Abstract
A high-performance liquid chromatography method coupled to tandem mass spectrometry was validated in order to study the pharmacokinetics of cefotaxime in shrimp hepatopancreases and plasma, as well as its withdrawal time related to a maximum residue limit (MRL) in shrimp muscle. Pharmacokinetics parameters were investigated through oral medication at a single dose of 25 mg/kg shrimp body weight and subsequent hepatopancreas and plasma cefotaxime concentration measurements at 0.5, 1, 2, 4, 8, 12 and 24 h after shrimp were fed with medication. The maximum concentration of cefotaxime was observed after one hour in the hepatopancreas (Cmax, 19.45 ± 2.10 mg/kg) and 4 h in plasma (0.184 ± 0.061 mg/L). Based on a minimum inhibitory concentration (MIC) of cefotaxime of 4.13 mg/L against Vibrio parahaemolyticus (known to cause acute hepatopancreatic necrosis disease (AHPND) in white leg shrimp), it was observed that the time during which the hepatopancreas cefotaxime concentration was above the MIC was 23 h. An every 24 h cefotaxime treatment could thus be effective in fighting against this bacterium in shrimp. The withdrawal time of cefotaxime was determined after shrimp were fed with medicated feed once a day and twice a day for three consecutive days. Shrimp muscle was collected on day 1 and day 3 during medication and 1, 3, 7, 14 and 21 days after medication was stopped. Considering an MRL of 50 μg/kg, the withdrawal times were 8.5 degree-days (corresponding to 6.9 h at 29.5 °C) after shrimp were fed with medicated feed once a day for 3 days and 95.5 degree-days (77.7 h at 29.5 °C) after shrimp were fed with medicated feed twice a day for 3 days. Moreover, histological analysis revealed that feeding shrimp with cefotaxime at the given dose in once- or twice-a-day treatments did not negatively impact the shrimp hepatopancreas.
Keywords:
cefotaxime; hepatopancreas; pharmacokinetics; Vibrio parahaemolyticus; white leg shrimp; withdrawal time Key Contribution:
This study has determined the pharmacokinetics parameters and withdrawal time of cefotaxime in white leg shrimp, supporting the rational use of this antibiotic in terms of shrimp disease treatment and food safety.
1. Introduction
Aquaculture is an important sector of Vietnam’s economy, with total annual production accounting for 5.4 million tons in 2023, with shrimp and striped catfish culture industries being responsible for most of this production [1]. Penaeid shrimp is a high-value export product produced mainly in the Mekong Delta in Vietnam and accounts for over 80% of shrimp production. Annually, the shrimp industry contributes about 40–45% to the total seafood export value, which is equivalent to USD 4.3 billion [2]. Like for other farmed aquatic animals, diseases, especially those caused by bacteria, frequently occur in the white leg shrimp culture industry with the development of intensive aquaculture. According to surveys performed previously in the Mekong Delta, Vietnam, common diseases causing heavy damage to shrimp reared in intensive systems are white feces disease, acute hepatopancreatic necrosis disease (AHPND), white spot syndrome virus, red body syndrome and black spot disease [3,4]. In order to control diseases in aquaculture, most farmers use antibiotics to prevent and treat bacterial infections [5,6,7]. The improper use of antibiotics led to residues found in exported shrimps. The Rapid Alert System for Food and Feed (RASFF) from the EU reported, from 2020 to 2022, residues of ciprofloxacin, oxytetracycline, sulfamethoxazole and nitrofuran metabolites in shrimp frozen products [8].
Pharmacokinetics (PK) and residues in shrimp were investigated in previous studies, for several antibiotic classes, such as tetracyclines, e.g., oxytetracycline [9,10,11,12,13,14]; phenicols, e.g., florfenicol [15,16], chloramphenicol, thiamphenicol [15]; sulfonamides, e.g., sulfamethoxazole [14]; quinolones, e.g., enrofloxacin [17,18,19]; nitrofurans [20]; and a combination of sulfamethoxazole and trimethoprim [21]. Cefotaxime (CFT) is a commonly used antibiotic in shrimp culture and plays an important role in disease treatment [22,23]. CFT is a third-generation cephalosporin, which belongs to the class of beta-lactams; it has a broad spectrum of activity against aerobic Gram-negative as well as Gram-positive bacteria, while it shows variable activity against anaerobic bacteria [24]. This antibiotic is used in aquaculture, and its residue can be found in countries where aquaculture is developed; i.e., in China, the detection frequency of CFT was 40% in fish ponds around Tai Lake, with a level exceeding 10,000 ng/L [25], and it was found in surface water of Xiangjiang River with a concentration of 830 ng/L [26]. In Egypt, CFT is used in bacterial pathogen management in tilapia [27]. In aquatic animals, studies related to side effects or pharmacokinetics of CFT are not common, and CFT was just investigated in tilapia (Oreochromis niloticus) in bacterial infectious treatment [27], but CFT PK has been studied in several terrestrial animals like goats (Capra hircus), buffalo calves (Bubalus bubalis), rats and camels (Camelus dromedarius) [28,29,30,31]. CFT is used to control susceptible bacterial infections in aquatic and terrestrial animals, such as tilapia, horses, birds, dogs, cats and reptiles at the dose of 10–80 mg/kg body weight via intraperitoneal, intramuscular, intravenous or subcutaneous injection [27].
Although antibiotics are very effective in fighting against bacterial infectious diseases, they result in negative effects on the internal organs of terrestrial animals [32,33] as well as aquatic animals [17,34,35,36,37,38,39]. Exposure to CFT in Artemia sinica, an aquatic invertebrate, showed oxidation stress and changes in intestinal cell morphology and gut microbiota [40]. Therefore, using CFT in disease management may result in a negative effect on cultured shrimp, especially in the hepatopancreas, which is responsible for chemical metabolism and the normal growth and development of shrimps and other crustaceans. The hepatopancreas (also called the midgut gland) is the most impacted organ during drug application or after chemical exposure. This organ, which is equivalent in vertebrates to the liver, pancreas and intestine, has been investigated by histologists and biologists for more than a century [41]. Because of its importance, any damage or change in the cell function of this organ could lead to negative effects, e.g., death, growth slowdown or immunodeficiency.
The outcomes of this research will, on the one hand, provide knowledge about CFT PK and withdrawal times in shrimp which will facilitate the establishment of guidelines for optimal use of this antibiotic in shrimp and, on the other hand, provide an improved understanding on the impact of CFT on hepatopancreas histology in white leg shrimp aquaculture during and after CFT medication.
2. Materials and Methods
2.1. Chemicals
Standards of CFT and penicillin V, with a purity greater than 98% (Dr. Ehrenstorfer, Augsburg, Germany), were purchased and stored at −20 °C. Stock solutions of CFT and penicillin V (1000 µg/mL) were prepared in a methanol and water mixture (1:1, v/v) and stored at −20 °C. These stock solutions were then diluted to a concentration of 1 µg/mL for use in sample fortification and standard curve preparation. CFT (99.9%) for medicated feed preparation was sourced from Phil Inter Pharma Co. Ltd. (Binh Duong, Vietnam). C18 Bondesil powder, with a particle size of 40 µm, was obtained from Agilent (Santa Clara, CA, USA). Ultra-high-performance liquid chromatography water, methanol, acetonitrile, dipotassium hydrogen sulfate and 98% formic acid were acquired from Merck (Darmstadt, Germany).
2.2. Experimental Shrimp
White leg shrimp were reared from post-larvae in the Faculty of Marine Science and Technology, College of Aquaculture and Fisheries, Can Tho University; the post-larvae were reared for approximately 45 days before experimental processing. Shrimp were checked for CFT residues before the experiment. For each pond, 200 shrimp (13.8 ± 1.1 g/shrimp) were stocked in 2 m3 tanks to reach the stocking density of 100 shrimp/m3 and acclimated for 1 week prior experiment. Experimental tanks were fully equipped with aeration to maintain dissolved oxygen above 5 mg/L. During the experiment, water salinity was at 10‰, and alkalinity was adjusted at 140–160 mg CaCO3/L; other parameters such as water temperature, pH and nitrite (NO2−) were measured every two days. During acclimation, shrimp were fed commercial pellet feed (40% crude protein) at 3% shrimp biomass, four times a day, 6 a.m., 10 a.m., 2 p.m. and 6 p.m. At each feeding time, after 45 min of feed provision, the excess feed (less than 5% of the total feed amount) was removed by siphoning.
2.3. Pharmacokinetics Experiment
To determine the pharmacokinetics of CFT, shrimp were fed a single dose of 25 mg CFT/kg body weight. The experiment was performed using 6 tanks stocked with shrimp as described above (corresponding to 6 replicates). The concentration of CFT in feed was calculated based on the amount of feed fed to 1 kg of shrimp biomass. Before every medication, medicated feed was prepared by spraying a solution of CFT over the feed. CFT (1 g) was dissolved in 30 mL of distilled water and mixed with 200 g feed, which corresponded to 5 mg CFT/g feed or 0.5% of CFT in feed. The medicated feed was then covered with 2% squid oil and left for 15 min before shrimp feeding at 6 a.m. Shrimp received then antibiotic-free feed at 10 a.m., 2 p.m. and 6 p.m. Analytical results in two samples showed that the content of CFT in feed was 0.48% and 0.54%, close to the theoretical concentration. Shrimp blood and hepatopancreas samples were collected at 0.5, 1, 2, 4, 8, 12 and 24 h after medication. At each sampling time, 3 shrimp were collected from one tank. Blood samples were collected from the pericardial cavity of shrimp using a 1 mL syringe soaked with anti-coagulation solution (EDTA 0.01 M, NaCl 0.338 M, glucose 0.115 M, trisodium citrate dihydrate 0.03 M). Blood samples from three shrimp in one tank were pooled, placed into 1.5 mL centrifuge tubes and centrifuged at 10,000 rpm for 5 min to collect plasma. All samples were stored at −80 °C until analysis. Meanwhile, hepatopancreases from 3 individual shrimp in each tank were collected into marked plastic bags and stored at −80 °C until analysis. During sampling, collected samples were preserved on ice to avoid CFT concentration changes in collected tissues.
2.4. Determination of the Withdrawal Times of Cefotaxime and Hepatopancreas Histological Observation
The withdrawal times of CFT in shrimp were determined after oral administration. Shrimp were fed medicated pellet feed (prepared as described above) at the dose of 25 mg CFT/kg body weight once a day at 6 a.m. (three tanks) and twice a day (three tanks) at 6 a.m. and 6 p.m. for three consecutive days. After three days of medication, shrimp were fed CFT-free feed for 21 days, four times a day, at 6 a.m., 10 a.m., 2 p.m. and 6 p.m. On days 1 and 3 during medication, samples were collected 1 h after medication for the “once-a-day treatment” group and 1 h after the second dose for the “twice-a-day treatment” group. Sampling was continued on days 1, 3, 7, 14 and 21 after the last medication. At each sampling time, 10 shrimp were randomly collected from one tank, and the shrimp muscle without shells was pooled, minced and kept in a −20 °C freezer until analysis.
The samples for hepatopancreatic histological observations were also collected in this experiment one day before medication, one hour after the last dose of medication (day 3 of medication) and 7 days after the medication was stopped to evaluate the impact on shrimp hepatopancreases. At each sampling time, three shrimp were collected from each tank. Histological sampling and preparation were performed as described by Lightner [42].
2.5. Cefotaxime Quantification
The analytical method for CFT residue analysis was adapted from the LC-MS/MS method for the determination of beta-lactam antimicrobials described by the United States Department of Agriculture [43].
2.5.1. Sample Extraction and Chromatographic and Mass Spectrometry Conditions
For the extraction of CFT, 3.0 ± 0.05 g of homogenized shrimp muscle, hepatopancreas or feed was introduced into a 50 mL Falcon tube and spiked with 60 µL of 1 µg/mL penicillin V solution as an internal standard (IS). Shrimp plasma (0.5 mL) was also fortified with 10 µL of 1 µg/mL penicillin V solution. The extraction, chromatographic and mass spectrometry conditions were as described by Phu et al. [44]. A Waters ACQUITY ultra-high-performance LC system (Waters, Milford, MA, USA) and a Hypersil GOLD C18 3 × 100 mm, 1.9 µm C18 column (Thermo Scientific, Waltham, MA, USA) were used. The mobile phases included two solvents: solvent A containing methanol/acetonitrile (1/1, v/v) and 0.1% formic acid, and solvent B containing water and 0.1% formic acid (the gradient elution is detailed in Table 1). Under such conditions, the retention times of CFT and penicillin V were 3.1 and 4.04 min, respectively. The following transitions (precursor > product ion, m/z) were used for quantitation and confirmation (collision energy indicated in brackets): cefotaxime 456.1 > 396.1 (10 eV) and 456.1 > 324.1 (14 eV); penicillin V (IS) 351.1 > 160.1 (16 eV) and 351.1 > 114.1 (35 eV).

Table 1.
The HPLC mobile phase conditions for cefotaxime analysis.
2.5.2. Quantification of Cefotaxime Residues
The concentrations of CFT residues were calculated using matrix-matched calibration curves prepared with CFT solutions at different concentrations and penicillin V, used as the internal standard. For the matrix-matched calibration curves, 3.0 ± 0.05 g of homogenized blank (antimicrobial free) shrimp muscle, hepatopancreas and feed samples and 0.5 mL of plasma were fortified with standard solutions of CFT at 6 concentrations (0, 10, 20, 40, 80 and 160 µg/kg for hepatopancreas and feed and µg/L for plasma) and 60 µL of a 1 µg/mL penicillin V solution, except for plasma where 10 µL of a 1 µg/mL penicillin V solution was added. The fortified samples were prepared on the same day as the analysis of the samples.
2.5.3. Method Validation
Since no certified reference material was available, the validation was conducted according to Commission Decision No. 2021/808/EC [45] by recovering known amounts of CFT added to a blank matrix. Validation for recovery, repeatability and within-laboratory reproducibility was carried out using 21 blank shrimp muscle samples fortified at three concentrations: 100 µg/kg (MRL) and 0.1 and 1.5 times the MRL, i.e., 10 and 150 µg/kg. Repeatability was assessed from seven repeated analyses performed on the same day, expressed as the coefficient of variation (CV) of the mean CFT concentration (µg/kg). Within-laboratory reproducibility was determined by calculating the mean and CV from results obtained over three independent days. Recovery was calculated by comparing the measured concentrations to the fortified concentrations for each sample. Mean recovery and CV, both expressed as %, were derived from all results obtained over the three days for each fortification level. The decision limit CCα (α = 5% for substances with an MRL) was determined as the average concentration of the blank samples fortified at the MRL concentration of CFT (100 µg/kg) under within-laboratory reproducibility conditions, plus 1.64 times the corresponding standard deviation. In addition, selectivity/specificity, matrix effect and ruggedness were determined according to Commission Decision No. 2021/808/EC [45]. The limit of detection (LOD) was calculated as 3.3 times and the limit of quantification (LOQ) was calculated as 10 times the standard deviation of the response divided by the slope of the matrix-matched calibration curve. The standard deviation of the response was determined following the ICH guidelines [46] via the responses of 21 blank samples, integrating the area of the chromatographic peak at the retention time corresponding to the expected compound.
2.6. Pharmacokinetics Data Analysis
CFT plasma and hepatopancreas concentration data were modeled using a naive pooled population approach based on a non-compartmental model with first-order absorption and elimination. The following pharmacokinetics parameters were calculated: maximal concentration (Cmax), time to maximal concentration (Tmax), area under the concentration–time curve from time 0 to 24 h (AUC0–24 h), elimination rate constant (kel), elimination half-life (T1/2el).
All PK data from shrimp plasma and hepatopancreases were computed using the linear-up log-down trapezoidal method using Phoenix 8.1 (Certara, Princeton, NJ, USA).
2.7. Withdrawal Times Calculation
European Medicine Agency (EMA) guidelines [47] were used to assess the withdrawal time. The concentration of CFT in muscle was measured at various time points post-treatment and analyzed using linear regression against time (degree-days) with the statistical software WT 1.4, developed by Hekman [48]. Degree-days were calculated by multiplying the average daily water temperature (in Celsius) by the total number of days the temperature was measured. The withdrawal period was defined as the time when the upper one-sided 95% tolerance limit for the CFT residue concentration fell below the MRL.
2.8. Histological Analysis
Shrimp hepatopancreases were collected and injected with Davidson’s AFA (ethanol–formalin–acetic acid) fixative for 24 h and then transferred and preserved in 70% ethanol solution. After fixation, the hepatopancreases were trimmed. Before we proceeded with casting, the samples were dehydrated by soaking in 70%, 80%, 95% and 100% ethanol and xylene before being put in paraffin. Then, the samples were cut into 6 μm thick sections and put in warm water at 45–50 °C to make the paraffin stretch and stick on the sections, and then the slides were stained with hematoxylin and eosin (H&E) dyes [49]. Histological sections of the hepatopancreases were examined by microscope (Novex, Arnhem, the Netherlands).
3. Results
3.1. LC-MS/MS Method Validation
The validation evaluated linearity, specificity, selectivity, signal per noise, stability, precision (repeatability and within-laboratory reproducibility), recovery and decision limit (CCα) according to Commission Decision No. 2021/808/EC [45] (Table 2).

Table 2.
Validation parameters of the LC-MS/MS method for quantification of CFT in shrimp muscle tissue.
LOD and LOQ of CFT were 1.5 µg/kg and 4 µg/kg for shrimp muscle, respectively. The comparison of CFT calibration curves prepared from blank shrimp tissue and standard solutions showed that the slope of the calibration curve in the solution was significantly lower (p = 0.002) than the one in the matrix (Figure 1), while the coefficients of determination (R2) were above 0.999 for both curves, in the matrix or in the solvent.
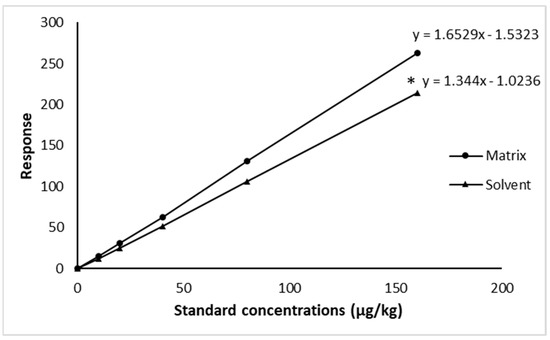
Figure 1.
CFT matrix-matched (using blank shrimp muscle) and solvent calibration curves obtained using the LC-MS/MS method; asterisk (*) value presented significant difference in slope values between matrix-matched and solvent calibration (p < 0.05).
The specificity of the method was evaluated by comparison of the chromatograms obtained when analyzing blank shrimp muscle samples with those obtained when analyzing the CFT–fortified samples. No peak was observed in the blank samples at the retention time of CFT (Figure 2A). The chromatogram of CFT obtained from the analysis of blank shrimp muscle fortified with 10 µg/kg is shown in Figure 2B.
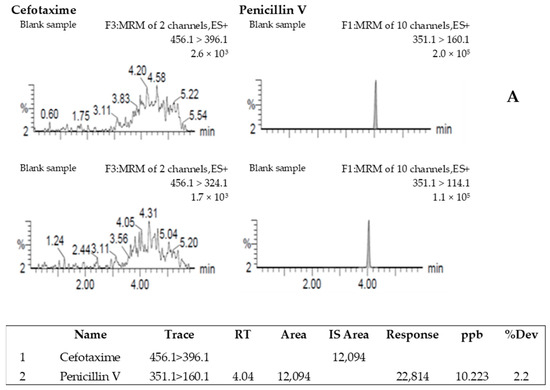
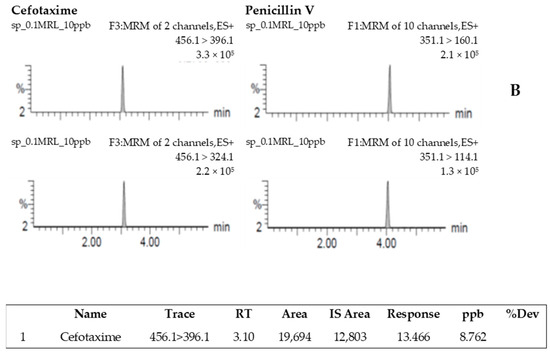
Figure 2.
Chromatogram resulting from the analysis of a blank shrimp sample spiked with internal standard (penicillin V) (A) and the analysis of a blank shrimp sample spiked with 10 µg/kg of cefotaxime and penicillin V (B).
Results for repeatability, within-laboratory reproducibility, recovery rate and decision limit (CCα) are presented in Table 2. The mean concentrations obtained for the repeatability were 9.39 ± 0.57 µg/kg, 94.23 ± 4.36 µg/kg and 141.89 ± 5.71 µg/kg at the fortification levels 0.1 MRL, MRL and 1.5 MRL, respectively, with a coefficient of variation of 5.95%, 4.97% and 3.64%, respectively. The average concentrations obtained from the within-laboratory reproducibility study at the fortification levels 0.1 MRL, MRL and 1.5 MRL were 9.62, 95.4 and 144 µg/kg for CFT with a coefficient of variation of 6.00%, 5.14% and 3.79%, respectively, with corresponding recoveries ranging between 88% and 107%. From the within-laboratory reproducibility study, the value of CCα was 108 µg/kg for CFT.
The analytical method was fully validated for shrimp muscle. For plasma and hepatopancreas, the LOD and LOQ were in the same order as those for muscle, i.e., 1.5 µg/L and 4 µg/L for plasma and 1.5 µg/kg and 4 µg/kg for hepatopancreas, respectively.
3.2. Pharmacokinetics (PK) Parameters of Cefotaxime in Plasma and Hepatopancreas of White Leg Shrimp
Non-compartment modeling was used to describe the PK of CFT in white leg shrimp after single oral dose administration. The results (Figure 3A,B) show large fluctuations in hepatopancreas and plasma CFT concentrations probably due to individual variations in antibiotic absorption between shrimp.
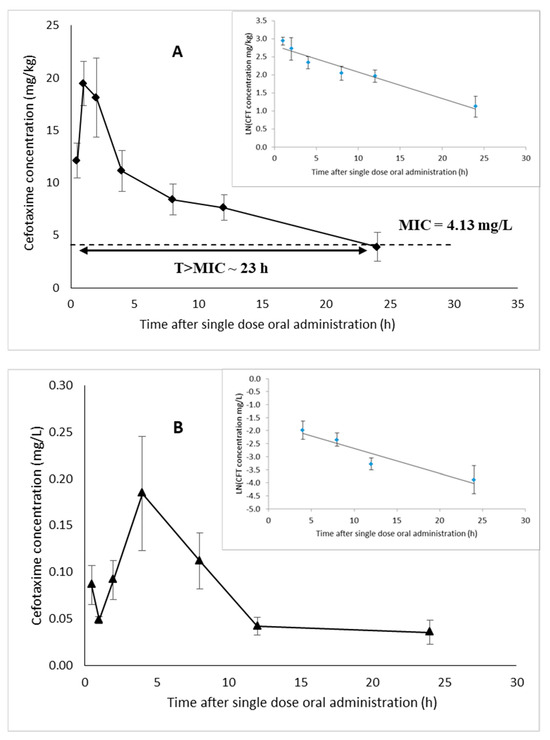
Figure 3.
Concentration of CFT versus time and elimination of CFT (insert) in hepatopancreas (A) and plasma (B) of white leg shrimp (n = 6) after administration of a single oral dose (25 mg/kg shrimp); the data are expressed as mean ± standard error; MIC, minimal inhibitory concentration (dotted line); T > MIC ~ 23 h, time during which the concentration remains above the MIC (A).
The results showed that the concentration of CFT in the hepatopancreas was much higher than that in plasma. The maximum hepatopancreas concentration (Cmax) was 19.45 mg/kg and was obtained after 1 h, while Cmax in plasma was 0.184 mg/L and was reached after 4 h of medication (Table 3). In addition, the AUC0−24 h value in shrimp hepatopancreas was 199.64 mg.h/kg, while plasma had an AUC0−24 h value of 2.15 mg.h/L, also showing that the distribution of CFT in shrimp hepatopancreas is much higher than in plasma. In addition, the elimination half-life (T1/2el) of CFT in plasma (8.78 h) was shorter than that in the hepatopancreas (11.23 h).

Table 3.
Main PK parameters of CFT in hepatopancreas and plasma after a single oral administration at a dose of 25 mg CFT/kg shrimp. Values of Cmax, Tmax and AUC0−24 h are presented as mean ± standard error, n = 6.
Regarding PK/pharmacodynamic modeling (PK/PD), based on a minimal inhibitory concentration (MIC) of CFT towards Vibrio parahaemolyticus (V. parahaemolyticus) strains of 4.13 mg/L (unpublished data), the time during which the CFT concentration in the hepatopancreas was above the MIC was 23 h (Figure 3A).
3.3. Depletion of Cefotaxime in Muscle of White Leg Shrimp
The residual concentrations of CFT in white leg shrimp muscle measured in “once a day” and “twice a day” treatment groups are shown in Figure 4. On the first day, after 1 h of medication, the CFT concentrations in shrimp muscle were 9.75 ± 8.64 µg/kg and 13.1 ± 4.09 µg/kg in the “once a day” and “twice a day” treatment groups, respectively. At 3 days of medication, the CFT concentration in shrimp muscle increased and reached a peak of 23.2 ± 8.02 µg/kg (“once a day” treatment group) and 93.52 ± 8.49 µg/kg (“twice a day” treatment group). After the medication had been stopped for 24 h, the cefotaxime concentration in shrimp muscle was below the LOD (1.5 µg/kg) in the treatment of feeding shrimp with medicated feed once a day and below the LOQ (4 µg/kg) in treatment of feeding shrimp with medicated feed twice a day (2.29 ± 0.80 µg/kg). Three days after medication, as well as after 7, 14 and 21 days after medication was stopped in both treatments, CFT concentration was not detected anymore in muscle.
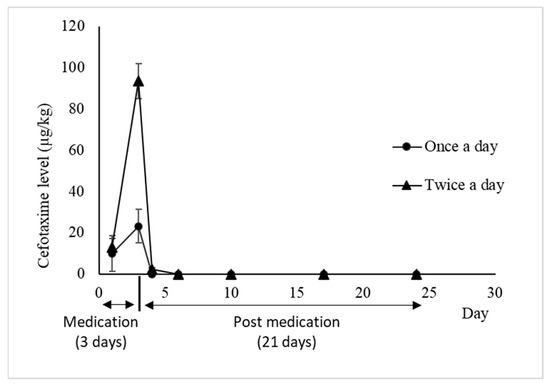
Figure 4.
Depletion of CFT (µg/kg) in white leg shrimp muscle during and after medication at a dose of 25 mg/kg shrimp when fed with CFT once a day and twice a day, during 3 consecutive days (mean ± SD, n = 3).
After three consecutive medications at an ambient temperature of 29.5 °C and at a dose of 25 mg/kg, the calculated withdrawal times (WTs) for an MRL of 50 μg/kg were 8.5 degree-days (corresponding to 6.9 h) and 95.5 degree-days (77.7 h) for once- and twice-a-day medication treatments, respectively (Figure 5A,C). For an MRL of 100 μg/kg, they were 2.5 degree-days (2.0 h) and 74.1 degree-days (60.3 h), respectively (Figure 5B,D).
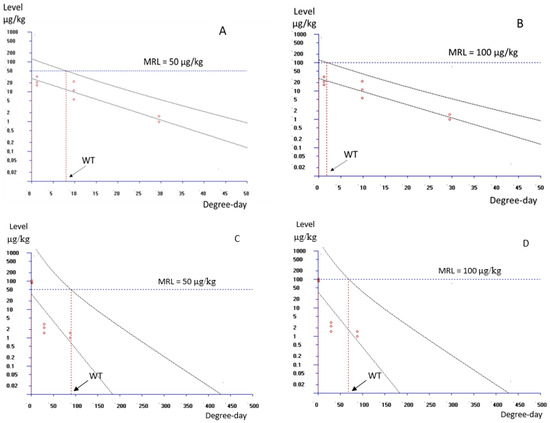
Figure 5.
Plot of the CFT residual concentrations in shrimp muscle recorded at 1, 8 and 24 h for once-a-day treatment and 1, 24 and 72 h for twice-a-day treatment as a function of degree-days. The withdrawal times (WTs) were calculated as the time when the one-sided 95% upper tolerance limit was below the MRL (50 μg/kg and 100 μg/kg) when shrimp were fed with CFT once a day (A,B) and twice a day (C,D) for 3 consecutive days at a dose of 25 mg/kg shrimp, at ambient temperature 29.5 °C.
3.4. Hepatopancreas Histology
The hepatopancreas histology before CFT medication showed no abnormalities. The hepatopancreatic tissue structure displayed the full presence of B, R and F cells (Figure 6A).
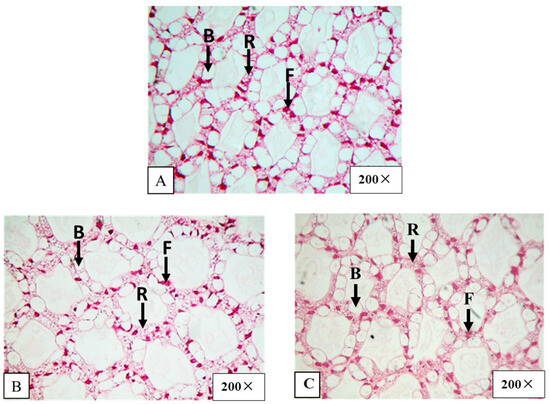
Figure 6.
Histological section of shrimp hepatopancreas before (A) and after shrimp were fed with CFT-medicated feed of twice-a-day treatment on day 3 of medication (B) and 7 days after the medication was stopped (C) (200×). B—blasenzellen cells, R—restzellen cells, F—fibrillenzellen cells.
After shrimp were fed with medicated feed once a day or twice a day for 3 consecutive days, the number of B, F and R cells remained unchanged on day 3 of medication (Figure 6B). Seven days after medication was stopped, the numbers of B, F and R cells in shrimp hepatopancreases were also normal (Figure 6C).
4. Discussion
4.1. Method Validation for CFT Analysis
The developed analytical method was validated for shrimp muscle. The coefficient of determination was high (R2 above 0.99), for both matrix and solvent CFT calibration curves, indicating a linear relationship between the responses and CFT concentrations. Slope values between matrix-matched and solvent calibration were 1.65 ± 0.04 and 1.34 ± 0.01, respectively, showing a significant matrix effect. Therefore, the method validation and the analysis of CFT in shrimp muscle were performed using calibration curves established in spiked blank shrimp muscle. This result is in agreement with the findings of previous studies on the validation of antibiotic drug analysis procedures in shrimp and aquaculture samples [50,51,52].
The repeatability and within-laboratory reproducibility (expressed as coefficients of variation on results obtained on the same day and on different days, respectively) at three fortified levels did not exceed 6% and were both much lower than the threshold set by Commission Decision No. 2021/808/EC [45] of 22%, respectively. The trueness, assessed through recovery at three different levels, ranged between 88% and 107% at the fortification levels of 0.1 MRL, MRL and 1.5 MRL, which meets the tolerance range for trueness of 80% to 120% of Commission Decision No. 2021/808/EC [45]. The decision limit (CCα) was 108 µg/kg, close to the corresponding MRL of 100 µg/kg. The parameters of selectivity/specificity and matrix effect were below the threshold set by Commission Decision No. 2021/808/EC [45].
4.2. Cefotaxime Pharmacokinetics in White Leg Shrimp
The results of the PK investigation showed that the concentrations of CFT in the hepatopancreas were very high, indicating a preferential distribution of CFT in this organ. The PK data of CFT in aquatic animals are very limited, but CFT PK has been studied extensively for some terrestrial animals. In this study, CFT concentration in shrimp plasma showed a longer elimination half-life (T1/2el = 8.78 h) compared with other animals, i.e., in buffalo calves (T1/2el = 0.94 h) [31] or goats (T1/2el = 1.03 h) [53]. However, the elimination of CFT in this study was faster than that in Arabian camels (15.46 h) [28]. In addition, the Cmax value of CFT in shrimp plasma (0.18 µg/mL) was much lower than that reported in some fish species when using antibiotics of the same group, i.e., cephalosporins. For example, the Cmax values of cephalosporins in dogfish treated with a dose of 6.6 mg/kg body weight [54], in tilapia treated with 5 mg/kg body weight [55], in olive flounder treated with 10 mg/kg body weight and in olive flounder treated with 20 mg/kg body weight [56] have been reported to be 3.75, 12.32, 7.61 and 13.34 µg/mL, respectively. Regarding the Tmax value, previous studies found that it strongly varies between species. For example, in white leg shrimp (in this study), the Tmax was 4 h; this value is shorter than that of dogfish (96 h), but it was longer in comparison with tilapia (0.74 h) and olive flounder (0.42 h for 10 mg/kg dose and 0.67 h for 20 mg/kg dose). The difference may be due to the difference in the circulatory systems of these species; i.e., in animals with an open circulatory system, all the internal organs and structures are constantly bathed in hemolymph [18]. In addition, the absorption phase and effective distribution are rapid, and both have a close relationship with an open circulation system [57]. In fish that have a closed circulatory system, blood from the gills goes to most organs before reaching the liver (where the drug can be metabolized) [58].
The results of this study showed that the Cmax and AUC0–24 h of CFT in hepatopancreases are much higher than those in plasma. A similar result was found in the experiment of Fang et al. [15], where the Cmax values of thiamphenicol and florfenicol in plasma (7.96 µg/mL and 5.53 µg/mL) of white leg shrimp (medicated at a dose of 10 mg/kg body weight) was much lower than the Cmax values in the shrimp hepatopancreas (204 µg/kg and 164 µg/kg, respectively). Fang et al. [18], who treated white leg shrimp by intramuscular injection of enrofloxacin at a dose of 10 mg/kg body weight, showed the same pattern in AUC values. These results indicate that there is an accumulation of CFT in the white leg shrimp hepatopancreas. The T1/2el value is an important PK parameter that describes the rate and duration of antibiotic elimination from the body [15]. Notably, the T1/2el value of CFT observed in this study was similar to that of sulfamethoxazole and trimethoprim in white leg shrimp after single-dose and multiple-dose oral administration [21].
CFT exhibits time-dependent bactericidal activity, and the time during which the tissue concentration is above the MIC (T > MIC) has been reported as an appropriate PK/PD index [59,60]. Craig [59] also stated that cephalosporins including CFT show maximal effectiveness in several animal pathogens when the time of tissue concentration is above the MIC for 60 to 70% of the dosing interval. In addition, Gustafsson et al. [60] reported that the average maximal antimicrobial effect was achieved when the concentration of CFT exceeded the MIC for 50% of the dosing interval. In the present study, based on an MIC for CFT towards V. parahaemolyticus strains of 4.13 mg/L, the PK/PD characteristics of CFT in the shrimp hepatopancreas toward this bacterium resulted in a T > MIC of approximately 23 h (Figure 3A). Generally, in shrimp farming, farmers apply antibiotics once a day during medication, suggesting a dosing interval of 24 h corresponding to approximately 95% of T > MIC. Hence, this illustrates the therapeutic efficiency of CFT in treating AHPND caused by V. parahaemolyticus in white leg shrimp. Generally, CFT must be used prudently as CFT is a third-generation cephalosporin which is not a first-line antibiotic. It is considered a critically important antibiotic, so it should be used only when necessary and must be combined with PK information. The results of this study illustrate that the dose which is proposed here is expected to be efficient in V. parahaemolyticus treatment in shrimp disease.
4.3. Cefotaxime Depletion in White Leg Shrimp
The withdrawal time is the time required to eliminate drug residues in edible tissues to a safe level for consumers [61]. MRLs for CFT have not been established in European legislation or elsewhere. In this study, both 50 µg/kg and 100 µg/kg were assumed as the MRL of CFT in edible shrimp muscle. On one hand, the MRL of 50 µg/kg of amoxicillin was chosen because both antibiotics are similar in terms of rapid elimination from muscle. On another hand, the MRL of 100 µg/kg of oxytetracycline was selected because CFT and oxytetracycline have relatively equal acute toxicity in mice (approximately 7000 mg/kg as LD50 value) [62,63].
In the current research, after 3 days of CFT medication at the dose of 25 mg/kg, the CFT WTs were lower than those of other groups of antibiotics. For example, the WT of oxytetracycline in white leg shrimp after oral administration at the dose of 4.5 g/kg feed for 14 consecutive days was 96 h at 29 °C (corresponding to 116 degree-days), for the MRL of 100 µg/kg set by the EU, Canada and India [61]; in another study of Wang et al. [14], the WTs in shrimp muscle of chloramphenicol, sulfamethoxazole and oxytetracycline at the dose of 2 g/kg feed after twice-a-day oral administration for 3 consecutive days were 139.7 h (139.7 degree-days), 30.6 h (30.6 degree-days) and 90.3 h (90.3 degree-days) at 24 °C, respectively.
4.4. The Effect of Cefotaxime on White Leg Shrimp Hepatopancreas Histology
Shrimp hepatopancreases are composed of numerous hepatopancreas tubules. The tubule walls are constituted of a simple epithelium, which contains four cell types including blasenzellen cells (B cells), embryonic cells (E cells), fibrillenzellen cells (F cells) and restzellen cells (R cells). The B, F and R cells are differentiated from E cells which are located at the narrow distal end of the tubule. Young R cells and F cells are located at a short distance away from the distal region, and B cells are at the middle and proximal regions of the tubules [64]. In this study, there was no negative impact of CFT on the B, F and R cells of shrimp hepatopancreases, contrary to what was observed for other antibiotics. Maftuch et al. [17] reported that hepatopancreatic cells were destroyed through shrinkage of microvilli, atrophy, inflammatory cell infiltration and plasma leakage from blood vessels when white leg shrimp were fed with enrofloxacin at a dose of 200 mg/kg body weight. Likewise, the hepatopancreatic cells of white leg shrimp exhibited degeneration and necrosis when oxytetracycline was used at doses of 9 and 18 g/kg feed [61]. Similarly, when feeding white leg shrimp at 13.5 and 22.5 g oxytetracycline/kg feed, Bray et al. [65] reported reduced levels of lipid droplet storage in the hepatopancreas R cells, some necrosis and sloughing of the hepatopancreas tubule epithelium, the general absence of hepatopancreas lipids, and moderate atrophy of the hepatopancreas tubules in the proximal portion, and at the higher dose, tubular atrophy in the hepatopancreatic cells was observed until 42 days; this research showed that the higher the dose of antibiotics used, the more severe the impact on the shrimp hepatopancreas. Besides the adverse effect of antibiotics, a decrease in the number of B cells in shrimp hepatopancreas was also found when feeding shrimp with other chemicals like an organic acid blend that consisted of formic, lactic, malic and citric acids [66].
5. Conclusions
A single-dose oral administration of 25 mg/kg in white leg shrimp revealed quick absorption and distribution. The concentrations of CFT were much higher in the shrimp hepatopancreas than in plasma, indicating that the antibiotic can be sufficient in V. parahaemolyticus infection control during shrimp culture. After CFT oral administration at a dose of 25 mg/kg, the withdrawal time was 78 h (at 29.5 °C water temperature) for all experimental regimes of administration, which will ensure consumer food safety. No negative effect was found in hepatopancreas tissue at the given dose. However, as a limitation, this study did not test the effectiveness of feeding shrimp with CFT in shrimp with Vibrio spp. infection challenge. Moreover, the withdrawal time of CFT should be determined in field practice.
Author Contributions
Conceptualization, T.K.D.H., M.-L.S., M.D., S.C., Q.T.N., C.D., T.H.O.D., Q.V.L. and M.P.T.; data curation, T.K.D.H., M.-L.S., M.D., S.C., Q.T.N., T.H.O.D. and M.P.T.; formal analysis, T.K.D.H., Q.T.N. and M.P.T.; investigation, T.K.D.H., M.-L.S., Q.T.N., C.D. and M.P.T.; methodology, T.K.D.H., M.-L.S., M.D., S.C., Q.T.N., C.D., T.H.O.D., Q.V.L. and M.P.T.; validation, T.K.D.H., M.-L.S., M.D., S.C., Q.T.N. and M.P.T.; visualization, T.K.D.H., M.-L.S., M.D., S.C., Q.T.N., C.D. and M.P.T.; supervision, M.-L.S., Q.T.N., C.D., T.H.O.D. and M.P.T.; writing—original draft, T.K.D.H., Q.T.N. and M.P.T.; writing—review and editing, T.K.D.H., M.-L.S., M.D., S.C., Q.T.N., C.D., T.H.O.D., Q.V.L. and M.P.T.; project administration and funding acquisition, M.-L.S. and M.P.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Wallonie-Bruxelles International (WBI), Project 6.1 “Reducing the antibiotic use in white leg shrimp aquaculture in the Mekong Delta, Vietnam”.
Institutional Review Board Statement
The animals used in this work were treated according to Decision No. 3965/QD-DHCT Date October 15 2021 “Can Tho University Regulation on Ethics in animal experimentation” URL: https://dra.ctu.edu.vn/images/upload/news/246.pdf (accessed on 11 April 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the first author.
Acknowledgments
We would like to thank the College of Aquaculture and Fisheries for facility support.
Conflicts of Interest
The authors all declare that they have no conflicts of interest.
References
- Vietnam Association of Seafood Exporters and Producers (VASEP). Vietnam Aquaculture and Fisheries Overview. 2024. Available online: https://vasep.com.vn/gioi-thieu/tong-quan-nganh (accessed on 30 May 2024).
- Vietnam Association of Seafood Exporters and Producers (VASEP). 2022. Available online: https://vasep.com.vn/san-pham-xuat-khau/tom/xuat-nhap-khau/xuat-khau-tom-dat-4-3-ty-usd-nam-2022-26128.html (accessed on 22 March 2023).
- Phu, T.M.; Vinh, P.Q.; Dao, N.L.A.; Viet, L.Q.; Thinh, N.Q. Chemical use in intensive white-leg shrimp aquaculture in Ben Tre province, Vietnam. Int. J. Sci. Res. Publ. 2019, 9, 812–815. [Google Scholar] [CrossRef]
- Thinh, N.Q.; Maita, M.; Phu, T.M. Chemical use in intensive white leg shrimp aquaculture in Tra Vinh province, Vietnam. Can Tho Univ. J. Sci. 2020, 56, 70–77. [Google Scholar] [CrossRef]
- Chi, T.T.K.; Clausen, J.H.; Van, P.T.; Tersbøl, B.; Dalsgaard, A. Use practices of antimicrobials and other compounds by shrimp and fish farmers in Northern Vietnam. Aquac. Rep. 2017, 7, 40–47. [Google Scholar] [CrossRef]
- Phu, T.M.; Phuong, N.T.; Dung, T.T.; Hai, D.M.; Son, V.N.; Rico, A.; Clausen, J.H.; Madsen, H.; Murray, F.; Dalsgaard, A. An evaluation of fish health-management practices and occupational health hazards associated with Pangasius catfish (Pangasianodon hypophthalmus) aquaculture in the Mekong Delta, Vietnam. Aquac. Res. 2016, 47, 2778–2794. [Google Scholar] [CrossRef]
- Rico, A.; Oliveira, R.; McDonough, S.; Matser, A.; Khatikarn, J.; Satapornvanit, K.; Nogueira, A.J.A.; Soares, A.M.V.M.; Domingues, I.; Van den Brink, P.J. Use, fate and ecological risks of antibiotics applied in tilapia cage farming in Thailand. Environ. Pollut. 2014, 191, 8–16. [Google Scholar] [CrossRef]
- Rapid Alert System for Food and Feed (RASFF). 2022. Available online: https://food.ec.europa.eu/safety/rasff_en (accessed on 10 March 2023).
- Bermúdez-Almada, M.C.; Pérez-Tello, M.G.; Valenzuela-Quintanar, A.I.; Vázquez-Moreno, L. Oxytetracycline residues in cultured white shrimp tissue by HPLC and a microbial receptor assay. J. Food Sci. 1999, 64, 638–640. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.; Zou, X.; Fu, G.; Li, C.; Fan, P.; Fang, W. Pharmacokinetics of oxytetracycline in Pacific white shrimp, Penaeus vannamei, after oral administration of a single-dose and multiple-doses. Aquaculture 2019, 512, 734348. [Google Scholar] [CrossRef]
- Nogueira-Lima, A.C.; Gesteira, T.C.V.; Mafezoli, J. Oxytetracycline residues in cultivated marine shrimp (Litopenaeus vannamei Boone, 1931) (Crustacea, Decapoda) submitted to antibiotic treatment. Aquaculture 2006, 254, 748–757. [Google Scholar] [CrossRef]
- Reed, L.A.; Siewicki, T.C.; Shah, J.C. Pharmacokinetics of oxytetracycline in the white shrimp, Litopenaeus setiferus. Aquaculture 2004, 232, 11–28. [Google Scholar] [CrossRef]
- Uno, K.; Aoki, T.; Kleechaya, W.; Tanasomwang, V.; Ruangpan, L. Pharmacokinetics of oxytetracycline in black tiger shrimp, Penaeus monodon, and the effect of cooking on the residues. Aquaculture 2006, 254, 24–31. [Google Scholar] [CrossRef]
- Wang, W.; Lin, H.; Xue, C.; Khalid, J. Elimination of chloramphenicol, sulphamethoxazole and oxytetracycline in shrimp, Penaeus chinensis following medicated-feed treatment. Environ. Int. 2004, 30, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Li, G.; Zhou, S.; Li, X.; Hu, L.; Zhou, J. Pharmacokinetics and tissue distribution of thiamphenicol and florfenicol in Pacific white shrimp Litopenaeus vannamei in freshwater following oral administration. J. Aquat. Anim. Health. 2013, 25, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Pan, L.; Wang, L. Tissue distribution, elimination of florfenicol and its effect on metabolic enzymes and related genes expression in the white shrimp Litopenaeus vannamei following oral administration. Aquac. Res. 2016, 47, 1584–1595. [Google Scholar] [CrossRef]
- Maftuch; Aziz, K.; Eslfitri, D.; Sanoesi, E.; Prihanto, A.A. Enrofloxacin stimulates cell death in several tissues of vannamei shrimp (Litopenaeus vannamei). Comp. Clin. Pathol. 2017, 26, 249–254. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, J.; Liu, X. Pharmacokinetics and tissue distribution of enrofloxacin after single intramuscular injection in Pacific white shrimp. J. Vet. Pharmacol. Ther. 2018, 41, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Huang, L.; Wei, W.; Wang, Y.; Zou, X.; Zhou, J.; Li, X.; Fang, W. Pharmacokinetics of enrofloxacin and its metabolite ciprofloxacin in Pacific white shrimp Litopenaeus vannamei after multiple-dose oral administration. Fish. Sci. 2018, 84, 869–876. [Google Scholar] [CrossRef]
- Douny, C.; Widart, J.; De Pauw, E.; Silvestre, F.; Kestemont, P.; Tu, H.T.; Phuong, N.T.; Maghuin-Rogister, G.; Scippo, M.L. Development of an analytical method to detect metabolites of nitrofurans: Application to the study of furazolidone elimination in Vietnamese black tiger shrimp (Penaeus monodon). Aquaculture 2013, 376, 54–58. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.; Zou, X.; Hu, K.; Sun, B.; Fang, W.; Fu, G.; Yang, X. Pharmacokinetics of sulfamethoxazole and trimethoprim in Pacific white shrimp, Litopenaeus vannamei, after oral administration of single-dose and multiple-dose. Environ. Toxicol. Pharmacol. 2017, 52, 90–98. [Google Scholar] [CrossRef]
- Plosker, G.L.; Foster, R.H.; Benfield, P. Cefotaxime. PharmacoEconomics 1998, 13, 91–106. [Google Scholar] [CrossRef]
- Thinh, N.Q.; Phu, T.M. Chapter 3 Drugs and chemicals use in white leg shrimp culture. In Drugs and Chemicals Use in Aquaculture; Can Tho University Publisher: Can Tho City, Vietnam, 2021; pp. 25–41. [Google Scholar]
- Carmine, A.A.; Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Cefotaxime. Drugs 1983, 25, 223–289. [Google Scholar] [CrossRef]
- Song, C.; Zhang, C.; Fan, L.; Qiu, L.; Wu, W.; Meng, S.; Hu, G.; Kamira, B.; Chen, J. Occurrence of antibiotics and their impacts to primary productivity in fishponds around Tai Lake, China. Chemosphere 2016, 161, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, L.; Li, H.; Luo, Z.; Lu, J.; Yang, Z. Pharmaceutically active compounds in the Xiangjiang River, China: Distribution pattern, source apportionment, and risk assessment. Sci. Total Environ. 2018, 636, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.G.; Ali, T.E.S.; Aboyadak, I.M.; Elbakry, M.A. Controlling Pseudomonas aeruginosa infection in Oreochromis niloticus spawners by cefotaxime sodium. Aquaculture 2021, 544, 737107. [Google Scholar] [CrossRef]
- Altayban, A.; Kandeel, M.; Tahoun, A.; Al-Nazawi, M. Cefotaxime pharmacokinetics in Arabian camel (Camelus dromedarius) calves after single intravenous injection. Trop. Anim. Health Prod. 2020, 52, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Atef, M.; Ramadan, A.; Afifi, N.A.; Youssef, S.A.H. Pharmacokinetic profile of cefotaxime in goats. Res. Vet. Sci. 1990, 49, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Golocorbin-Kon, S.; Mikov, M.; Arafat, M.; Lepojevic, Z.; Mikov, I.; Sahman-Zaimovic, M.; Tomic, Z. Cefotaxime pharmacokinetics after oral application in the form of 3α,7α-dihydroxy-12-keto-5β-cholanate microvesicles in rat. Eur. J. Drug Metab. Pharmacokinet. 2009, 34, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Srivastava, A.K.; Deore, M.D. Pharmacokinetics of cefotaxime in hepatic-dysfunctioned buffalo calves. Vet. Arh. 2005, 75, 339–348. [Google Scholar]
- Attia, Y.A.; Bovera, F.; Abd-Elhamid, A.E.-H.E.; Calabrò, S.; Mandour, M.A.; Al-Harthi, M.A.; Hassan, S.S. Evaluation of the carryover effect of antibiotic, bee pollen and propolis on growth performance, carcass traits and splenic and hepatic histology of growing rabbits. J. Anim. Physiol. Anim. Nutr. 2019, 103, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Nale, L.P.; More, P.R.; More, B.K.; Ghumare, B.C.; Shendre, S.B.; Mote, C.S. Protective effect of Carica papaya L. seed extract in gentamicin induced hepatotoxicity and nephrotoxicity in rats. Int. J. Pharm. Bio. Sci. 2012, 3, 508–515. [Google Scholar]
- Augusto, J.; Smith, B.; Smith, S.; Robertson, J.; Reimschuessel, R. Gentamicin-induced nephrotoxicity and nephroneogenesis in Oreochromis nilotica, a tilapian fish. Dis. Aquat. Organ. 1996, 26, 49–58. [Google Scholar] [CrossRef]
- Bojarski, B.; Kot, B.; Witeska, M. Antibacterials in Aquatic Environment and Their Toxicity to Fish. Pharmaceuticals 2020, 13, 189. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Mostakim, G.M.; Azom, M.G.; Rahman, U.O.; Khan, M.M.; Quader Khan, M.G.; Islam, M.S. Effect of an amalgamated antibiotic and its connection to cyto-genotoxicity and histo-architectural malformations in stinging catfish. Emerg. Contam. 2022, 8, 381–390. [Google Scholar] [CrossRef]
- Manna, S.K.; Bera, A.K.; Das, N.; Bandopadhyay, C.; Baitha, R.; Sen Ghadei, S.; Das, B.K.; Kumar, A.; Ravindran, R.; Krishna, N.; et al. Determination of biosafety of the antibiotic oxytetracycline hydrochloride in Pangasianodon hypophthalmus. Aquac. Res. 2021, 52, 2470–2480. [Google Scholar] [CrossRef]
- Rodrigues, S.; Antunes, S.C.; Nunes, B.; Correia, A.T. Histopathological effects of the antibiotic erythromycin on the freshwater fish species Oncorhynchus mykiss. Ecotoxicol. Environ. Saf. 2019, 181, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Zhuang, H.; Li, X.; Gao, X.; An, Z.; Liu, X.; Yang, H.; Wei, W.; Zhang, X. Excessive use of enrofloxacin leads to growth inhibition of juvenile giant freshwater prawn Macrobrachium rosenbergii. Ecotoxicol. Environ. Saf. 2019, 169, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Zheng, K.; Wang, W.; Zheng, M.; Liu, Y.; Yin, H.; Zhang, D. Cefotaxime Exposure-Caused Oxidative Stress, Intestinal Damage and Gut Microbial Disruption in Artemia sinica. Microorganisms 2024, 12, 675. [Google Scholar] [CrossRef] [PubMed]
- Caceci, T.; Neck, K.F.; Lewis, D.D.H.; Sis, R.F. Ultrastructure of the hepatopancreas of the Pacific white shrimp, Penaeus vannamei (Crustacea: Decapoda). J. Mar. Biol. Assoc. U. K. 1988, 68, 323–337. [Google Scholar] [CrossRef]
- Lightner, D.V. A Handbook of Shrimp Pathology and Diagnostic Procedures for Diseases of Cultured Penaeid Shrimp; World Aquaculture Society: Baton Rouge, LA, USA, 1996. [Google Scholar]
- USDA. Screening and Confirmation of β-Lactam Antibiotics by HPLC-MS/MS; CLG-BLAC; United States Department of Agriculture, Food Safety and Inspection Service, Office of Public Health Science: Washington, DC, USA, 2011; Volume 2, pp. 1–17. [Google Scholar]
- Phu, T.M.; Em, N.T.; Thinh, N.Q.; Phuong, N.T.; Dalgaard, A.; Scippo, M.-L.; Devreese, M.; Croubels, S. Pharmacokinetics and muscle residue depletion of amoxicillin in cage cultured hybrid red tilapia (Oreochromis mossambicus × Oreochromis niloticus). Aquaculture 2019, 505, 206–211. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the Performance of Analytical Methods for Residues of Pharmacologically Active Substances Used in Food-Producing Animals and on the Interpretation of Results as Well as on the Methods to be Used for Sampling and Repealing Decisions 2002/657/EC and 98/179/EC; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- ICH. Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology; ICH: Montréal, Canada, 2005; p. 17. [Google Scholar]
- European Medicines Agency (EMA). 2022. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/adopted-guideline-determination-withdrawal-periods-edible-tissues-revision-2_en.pdf (accessed on 10 May 2023).
- Hekman, P. Withdrawal-Time Calculation Program WT1. 4; BRD Agency for the Registration of Veterinary Medicinal Products: Wageningen, The Netherlands, 2004; pp. 1–8. [Google Scholar]
- Yang, Z.B.; Zhao, Y.L.; Li, N.; Yang, J. Effect of waterborne copper on the microstructures of gill and hepatopancreas in Eriocheir sinensis and its induction of metallothionein synthesis. Arch. Environ. Contam. Toxicol. 2007, 52, 222–228. [Google Scholar] [CrossRef]
- Fedorova, G.; Nebesky, V.; Randak, T.; Grabic, R. Simultaneous determination of 32 antibiotics in aquaculture products using LC-MS/MS. Chem. Pap. 2014, 68, 29–36. [Google Scholar] [CrossRef]
- Lopes, R.P.; Reyes, R.C.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Multiresidue determination of veterinary drugs in aquaculture fish samples by ultra high performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B 2012, 895, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.K.; Rangasamy, R.; Krishnan, A.A.; Singh, D.P.; Uke, S.P.; Malekadi, P.K.; Sengar, A.S.; Mohamed, D.P.; Gupta, A. Simultaneous determination of multi-residue and multi-class antibiotics in aquaculture shrimps by UPLC-MS/MS. Food Chem. 2018, 260, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Dutta, B.P.; Debnath, S.C.; Mandal, T.K.; Chakraborty, A.K. Modification of pharmacokinetics of cefotaxime in uranyl nitrate-induced renal damage in black bengal goats. J. Vet. Sci. 2019, 5, 1–3. [Google Scholar] [CrossRef]
- Fayette, M.A.; Rose, J.B.; Hunter, R.P.; Bowman, M.R.; Proudfoot, J.S. Naïve-pooled pharmacokinetics of ceftiofur crystalline free acid after single intramuscular administration in smooth dogfish (Mustelus canis). J. Zoo Wildl. Med. 2019, 50, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Khalil, W.F.; Shaheen, H.M.; Abdou, R.H. Ceftiofur pharmacokinetics in Nile tilapia Oreochromis niloticus after intracardiac and intramuscular administrations. Dis. Aquat. Organ. 2016, 121, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.S.; Kwon, M.G.; Hwang, J.Y.; Hwang, S.D.; Kim, D.H.; Bae, J.S.; Park, K.H.; Lee, J.H. Estimation of pharmacological properties of ceftiofur, an injectable cephalosporin antibiotic, for treatment of streptococcosis in cultured olive flounder Paralichthys olivaceus. Aquac. Res. 2021, 52, 831–841. [Google Scholar] [CrossRef]
- Wu, G.; Meng, Y.; Zhu, X.; Huang, C. Pharmacokinetics and tissue distribution of enrofloxacin and its metabolite ciprofloxacin in the Chinese mitten-handed crab, Eriocheir sinensis. Anal. Biochem. 2006, 358, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.F.; Giltrow, E.; Huggett, D.B.; Hutchinson, T.H.; Saye, J.; Winter, M.J.; Sumpter, J.P. Comparative physiology, pharmacology and toxicology of β-blockers: Mammals versus fish. Aquat. Toxicol. 2007, 82, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 1995, 22, 89–96. [Google Scholar] [CrossRef]
- Gustafsson, I.; Löwdin, E.; Odenholt, I.; Cars, O. Pharmacokinetic and pharmacodynamic parameters for antimicrobial effects of cefotaxime and amoxicillin in an in vitro kinetic model. Antimicrob. Agents Chemother. 2001, 45, 2436–2440. [Google Scholar] [CrossRef]
- Avunje, S.; Patil, P.K.; Ezaz, W.; Praveena, E.; Ray, A.; Viswanathan, B.; Alavandi, S.V.; Puthiyedathu, S.K.; Vijayan, K.K. Effect of oxytetracycline on the biosafety, gut microbial diversity, immune gene expression and withdrawal period in Pacific whiteleg shrimp, Penaeus vannamei. Aquaculture 2021, 543, 736957. [Google Scholar] [CrossRef]
- Berezhinskaia, V.V.; Dolgova, G.V.; Egorenko, G.G.; Svinogeeva, T.P.; Zebrev, A.I.; Smolkina, T.V.; Shtegel’man, L.A.; Nikitin, A.V. General toxic and organotropic properties of cefotaxime in acute and chronic experiments. Antibiot. Khimioterapiia Antibiot. Chemoterapy Sic. 1990, 35, 25–27. [Google Scholar]
- US EPA. Oxytetracycline Hydrochloride: Human Health Risk Assessment for New Uses on Fruiting Vegetables (CG 8) and Cucurbit Vegetables (CG 9); US EPA: Washington, DC, USA, 2012. [Google Scholar]
- Pathak, M.; Krishna Reddy, A.; Kulkarni, M.; Harikrishna, V.; Srivastava, P.; Chadha, N.; Lakra, W. Histological Alterations in the Hepatopancreas and Growth Performance of Pacific White Shrimp (Litopenaeus vannamei, Boone 1931) Reared in Potassium Fortified Inland Saline Ground Water. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3531–3542. [Google Scholar] [CrossRef]
- Bray, W.A.; Williams, R.R.; Lightner, D.V.; Lawrence, A.L. Growth, survival and histological responses of the marine shrimp, Litopenaeus vannamei, to three dosage levels of oxytetracycline. Aquaculture 2006, 258, 97–108. [Google Scholar] [CrossRef]
- Romano, N.; Koh, C.B.; Ng, W.K. Dietary microencapsulated organic acids blend enhances growth, phosphorus utilization, immune response, hepatopancreatic integrity and resistance against Vibrio harveyi in white shrimp, Litopenaeus vannamei. Aquaculture 2015, 435, 228–236. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).