Abstract
The purpose of this study was to measure organ blood flow (OBF) in yellow catfish (YC, Pelteobagrus fulvidraco), largemouth bass (LB, Micropterus salmoides), and grass carp (GC, Ctenopharyngodon idella) using the method of fluorescent microspheres. Yellow–green microspheres were injected into the fish via cardiac catheterization using a syringe pump at a rate of 0.8 mL/min. Reference blood samples were collected from the dorsal aorta, and fish tissues were harvested after 5 min and processed for fluorescence spectrophotometric analysis. The results showed that the OBF of the heart increased significantly with the increase in temperature from 20 to 30 °C, while there was no significant difference in the OBF of other organs/tissues in YC. The OBFs of different species of LB and GC were also determined at 25 °C. In GC, the blood flow rates of the heart, spleen, kidney, liver, others, gills, swim bladder, intestines, muscles, and skin were 9.55, 1.00, 10.3, 6.92, 6.70, 6.04, 2.06, 2.81, 1.78, and 3.72 (mL/min/g), respectively. In LB, the blood flow rates of the same organs were 8.80, 2.33, 1.01, 0.71, 4.11, 2.72, 1.22, 0.54, 9.47, and 0.40 (mL/min/g), respectively. Compared to the OBFs of YC at 25 °C, the OBFs in GC were the highest, followed by LB. These results reflect that OBF in fish has significant species differences. These studies provide fundamental physiological data on OBFs in YC, GC, and LB, which has practical implications for improving the development of disciplines associated with fish physiology.
Keywords:
organ blood flow; fluorescent microspheres; Pelteobagrus fulvidraco; Ctenopharyngodon idella; Micropterus salmoides Key Contribution:
The information on organ blood flow for fish was scarce. The present study first determined the organ blood flow in different three fish species to help build a physiologically based pharmacokinetic model.
1. Introduction
The disposition of medicines in the bodies of humans and animals is influenced by many physiological parameters [1,2]. Organ blood flow (OBF) is one of the most important parameters that can transport various nutrients, waste, or medicine to enter or leave organs. Its changes easily affect the distribution, metabolism, and elimination of medicines in the body to further influence pharmacological and toxicological activities. In aquaculture, fish are heterothermic animals whose OBF may be easily influenced by environmental temperature and fish species. So, a better understanding of the influence of both factors on OBF is beneficial for pharmacological study and other scientific fields. In previous studies, OBFs have been determined in humans, cattle, pigs, and chickens [3,4,5,6,7], but few studies have been conducted in fish [8].
With the advancement of OBF measurement technology, new blood flow monitoring methods have been continually developed. Different methods for blood flow monitoring have their advantages and shortcomings due to different technical principles. Common methods used to monitor blood flow in the laboratory include radioactive microspheres (RM) [9], fluorescent microspheres (FM) [10], ultrasound flowmeter (UF) [11], laser Doppler flowmetry (LDF) [12], and functional magnetic resonance imaging (FMRI) [13]. Among them, LDF and FMRI are widely popular in academic research and clinical diagnosis in human medicine due to their non-invasiveness and simple operation [14]. However, they have limitations such as a long scanning time, inability to measure quietly in awake experimental animals, unclear imaging effects on moving organs (such as the gastrointestinal tract and lungs), and high costs for ordinary laboratories. Compared to LDF and FMRI, FM and UF methods are usually used for the measurement of OBF in experimental animals. They can precisely position organs and repeatedly non-invasively measure OBFs [15]. Moreover, compared to RM, FM does not have radioactivity to prevent harm to operators and the environment from radio substances [16], and it has the advantages of high sensitivity, excellent color separation, and easier measurement [17]. The technology of FM also provides more detailed information on inter-organ and intra-organ perfusion. Therefore, FM possesses more merits than other methods in determining OBF.
In the present study, we selected yellow catfish (YC, Pelteobagrus fulvidraco), largemouth bass (LB, Micropterus salmoides), and grass carp (GC, Ctenopharyngodon idella) to conduct experiments because they are all commercially important fish species in China. In 2019, their production reached 509,610, 432,058, and 5,504,301 tons, respectively [18]. We aimed to (1) determine OBF in YC at different temperatures; (2) assay OBF in different species of YC, LB, and GC at the same temperature; and (3) assay organ weight in YC, LB, and GC. These determinations of parameters can help to better understand the relation of medicine disposition to physiological activities in the body, thereby optimizing drug treatment plans and predicting the withdrawal time of drugs.
2. Materials and Methods
2.1. Chemicals and Reagents
Thermo Fisher Scientific (Waltham, MA, USA) provided fluosphere beads, which are polystyrene microspheres sized at 15 μm with a purity of >99%. Shanghai Mclean Biochemical Technology Co., Ltd. (Shanghai, China) provided Triton X-100, potassium hydroxide (95%), potassium dihydrogen phosphate (99.5%), dihydrogen potassium phosphate (98%), and ethylene glycol diacetate (99%). Tween 80 was provided by Biofroxx (Einhausen, Germany). Heparin sodium was obtained from Shanghai Yuan Ye Biological Technology Co., Ltd. (Shanghai, China). Sodium chloride was purchased from National Pharmaceutical Group Chemical Reagent Co., Ltd. (Shanghai, China). The 22 G needles, 23 G needles, PE-40 and PE-20 polyethylene tubes, 1 mL syringes, surgical scissors, surgical needles, scalpels, tweezers, surgical suture, heparin, and 0.9% saline were obtained from Provence Biotechnology Co., Ltd. (Wuhan, China). The 1.5 mL vials and centrifugal tubes were purchased from Shanghai CNW Technologies (Shanghai, China).
2.2. Animals
Thirty healthy YC (112.3 ± 45.7 g, 12 months old, mixed sex), twenty LB (338.6 ± 44.6 g, 12 months old, mixed sex), and twenty healthy GC (941.5 ± 155.1 g, 8 months old, mixed sex) were purchased from the facility of Yangtze River Fisheries Research Institute in Wuhan, China. The fish were placed in aquariums with a capacity of 480 L (5 fish per tank) and gradually acclimated to the temperatures of 20 °C, 25 °C, and 30 °C for two weeks. Water heaters and air conditioning were used to keep constant temperatures. Water quality parameters were maintained at a benign level, including dissolved oxygen concentration ≥ 8 µg/mL, total ammonia nitrogen level ≤ 0.75 μg/mL, nitrite nitrogen level ≤ 0.06 μg/mL, and pH being approximately 7.1 ± 0.3. All animal-related protocols were approved by the Fish Ethics Committee of the Yangtze River Fisheries Research Institute at the Chinese Academy of Fishery Sciences, Wuhan, China.
2.3. Dorsal Aorta and Heart Cannulation
The fish was weighed and anesthetized by eugenol with a concentration of 50 µg/mL. A 23 G needle was connected to a 50 cm PE-50 polyethylene tube to prepare a dorsal aorta catheter. The puncture needle was inserted into the dorsal aorta, and the catheter was fixed in the appropriate position. An appropriate amount of 1% heparinized solution was injected to prevent blood reflux and clotting inside the catheter. The other end of the catheter was passed through a hole made by surgical scissors and then fixed with a suture and connected to a 1 mL syringe filled with heparinized 0.9% saline solution (containing 10 IU of sodium heparin), pushed or drawn by a suction pump for blood sampling [19]. The fish was not fed during the experiment.
The heart catheter included a 22 G needle (2.54 cm) with a 90° bend and 15 cm of PE-20 tubing [20]. The catheter was also filled with a 1% heparinized solution. After injecting 100 μL of microsphere preparation liquid (containing approximately 100,000 beads) at a uniform rate of 0.8 mL/min into the ventricle using a suction pump, approximately 1 mL of a reference blood sample was collected immediately from the dorsal aorta at a uniform rate of 10 mL/min by another suction pump. Blood samples were stored in a heparinized 10 mL centrifuge tube. After 5 min, fish plasma was drawn by a 5 mL syringe from caudal vessels. Skin, muscle, heart, liver, spleen, gill, kidney, swim bladder, intestine, and other tissues were also collected from each fish. As for YC, we examined the OBF at disparate temperatures of 20 °C, 25 °C, and 30 °C, respectively. The experiments of LB and GC were conducted only at a temperature of 25 °C.
2.4. Sample Preparation
One gram or 1 mL of sample was placed in a 15 mL centrifuge tube with a cap. A total of 8 mL ethanol KOH solution with a concentration of 2 mol/L was added to each centrifuge tube, shaken, and placed in a 50 °C water bath overnight (48 h). During this period, the cap should be opened to release pressure. After the tissues or organs were completely digested and dissolved, samples were centrifuged at 2000× g for 20 min. The obtained supernatant was discarded by a suction tube to a height of 1.5 cm from the bottom of the tube. Then, 8 mL of 1% Triton X-100 was added and shaken for 1 min, and then centrifuged at 2000× g for 20 min. The resulting supernatant was also discarded. Finally, 7 mL of phosphate buffer was added to the tube shaken for 1 min, and continuedly centrifuged at 2000× g for 20 min. The resulting supernatant was discarded to 0.5 mL at the bottom of the tube. Afterward, 3 mL of 2-ethoxy ethyl acetate was added and mixed. All samples were placed in the darkroom. On the fifth day, the tubes were gently shaken for 20 s until the pellet ruptured. On the eighth day, the tubes were shaken vigorously for 30 s until the pellet broke up and then centrifuged at 2000× g for 20 min. The final supernatant was detected by a fluorescence spectrophotometer.
2.5. Standard Curve and Recovery Rate
One hundred microliters of fluorescent microspheres (1 × 106 beads/mL) were used to prepare standard solutions with quantities of 10,000, 2000, 400, 80, 16, 3.2, and 0.64 beads/mL. The fluorescence was measured by utilizing a fluorescence spectrophotometer (Cary Eclipse, Varian Inc., Palo Alto, CA, USA) with an excitation wavelength of 505 nm and an emission wavelength of 515 nm. The microsphere quantity was plotted on the x-axis, and the fluorescence intensity was plotted on the y-axis to construct a standard curve. The linear correlation coefficient of the curve was calculated.
Six standard tubes were filled with 100 μL of microsphere solution containing 10,000 microspheres. Then, 3 mL of ethylene glycol ether acetate was added to each tube, which was shaken and poured into a glass vessel. The fluorescence intensity of the blue microspheres in the standard tube was measured using a fluorescence spectrophotometer. Six spiked samples were prepared using the same number of microspheres. After the above-mentioned sample preparation, the fluorescence intensity was also detected and compared with that of the standard tube to calculate the recovery rate of the microspheres.
2.6. Calculation of Regional Organ Perfusion
The blood flow distribution of each tissue was determined based on the proportion of microspheres that remained in each tissue. An arterial reference sample was extracted using an injection pump, and the sample velocity (3–5 mL/min) was calculated by weighing the blood sample. The flow rate to each tissue was calculated based on the following equation.
where i is the sample number, fli is the fluorescence value of each organ, flref is the fluorescence value of the reference blood sample, R is the extraction rate of the reference blood sample, and Qi is the flow to each tissue.
2.7. Statistical Analysis
All data are expressed as x ± s (n = 6) and analyzed using Excel 2019 software and SPSS (IBM SPSS statistic 19.0). A one-way ANOVA was used to compare differences among multiple groups. If the p value was less than 0.05, it indicated statistical significance. If the p value was more than 0.05, it indicated statistical insignificance.
3. Results
3.1. Methodology
A standard curve was constructed for each batch of microspheres to confirm linearity and concentration range. Figure 1 shows that the standard curve equation for yellow–green fluorescent microspheres is Y = 0.8532x − 69.016 (R2 = 0.9991) across microsphere quantities from 1 to 10,000. As shown in Figure 2, the absorbances of both the standard tube and the microspheres recovered from each tissue sample were recorded. The mean absorbance value of the recovered microspheres was 0.2490 AU. The microsphere recovery rate for the tissue samples ranged from 82.16% to 97.63%, which reached the experimental requirements.
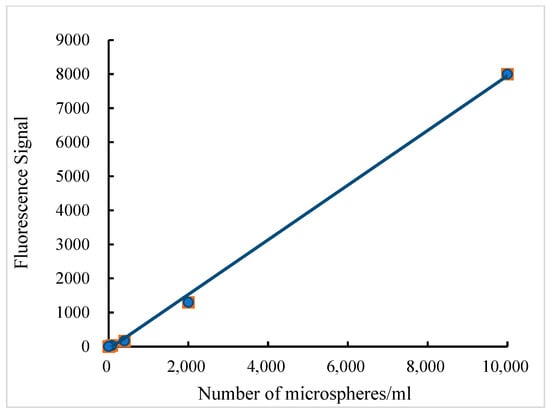
Figure 1.
Relationship between fluorescence intensity and the number of yellow–green fluorescent microspheres.
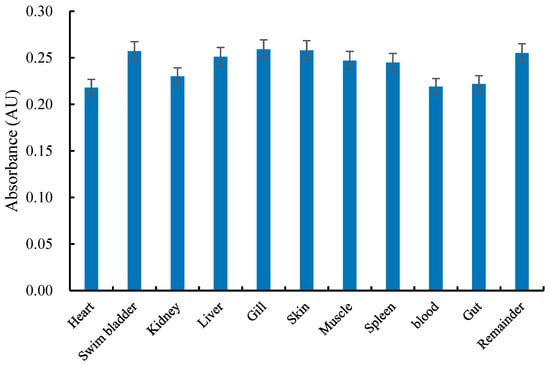
Figure 2.
Mean absorbance of dye extracted from blue microspheres isolated from tissues of fish.
3.2. Organs/Tissues Weight
Table 1 shows the organ/tissue percentage ratios to the total body weight of YC, GC, and LB, respectively. The remainder tissue had the largest proportion compared to other organs or tissues in the three fish species. The proportion values of the remainder tissue were 52.59%, 43.45%, and 50.79% in YC, GC, and LB, respectively. The second percentage was for muscle, with values of 32.26% in YC, 41.85% in GC, and 39.64% in LB, respectively. Thereafter, the percentages of skin ranged from 2.73% to 5.88%, and their order was YC > GC > LB. The percentages of blood ranged from 1.18% to 5.80%, and their order was GC > YC > LB. The percentages of liver ranged from 1.02% to 1.58%, and their order was YC > LB > GC. The percentages of intestines ranged from 0.90% to 1.51%, and their order was LB > GC > YC. Finally, the proportions of the heart, spleen, kidney, and swim bladder were all below 1.00%.

Table 1.
Experimentally measured percentages of blood and organs/tissues in total body weight in yellow catfish (Pelteobagrus fulvidraco), largemouth bass (Micropterus salmoides), and grass carp (Ctenopharyngodon idella) (n = 6).
3.3. OBFs of YC at Different Temperatures
Table 2 shows the OBFs of various organs/tissues in YC at 20, 25, and 30 °C. Their differences at different temperatures were compared by the ANOVA. In the heart, although the OBF of the heart increased with increasing temperature, the difference at disparate temperatures was not significant. In other organs, the OBF did not display a temperature dependence. The spleen had the highest blood flow rate at 20 °C. The highest blood flow rates of the muscles, liver, kidney, swim bladder, intestines, and other tissues were presented at 25 °C. The gills had the highest blood flow rate at 30 °C. At 20 °C, the order of the OBF rate in YC was as follows: spleen > heart > gills > liver = remainder > skin > kidney > swim bladder > muscle > intestine. At 25 °C, the order of the OBF rate in YC was as follows: heart > spleen > kidney > liver > remainder > gills > swim bladder > intestine > muscle > skin. At 30 °C, the arrangement of the OBF in YC was as follows: heart > spleen > gills > liver > kidney > intestine > swim bladder > muscle > remainder > skin.

Table 2.
Percentages of organs/tissues in total body weight and perfusion volume (Vb) of organs/tissues of yellow catfish (Pelteobagrus fulvidraco) at different temperatures (n = 6).
3.4. OBFs of YC, LB, and GC at the Same Temperature
The OBF of YC, GC, and LB at 25 °C are shown in Table 3. Compared to the overall OBF of the three fish species, GC had the highest OBF, except for the organ of the spleen. In YC, the blood flow rates of the heart, spleen, kidney, liver, others, gills, swim bladder, intestines, muscles, and skin were 6.40, 2.23, 0.85, 0.76, 0.59, 0.54, 0.44, 0.28, 0.24, and 0.21 mL/min/g, respectively. In GC, the blood flow rates of the heart, spleen, kidney, liver, others, gills, swim bladder, intestines, muscles, and skin were 9.55, 1.00, 10.3, 6.92, 6.70, 6.04, 2.06, 2.81, 1.78, and 3.72 mL/min/g, respectively. In largemouth black bass, the blood flow rates of the same organs were 8.80, 2.33, 1.01, 0.71, 4.11, 2.72, 1.22, 0.54, 9.47, and 0.40 mL/min/g, respectively. Through statistical ANOVA, the OBF of the same organ in disparate species showed statistical differences.

Table 3.
Percentages of organs/tissues in total body weight and perfusion volume (Vb) of organs/tissues of yellow catfish (Pelteobagrus fulvidraco), grass carp (Ctenopharyngodon idella), and largemouth bass (Micropterus salmoides) at 25 °C (n = 6).
4. Discussion
4.1. Organs Weight
From the perspective of organ weight except for the remainder tissues, the muscles of the YC, GC, and LB had the highest proportion in a single organ/tissue, accounting for 32.26%, 41.85%, and 39.64%, respectively. Meanwhile, the percentages of blood in YC, GC, and LB were 2.21%, 5.80%, and 1.18%, respectively. These may be related to the physiological characteristics of the three species, which belong to mid- to upper-level migratory fish that require strong swimming and metabolic activity capabilities [21]. Therefore, the high proportion of muscle and blood in these fish satisfies their body’s needs for metabolism and movement. The weight proportion of organs such as the heart, liver, spleen, gills, kidneys, and swim bladders in the fish is relatively small, but still crucial for their survival and normal physiological function. Additionally, the gills account for a certain proportion because they are an important organ for the fish to breathe and maintain salinity balance. Moreover, there may be some correlation between organs/tissue weight and a fish’s blood flow and metabolic activity. Larger organs/tissues may require more blood and oxygen to maintain their normal metabolism and physiological function. From these viewpoints, measurement of the weight of fish organs/tissues will help to understand their physiological properties and provide a certain reference for evaluating the fish’s health status and growth.
4.2. OBFs of YC at Different Temperatures
In the present study, we found that the OBF of the heart increased with increasing temperature. This may be attributable to the heart being the most predominant organ for animals, being responsible for delivering oxygen and nutrients to all tissues and organs. However, the OBFs of other organs did not present temperature dependence. A previous study had similar findings. The blood perfusion of the intestine showed an elevated trend with the increase in temperature, but other organs and red muscles were not affected by acclimation temperature in rainbow trout [8]. These presented phenomena may exist to maintain stable blood pressure. When cardiac output increases with enhanced temperature, the OBF might be redistributed by the alteration in coeliacomesenteric constriction or peripheral vascular tone to restrict the increase in vascular resistance [22,23], thereby avoiding excessive blood pressure. Hence, this phenomenon is a self-protection measure for the fish body to acclimatize to a change in temperature. During this procedure of adjustment, the OBF did not increase with the temperature rise, but the fish body did show corresponding biochemical changes to adapt to the elevated temperature. The published results demonstrated that the reduction in blood flow in muscle induced the increase in mitochondrial enzyme activity to adapt to the reduction of oxygen volume [24].
4.3. Comparison of OBFs among YC, LB, and GC at the Same Temperature
In this study, we found that the heart and spleen had a higher blood flow in YC; the skin, muscle, heart, liver, gill, and kidney had a higher blood flow in GC; and the muscle, heart, spleen, gill, kidney had a higher blood flow in LB. In dogfish, the highest tissue organ blood perfusion was presented in the kidney, red muscle, and spleen [25]. Barron et al. [8] reported that the highest organ blood perfusion rate was obtained in the intestine, red muscle, kidney, and pyloric caeca in rainbow trout. These results reveal that the kidney and the muscle commonly had a relatively high blood flow. The kidney is the main excretion organ that needs sufficient blood volume to filter nutrient compounds and exclude metabolic waste. Muscle is one of the most important energy-consuming tissues in fish, requiring a relatively large amount of oxygen and blood flow during activity. Hence, they need higher blood flow. Additionally, although the liver is also an important metabolic organ in fish, it has less blood flow. This is because its blood flow does not represent the total liver perfusion and does not include the visceral circulation entering the liver through the portal vein. The hepatic portal blood flow can be estimated by combining part of the visceral OBF but not including the kidneys.
According to the experimental results in the present study, at 25 °C the highest OBF rate was observed in GC, followed by LB and YC. These differences may be partly related to fish body size and metabolism. Authors have speculated that larger fish require more blood to deliver oxygen and nutrients to various parts of the body, hence their OBF rates are relatively high. Smaller fish have smaller body sizes, requiring less energy and oxygen, hence their OBF rates are relatively small. Apart from temperature, differences in OBF for different fish may be related to various factors, including body size, metabolic rate, behavior, and physiological state.
4.4. Comparison of OBF between Fish and Other Animals
OBF has been determined in chickens, cattle, swine, and sheep [4,5,7]. In the present study, we determined the OBFs of three fish species and calculated average values for comparison to those of chickens, cattle, swine, and sheep. The average percentage of muscle to total cardiac output in fish is 41.59%, which is far higher than chickens (7.64%) and sheep (10.09%), and higher than adult cattle (28.00%) and swine (34.20%). In the liver, the average percentage (1.38%) in fish is close to that in sheep (1.20%) but far less than that in chickens (25.26%) and adult cattle (46.00%). In the kidney, the average ratio in fish is 0.86% far less than that in chickens (20.12%), adult cattle (28.00%), and swine (11.40%). In skin, the percentage value (1.76%) in fish is less than that in chickens (15.05%), sheep (10.93%), and swine (3.50%). In the intestine, the ratio value (0.48%) in fish is less than that in chicken (11.61%), swine (20.40%), and sheep (19.23%). In the spleen, the ratio value (0.17%) in fish is also less than that in chickens (4.03%), sheep (4.54%), and swine (3.10%). Overall, only the proportion of blood flow in the muscle of fish is more than in chickens, sheep, cattle, and swine, but these in other organs are less than in chickens, sheep, cattle, and swine. The detailed reason may be related to different physiological properties and life environments.
5. Conclusions
In this study, body weight and OBFs were detected in YC, GC, and LB. Percentage ratios of remainder tissues and muscle to the total body weight had higher values than other organs or tissues. The OBF in the heart of the YC increases with temperature changes, while other tissue blood flow rates do not show significant changes. This indicates that the organ perfusion of fish is unaffected by the change in temperature to keep physiological balance. At the same temperature, the GC has the largest OBF rate, followed by the LB and YC, suggesting that OBFs show intra-species differences. This study is beneficial to better understanding the physiological role of OBF in fish and provides fundamental data for other study fields.
Author Contributions
Conceptualization, N.X.; methodology, N.X.; software, H.Z.; validation, Q.Y. and S.Z.; formal analysis, H.Z.; investigation, Q.Y.; resources, X.A.; data curation, H.Z.; writing—original draft preparation, N.X.; writing—review and editing, N.X.; visualization, S.Z.; supervision, X.A.; project administration, H.Z.; funding acquisition, N.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the financial support from the National Natural Science Foundation of China (32173022); the Central Public-interest Scientific Institution Basal Research Fund, CAFS (YFI20240404), the Central Public-interest Scientific Institution Basal Research Fund, CAFS (2023TD47), and the National Key R&D Program of China (2019YFD0901701).
Institutional Review Board Statement
This study was approved by the Fish Ethics Committee of Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences, Wuhan, China (YFI2022xuning03, 4 July 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
FM, fluorescent microspheres; FMRI, functional magnetic resonance imaging; GC, grass carp; LB, largemouth bass; LDF, laser doppler flowmetry; OBF, organ blood flow; RM, radioactive microspheres; UF, ultrasound flowmeter; YC, yellow catfish.
References
- Clarke, A.; Johnston, N.M. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 1999, 68, 893–905. [Google Scholar] [CrossRef]
- Killen, S.S.; Atkinson, D.; Glazier, D.S. The intraspecific scaling of metabolic rate with body mass in fishes depends on lifestyle and temperature. Ecol. Lett. 2010, 13, 184–193. [Google Scholar] [CrossRef]
- Hu, Q.; Nelson, T.J.; Seymour, R.S. Regional femoral bone blood flow rates in laying and non-laying chickens estimated with fluorescent microspheres. J. Exp. Biol. 2021, 224, jeb242597. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.-S.; Elwell-Cuddy, T.; Baynes, R.; Tell, L.; Davis, J.; Maunsell, F.; Riviere, J.; Lin, Z. Physiological parameter values for physiologically based pharmacokinetic models in food-producing animals. Part III: Sheep and goat. J. Vet. Pharmacol. Ther. 2020, 44, 456–477. [Google Scholar] [CrossRef]
- Lin, Z.; Li, M.; Wang, Y.S.; Tell, L.A.; Baynes, R.E.; Davis, J.L.; Vickroy, T.W.; Riviere, J.E. Physiological parameter values for physiologically based pharmacokinetic models in food-producing animals. Part I: Cattle and swine. J. Vet. Pharmacol. Ther. 2020, 43, 385–420. [Google Scholar] [CrossRef]
- Meng, L.; Wang, Y.; Zhang, L.; McDonagh, D.L. Heterogeneity and Variability in Pressure Autoregulation of Organ Blood Flow: Lessons Learned over 100+ Years. Crit. Care Med. 2019, 47, 436–448. [Google Scholar] [CrossRef]
- Wang, Y.S.; Li, M.; Tell, L.A.; Baynes, R.E.; Davis, J.L.; Vickroy, T.W.; Riviere, J.E.; Lin, Z. Physiological parameter values for physiologically based pharmacokinetic models in food-producing animals. Part II: Chicken and turkey. J. Vet. Pharmacol. Ther. 2020, 44, 423–455. [Google Scholar] [CrossRef]
- Barron, M.G.; Tarr, B.D.; Hayton, W.L. Temperature-dependence of cardiac output and regional blood flow in rainbow trout, Salmo gairdneri Richardson. J. Fish Biol. 1987, 31, 735–744. [Google Scholar] [CrossRef]
- Neutze, J.; Wyler, F.; Rudolph, A. Use of radioactive microspheres to assess distribution of cardiac output in rabbits. J. Am. J. Physiol. 1968, 215, 486–495. [Google Scholar] [CrossRef]
- Schimmel, C.; Frazer, D.; Glenny, R.W.; Physiology, C. Extending fluorescent microsphere methods for regional organ blood flow to 13 simultaneous colors. Am. J. Physiol.-Heart C 2001, 280, H2496–H2506. [Google Scholar] [CrossRef]
- Forsberg, F.; Liu, J.-B.; Russell, K.M.; Guthrie, S.L.; Goldberg, B.B. Volume flow estimation using time domain correlation and ultrasonic flowmetry. J. Ultras. Med. 1995, 21, 1037–1045. [Google Scholar] [CrossRef]
- Eun, H.C. Evaluation of skin blood flow by laser Doppler flowmetry. Clin. Dermatol. 1995, 13, 337–347. [Google Scholar] [CrossRef]
- Henson, R. Introduction to Functional Magnetic Resonance Imaging: Principles and Techniques; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Smits, G.J.; Roman, R.J.; Lombard, J.H. Evaluation of laser-Doppler flowmetry as a measure of tissue blood flow. J. Appl. Physiol. 1986, 61, 666–672. [Google Scholar] [CrossRef]
- Tabrizchi, R.; Pugsley, M.K. Methods of blood flow measurement in the arterial circulatory system. J. Pharmacol. Toxicol. Meth. 2000, 44, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Glenny, R.W. Blood flow measurements using fluorescent microspheres. In Analytical Spectroscopy Library; Elsevier: Amsterdam, The Netherlands, 1995; Volume 6, pp. 255–280. [Google Scholar]
- Prinzen, F.W.; Bassingthwaighte, J.B. Blood flow distributions by microsphere deposition methods. Cardiovasc. Res. 2000, 45, 13–21. [Google Scholar] [CrossRef]
- CFFA. China Fishery Statistics Yearbook 2019; China Agriculture Press: Beijing, China, 2023. [Google Scholar]
- Lucchetti, D.; Fabrizi, L.; Guandalini, E.; Podestà, E.; Marvasi, L.; Zaghini, A.; Coni, E. Long depletion time of enrofloxacin in rainbow trout (Oncorhynchus mykiss). Antimicrob. Agents Chemother. 2004, 48, 3912–3917. [Google Scholar] [CrossRef]
- Xu, N.; Li, M.; Chou, W.C.; Lin, Z. A physiologically based pharmacokinetic model of doxycycline for predicting tissue residues and withdrawal intervals in grass carp (Ctenopharyngodon idella). Food Chem. Toxicol. 2020, 137, 111127. [Google Scholar] [CrossRef]
- Qiang, J.; Zhong, C.Y.; Bao, J.W.; Liang, M.; Liang, C.; Li, H.X.; He, J.; Xu, P. The effects of temperature and dissolved oxygen on the growth, survival and oxidative capacity of newly hatched hybrid yellow catfish larvae (Tachysurus fulvidraco♀ × Pseudobagrus vachellii♂). J. Therm. Biol. 2019, 86, 102436. [Google Scholar] [CrossRef]
- Canty, A.A.; Farrell, A.P. Intrinsic regulation of flow in an isolated tail preparation of the ocean pout (Macrozoarces americanus). Can. J. Zool. 1985, 63, 2013–2020. [Google Scholar] [CrossRef]
- Randall, D.J.; Daxboeck, C. Cardiovascular changes in the rainbow trout (Salmo gairdneri Richardson) during exercise. Can. J. Zool. 1982, 60, 1135–1140. [Google Scholar] [CrossRef]
- Elander, A.; Idström, J.P.; Holm, S.; Scherstén, T.; Bylund-Fellenius, A.C. Metabolic adaptation to reduced muscle blood flow. II. Mechanisms and beneficial effects. Amer. J. Physiol. 1985, 249, E70–E76. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.; Pierce, M.; Pierce, E. Blood flow distribution in Squalus acanthias: A sequel. J. Bull. Mt Desert Biol. Lab. 1973, 13, 64–66. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).