg diet. This level significantly improved growth, the feed conversion ratio, hematological parameters (hemoglobin, red blood cells, hematocrit, white blood cells), and serum enzyme levels (aspartate transaminase and alanine aminotransferase). Specifically, the white blood cell count decreased by 16%—from 2.71 × 104/mm3 in the control group (0 g of retinyl acetate) to 2.27 × 104/mm3—with 0.11 g of dietary retinyl acetate. The study concludes that the optimal vitamin A range for growth and health in these carp lies between 0.10 and 0.12 g/kg diet. The findings are crucial for developing vitamin A balanced feed for intensive culture of C. carpio var. communis.
Thompson et al. [56] conducted a study on Atlantic salmon, feeding them diets containing low (0.37 mg/kg diet), normal (1.95 mg/kg diet), and high (15 mg/kg diet) levels of vitamin A for four months, with liver vitamin A levels corresponding to dietary intake. While fish on the low-vitamin-A diet exhibited reduced kidney leucocyte migration and serum bactericidal activity, those on the high-vitamin-A diet showed enhanced serum antiprotease activity compared to other groups. Importantly, high dietary levels of vitamin A did not adversely affect phagocyte respiratory burst activity, bactericidal activity, eicosanoid production, lymphocyte functions, serum lysozyme, or complement activity.
In summary, the body of research clearly highlights the indispensable role of vitamin A in the immunology of various fish species. Across different studies, dietary vitamin A supplementation has been shown to significantly enhance immune responses and increase disease resistance. Conversely, deficiency in vitamin A leads to marked impairments in health and immune function. These findings underscore the critical importance of carefully balancing dietary vitamin A levels in aquaculture to optimize fish health and ensure resilience against diseases. This collective evidence reinforces the need for tailored nutritional strategies that incorporate adequate vitamin A to sustain and enhance fish immunity in both natural and controlled environments. Table 3 presents the effects of vitamin A on immune parameters across various fish species.

Table 3.
Effects of vitamin A on immune function across various fish species.
3. Vitamin A Status and Oxidative Stress
3.1. Oxidative Stress in Fish
Oxidative stress is characterized by an imbalance between the production of reactive oxygen species (ROS) and the biological system’s capacity to detoxify these reactive intermediates or repair resultant damage [79]. In aquatic environments and fish farms, fish are frequently exposed to various stressors such as pollutants, temperature fluctuations, hypoxia, unbalanced diets, antinutrients in feeds, handling and transportation stresses, high stocking densities, suboptimal management practices, specific medications, oxidative disinfectants, and genetic predispositions, all of which can exacerbate ROS production [80] (Table 4).

Table 4.
Some common oxidative stressors for fish and their permissible limits [80].
ROS include free radicals like superoxide anions, hydroxyl radicals, and non-radical species such as hydrogen peroxide [81,82]. Excessive ROS presence leads to oxidative damage to cellular components, including lipids, proteins, and nucleic acids [83]. Lipid peroxidation, for example, yields malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE), compromising membrane integrity and function [84,85,86,87]. Protein oxidation affects enzyme activities and structural proteins, while oxidative damage to DNA can lead to mutagenesis and impaired cellular function [5].
Fish, like other vertebrates, have evolved a complex antioxidant defense system to mitigate oxidative damage. This system includes enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), as well as non-enzymatic antioxidants like vitamins E, C, and A, glutathione, and carotenoids [88,89,90]. However, long-term or intense stress can break down these defenses, leading to problems like poor growth, a weakened immune system, and lower chances of successful reproduction [80].
Understanding how oxidative stress affects fish is very important for the aquaculture industry. This knowledge helps improve fish health and growth while reducing the effects of environmental stress. Better management practices and adding antioxidant vitamins to fish diets are key ways to help fish cope with oxidative stress.
3.2. Importance of Vitamin A in Combating Oxidative Stress
Vitamin A is widely acknowledged as being crucial for promoting optimal health, growth, and development in fish [2]. While its essential roles are well-documented, recent studies suggest that vitamin A status significantly affects oxidative stress in aquaculture [4,15,16,77,91]. Vitamin A acts as a systemic antioxidant, influencing various biological processes in fish [5,16].
The main mechanisms through which vitamin A combats oxidative stress include
- Scavenging Free Radicals: Retinol exhibits direct antioxidant properties owing to its hydrophobic polyene chains, allowing it to quench singlet oxygen and neutralize radicals [10,12]. Despite being prone to auto-oxidation in high-oxygen environments, retinol remains effective under physiological oxygen levels [5,92,93]. Palace et al. [94] detailed how retinol acts as a chain-breaking antioxidant by intercepting peroxyl radicals, thereby halting lipid peroxidation and the formation of hydroperoxides. It efficiently scavenges peroxyl radicals in various lipid models, including liposomes mimicking cell membranes.
- Enhancing Antioxidant Enzyme Activity: ATRA, a metabolite of vitamin A, serves as a potent transcriptional regulator that influences the expression of genes involved in antioxidant processes [95,96] (Figure 3). Specifically, ATRA has been shown to upregulate the expression and activity of key antioxidant enzymes such as SOD, CAT, and GPx [5,90,97,98].
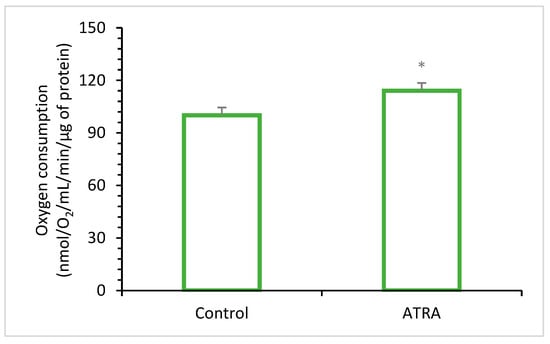 Figure 3. Oxygen consumption in adipocytes exposed to 2 µM of ATRA (all-trans-retinoic acid) was measured using Clarke’s electrode (adapted from Tourniaire et al. [99]). Control refers to control cells, which received the vehicle (dimethyl sulfoxide). Data are the mean ± SEM of three independent cultures per treatment condition. The assessment compared ATRA-treated cells to untreated cells and measured their oxygen consumption rates to determine if ATRA-induced gene expression changes altered cellular metabolism. ATRA increased oxygen consumption by 15% (* p < 0.05).
Figure 3. Oxygen consumption in adipocytes exposed to 2 µM of ATRA (all-trans-retinoic acid) was measured using Clarke’s electrode (adapted from Tourniaire et al. [99]). Control refers to control cells, which received the vehicle (dimethyl sulfoxide). Data are the mean ± SEM of three independent cultures per treatment condition. The assessment compared ATRA-treated cells to untreated cells and measured their oxygen consumption rates to determine if ATRA-induced gene expression changes altered cellular metabolism. ATRA increased oxygen consumption by 15% (* p < 0.05).
- 3.
- Inducing Autophagy: ATRA has been found to induce autophagy, a critical cellular mechanism that helps cells cope with nutrient scarcity or oxidative stress, as observed in fish and other organisms [100,101]. Research by Rajawat et al. [100] suggests that ATRA promotes the formation of autophagosomes via a pathway that does not involve conventional nuclear retinoid receptors. Specifically, ATRA triggers the relocation of the cation-independent mannose-6-phosphate receptor from the trans-Golgi region to maturing autophagosomes, leading to their acidification. This autophagic process plays a crucial role in managing redox balance and maintaining cellular stability [102].
3.3. Case Studies: Effects of Vitamin A Supplementation on Antioxidant Capacity
Many studies have looked closely at how vitamin A supplements affect the antioxidant levels in fish and found positive results. Wu et al. [16] conducted a study focusing on grass carp to investigate how different levels of dietary vitamin A (0, 600, 1200, 1800, 2800, and 3800 IU/kg) influence flesh quality through enhanced antioxidative ability via Nrf2/Keap1 signaling. Over a 10-week feeding period, vitamin A supplementation reduced oxidative damage and increased the activities of antioxidant enzymes such as MnSOD (by up to 17%), CAT (by up to 55%), and GPx (by up to 23%), likely through the activation of Nrf2 signaling pathways.
Fontagne et al. [103] explored the dynamics of antioxidant enzymes during the early development of rainbow trout, particularly examining the influence of retinoids on lipid peroxidation. They observed a progressive decrease in retinoid concentrations as development advanced, notably in fry fed oxidized lipid diets, which correlated with elevated levels of lipid-soluble fluorescent products, indicating increased lipid peroxidation.
Jiang et al. [104] investigated the effects of dietary vitamin A on antioxidant mechanisms and intestinal barrier function in young grass carp. Over a 10-week period, fish were fed varying levels of vitamin A (0, 600, 1200, 1800, 2800, and 3800 IU/kg), followed by a challenge with Aeromonas hydrophila. They observed that vitamin A deficiency led to oxidative damage in the intestines, characterized by reduced levels of non-enzymatic antioxidants such as glutathione and vitamin A, as well as decreased activities of antioxidant enzymes. These changes were attributed to altered mRNA levels regulated by NF-E2-related factor 2 (Nrf2) and the increased expression of kelch-like-ECH-associated protein (Keap1), contributing to enhanced apoptosis signaling and the disruption of intestinal tight-junction complexes across different intestinal segments. These findings underscore the critical role of dietary vitamin A in maintaining antioxidant capacity and structural integrity in fish intestines.
In another comprehensive study by Wu et al. [105] involving grass carp, various dietary levels of vitamin A (ranging from 68 to 4769 IU/kg) were tested over a 12-week period to assess their impact on growth performance and blood biochemical indices. Higher levels of vitamin A significantly enhanced the specific growth rate, with the most substantial improvement observed at 4769 IU/kg. Notably, while vitamin A levels did not affect the feed conversion ratio, feeding rate, or survival rate, serum SOD activity increased proportionally, with up to a 35% enhancement compared to the control observed at 68 IU/kg of vitamin A supplementation.
Overall, vitamin A supplementation consistently supports antioxidant defense mechanisms and enhances overall health and growth in fish, though the optimal levels and effects can vary depending on specific conditions and species. Table 5 presents the effects of vitamin A on oxidative stress status in fish.

Table 5.
Effects of vitamin A on oxidative stress status in fish.
4. Impact of Vitamin A on Fish Growth
4.1. Growth Metrics in Fish
Fish growth is a complex process influenced by genetic, nutritional, and environmental factors [109]. Accurately measuring growth is crucial for understanding how micronutrients, like vitamin A, affect fish development. Key growth metrics include length, weight, condition factors, the specific growth rate (SGR), the feed conversion ratio (FCR), and body composition [110,111].
Length and weight are the most commonly used metrics due to their straightforwardness [112]. Regular measurements of these parameters provide insights into overall growth trends and the health of fish. The condition factor, calculated as the ratio of weight to the cube of length, helps assess fish robustness and well-being [113].
The specific growth rate (SGR) expresses growth as a percentage increase in body weight per day, offering a normalized measure that adjusts for initial size differences [114]. The feed conversion ratio (FCR) is another critical parameter, indicating how efficiently fish convert feed into body mass. A lower FCR indicates better feed efficiency and growth performance.
Body composition analysis, including protein, lipid, and moisture content measurements, provides detailed information on fish nutritional status and overall health [110,115]. These measurements help determine how dietary changes, such as adding vitamin A, affect fish growth and development.
4.2. Vitamin A and Growth Regulation
Vitamin A plays a crucial role in regulating growth and development in fish by influencing various physiological processes. It acts through its metabolites as a steroid hormone that governs growth, cell differentiation, and overall physiological development [2,116,117]. Specifically, vitamin A and its metabolites, including ATRA, are essential for glycoprotein and glycosaminoglycan synthesis, which are pivotal for the differentiation of epithelial tissues. This function underscores the key role of retinoids in fish physiology [4].
ATRA plays a critical role in cellular differentiation and development [18]. It functions by binding to nuclear receptors such as RARs and RXRs, which regulate the transcription of genes involved in cell growth and differentiation [118]. ATRA exerts its influence on cellular proliferation and differentiation by promoting cells through the G1 phase of the cell cycle. This process involves activating genes that support cell division while suppressing those associated with cell cycle arrest [119], thus ensuring proper tissue and organ development, including the skeletal system, which is crucial for fish growth and body structure [120,121].
Moreover, ATRA signaling significantly contributes to morphogenesis in fish [6,122,123]. It plays a vital role in organizing the trunk through essential morphogenetic processes such as mesoderm segmentation, axial elongation, and the establishment of anterior–posterior identity within segments [124,125,126,127,128,129].
Additionally, vitamin A is crucial for maintaining the integrity of intestinal epithelial tissues in fish, which are essential for nutrient absorption and overall health [2]. Adequate levels of vitamin A ensure the proper functioning of the digestive and respiratory systems, thereby supporting efficient nutrient and oxygen uptake necessary for growth.
4.3. Effects of Vitamin A Supplementation on Growth Rates and Body Composition
Many studies have looked at how vitamin A affects fish growth, showing that it helps fish grow better and improves their body composition.
In a 12-week study by Wu et al. [104], the impact of dietary vitamin A levels on grass carp was examined. Fish were fed diets containing vitamin A concentrations ranging from 68 to 4769 IU/kg. Higher vitamin A levels significantly increased the specific growth rate, with the maximum effect observed at 4769 IU/kg, which led to a 52% improvement compared to the control group receiving 68 IU/kg. However, the feed conversion ratio, feeding rate, and survival rate remained unaffected. Overall body composition did not vary significantly across the dietary treatments.
Similarly, Hassan et al. [4] explored the effects of different vitamin A supplementation levels on common carp over 10 weeks. Diets ranged from 0 to 0.19 g/kg diet of vitamin A (as retinyl acetate). Increasing vitamin A levels resulted in significant enhancements in growth parameters, including live weight gain (up to 68%), feed conversion ratio (up to 59%), protein efficiency ratio (up to 70%), specific growth rate (up to 33%), and body protein deposition (up to 163%), compared to the control group with 0 g retinyl acetate. Optimal responses were noted at 0.11 g/kg diet, accompanied by enhancements in hematological parameters like hemoglobin, erythrocyte count, and hematocrit.
Udo [130] investigated the impact of dietary vitamin A supplementation on African catfish over 113 days. Fish fed diets with 833 IU/kg and 1666 IU/kg of vitamin A exhibited significantly higher final weights compared to those on the control diet (no vitamin A supplementation). The diet containing 1666 IU/kg of vitamin A resulted in the highest daily weight gain, showing a 42% increase, and the greatest specific growth rate, which improved by 22% compared to the unsupplemented group.
Campeche et al. [33] determined the vitamin A requirements of Nile tilapia in a study comparing all-male and mixed-sex groups over 75 days. Dietary supplementation levels of retinyl palmitate ranged from 0 to 5400 IU/kg. Fish showed deficiency signs at lower levels (0–1200 IU/kg), with moderate signs at higher levels (1800–3600 IU/kg). Significant effects on final weight and weight gain were observed up to 5400 IU/kg, with confirmation of vitamin A uptake and storage in the liver.
Hu et al. [131] conducted a 10-week feeding trial on hybrid tilapia to evaluate the effects of dietary vitamin A supplementation. Diets containing 0 to 50,000 IU vitamin A (as retinyl acetate) per kg diet were tested. Growth performance significantly improved in supplemented groups (up to 97%) compared to those without vitamin A, with optimal dietary levels ranging from 5850 to 6970 IU/kg determined by broken-line regression analysis based on weight gain and liver vitamin A retention.
These studies collectively underscore the significant role of vitamin A in enhancing growth and health parameters across various species of cultured fish. Table 6 presents the effects of vitamin A on growth parameters across various fish species.

Table 6.
Effects of vitamin A on growth parameters across various fish species.
Across the studies reviewed, vitamin A consistently demonstrates a positive impact on fish growth and health, with optimal effects varying by species and supplementation levels. Higher concentrations of vitamin A generally lead to increased growth rates and improved feed conversion, though the specific optimal levels differ. For example, the most significant growth improvements are seen at levels ranging from 4769 IU/kg in grass carp to 6970 IU/kg in hybrid tilapia, while lower levels might cause deficiencies or suboptimal growth. Overall, the findings suggest that vitamin A is crucial for enhancing growth performance in cultured fish, but the optimal dietary levels should be carefully calibrated for each species to maximize benefits without causing adverse effects.
5. Integrative Discussion
Vitamin A is essential for keeping fish healthy and productive. It helps with many important functions, including immune support, antioxidant protection, and growth. Because of its crucial role, ensuring fish obtain enough vitamin A is very important in aquaculture for successful and sustainable fish farming.
5.1. Interconnectedness of Immunology, Antioxidant Capacity, and Growth
Vitamin A has a major impact on fish health in many different ways. Primarily, vitamin A enhances immune defenses by regulating gene expression, crucial for immune cell differentiation and function [25]. This regulatory role not only strengthens the immune system’s ability to combat pathogens but also maintains mucosal barriers, vital for protecting mucosal tissues such as the gut and respiratory tract [57,58].
Furthermore, vitamin A’s antioxidant properties are pivotal in shielding cells from oxidative stress—a state that can impair immune function and overall health [90,100]. By scavenging free radicals and synergizing with antioxidants like vitamin E, vitamin A helps preserve cellular integrity and function, promoting longevity and resilience in fish.
At the same time, vitamin A helps with growth and development by managing important processes like cell growth and specialization [2]. This helps tissues and organs develop properly, leading to strong growth and the better use of nutrients.
Cellular metabolism naturally produces ROS, which serve critical roles in signaling pathways and as defense mechanisms against pathogens [79,132]. Immune cells such as macrophages and neutrophils rely on ROS for proper function [133], yet sufficient antioxidant defenses are necessary to mitigate the potential harm from excessive free radical production [134]. This interplay between immunity and oxidative capacity highlights their interconnectedness, crucial for optimal growth metrics. Only fish with a healthy immune system and robust antioxidant potential can achieve their genetic potential for growth and development.
5.2. Interaction of Vitamin A with Other Vitamins
The effectiveness of vitamin A in fish health is significantly influenced by its interactions with other vitamins. For instance, the interplay between vitamins A and E is crucial in various physiological contexts, where they may synergistically support cellular health and development. Research using a unilamellar liposomal system composed of phosphatidylcholine shows that α-tocopherol (vitamin E) enhances the antioxidant efficacy of all-trans-retinol (vitamin A) by reducing its auto-oxidation [135]. This enhancement likely results from the combined scavenging of radicals, providing synergistic protection of the lipid system against peroxidative stress [136]. Furthermore, vitamin E preserves vitamin A through several mechanisms: protecting it from oxidation in the intestinal lumen, increasing its intestinal absorption, and enhancing its storage [19]. Animals fed a diet containing vitamin E exhibited a tenfold increase in liver vitamin A content compared to those on a diet without vitamin E [137]. Additionally, vitamin A is indirectly essential for the absorption and utilization of fat-soluble vitamins as it is crucial for maintaining intestinal functionality [138].
Similarly, vitamins A and D play integral roles in various physiological processes, including immune regulation, bone metabolism, and cellular differentiation [139,140,141]. Their interplay is complex, involving the binding of their active forms to specific nuclear receptors. Active forms of vitamin A, such as ATRA and 9-cis retinoic acid (9-cis RA), interact with RARs and RXRs, while 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) binds to the vitamin D receptor and RXRs [19]. These receptors form heterodimers that bind to response elements such as the vitamin D response element and the retinoic acid response element. Research indicates that 9-cis RA can modulate the effects of 1,25(OH)2D3, resulting in outcomes that range from antagonistic to synergistic [55,142,143]. Notably, ATRA can influence the expression of the vitamin D-binding protein complex, which is critical for the cellular uptake and actions of vitamin D, implicating vitamin A in the modulation of vitamin D metabolism within specific cell types [19]. Moreover, vitamins A and D collaboratively regulate immune responses in innate lymphoid cells (ILCs) [144] (Figure 4). However, ATRA and 1,25(OH)2D3 may also exert antagonistic effects on the expression of effector cytokines and gut-homing integrin by ILCs. The balance between these vitamins could be a key determinant in ILC activity and associated diseases, including inflammation [145].
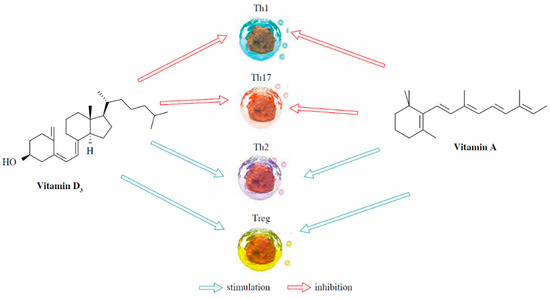
Figure 4.
Immunomodulatory capacity of vitamin A and D3 [144]. Th = T helper cells: Th1 (they primarily produce cytokines such as interferon-gamma (IFN-γ) and interleukin-2 (IL-2) and are involved in cell-mediated immunity), Th17 (they produce cytokines such as interleukin-17 (IL-17) and interleukin-22 (IL-22) and are involved in the defense against extracellular pathogens), Th2 (they produce cytokines such as interleukin-4 (IL-4), interleukin-5 (IL-5), and interleukin-13 (IL-13) and are involved in humoral immunity and allergic responses); Treg = regulatory T cells (they express the transcription factor Foxp3 and play a crucial role in immune tolerance and regulation).
Vitamin C is synthesized endogenously within the liver of many species. However, teleost fishes have lost this ability [146,147]. This important micronutrient is crucial for different processes in the body, such as helping with tissue growth and repair, reducing oxidative stress, and supporting the immune system [147,148]. Administering vitamins A and C, either before or after inducing stress in fish or other animals, has been shown to significantly enhance the activities of crucial antioxidant enzymes such as SOD, glutathione-S-transferase, and CAT [16,149,150]. Importantly, combined treatment with both vitamins yields markedly superior outcomes compared to the use of either vitamin alone.
5.3. Recommendations for Integrated Management Practices in Aquaculture
To obtain the most benefit from vitamin A and keep fish healthy, it is essential to use integrated management practices:
- Nutritionally Balanced Diets: Formulating diets that contain sufficient vitamin A and account for its interactions with vitamins E, D, and C is essential. Using stable and bioaccessible forms of vitamin A in high-quality feeds enhances bioavailability, supporting immune function, antioxidant capacity, and growth [151,152].
- Water Quality Management: Maintaining optimal water conditions is fundamental for nutrient absorption and metabolic processes [153,154]. The regular monitoring and adjustment of water parameters prevent conditions that could impair vitamin A metabolism, ensuring efficient utilization and maximizing fish health [155,156].
- Stress-Reduction Strategies: Implementing measures to reduce stress, such as optimizing stocking densities and minimizing handling stress, lowers the demand for vitamin A during stressful periods. This promotes improved immune responses and growth performance [157,158,159].
- Environmental Enrichment: Providing enriched environments resembling natural habitats encourages behavioral and physiological adaptations that reduce stress and enhance overall well-being [160,161,162]. This supports vitamin A’s efficacy in maintaining robust immune function and promoting growth.
- Monitoring and Adjustment: The continuous monitoring of fish health indicators and environmental parameters enables proactive adjustments in management [163,164]. Biomarker assessments of vitamin A levels and immune function offer insights into the effectiveness of dietary strategies, facilitating timely interventions to optimize fish health.
- Integrated Health Management: Integrating nutrition, water quality management, and disease prevention measures ensures a comprehensive approach to maximizing the benefits of vitamin A. Strategies such as vaccination programs, biosecurity measures, and probiotic use enhance immune competence and overall resilience in fish populations [165,166].
6. Future Directions
Although much has been learned about how vitamin A affects fish health, there are still many unanswered questions, so more research is needed. Looking into these areas could help us learn more and improve fish farming.
One important area that needs more research is how vitamin A affects the immune system at the molecular level. While it is established that vitamin A impacts immune-related gene expression, the intricate pathways and interactions with other immune-modulating nutrients in fish are not fully elucidated [54,167]. Advanced molecular and genomic techniques offer potential for uncovering these mechanisms.
Another important area that needs more research is how vitamin A needs differ between various fish species and at different stages of their growth. These requirements may range from 2000 to 20,000 IU or higher of vitamin A per kilogram of fish feed. While some data are available for commonly cultivated species, comprehensive studies spanning a broader spectrum of species and life stages—from larvae to adults—are needed. It is logical to assume that the vitamin A requirement of fish will depend not only on species but also on life stage, nutritional status, reproductive status, and the presence of antinutritional factors in feed. Furthermore, stress, infection, and illness can significantly increase the vitamin A needs. Such research is essential for refining dietary guidelines and ensuring optimal vitamin A levels across diverse aquaculture systems. For more detailed information on vitamin A requirements in various fish species, readers should refer to the paper by Hernandez and Hardy [2].
Additionally, it is very important to study how environmental stress affects vitamin A metabolism and its effectiveness. Understanding how factors like fluctuations in water temperature, pH variations, and exposure to pollutants affect the stability and absorption of vitamin A could lead to enhanced management strategies [168]. Long-term studies examining the combined effects of multiple environmental stressors on vitamin A metabolism would be particularly valuable [169].
Research gaps also extend to interactions between vitamin A and other micronutrients, especially within the context of fortified feeds. Investigating how different combinations of micronutrients influence the effectiveness of vitamin A can guide the development of more balanced and efficient diets [170]. Additionally, the role of the gut microbiota in vitamin A metabolism and its implications for fish health is a burgeoning area that warrants further exploration [171,172].
Recent improvements in how fish feed is formulated and delivered offer great potential for better managing vitamin A supplementation in fish farming [173]. Advanced microencapsulation techniques effectively protect vitamin A from degradation during feed processing and storage, ensuring that fish receive stable and bioavailable forms of the vitamin [174,175]. Furthermore, nanotechnology can enhance the delivery and absorption of vitamin A, improving its efficacy and minimizing wastage [176].
Additionally, the progress in precision aquaculture, which integrates data from various sources such as water quality sensors, growth monitors, and health assessments, facilitates more informed decision-making [177]. By combining these data with advanced analytics and machine learning algorithms, it is possible to optimize feeding strategies and environmental management, ensuring that vitamin supplementation is precisely tailored to the specific needs of the fish.
Finally, promoting research and collaboration among scientists, industry stakeholders, and policymakers can drive the development and adoption of sustainable practices. Public–private partnerships and funding for research on optimal vitamin supplementation, methods and technologies can accelerate progress and lead to more resilient aquaculture systems.
7. Conclusions
This review has combined information on how vitamin A affects fish health, highlighting its key roles in supporting the immune system, controlling growth, and reducing oxidative stress. The integrative approach highlights vitamin A as a cornerstone in fish diets, essential for enhancing productivity and health in aquaculture. By modulating immune responses, supporting antioxidant defenses, and facilitating growth and development, vitamin A contributes significantly to fish resilience and performance.
The interplay of vitamin A with other vitamins, such as E, D, and C, underscores the need for balanced nutritional strategies to optimize fish health. Effective aquaculture practices should incorporate these insights to formulate nutritionally adequate diets, maintain optimal water quality, and implement stress-reduction and environmental enrichment strategies. Overall, the findings advocate for a holistic approach to fish nutrition and management, ensuring sustainable and efficient aquaculture production. Future research directions should focus on further exploring vitamin A’s molecular mechanisms and its synergistic effects with other nutrients to refine dietary formulations and management practices for various fish species.
Author Contributions
Conceptualization, Y.S.; methodology, Y.S. and W.P.; software, Y.S.; validation, W.P.; formal analysis, W.P.; investigation, Y.S.; resources, Y.S. and W.P.; data curation, Y.S.; writing—original draft preparation, Y.S.; writing—review and editing, W.P.; visualization, Y.S.; supervision, W.P.; project administration, Y.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Both authors of this work are affiliated with BASF, a manufacturer of vitamins and carotenoids, including vitamin A. Nevertheless, it is crucial to underscore that the content of this manuscript has been sourced exclusively from scientific peer-reviewed data. Our unwavering commitment lies in upholding transparency and adhering to ethical research principles.
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| APCs | Antigen-presenting cells |
| ATRA | All-trans-retinoic acid |
| CAT | Catalase |
| FCR | Feed conversion ratio |
| FR | Feeding rate |
| GPx | Glutathione peroxidase |
| IFN-γ | Interferon-gamma |
| IL | Interleukin |
| ILCs | Innate lymphoid cells |
| IU | International Unit |
| Keap1 | Kelch-like ECH-associated protein 1 |
| MDA | Malondialdehyde |
| MPO | Myeloperoxidase |
| Nrf 2 | Nuclear factor-erythroid 2 p45-related factor 2 |
| RAREs | Retinoic acid response elements |
| RARs | Retinoic acid receptors |
| ROS | Reactive oxygen species |
| RXRs | Retinoid X receptors |
| SGR | Specific growth rate |
| SOD | Superoxide dismutase |
| SR | Survival rate |
| Tregs | T cells |
References
- Clugston, R.D.; Blaner, W.S. Vitamin A (retinoid) metabolism and actions: What we know and what we need to know about amphibians. Zoo Biol. 2014, 33, 527–535. [Google Scholar] [CrossRef]
- Hernandez, L.H.; Hardy, R.W. Vitamin A functions and requirements in fish. Aquac Res. 2020, 51, 3061–3071. [Google Scholar] [CrossRef]
- Martin, S.A.M.; Krol, E. Nutrigenomics and immune function in fish: New insights from omics technologies. Dev. Comp. Immunol. 2017, 75, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Ahmed, I.; Wani, G.B. Effect of Supplementation of Vitamin A on Growth, Haemato-Biochemical Composition, and Antioxidant Ability in Cyprinus carpio var. communis. Aquac. Nutr. 2022, 2022, 8446092. [Google Scholar] [CrossRef] [PubMed]
- Shastak, Y.; Gordillo, A.; Pelletier, W. The relationship between vitamin A status and oxidative stress in animal production. J. Appl. Anim. Res. 2023, 51, 546–553. [Google Scholar] [CrossRef]
- Samrani, L.M.M.; Pennings, J.L.A.; Hallmark, N.; Bars, R.; Tinwell, H.; Pallardy, M.; Piersma, A.H. Dynamic regulation of gene expression and morphogenesis in the zebrafish embryo test after exposure to all-trans retinoic acid. Reprod. Toxicol. 2023, 115, 8–16. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. The role of vitamin A in non-ruminant immunology. Front. Anim. Sci. 2023, 4, 1197802. [Google Scholar] [CrossRef]
- Wold, H.L.; Wake, K.; Higashi, N.; Wang, D.; Kojima, N.; Imai, K.; Blomhoff, R.; Senoo, H. Vitamin A distribution and content in tissues of the lamprey, Lampetra japonica. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004, 276, 134–142. [Google Scholar] [CrossRef]
- Shidoji, Y.; Muto, Y. Vitamin A transport in plasma of the non-mammalian vertebrates: Isolation and partial characterization of piscine retinol-binding protein. J. Lipid Res. 1977, 18, 679–691. [Google Scholar] [CrossRef]
- Landete, J.M. Dietary intake of natural antioxidants: Vitamins and polyphenols. Crit. Rev. Food Sci. Nutr. 2013, 53, 706–721. [Google Scholar] [CrossRef]
- Roos, N.; Chamnan, C.; Loeung, D.; Jakobsen, J.; Thilsted, S.H. Freshwater fish as a dietary source of vitamin A in Cambodia. Food Chem. 2007, 103, 1104–1111. [Google Scholar] [CrossRef]
- Alsop, D.; Van Der Kraak, G.J.; Brown, S.B.; Eales, J.G. The biology and toxicology of retinoids in fish. In Biocheminstry and Molecular Biology of Fishes; Mommsen, T.P., Moon, T.W., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2005; Volume 6 Environmental toxicology; pp. 413–428. [Google Scholar] [CrossRef]
- Wagner, H.J. Retinal structure of fishes. In The Visual System of Fish; Douglas, R., Djamgoz, M., Eds.; Springer: Dordrecht, The Netherlands, 1990. [Google Scholar] [CrossRef]
- Jami, R.; Mérour, E.; Lamoureux, A.; Bernard, J.; Millet, J.K.; Biacchesi, S. Deciphering the Fine-Tuning of the Retinoic Acid-Inducible Gene-I Pathway in Teleost Fish and Beyond. Front. Immunol. 2021, 12, 679242. [Google Scholar] [CrossRef]
- Jiang, W.D.; Zhang, L.; Feng, L.; Wu, P.; Liu, Y.; Kuang, S.Y.; Li, S.W.; Tang, L.; Mi, H.F.; Zhang, L.; et al. New Insight on the Immune Modulation and Physical Barrier Protection Caused by Vitamin A in Fish Gills Infected with Flavobacterium columnare. Front Immunol. 2022, 13, 833455. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, L.; Jiang, W.; Liu, Y.; Jiang, J.; Kuang, S.; Li, S.; Tang, L.; Tang, W.; Zhou, X.; et al. Dietary Vitamin A Improved the Flesh Quality of Grass Carp (Ctenopharyngodon idella) in Relation to the Enhanced Antioxidant Capacity through Nrf2/Keap 1a Signaling Pathway. Antioxidants 2022, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Yeung, K.W.Y.; Lai, R.W.S.; Zhou, G.J.; Leung, K.M.Y. Concentration-response of six marine species to all-trans-retinoic acid and its ecological risk to the marine environment. Ecotoxicol. Environ. Saf. 2022, 235, 113455. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.R.; Wingert, R.A. Zebrafish as a Model to Study Retinoic Acid Signaling in Development and Disease. Biomedicines 2023, 11, 1180. [Google Scholar] [CrossRef]
- Guilland, J.-C. Les interactions entre les vitamines A, D, E et K: Synergie et/ou competition. OCL 2011, 18, 59–67. [Google Scholar] [CrossRef]
- Kane, M.A. Analysis, occurrence, and function of 9-cis-retinoic acid. Biochim. Biophys. Acta 2012, 1821, 10–20. [Google Scholar] [CrossRef]
- Buck, J.; Grun, F.; Derguini, F.; Chen, Y.; Kimura, S.; Noy, N.; Hammerling, U. Anhydroretinol: A naturally occur-ring inhibitor of lymphocyte physiology. J. Exp. Med. 1993, 178, 675–680. [Google Scholar] [CrossRef]
- Blomhoff, R.; Blomhoff, H.K. Overview of retinoid metabolism and function. Overview of retinoid metabolism and function. J. Neurobiol. 2006, 66, 606–630. [Google Scholar] [CrossRef]
- Fernández, I.; Gisbert, E. The effect of vitamin a on flatfish development and skeletogenesis: A review. Aquaculture 2011, 315, 34–48. [Google Scholar] [CrossRef]
- Hernandez de-Dios, M.A.; Tovar-Ramírez, D.; Maldonado García, D.; Galaviz-Espinoza, M.A.; Spanopoulos Zarco, M.; Maldonado-García, M.C. Functional Additives as a Boost to Reproductive Performance in Marine Fish: A Review. Fishes 2022, 7, 262. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, L.; Jiang, W.-D.; Liu, Y.; Wu, P.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; Zhou, X.-Q. Vitamin A deficiency suppresses fish immune function with differences in different intestinal segments: The role of transcriptional factor NF-κB and p38 mitogen-activated protein kinase signalling pathways. Br. J. Nutr. 2017, 117, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J.W. Hypervitaminosis A in rainbow trout (Salmo gairdneri): Toxicity signs and maximum tolerable level. J. Nutr. 1983, 113, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Furuita, H.; Tanaka, H.; Yamamoto, T.; Shiraishi, M.; Takeuchi, T. Effects of high dose of vitamin A on reproduction and egg quality of Japanese flounder Paralichthys olivaceus. Fish. Sci. 2001, 67, 606–613. [Google Scholar] [CrossRef]
- NASEM (National Academies of Sciences, Engineering, and Medicine). Nutrient Requirements of Fish and Shrimp; National Academic Science: Washington, DC, USA, 2011. [Google Scholar]
- Taveekijakarn, P.; Miyazaki, T.; Matsumoto, M.; Arai, S. Vitamin A Deficiency in Cherry Salmon. J. Aquat. Anim. Health 1994, 6, 251–259. [Google Scholar] [CrossRef]
- Saleh, G.; Eleraku, W.; Gropp, J.M. A short note on the effects of vitamin A hypervitaminosis on health and growth of Tilapia nilotica (Oreochromis niloticus). J. Appl. Ichthyol. 1995, 11, 382–385. [Google Scholar] [CrossRef]
- Dedi, J.; Takeuchi, T.; Seikai, T.; Watanabe, T.; Hosoya, K. Hyper vitaminosis A during vertebral morphogenesis in larval Japanese flounder. Fish. Sci. 1997, 63, 466–473. [Google Scholar] [CrossRef]
- Ørnsrud, R.; Graff, I.E.; Høie, S.; Totland, G.K.; Hemre, G.-I. Hypervitaminosis A in first-feeding fry of the Atlantic salmon (Salmo salar L.). Aquac. Nutr. 2002, 8, 7–13. [Google Scholar] [CrossRef]
- Campeche, D.F.B.; Catharino, R.R.; Godoy, H.T.; Cyrino, J.E.P. Vitamin A in diets for Nile tilapia. Sci. Agric. 2009, 66, 751–756. [Google Scholar] [CrossRef]
- Dominguez, D.; Montero, D.; Zamorano, M.J.; Castro, P.L.; da Silva, J.; Fontanillas, R.; Izquierdo, M. High Levels of Vitamin A in Plant-Based Diets for Gilthead Seabream (Sparus aurata) Juveniles, Effects on Growth, Skeletal Anomalies, Bone Molecular Markers, and Histological Morphology. Aquac. Nutr. 2023, 2023, 5788432. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Jameel, F.; Rani, D.; Serajuddin, M. Deficiency of protein, fat and vitamins in freshwater catfish, Clarias batrachus: Morphological symptoms and impact on growth performance. Borneo J. Mar. Sci. Aquac. 2019, 3, 9–12. [Google Scholar]
- Yang, Q.-H.; Zhou, X.-Q.; Jiang, J.; Liu, Y. Effect of dietary vitamin A deficiency on growth performance, feed utilization and immune responses of juvenile Jian carp (Cyprinus carpio var. Jian). Aquac. Res. 2008, 39, 902–906. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Li, Y.; Gu, J.; Liu, J.; Tang, J.; Wang, J.; Ryffel, B.; Shen, Y.; Brand, D.; et al. Differential role of all-trans retinoic acid in promoting the development of CD4+ and CD8+ regulatory T cells. J. Leukoc. Biol. 2014, 95, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Mariu, A.; Chatha, A.M.M.; Naz, S.; Khan, M.F.; Safdar, W.; Ashraf, I. Effect of Temperature, pH, Salinity and Dissolved Oxygen on Fishes. J. Zool. Syst. 2023, 1, 1–12. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Delving into Vitamin A Supplementation in Poultry Nutrition: Current Knowledge, Functional Effects, and Practical Implications. Worlds Poult. Sci. J. 2023, 79, 109–131. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organisation of the United Nations). Record Fisheries and Aquaculture Production Makes Critical Contribution to Global Food Security. 2022. Available online: https://www.fao.org/newsroom/detail/record-fisheries-aquaculture-production-contributes-food-security-290622/en (accessed on 6 July 2024).
- Palace, V.P.; Werner, J. Vitamins A and E in the maternal diet influence egg quality and early life stage development in fish: A review. Sci. Mar. 2006, 70, 41–57. [Google Scholar] [CrossRef]
- Firdaus-Nawi, M.; Zamri-Saad, M. Major Components of Fish Immunity: A Review. Pertanika J. Trop. Agric. Sci. 2016, 39, 393–420. [Google Scholar]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K.A. Main Components of Fish Immunity: An Overview of the Fish Immune System. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A Comparison of the Innate and Adaptive Immune Systems in Cartilaginous Fish, Ray-Finned Fish, and Lobe-Finned Fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef]
- Semple, S.L.; Dixon, B. Salmonid Antibacterial Immunity: An Aquaculture Perspective. Biology 2020, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, F.A.; Oettgen, H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010, 125 (Suppl. S2), S33–S40. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xu, H.; Yu, Y.; Xu, Z. Regulatory roles of cytokines in T and B lymphocytes-mediated immunity in teleost fish. Dev. Comp. Immunol. 2023, 144, 104621. [Google Scholar] [CrossRef]
- Magnadottir, B. Immunological Control of Fish Diseases. Mar. Biotechnol. 2010, 12, 361–379. [Google Scholar] [CrossRef]
- Barbosa, L.M.G.; Moraes, G.; Anibal, F.F.; Machado, C.M.M. Effect of environmental thermal fluctuations on innate immune responses in pacu Piaractus mesopotamicus juveniles. Aquac. Rep. 2020, 17, 100303. [Google Scholar] [CrossRef]
- Magadan, S.; Sunyer, O.J.; Boudinot, P. Unique Features of Fish Immune Repertoires: Particularities of Adaptive Immunity within the Largest Group of Vertebrates. Results Probl. Cell Differ. 2015, 57, 235–264. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Banu, H.; Prakash, A.; Tripathi, G. Immune System of Fish: An Evolutionary Perspective. In Antimicrobial Immune Response; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Green, H.N.; Mellanby, E. Vitamin A as an anti-infective agent. Brit. Med. J. 1928, 2, 691. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Vitamin A supply in swine production: A review of current science and practical considerations. Appl. Anim. Sci. 2023, 39, 289–305. [Google Scholar] [CrossRef]
- Vijayaram, S.; Ringø, E.; Zuorro, A.; van Doan, H.; Sun, Y. Beneficial roles of nutrients as immunostimulants in aquaculture: A review. Aquac. Fish. 2023, 9, 707–720. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Pet Wellness and Vitamin A: A Narrative Overview. Animals 2024, 14, 1000. [Google Scholar] [CrossRef]
- Thompson, I.; Fletcher, T.C.; Houlihan, D.F.; Secombes, C.J. The effect of dietary vitamin A on the immunocompetence of Atlantic salmon (Salmo salar L.). Fish Physiol. Biochem. 1994, 12, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish. Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Speirs, Z.C.; Loynes, C.A.; Mathiessen, H.; Elks, P.M.; Renshaw, S.A.; Jørgensen, L.V.G. What can we learn about fish neutrophil and macrophage response to immune challenge from studies in zebrafish. Fish Shellfish. Immunol. 2024, 148, 109490. [Google Scholar] [CrossRef]
- Jiang, W.-D.; Zhang, L.; Feng, L.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Zhou, X.-Q. Inconsistently impairment of immune function and structural integrity of head kidney and spleen by vitamin A deficiency in grass carp (Ctenopharyngodon idella). Fish Shellfish. Immunol. 2020, 99, 243–256. [Google Scholar] [CrossRef]
- Guimaraes, I.G.; Lim, C.; Yildirim-Aksoy, M.; Li, M.H.; Klesius, P.H. Effects of dietary levels of vitamin A on growth, hematology, immune response and resistance of Nile tilapia (Oreochromis niloticus) to Streptococcus iniae. Anim. Feed. Sci. Technol. 2014, 188, 126–136. [Google Scholar] [CrossRef]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin Effects on the Immune System: Vitamins A and D Take Centre Stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef]
- Nagpal, I.; Wei, L.N. All-trans Retinoic Acid as a Versatile Cytosolic Signal Modulator Mediated by CRABP1. Int. J. Mol. Sci. 2019, 20, 3610. [Google Scholar] [CrossRef]
- White, J.A.; Boffa, M.B.; Jones, B.; Petkovich, M. A zebrafish retinoic acid receptor expressed in the regenerating caudal fin. Development 1994, 120, 1861–1872. [Google Scholar] [CrossRef]
- Balmer, J.E.; Blomhoff, R. A robust characterization of retinoic acid response elements based on a comparison of sites in three species. J. Steroid Biochem. Mol. Biol. 2005, 96, 347–354. [Google Scholar] [CrossRef]
- Fernández, I.; Tiago, D.M.; Laizé, V.; Leonor Cancela, M.; Gisbert, E. Retinoic acid differentially affects in vitro proliferation, differentiation and mineralization of two fish bone-derived cell lines: Different gene expression of nuclear receptors and ECM proteins. J. Steroid Biochem. Mol. Biol. 2014, 140, 34–43. [Google Scholar] [CrossRef]
- Lu, L.; Ma, J.; Li, Z.; Lan, Q.; Chen, M.; Liu, Y.; Xia, Z.; Wang, J.; Han, Y.; Shi, W.; et al. All-Trans Retinoic Acid Promotes TGF-β-Induced Tregs via Histone Modification but Not DNA Demethylation on Foxp3 Gene Locus. PLoS ONE 2011, 6, e24590. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-M.; Wang, K.-P.; Ma, J.; Zheng, S.G. The role of all-trans retinoic acid in the biology of Foxp3+ regulatory T cells. Cell. Mol. Immunol. 2015, 12, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Indrevær, R.L.; Moskaug, J.; Paur, I.; Bøhn, S.K.; Jørgensen, S.F.; Blomhoff, R.; Aukrust, P.; Fevang, B.; Blomhoff, H.K. IRF4 Is a Critical Gene in Retinoic Acid-Mediated Plasma Cell Formation and Is Deregulated in Common Variable Immunodeficiency-Derived B Cells. J. Immunol. 2015, 195, 2601–2611. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Fu, S.; Yin, X.; Guo, Z.; Wang, A.; Ye, J. Long-Lived Plasma Cells Secrete High-Affinity Antibodies Responding to a T-Dependent Immunization in a Teleost Fish. Front. Immunol. 2019, 10, 2324. [Google Scholar] [CrossRef]
- Bono, M.R.; Tejon, G.; Flores-Santibañez, F.; Fernandez, D.; Rosemblatt, M.; Sauma, D. Retinoic Acid as a Modulator of T Cell Immunity. Nutrients 2016, 8, 349. [Google Scholar] [CrossRef]
- Duriancik, D.M.; Lackey, D.E.; Hoag, K.A. Vitamin A as a regulator of antigen presenting cells. J. Nutr. 2010, 140, 1395–1399. [Google Scholar] [CrossRef]
- Iliev, D.B.; Thim, H.; Lagos, L.; Olsen, R.; Jørgensen, J.B. Homing of Antigen-Presenting Cells in Head Kidney and Spleen—Salmon Head Kidney Hosts Diverse APC Types. Front. Immunol. 2013, 4, 137. [Google Scholar] [CrossRef]
- Lewis, K.L.; Del Cid, N.; Traver, D. Perspectives on antigen presenting cells in zebrafish. Dev. Comp. Immunol. 2014, 46, 63–73. [Google Scholar] [CrossRef]
- Conserva, M.R.; Anelli, L.; Zagaria, A.; Specchia, G.; Albano, F. The Pleiotropic Role of Retinoic Acid/Retinoic Acid Receptors Signaling: From Vitamin A Metabolism to Gene Rearrangements in Acute Promyelocytic Leukemia. Int. J. Mol. Sci. 2019, 20, 2921. [Google Scholar] [CrossRef]
- Sakai, M.; Hikima, J.; Kono, T. Fish cytokines: Current research and applications. Fish. Sci. 2021, 87, 1–9. [Google Scholar] [CrossRef]
- Hernandez, L.H.; Teshima, S.-I.; Koshio, S.; Ishikawa, M.; Tanaka, Y.; Alam, S. Effects of vitamin A on growth, serum anti-bacterial activity and transaminase activities in the juvenile Japanese flounder, Paralichthys olivaceus. Aquaculture 2007, 262, 444–450. [Google Scholar] [CrossRef]
- Cuesta, A.; Ortuno, J.; Rodrigues, A.; Esteban, M.A.; Meseguer, J. Changes in some innate defense parameters of seabream (Sparus aurata L.) induced by retinol acetate. Fish Shellfish. Immunol. 2002, 13, 279–291. [Google Scholar] [CrossRef]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Da Acad. Bras. De Ciênc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef] [PubMed]
- Shastak, Y.; Pelletier, W. Captivating Colors, Crucial Roles: Astaxanthin’s Antioxidant Impact on Fish Oxidative Stress and Reproductive Performance. Animals 2023, 13, 3357. [Google Scholar] [CrossRef] [PubMed]
- Filho, D.W. Reactive oxygen species, antioxidants and fish mitochondria. Front. Biosci. 2007, 12, 1229–1237. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Li, X.; Naseem, S.; Hussain, R.; Ghaffar, A.; Li, K.; Khan, A. Evaluation of DNA Damage, Biomarkers of Oxidative Stress, and Status of Antioxidant Enzymes in Freshwater Fish (Labeo rohita) Exposed to Pyriproxyfen. Oxidative Med. Cell. Longev. 2022, 2022, 5859266. [Google Scholar] [CrossRef]
- Alghazeer, R.; Howell, N.K. Formation of 4-hydroxynonenal (4-HNE) in frozen mackerel (Scomber scombrus) in the presence and absence of green tea. J. Sci. Food Agric. 2008, 88, 1128–1134. [Google Scholar] [CrossRef]
- Bastos, F.F.; Tobar, S.A.; Dantas, R.F.; Silva, E.S.; Nogueira, N.P.; Paes, M.C.; Righi, B.D.; Bastos, J.C.; Bastos, V.L. Melatonin affects conjugation of 4-hydroxynonenal with glutathione in liver of pacu, a hypoxia-tolerant fish. Fish Physiol. Biochem. 2013, 39, 1205–1214. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Garcia, D.; Lima, D.; da Silva, D.G.H.; de Almeida, E.A. Decreased malondialdehyde levels in fish (Astyanax altiparanae) exposed to diesel: Evidence of metabolism by aldehyde dehydrogenase in the liver and excretion in water. Ecotoxicol. Environ. Saf. 2020, 190, 110107. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant Defenses in Fish: Biotic and Abiotic Factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Zommara, M.; Eweedah, N.M.; Helal, A.I.; Aboel-Darag, M.A. The potential role of nano-selenium and vitamin C on the performances of Nile tilapia (Oreochromis niloticus). Environ. Sci. Pollut. Res. 2020, 27, 9843–9852. [Google Scholar] [CrossRef] [PubMed]
- Zengin, H. The effects of feeding and starvation on antioxidant defence, fatty acid composition and lipid peroxidation in reared Oncorhynchus mykiss fry. Sci. Rep. 2021, 11, 16716. [Google Scholar] [CrossRef] [PubMed]
- Battisti, E.K.; Marasca, S.; Durigon, E.G.; Villes, V.S.; Schneider, T.L.S.; Uczay, J.; Peixoto, N.C.; Lazzari, R. Growth and oxidative parameters of Rhamdia quelen fed dietary levels of vitamin A. Aquaculture 2017, 474, 11–17. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Dao, D.Q.; Ngo, T.C.; Thong, N.M.; Nam, P.C. Is vitamin A an antioxidant or a pro-oxidant? J. Phys. Chem. B 2017, 121, 9348–9357. [Google Scholar] [CrossRef]
- Palace, V.P.; Khaper, N.; Qin, Q.; Singal, P.K. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free Radic. Biol. Med. 1999, 26, 746–761. [Google Scholar] [CrossRef]
- Blaner, W.S.; Shmarakov, I.O.; Traber, M.G. Vitamin A and vitamin E: Will the real antioxidant please stand up? Annu. Rev. Nutr. 2021, 41, 105–131. [Google Scholar] [CrossRef]
- Wang, J.; Zou, L.; Jiang, P.; Yao, M.; Xu, Q.; Hong, Q.; Zhu, J.; Chi, X. Vitamin A ameliorates valproic acid-induced autism-like symptoms in developing zebrafish larvae by attenuating oxidative stress and apoptosis. Neurotoxicology 2024, 101, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Park, U.-H.; Han, H.S.; Um, E.; An, X.-H.; Kim, E.-J.; Um, S.J. Redox regulation of transcriptional activity of retinoic acid receptor by thioredoxin glutathione reductase (TGR). Biochem. Biophys. Res. Commun. 2009, 390, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Flohé, L. Regulatory phenomena in the glutathione peroxidase superfamily. Antioxid. Redox Signal. 2020, 33, 498–516. [Google Scholar] [CrossRef] [PubMed]
- Tourniaire, F.; Musinovic, H.; Gouranton, E.; Astier, J.; Marcotorchino, J.; Arreguin, A.; Bernot, D.; Palou, A.; Bonet, M.L.; Ribot, J.; et al. All-trans retinoic acid induces oxidative phosphorylation and mitochondria biogenesis in adipocytes. J. Lipid Res. 2015, 56, 1100–1109. [Google Scholar] [CrossRef]
- Rajawat, Y.; Hilioti, Z.; Bossis, I. Autophagy: A target for retinoic acids. Autophagy 2010, 6, 1224–1226. [Google Scholar] [CrossRef]
- Valenzuela, C.A.; Azúa, M.; Álvarez, C.A.; Schmitt, P.; Ojeda, N.; Mercado, L. Evidence of the Autophagic Process during the Fish Immune Response of Skeletal Muscle Cells against Piscirickettsia salmonis. Animals 2023, 13, 880. [Google Scholar] [CrossRef]
- Zhou, Z.; He, Y.; Wang, S.; Wang, Y.; Shan, P.; Li, P. Autophagy regulation in teleost fish: A double-edged sword. Aquaculture 2022, 558, 738369. [Google Scholar] [CrossRef]
- Fontagné, S.; Lataillade, E.; Brèque, J.; Kaushik, S. Lipid peroxidative stress and antioxidant defence status during ontogeny of rainbow trout (Oncorhynchus mykiss). Br. J. Nutr. 2008, 100, 102–111. [Google Scholar] [CrossRef]
- Jiang, W.D.; Zhou, X.Q.; Zhang, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Vitamin A deficiency impairs intestinal physical barrier function of fish. Fish Shellfish. Immunol. 2019, 87, 546–558. [Google Scholar] [CrossRef]
- Wu, F.; Zhu, W.; Liu, M.; Chen, C.; Chen, J.; Tan, Q. Effects of Dietary Vitamin A on Growth Performance, Blood Biochemical Indices and Body Composition of Juvenile Grass Carp (Ctenopharyngodon Idellus). Turk. J. Fish. Aquat. Sci. 2016, 16, 339–345. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, M.; Liang, X.-F.; Peng, D.; Xie, R.; Wu, D. Dietary supplementation of VA enhances growth, feed utilization, glucose and lipid metabolism, appetite, and antioxidant capacity of Chinese perch (Siniperca chuatsi). Fish Physiol. Biochem. 2024, 50, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Deering, M.J.; Paradis, H.; Ahmad, R.; Al-Mehiawi, A.S.; Gendron, R.L. The role of dietary vitamin A in mechanisms of cataract development in the teleost lumpfish (Cyclopterus lumpus L). J. Fish Dis. 2024, 47, e13899. [Google Scholar] [CrossRef]
- Liang, D.; Yang, Q.; Tan, B.; Dong, X.; Chi, S.; Liu, H.; Zhang, S. Dietary vitamin A deficiency reduces growth performance, immune function of intestine, and alters tight junction proteins of intestine for juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Fish Shellfish. Immunol. 2020, 107 Pt A, 346–356. [Google Scholar] [CrossRef]
- Devlin, R.H.; Leggatt, R.A.; Benfey, T.J. Chapter 7—Genetic modification of growth in fish species used in aquaculture: Phenotypic and physiological responses. In Fish Physiology; Benfey, T.J., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 38, pp. 237–272. [Google Scholar] [CrossRef]
- Breck, J.E. Body composition in fishes: Body size matters. Aquaculture 2014, 433, 40. [Google Scholar] [CrossRef]
- Young, T.; Laroche, O.; Walker, S.P.; Miller, M.R.; Casanovas, P.; Steiner, K.; Esmaeili, N.; Zhao, R.; Bowman, J.P.; Wilson, R.; et al. Prediction of Feed Efficiency and Performance-Based Traits in Fish via Integration of Multiple Omics and Clinical Covariates. Biology 2023, 12, 1135. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organisation of the United Nations). The Measurement of Fish and Shellfish. 2022. Available online: https://www.fao.org/4/F0752E/F0752E03.htm (accessed on 7 July 2024).
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Crane, D.P.; Ogle, D.H.; Shoup, D.E. Use and misuse of a common growth metric: Guidance for appropriately calculating and reporting specific growth rate. Rev. Aquacult. 2020, 12, 1542–1547. [Google Scholar] [CrossRef]
- Ahmed, I.; Jan, K.; Fatma, S.; Dawood, M.A.O. Muscle proximate composition of various food fish species and their nutritional significance: A review. J. Anim. Physiol. Anim. Nutr. 2022, 106, 690–719. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Q.; Chen, J.; Yang, H.; Zhang, W.; Wang, D.; Li, S.; Tao, M.; Shi, H.; Lin, H.; et al. Retinoic acid and androgen influence germ cells development and meiotic initiation in juvenile orange-spotted grouper, Epinephelus coioides. Gen. Comp. Endocrinol. 2020, 289, 113379. [Google Scholar] [CrossRef]
- Fraher, D.; Mann, R.J.; Dubuisson, M.J.; Ellis, M.K.; Yu, T.; Walder, K.; Ward, A.C.; Winkler, C.; Gibert, Y. The endocannabinoid system and retinoic acid signaling combine to influence bone growth. Mol. Cell. Endocrinol. 2021, 529, 111267. [Google Scholar] [CrossRef]
- Brown, G. Retinoic acid receptor regulation of decision-making for cell differentiation. Front. Cell Dev. Biol. 2023, 11, 1182204. [Google Scholar] [CrossRef]
- Qiu, J.; Nordling, S.; Vasavada, H.H.; Butcher, E.C.; Hirschi, K.K. Retinoic Acid Promotes Endothelial Cell Cycle Early G1 State to Enable Human Hemogenic Endothelial Cell Specification. Cell Rep. 2020, 33, 108465. [Google Scholar] [CrossRef] [PubMed]
- Haga, Y.; Du, S.-J.; Satoh, S.; Kotani, T.; Fushimi, H.; Takeuchi, T. Analysis of the mechanism of skeletal deformity in fish larvaeusing a vitamin A-induced bone deformity model. Aquaculture 2011, 315, 26–33. [Google Scholar] [CrossRef]
- Jackman, W.R.; Gibert, Y. Retinoic Acid Signaling and the Zebrafish Dentition during Development and Evolution. Subcell. Biochem. 2020, 95, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Mackowetzky, K.; Dicipulo, R.; Fox, S.C.; Philibert, D.A.; Todesco, H.; Doshi, J.D.; Kawakami, K.; Tierney, K.; Waskiewicz, A.J. Retinoic acid signaling regulates late stages of semicircular canal morphogenesis and otolith maintenance in the zebrafish inner ear. Dev. Dyn. 2022, 251, 1798–1815. [Google Scholar] [CrossRef]
- Paulissen, E.; Palmisano, N.J.; Waxman, J.S.; Martin, B.L. Somite morphogenesis is required for axial blood vessel formation during zebrafish embryogenesis. eLife 2022, 11, e74821. [Google Scholar] [CrossRef]
- Durbin, L.; Sordino, P.; Barrios, A.; Gering, M.; Thisse, C.; Thisse, B.; Brennan, C.; Green, A.; Wilson, S.; Holder, N. Anteroposterior patterning is required within segments for somite boundary formation in developing zebrafish. Development 2000, 27, 1703–1713. [Google Scholar] [CrossRef]
- Stickney, H.L.; Barresi, M.J.F.; Devoto, S.H. Somite development in zebrafish. Dev. Dyn. 2000, 219, 287–303. [Google Scholar] [CrossRef]
- Ward, A.B.; Mehta, R.S. Axial Elongation in Fishes: Using Morphological Approaches to Elucidate Developmental Mechanisms in Studying Body Shape. Integr. Comp. Biol. 2010, 50, 1106–1119. [Google Scholar] [CrossRef]
- Lleras Forero, L.; Narayanan, R.; Huitema, L.F.; Van Bergen, M.; Apschner, A.; Peterson-Maduro, J.; Logister, I.; Valentin, G.; Morelli, L.G.; Oates, A.C.; et al. Segmentation of the zebrafish axial skeleton relies on notochord sheath cells and not on the segmentation clock. eLife 2018, 7, e33843. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Detailed Review Paper on the Retinoid System. OECD Series on Testing and Assessment, Nr.343, Paris. 2021. Available online: https://one.oecd.org/document/ENV/CBC/MONO(2021)20/en/pdf (accessed on 11 February 2024).
- Isabella, A.J.; Barsh, G.R.; Stonick, J.A.; Dubrulle, J.; Moens, C.B. Retinoic Acid Organizes the Zebrafish Vagus Motor Topographic Map via Spatiotemporal Coordination of Hgf/Met Signaling. Dev. Cell 2020, 53, 344–357.e5. [Google Scholar] [CrossRef]
- Udo, I.U. Effects of dietary vitamin a level on growth, feed utilization and survival of juvenile North African catfish (Clarias gariepinus). Livest. Res. Rural. Dev. 2017, 29, 1–8. Available online: https://www.lrrd.org/lrrd29/2/udo29022.htm#:~:text=gariepinus%20should%20contain%20vitamin%20A,growth%20and%20efficient%20feed%20utilization (accessed on 7 July 2024).
- Hu, C.-J.; Chen, S.-M.; Pan, C.-H.; Huang, C.-H. Effects of dietary vitamin A or β-carotene concentrations on growth of juvenile hybrid tilapia, Oreochromis niloticus × O. aureus. Aquaqulture 2006, 253, 602–607. [Google Scholar] [CrossRef]
- Paiva, C.N.; Bozza, M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef] [PubMed]
- Shekhova, E. Mitochondrial Reactive Oxygen Species as Major Effectors of Antimicrobial Immunity. PLoS Pathog. 2020, 16, e1008470. [Google Scholar] [CrossRef]
- Victor, V.M.; Rocha, M.; De la Fuente, M. Immune Cells: Free Radicals and Antioxidants in Sepsis. Int. Immunopharmacol. 2004, 4, 327–347. [Google Scholar] [CrossRef]
- Tesoriere, L.; Bongiorno, A.; Pintaudi, A.M.; D’Anna, R.; D’Arpa, D.; Livrea, M.A. Synergistic interactions between vitamin A and vitamin E against lipid peroxidation in phosphatidylcholine liposomes. Arch. Biochem. Biophys. 1996, 326, 57–63. [Google Scholar] [CrossRef]
- Tesoriere, L.; Ciaccio, M.; Bongiorno, A.; Riccio, A.; Pintaudi, A.; Livrea, M. antioxidant activity of all-trans-retinol in homogeneous solution and in phosphatidylcholine liposomes. Arch. Biochem. Biophys. 1993, 307, 217–223. [Google Scholar] [CrossRef]
- Moore, T. The effect of vitamin E deficiency on vitamin A reserves of the rat. Biochem. J. 1940, 34, 1321. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Pang, S.-J.; Zhang, K.-W.; Li, P.-G.; Yang, C. Dietary vitamin A modifies the gut microbiota and intestinal tissue transcriptome, impacting intestinal permeability and the release of inflammatory factors, thereby influencing Aβ pathology. Front. Nutr. 2024, 11, 1367086. [Google Scholar] [CrossRef]
- Fernández, I.; Ortiz-Delgado, J.B.; Sarasquete, C.; Gisbert, E. Vitamin A effects on vertebral bone tissue homeostasis in gilthead sea bream (Sparus aurata) juveniles. J. Appl. Ichthyol. 2012, 28, 419–426. [Google Scholar] [CrossRef]
- Lucock, M.; Jones, P.; Martin, C.; Beckett, E.; Yates, Z.; Furst, J.; Veysey, M. Vitamin D: Beyond Metabolism. J. Evid. Based Complement. Altern. Med. 2015, 20, 310–322. [Google Scholar] [CrossRef]
- Cheng, K.; Huang, Y.; Wang, C.; Ali, W.; Karrow, N.A. Physiological function of vitamin D3 in fish. Rev. Aquac. 2023, 15, 1732–1748. [Google Scholar] [CrossRef]
- MacDonald, P.N.; Dowd, D.R.; Nakajima, S.; Galligan, M.A.; Reeder, M.C.; Haussler, C.A.; Ozato, K.; Haussler, M.R. Retinoid X receptors stimulate and 9-cis retinoic acid inhibits 1,25-dihydroxyvitamin D3-activated expression of the rat osteocalcin gene. Mol. Cell. Biol. 1993, 13, 5907–5917. [Google Scholar]
- Pierens, S.L.; Fraser, D.R. The origin and metabolism of vitamin D in rainbow trout. J. Steroid Biochem. Mol. Biol. 2015, 145, 58–64. [Google Scholar] [CrossRef]
- Džopalić, T.; Božić-Nedeljković, B.; Jurišić, V. The role of vitamin A and vitamin D in modulation of the immune response with a focus on innate lymphoid cells. Cent. Eur. J. Immunol. 2021, 46, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Ruiter, B.; Patil, S.U.; Shreffler, W.G. Vitamins A and D have antagonistic effects on expression of effector cytokines and gut-homing integrin in human innate lymphoid cells. Clin. Exp. Allergy 2015, 45, 1214–1225. [Google Scholar] [CrossRef]
- De Tullio, M.C. The Mystery of Vitamin C. Nat. Educ. 2010, 3, 48. [Google Scholar]
- Ching, B.; Chew, S.F.; Ip, Y.K. Ascorbate synthesis in fishes: A review. IUBMB Life 2015, 67, 69–76. [Google Scholar] [CrossRef]
- Lee, K.W.; Yoo, H.K.; Kim, S.-S.; Han, G.S.; Jung, M.M.; Kim, H.S. Effect of Dietary Vitamin C Supplementation on Growth Performance and Biochemical Parameters in Grower Walleye Pollock, Gadus chalcogrammus. Animals 2024, 14, 1026. [Google Scholar] [CrossRef]
- Kanter, M.; Coskun, O.; Armutcu, F.; Uz, Y.H.; Kizilay, G. Protective effects of vitamin C, alone or in combination with vitamin A, on endotoxin-induced oxidative renal tissue damage in rats. Tohoku J. Exp. Med. 2005, 206, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.M.; Yu, H.R.; Li, L.Y.; Li, M.; Qiu, X.Y.; Fan, X.Q.; Fan, Y.L.; Shan, L.L. Effects of Dietary Vitamin C on the Growth Performance, Biochemical Parameters, and Antioxidant Activity of Coho Salmon Oncorhynchus kisutch (Walbaum, 1792) Postsmolts. Aquac. Nutr. 2022, 2022, 6866578. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; To, V.P.T.H.; Santigosa, E.; Dumas, A.; Hernandez, J.M. Vitamin nutrition in salmonid aquaculture: From avoiding deficiencies to enhancing functionalities. Aquaculture 2022, 561, 738654. [Google Scholar] [CrossRef]
- Manam, V.K. Fish feed nutrition and its management in aquaculture. Int. J. Fish Aquat. Stud. 2023, 11, 58–61. [Google Scholar] [CrossRef]
- Terech-Majewska, E.; Pajdak, J.; Siwicki, A.K. Water as a source of macronutrients and micronutrients for fish, with special emphasis on the nutritional requirements of two fish species: The common carp (Cyprinus carpio) and the rainbow trout (Oncorhynchus mykiss). J. Elem. 2016, 21, 947–961. [Google Scholar]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef]
- Akhter, F.; Siddiquei, H.R.; Alahi, M.E.E.; Mukhopadhyay, S.C. Recent Advancement of the Sensors for Monitoring the Water Quality Parameters in Smart Fisheries Farming. Computers 2021, 10, 26. [Google Scholar] [CrossRef]
- Lindholm-Lehto, P. Water quality monitoring in recirculating aquaculture systems. Aquac. Fish Fish. 2023, 3, 113–131. [Google Scholar] [CrossRef]
- Sneddon, L.U.; Wolfenden, D.C.C.; Thomson, J.S. Stress management and welfare. Fish. Physiol. 2016, 35, 463–539. [Google Scholar]
- Long, L.; Zhang, H.; Ni, Q.; Liu, H.; Wu, F.; Wang, X. Effects of stocking density on growth, stress, and immune responses of juvenile Chinese sturgeon (Acipenser sinensis) in a recirculating aquaculture system. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 219, 25–34. [Google Scholar] [CrossRef]
- Dara, M.; Carbonara, P.; La Corte, C.; Parrinello, D.; Cammarata, M.; Parisi, M.G. Fish Welfare in Aquaculture: Physiological and Immunological Activities for Diets, Social and Spatial Stress on Mediterranean Aqua Cultured Species. Fishes 2023, 8, 414. [Google Scholar] [CrossRef]
- Gerber, B.; Stamer, A.; Stadtlander, T. Environmental Enrichment and Its Effects on Welfare in Fish. FiBL Schweiz. 2015. Available online: https://orgprints.org/id/eprint/29142/1/Gerber-etal-2015-Environmental-Enrichment-and-its-effects-on-welfare-in-fish-FiBL-Review.pdf (accessed on 9 July 2024).
- Näslund, J.; Johnsson, J.I. Environmental enrichment for fish in captive environments: Effects of physical structures and substrates. Fish Fish. 2016, 17, 1–30. [Google Scholar] [CrossRef]
- Arechavala-Lopez, P.; Caballero-Froilán, J.C.; Jiménez-García, M.; Capó, X.; Tejada, S.; Saraiva, J.L.; Sureda, A.; Moranta, D. Enriched environments enhance cognition, exploratory behaviour and brain physiological functions of Sparus aurata. Sci. Rep. 2020, 10, 11252. [Google Scholar] [CrossRef] [PubMed]
- Browning, H. Improving welfare assessment in aquaculture. Front. Vet. Sci. 2023, 10, 1060720. [Google Scholar] [CrossRef]
- Hemal, M.M.; Rahman, A.; Nurjahan; Islam, F.; Ahmed, S.; Kaiser, M.S.; Ahmed, M.R. An Integrated Smart Pond Water Quality Monitoring and Fish Farming Recommendation Aquabot System. Sensors 2024, 24, 3682. [Google Scholar] [CrossRef] [PubMed]
- Gudding, R.; Lillehaug, A.; Tavornpanich, S. Immunoprophylaxis in Biosecurity Programs. J. Appl. Aquac. 2015, 27, 220–227. [Google Scholar] [CrossRef]
- Wang, B.; Thompson, K.D.; Wangkahart, E.; Yamkasem, J.; Bondad-Reantaso, M.G.; Tattiyapong, P.; Jian, J.; Surachetpong, W. Strategies to enhance tilapia immunity to improve their health in aquaculture. Rev. Aquac. 2023, 15 (Suppl. S1), 41–56. [Google Scholar] [CrossRef]
- Varghese, T.; Gopan, A.; Rejish Kumar, V. Reinventing the Micronutrients beyond Nutrition: Functions in Immune Modulation and Stress Mitigation of Fish. In Biotechnological Advances in Aquaculture Health Management; Gupta, S.K., Giri, S.S., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organisation of the United Nations). Causes and Effects of Pollution on Fish. 2024. Available online: https://www.fao.org/fishery/docs/CDrom/aquaculture/a0844t/docrep/009/T1623E/T1623E03.htm (accessed on 11 July 2024).
- Brooks, P.R.; Crowe, T.P. Combined Effects of Multiple Stressors: New Insights Into the Influence of Timing and Sequence. Front. Ecol. Evol. 2019, 7, 387. [Google Scholar] [CrossRef]
- Keleştemur, G.T.; Özdemir, Y. Effect of Dietary Vitamin A and E on Tissues Vitamin Concentrations and Lipid Peroxidation of Juvenile Rainbow Trout at Different Flow Rates. Turk. J. Sci. Technol. 2016, 11, 21–29. [Google Scholar]
- Zhou, C.; Yang, S.; Ka, W.; Gao, P.; Li, Y.; Long, R.; Wang, J. Association of Gut Microbiota With Metabolism in Rainbow Trout Under Acute Heat Stress. Front. Microbiol. 2022, 13, 846336. [Google Scholar] [CrossRef]
- Kong, M.; Zhao, W.; Wang, C.; Qi, J.; Liu, J.; Zhang, Q. A Well-Established Gut Microbiota Enhances the Efficiency of Nutrient Metabolism and Improves the Growth Performance of Trachinotus ovatus. Int. J. Mol. Sci. 2024, 25, 5525. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.K.; Shakya, A.; Bashir, K.; Kushwaha, S.C.; McClements, D.J. Vitamin A fortification: Recent advances in encapsulation technologies. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2772–2819. [Google Scholar] [CrossRef] [PubMed]
- Shastak, Y.; Pelletier, W.; Kuntz, A. Insights into Analytical Precision: Understanding the Factors Influencing Accurate Vitamin A Determination in Various Samples. Analytica 2024, 5, 54–73. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Nutritional Balance Matters: Assessing the Ramifications of Vitamin A Deficiency on Poultry Health and Productivity. Poultry 2023, 2, 493–515. [Google Scholar] [CrossRef]
- Arshad, R.; Gulshad, L.; Haq, I.U.; Farooq, M.A.; Al-Farga, A.; Siddique, R.; Manzoor, M.F.; Karrar, E. Nanotechnology: A novel tool to enhance the bioavailability of micronutrients. Food Sci. Nutr. 2021, 9, 3354–3361. [Google Scholar] [CrossRef]
- Vettom, E.J.; Danve, A.; Khalasi, Y. Precision Aquaculture: Integrating Technology for Enhanced Production and Sustainability. Agric. Mag. 2024, 3, 203–211. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).