Exploring Growth Patterns of Maurolicus muelleri across Three Northeast Atlantic Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Area
2.2. Sample Collection
| Area | Survey | Period | TS (°C) | T100m (°C) | Nb. Individuals |
|---|---|---|---|---|---|
| BoB | MEGS | 1–6 Apr19 | 12.6 | 12.6 | 62 |
| BIOMAN | 4–10 May20 | 12.7 | 12.7 | 138 | |
| JUVENA | 1–30 Sep19 | 20.01 | 17.4 | 874 | |
| JUVENA | 1–30 Sep20 | 21.13 | 17.6 | 500 | |
| CS | WESPAS | 13-Jun20 | 14 | 9.9 | 152 |
| IBWSS | 4-Apr21 | 8–11.5 | 8.9 | 94 | |
| WESPAS | 24-Jun21 | 13 | 11.1 | 149 | |
| NS | MEESO-SINTEF | 7–19Nov19 | 10 | 7.5 2 | 199 |
| MEESO-IMR | 11–16 Mar20 | 6.2 | NA | 113 | |
| CS 1 | SINTEF 1 | 4-Jul16 | 5.2 3 | NA | 95 |
| SINTEF 2 | 18–21May17 | 12.3 3 | 14.8 3 | 50 |
| Survey | Type/Vessel | Region | Objective | Net Description |
|---|---|---|---|---|
| JUVENA MEGS BIOMAN | Opportunistic/R/V Ramom Margalef R/V Emma Bardan | BoB | Anchovy juveniles Mackerel/horse mackerel Anchovy adults and sardine | Pelagic trawl, 12 × 25 m, mesh of 4–8 mm |
| WESPAS | Opportunistic/RV Celtic Explore | CS | Pelagic species | Pelagic midwater trawl (85 m length, fishing circle of 420 m, wing mesh size of 2.4 m graded through to codend of 10 cm) |
| IBWSS | Opportunistic/RV Celtic Explore | CS | Blue whiting | Pelagic trawl, 82 × 73 m, and Macroplankton trawl |
| SINTEF | “Ad hoc” MS Birkeland | CS | Mesopelagic fish | 800 m circumference mesopelagic fish trawl, 10 mm innernet codend, 16 mm innernet extension piece, 20 mm innernet in the trawl belly. Small-meshed (10 mm) covers in the belly and extension piece |
| SINTEF | “Ad hoc”/MS Birkeland | NS | Mesopelagic fish | |
| IMR | “Ad hoc”/R/V Kristine Bonnevie | NS | Mesopelagic fish | Fine-meshed mesopelagic trawl, 8 mm mesh size |
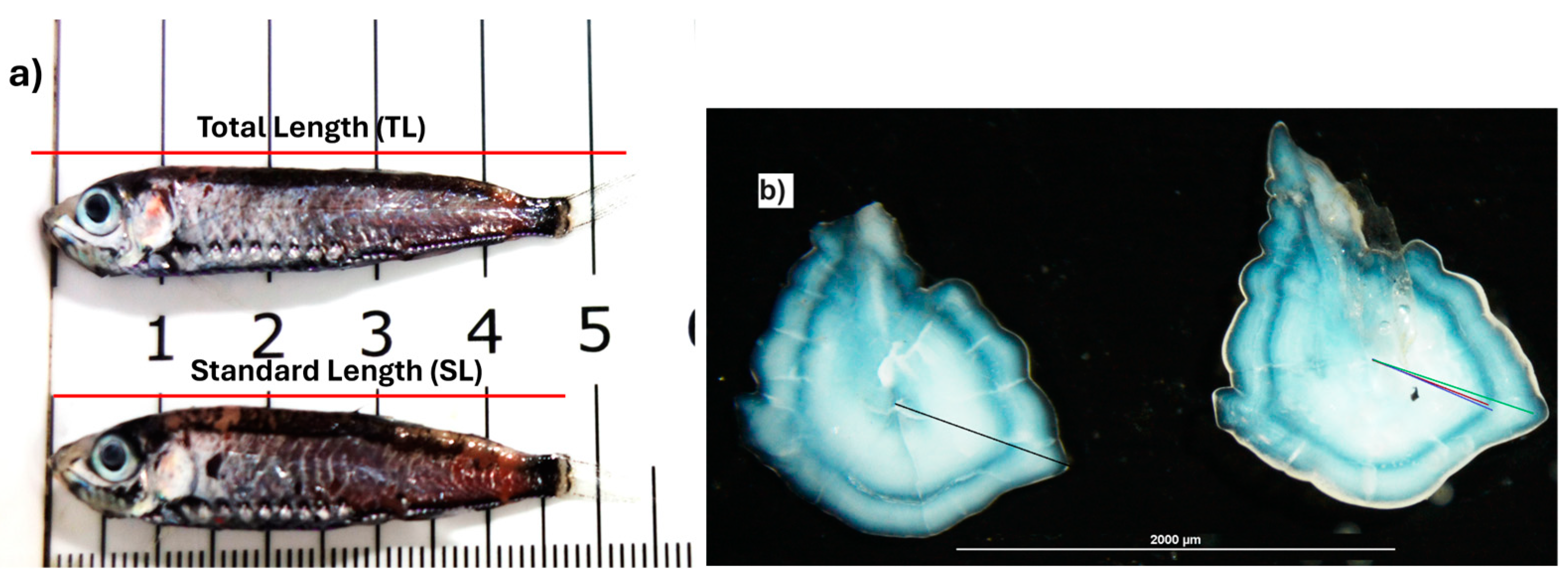
2.3. Laboratory Procedures
2.4. Statistical Analysis
3. Results
3.1. Length Size Structure
3.2. Stomach Somatic Index (SSI)
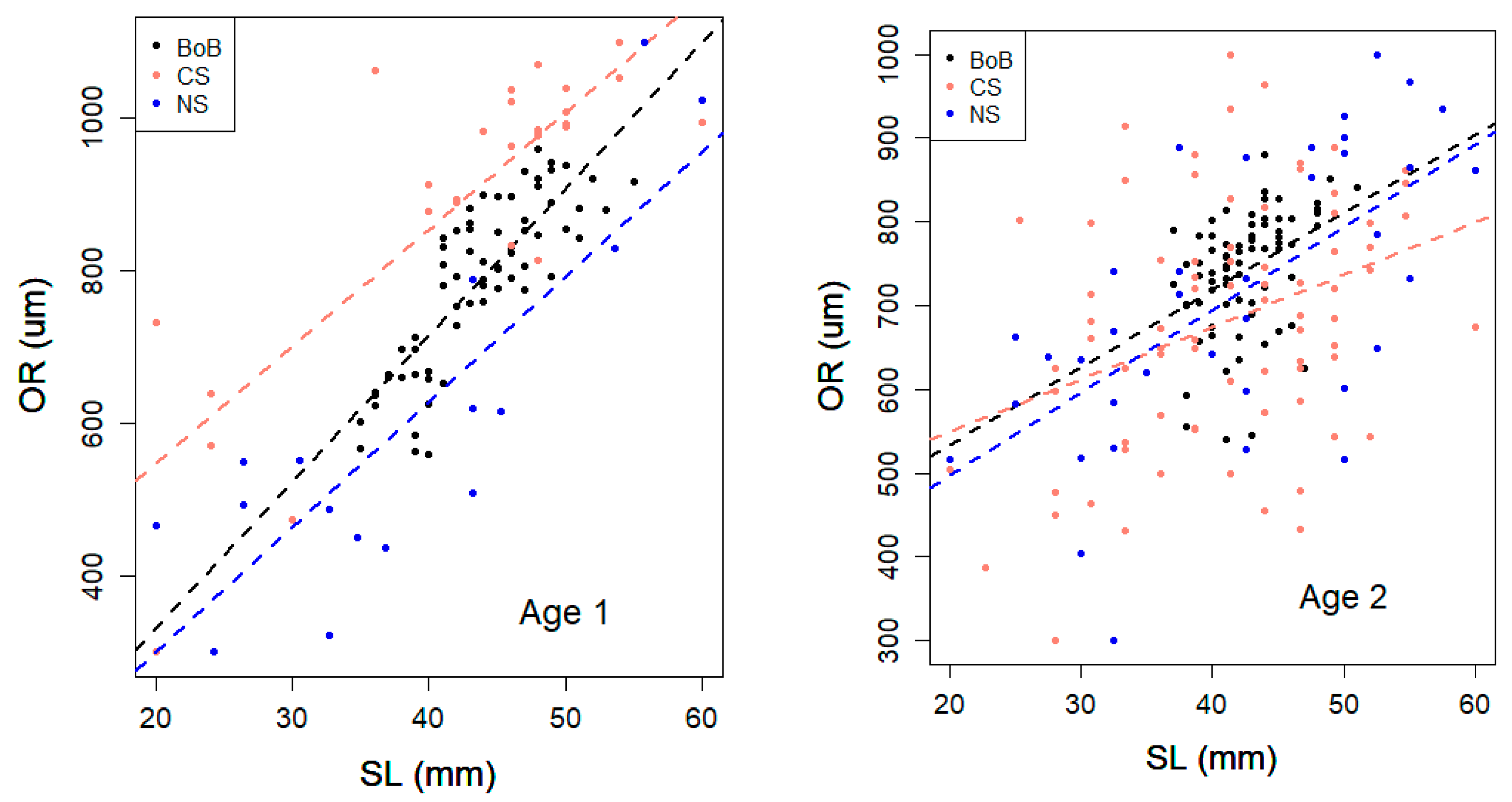
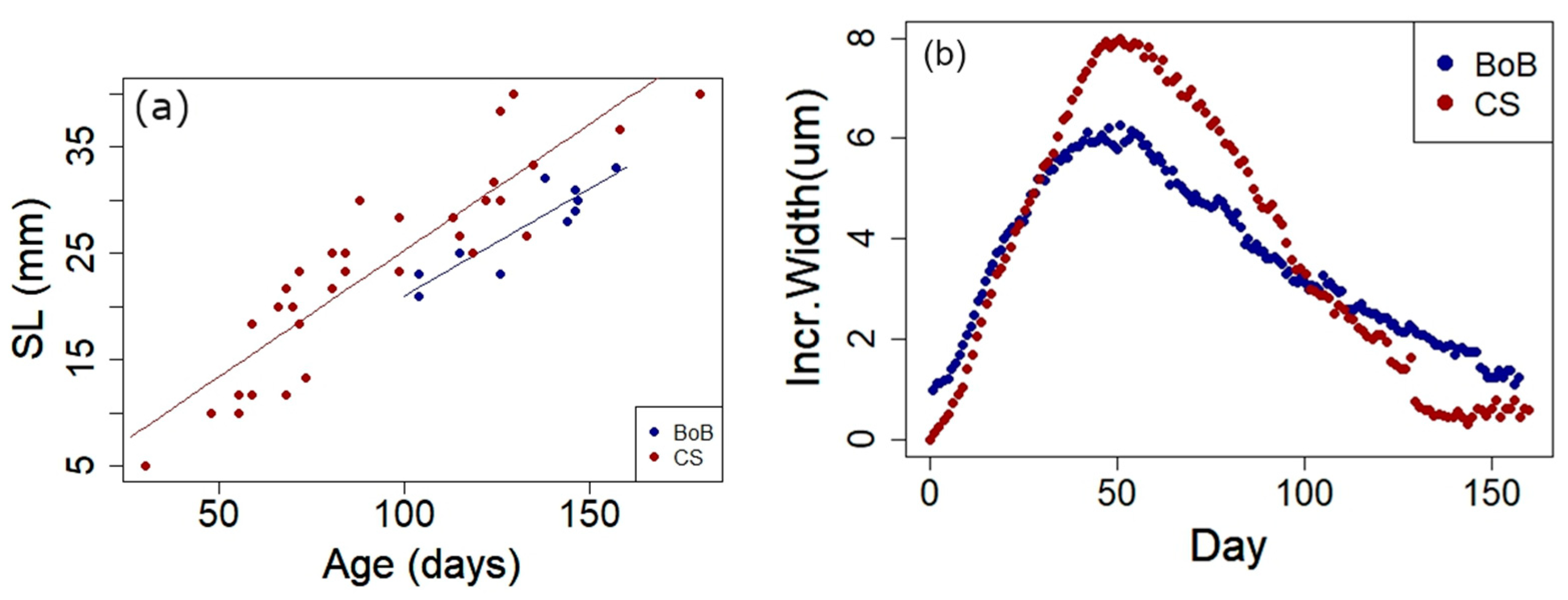
3.3. Otolith Analysis
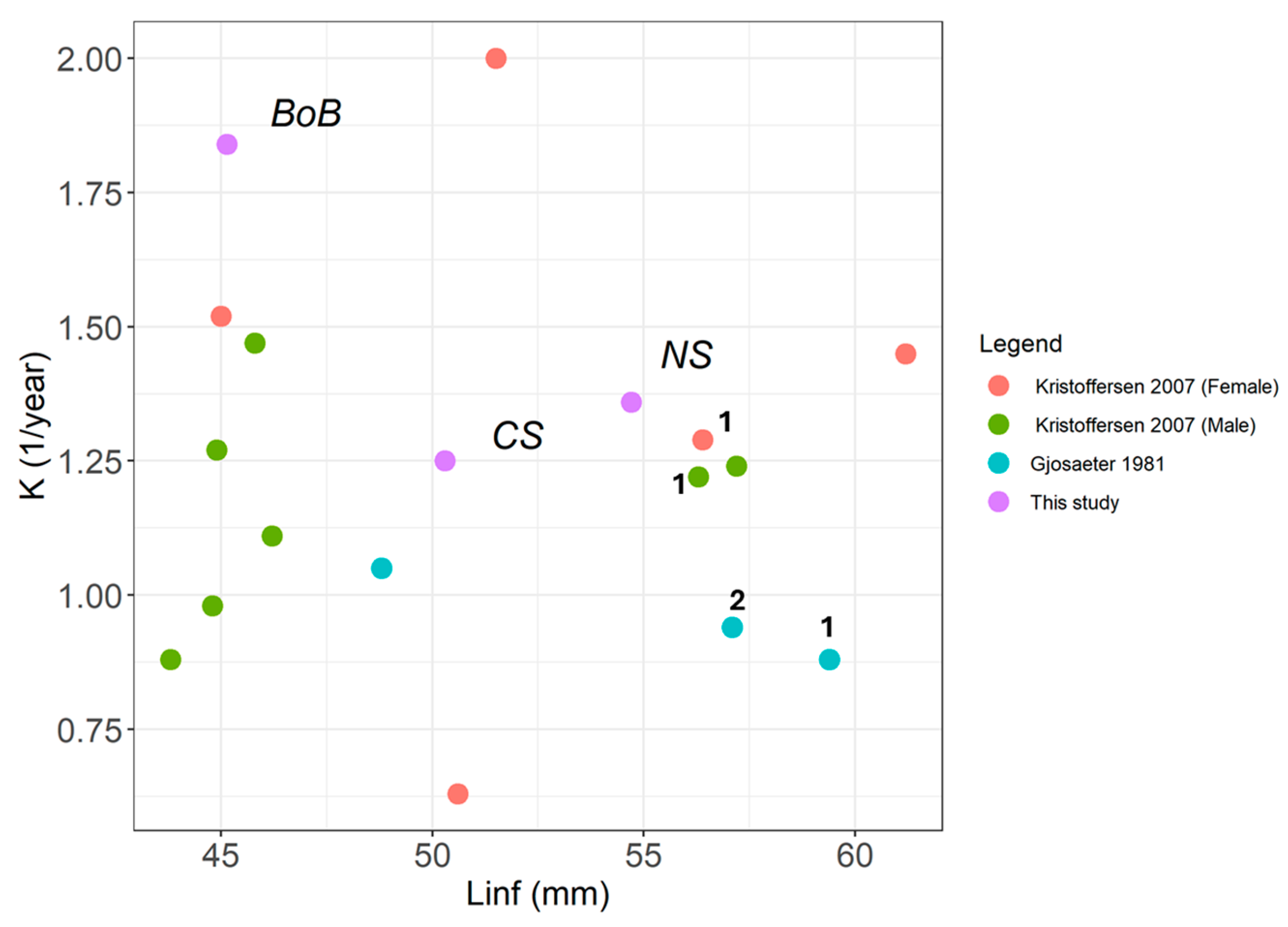
3.4. Von Bertalanffy Growth Models (VBGM)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rees, D.J.; Poulsen, J.Y.; Sutton, T.T.; Costa, P.A.S.; Landaeta, M.F. Global phylogeography suggests extensive eucosmopolitanism in Mesopelagic Fishes (Maurolicus: Sternoptychidae). Sci. Rep. 2020, 10, 20544. [Google Scholar] [CrossRef]
- Sobradillo, B.; Boyra, G.; Martinez, U.; Carrera, P.; Peña, M.; Irigoien, X. Target strength and swimbladder morphology of Mueller’s pearlside (Maurolicus muelleri). Sci. Rep. 2019, 9, 17311. [Google Scholar] [CrossRef] [PubMed]
- Caiger, P.E.; Lefebve, L.S.; Llopiz, K. Growth and reproduction in mesopelagic fishes: A literature review. ICES J. Mar. Sci. 2021, 78, 765–781. [Google Scholar] [CrossRef]
- Gjøsæter, J. Life history and ecology of Maurolicus muelleri (Gonostimatidae) in Norwegian waters. Fisk. Skr. Ser. Havunders. 1981, 17, 109–131. [Google Scholar]
- Kristoffersen, J.B.; Salvanes, A.G.V. Life history of Maurolicus muelleri in fjordic and oceanic environments. J. Fish Biol. 1998, 53, 1324–1341. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Mauchline, J. Biology of Sternoptychid Fishes Rockall Trough; Northeastern Atlantic Ocean. Biol. Oceanogr. 1987, 4, 99–120. [Google Scholar]
- Carbonara, P.; Follesa, M.C. (Eds.) Handbook on Fish Age Determination: A Mediterranean Experience; Studies and Reviews 2019, No. 98; FAO: Rome, Italy, 2019; 192p.
- Casas, M.C. Increment formation in otoliths of slow-growing winter flounder (Pleuronectes americanus) larvae in cold water. Can. J. Fish. Aquat. Sci. 1998, 55, 162–169. [Google Scholar] [CrossRef]
- Cassoff, R.M.; Campana, S.E.; Myklevoll, S. Changes in baseline growth and maturation parameters of Northwest Atlantic porbeagle; Lamna nasus; following heavy exploitation. Can. J. Fish. Aquat. Sci. 2007, 64, 19–29. [Google Scholar] [CrossRef]
- Kristoffersen, J.B. Growth rate and relative otolith size in populations of adult Muller’s pearlside Maurolicus muelleri. J. Fish Biol. 2007, 71, 1317–1330. [Google Scholar] [CrossRef]
- Lee, R.M. An investigation into the methods of growth determination in fishes. Cons. Perm. Int. Explor. Mer Hbl. Circonstance 1912, 63, 35. [Google Scholar]
- Salvanes, A.G.V.; Stockley, B.M. Spatial variation of growth and gonadal developments of Maurolicus muelleri in the Norwegian Sea and in a Norwegian fjord. Mar. Biol. 1996, 126, 321–332. [Google Scholar] [CrossRef]
- ICES. Working group on Mackerel and horse mackerel eggs surveys (WGMEGS). ICES Sci. Rep. 2023, 5, 118. [Google Scholar] [CrossRef]
- Racault, M.-F.; Le Quéréc, C.; Buitenhuis, E.; Sathyendranath, S.; Platt, T. Phytoplankton phenology in the global ocean. Ecol. Indic. 2012, 14, 152–163. [Google Scholar] [CrossRef]
- Ellingsen, I.H.; Dalpadado, P.; Slagstad, D.; Loeng, H. Impact of climatic change on the biological production in the Barents Sea. Clim. Chang. 2008, 87, 155–175. [Google Scholar] [CrossRef]
- Boyra, G.; Martinez, U.; Cotano, U.; Santos, M.; Irigoien, X.; Uriarte, A. Acoustic surveys for juvenile anchovy in the Bay of Biscay: Abundance estimate as an indicator of the next year’s recruitment and spatial distribution patterns. ICES J. Mar. Sci. 2013, 70, 1354–1368. [Google Scholar] [CrossRef]
- Alvarez, P.; Korta, M.; Garcia, D.; Boyra, G. Life-History Strategy of Maurolicus muelleri (Gmenlin; 1789) in the Bay of Biscay. Hydrobiology 2023, 2, 289–313. [Google Scholar] [CrossRef]
- O’Donnell, C.; O´Malley, M.; Smith, T.; O´Brien, S.; Mullins, E.; Connaughton, P.; Tadeo, M.P.; Barile, C. Western European Shelf Pelagic Acoustic Survey (WESPAS). 3 June–12 July 2020. FEAS Survey Series: 2020/03; Marine Institute: Galway, Ireland, 2020; Available online: http://hdl.handle.net/10793/1659 (accessed on 17 June 2024).
- O’Donnell, C.; O’Malley, M.; Mullins, E.; Connaughton, P.; Keogh, N.; Croot, P. Western European Shelf Pelagic Acoustic Survey (WESPAS), 9 June–20 July 2021. FEAS Survey Series: 2021/03; Marine Institute: Galway, Ireland, 2021; Available online: http://hdl.handle.net/10793/1720 (accessed on 17 June 2024).
- ICES. Report on the Working Group on International Blue whiting spawning stock survey (IBWSS) spring 2021. In WD on Widely Distributed Stocks; International Council for the Exploration of the Sea (ICES): Copenhagen, Denmark, 2021. [Google Scholar]
- Grimaldo, E.; Grimsmo, L.; Alvarez, P.; Herrmann, B.; Møen Tveit, G.; Tiller, R.; Slizyte, R.; Aldanondo, N.; Guldberg, T.; Toldnes, B.; et al. Investigating the potential for a commercial fishery in the Northeast Atlantic utilizing mesopelagic species. ICES J. Mar. Sci. 2020, 77, 2541–2556. [Google Scholar] [CrossRef]
- Ottersen, G. A digital temperature atlas for the Norwegian Sea. ICES J. Mar. Sci. 2010, 67, 1525–1537. [Google Scholar] [CrossRef]
- ICES. Report of the Workshop on Age Reading of European Anchovy (WKARA) 2010, 9–13 November 2009; ICES CM 2009/ACOM:43 2010; ICES: Sicily, Italy; pp. 9–13. [CrossRef]
- Aldanondo, N.; Cotano, U.; Tiepolo, M.; Boyra, G.; Irigoien, X. Growth and movement patterns of early juvenile European anchovy (Engraulis encrasicolus L.) in the Bay of Biscay based on otolith microstructure and chemistry. Fish. Oceanogr. 2010, 19, 196–208. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 4th ed.; W.H. Freeman and Company: New York, NY, USA, 2012. [Google Scholar]
- Sachs, L. Applied Statistics: A Handbook of Techniques; Springer: Berlin/Heidelberg, Germany, 1982; 706p. [Google Scholar]
- Le Cren, E.D. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J. Anim. Ecol. 1951, 20, 201–219. [Google Scholar] [CrossRef]
- Sampedro, P.; Saínza, M.; Trujillo, V. Inbio 2.0 Manual. A simple tool to calculate biological parameter’s uncertainty. 2013. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2021. Available online: https://www.R-project.org/ (accessed on 15 January 2024).
- Campana, S.E. Measurement and interpretation of the microstructure of fish otoliths. Can. Spec. Publ. Fish. Aquat. Sci. 1992, 117, 59–71. [Google Scholar]
- Schnute, J.T.; Fournier, D.A. A new approach to length-frequency analysis: Growth structure. Can. J. Fish. Aquat. Sci. 1980, 37, 1337–1351. [Google Scholar] [CrossRef]
- Rypel, A.L. The cold-water connection: Bergmann’s in North American freshwater fishes. Am. Nat. 2014, 183, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Kaartvedt, S.; Knutsen, T.; Holstz, J.C. Schooling of the vertically migrating mesopelagic fish Maurolicus muelleri in light summer nights. Mar. Ecol. Prog. Ser. 1998, 170, s287–s290. [Google Scholar] [CrossRef]
- deBusserolles, F.; Cortesi, F.; Helvik, J.V.; Davies, W.I.L.; Templin, R.M.; Sullivan, R.K.P.; Michell, C.T.; Mountford, J.K.; Collin, S.P.; Irigoien, X.; et al. Pushing the limits of photoreception in twilight conditions: The rod-like cone retina of the deep-sea pearlsides. Sci. Adv. 2017, 3, eaao4709. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.; Klevjer, T.A.; Røstad, A.; Aksnes, D.L.; Kaartvedt, S. Flexible behaviour in a mesopelagic fish (Maurolicus muelleri). ICES J. Mar. Sci. 2021, 78, 1623–1635. [Google Scholar] [CrossRef]
- Folkvord, A.; Gundersen, G.; Albretsen, J.; Asplin, L.; Kaartvedt, S.; Giske, J. Impact of hatch date on early life growth and survival of Mueller’s pearlside (Maurolicus muelleri) larvae and life-history consequences. Can. J. Fish. Aquat. Sci. 2016, 73, 163–176. [Google Scholar] [CrossRef]
- Bellucco, A.; Hara, A.; Machado Almeida, E.; del Bianco Rossi-Wongtschowski, C.L. Growth parameters estimates of Maurolicus stehmanni Parin and Kobyliansky 1996 (Teleostei; Sternoptichydae) from south and southeastern Brazilian waters. Braz. J. Oceanogr. 2004, 52, 195–205. [Google Scholar] [CrossRef]
- Landaeta, M.F.; Bustos, C.A.; Contreras, J.E.; Salas-Berríos, F.; Palacios-Fuentes, P.; Alvarado-Niño, M.; Balbontín, F. Larval fish feeding ecology; growth and mortality from two basins with contrasting environmental conditions of an inner sea of northern Patagonia; Chile. Mar Environ. Res 2015, 106, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Aldanondo, N.; Kaartvedt, S.; Irigoien, X. Growth patterns of two Read Sea mesopelagic fishes. Mar. Biol. 2023, 170, 8. [Google Scholar] [CrossRef]
- Beverton, R.J.H.; Holt, S.J. On the dynamics of exploited fish populations. Fish. Investig. 1957, 19, 1–533. [Google Scholar]
- Beverton, R.J. Longevity in fish: Some ecological and evolutionary considerations. Basic Life Sci. 1987, 42, 161–185. [Google Scholar] [PubMed]
- Walters, C.J.; Post, J.R. Density-dependent growth and competitive asymmetries in size-structured fish populations: A theoretical model and recommendations for field experiments. Trans. Am. Fish. Soc. 1993, 122, 34–45. [Google Scholar] [CrossRef]
- Froese, R.; Binohlan, C. Empirical relationships to estimate asymptotic length; length at first maturity and length at maximum yield per recruit in fishes; with a simple method to evaluate length frequency data. J. Fish Biol. 2000, 56, 758–773. [Google Scholar] [CrossRef]
- Pilling, G.M.; Kirkwood, G.P.; Walker, S.G. An improved method for estimating individual growth variability in fish; and the correlation between von Bertalanffy growth parameters. Can. J. Fish. Aquat. Sci. 2002, 59, 424–432. [Google Scholar] [CrossRef]






| Area | b | SE_b | a | SE_a | R2 adj | p | TW40mm | TW60mm |
|---|---|---|---|---|---|---|---|---|
| ALL | 3.03 | 1.02 × 10−2 | 9.92 × 10−6 | 3.67 × 10−2 | 0.96 | <0.001 | 0.71 | 2.42 |
| NS | 3.17 | 3.99 × 10−2 | 5.71 × 10−6 | 1.46 × 10−1 | 0.96 | <0.001 | 0.68 | 2.48 |
| CS | 2.93 | 2.36 × 10−2 | 1.39 × 10−5 | 8.77 × 10−2 | 0.96 | <0.001 | 0.69 | 2.25 |
| BoB | 3.11 | 1.13 × 10−2 | 7.85 × 10−6 | 4.00 × 10−2 | 0.97 | <0.001 | 0.76 | 2.70 |
| Region | Mean | Median | N |
|---|---|---|---|
| BoB | 5.86 ± 3.51 | 5.08 | 1365 |
| CS | 5.36 ± 5.36 | 3.87 | 226 |
| NS | 3.24 ± 1.86 | 3.05 | 263 |
| BoB | CS | NS | ||||
|---|---|---|---|---|---|---|
| Age | SL | SD | SL | SD | SL | SD |
| 0 | 27.47 | 4.4 | 22.26 | 3.02 | 31.53 | 2.81 |
| 1 | 41.92 | 4.95 | 38.87 | 6.81 | 48.6 | 4.95 |
| 2 | 44.21 | 3.31 | 45.77 | 4.99 | 52.27 | 5.1 |
| 3 | na | na | 53.94 | 3.87 | ||
| 4 | na | na | 63 | na | ||
| Dunn Test | Age 0 | Age 1 | Age 2 | |||
|---|---|---|---|---|---|---|
| Regions | Z | P-adj | Z | P-adj | Z | P-adj |
| BoB-CS | 7.185 | <0.001 | 7.337 | <0.001 | −8.838 | <0.001 |
| BoB-NS | −1.543 | 0.368 | 2.582 | 0.0294 | −4.969 | <0.001 |
| CS-BoB | −7.75 | <0.001 | −5.899 | <0.001 | 5.673 | <0.001 |
| Region | Mean Residual | SE | N |
|---|---|---|---|
| BoB | −1.69 | 7.599 | 143 |
| CS | 0.78 | 6.304 | 110 |
| NS | 2.13 | 6.201 | 75 |
| Region | SLinf (mm) | CV | K | CV | to | CV | N |
|---|---|---|---|---|---|---|---|
| BoB | 45.14 | 0.007 | 1.84 | 0.092 | −0.025 | 1.745 | 1574 |
| CS | 50.3 | 0.018 | 1.25 | 0.14 | −0.075 | 0.981 | 335 |
| NS | 54.7 | 0.019 | 1.36 | 0.178 | −0.145 | 0.063 | 262 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez, P.; Aldanondo, N.; Wieczorek, A.M.; Cariou, T.; Boyra, G.; Grimaldo, E.; Melle, W.; Klevjer, T. Exploring Growth Patterns of Maurolicus muelleri across Three Northeast Atlantic Regions. Fishes 2024, 9, 250. https://doi.org/10.3390/fishes9070250
Alvarez P, Aldanondo N, Wieczorek AM, Cariou T, Boyra G, Grimaldo E, Melle W, Klevjer T. Exploring Growth Patterns of Maurolicus muelleri across Three Northeast Atlantic Regions. Fishes. 2024; 9(7):250. https://doi.org/10.3390/fishes9070250
Chicago/Turabian StyleAlvarez, Paula, Naroa Aldanondo, Alina M. Wieczorek, Thibault Cariou, Guillermo Boyra, Eduardo Grimaldo, Webjørn Melle, and Thor Klevjer. 2024. "Exploring Growth Patterns of Maurolicus muelleri across Three Northeast Atlantic Regions" Fishes 9, no. 7: 250. https://doi.org/10.3390/fishes9070250
APA StyleAlvarez, P., Aldanondo, N., Wieczorek, A. M., Cariou, T., Boyra, G., Grimaldo, E., Melle, W., & Klevjer, T. (2024). Exploring Growth Patterns of Maurolicus muelleri across Three Northeast Atlantic Regions. Fishes, 9(7), 250. https://doi.org/10.3390/fishes9070250







