Acoustic Characteristics of Spawning Biological Sounds of Brown Croaker (Miichthys miiuy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Measurement of Biological Fish Sounds
2.2. Signal Processing and Acoustic Characteristic Analysis Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tyack, P.L. Animal Acoustic Communication; Springer: Berlin, Germany, 1998; pp. 163–220. ISBN 9780128030097. [Google Scholar]
- Popper, A.N.; Fay, R.R.; Platt, C.; Sand, O. Sensory Processing in Aquatic Environments; Springer: Berlin, Germany, 2003; pp. 3–28. ISBN 9780387955278. [Google Scholar]
- Nilsson, J. Acoustic behaviour of spawning cod (Gadus morhua L.). Candidatus. Scientiarum Thesis, University of Bergen, Bergen, Norway, 2004; pp. 1–135. [Google Scholar]
- Hawkins, A.D.; Amorim, M.C.P. Spawning sounds of the male haddock Melanogrammus aeglefinus. Environ. Biol. Fish. 2000, 59, 29–41. [Google Scholar] [CrossRef]
- Locascio, J.V.; Mann, D.A. Diel and seasonal timing of sound production by black drum (Pogonias cromis). Fish. Bull. 2011, 109, 327–339. [Google Scholar]
- Rice, A.; Soldevilla, M.; Quinlan, J. Nocturnal patterns in fish chorusing off the coasts of Georgia and eastern Florida. Bull. Mar. Sci. 2017, 93, 455–474. [Google Scholar] [CrossRef]
- Hildebrand, J. Anthropogenic and natural sources of ambient noise in the ocean. Mar. Ecol. Prog. Ser. 2009, 395, 5–20. [Google Scholar] [CrossRef]
- McWilliam, J.N.; Hawkins, A.D. A comparison of inshore marine soundscapes. J. Exp. Mar. Biol. Ecol. 2013, 446, 166–176. [Google Scholar] [CrossRef]
- Aaron, N.R.; Stacy, C.F.; Andrea, J.M.; Ingrid, M.K.; Phillip, S.L.; William, E.B.; Andrew, H.B. Evolutionary Patterns in Sound Production across Fishes. Ichthyol. Herpetol. 2022, 110, 1–12. [Google Scholar] [CrossRef]
- Aaron, N.R.; Marissa, L.G.; Laurel, B.S.; Holger, K. Conservation Bioacoustics-Listening to the Heartbeat of the Earth. Acoust. Today 2023, 19, 46–53. Available online: https://acousticstoday.org/conservation-bioacoustics-listening-to-the-heartbeat-of-the-earth-aaron-n-rice-marissa-l-garcia-laurel-b-symes-and-holger-klinck (accessed on 15 November 2023).
- Fish, M.P.; Mowbray, W.H. Biological Underwater Sounds; Johns Hopkins: London, UK, 1970; pp. 1–231. ISBN 9780801811302. [Google Scholar]
- Fine, M.L.; Parmentier, E. Sound Communication in Fishes; Springer: Wien, Austria, 2015; pp. 77–126. ISBN 9783709118450. [Google Scholar]
- Rountree, R.A.; Gilmore, R.G.; Goudey, C.A.; Hawkins, A.D.; Luczkovich, J.J.; Mann, D.A. Listening to fish. Fisheries 2006, 31, 433–446. [Google Scholar] [CrossRef]
- Marques, T.A.; Thomas, L.; Martin, S.W.; Mellinger, D.K.; Ward, J.A.; Moretti, D.J.; Harris, D.; Tyack, P.L. Estimating animal population density using passive acoustics. Biol. Rev. 2012, 88, 287–309. [Google Scholar] [CrossRef]
- Kang, T.S. Identification and Authentication of Commercial Brown Croaker (Miichthys miiuy) Products by Two PCR-Based Methods. J. Food Prot. 2021, 84, 463–471. [Google Scholar] [CrossRef]
- FishSounds. 2024. Available online: https://fishsounds.net/fish.js?id=2f613f68-982d-4c56-8522-f98b7c58dabb (accessed on 1 January 2024).
- Gwak, W.S.; Roy, A. Genetic Diversity and Population Structure of Brown Croaker (Miichthys miiuy) in Korea and China Inferred from mtDNA Control Region. Genes 2023, 14, 1692. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Park, M.H. Taxonomic revision of the family Sciaenidae (Pisces, Perciformes) from Korea. Korean J. Icthoyol. 1992, 4, 29–53. Available online: https://koreascience.kr/article/JAKO201030853091333.page (accessed on 30 June 2010). (In Korean).
- Kim, Y.U.; Kim, Y.M.; Kim, Y.S. Commercial fish of the coastal and offshore water in Korea. Nat’l. Fish Res. Dev. Agency Korea 2004, 1–299. (In Korean) [Google Scholar]
- Li, M.Y.; Zheng, Z.M.; Zhu, J.Q. Bloodstock culture and artificial propagation of Miichthys miiuy (Basilewsky). J. Fish Sci. China 2005, 24, 32–34. [Google Scholar]
- Song Meter SM3M. Submersible and Deep Water. 2024. Available online: https://www.wildlifeacoustics.com/uploads/user-guides/SM3M-USER-GUIDE.pdf (accessed on 1 January 2024).
- KMA (Korea Meteorological Administration). Weather, Sunset and Sunrise Times. 2024. Available online: https://www.kma.go.kr/neng/index.do (accessed on 1 January 2024).
- Welch, P. The use of fast Fourier transform for the estimation of power spectra: A method based on time averaging over short, modified periodograms. IEEE Trans. Audio 1967, 15, 70–73. [Google Scholar] [CrossRef]
- Ainslie, M.A.; Halvorsen, M.B.; Robinson, S.P. A Terminology Standard for Underwater Acoustics and the Benefits of International Standardization. IEEE J. Oceanic Eng. 2022, 47, 179–200. [Google Scholar] [CrossRef]
- Carrico, R.; Silva, M.A.; Menezes, G.M.; Vieira, M.; Bolgan, M.; Fonseca, P.J.; Amorim, M.C.P. Temporal dynamics in diversity patterns of fish sound production in the Condor seamount (Azores, NE Atlantic). Deep. Sea Res. Part I Oceanogr. Res. Pap. 2020, 164, 103357. [Google Scholar] [CrossRef]
- Boyle, K.S.; Hightower, C.L.; Nelson, T.R.; Powers, S.P. Diel, temporal, and spatial patterns of biotic soundscapes among Alabama artificial reefs in late spring and summer. Front. Mar. Sci. 2022, 30, 954974. [Google Scholar] [CrossRef]
- Erisman, B.E.; Rowell, T.J. A sound worth saving: Acoustic characteristics of a massive fish spawning aggregation. Biol. Lett. 2017, 13, 20170656. [Google Scholar] [CrossRef]
- Mok, H.K.; Wu, S.C.; Sirisuary, S.; Fine, M.L. A sciaenid swim bladder with long skinny fingers produces sound with an unusual frequency spectrum. Sci. Rep. 2020, 10, 18619. [Google Scholar] [CrossRef]
- Tellechea, J.S.; Martinez, C.; Fine, M.L.; Norbis, N. Sound production in the whitemouth croaker and relationship between fish size and disturbance call characteristics. Environ. Biol. Fish. 2010, 89, 163–172. [Google Scholar] [CrossRef]
- Ladich, F. Acoustic communication in fishes: Temperature plays a role. Fish Fish. 2018, 18, 598–612. [Google Scholar] [CrossRef]
- Ladich, F.; Maiditsch, I.P. Temperature affects sound production in fish with two sets of sonic organs: The Pictus cat. Comp. Biochem. Physiol. Part A 2020, 240, 110589. [Google Scholar] [CrossRef] [PubMed]
- Montie, E.W.; Vega, S.; Powell, M. Seasonal and Spatial Patterns of Fish Sound Production in the May River, South Carolina. T. Am. Fish. Soc. 2015, 144, 705–716. [Google Scholar] [CrossRef]

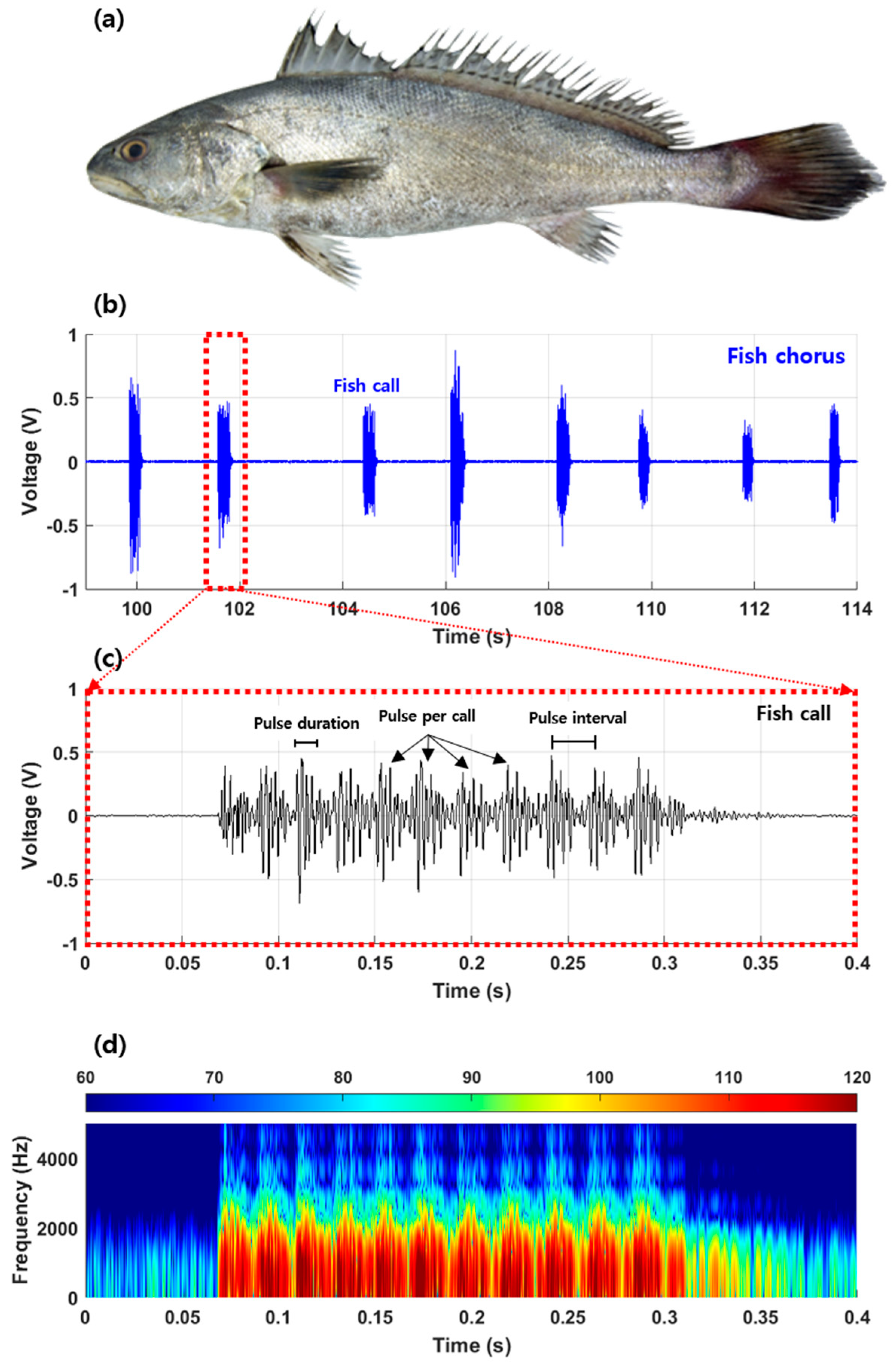
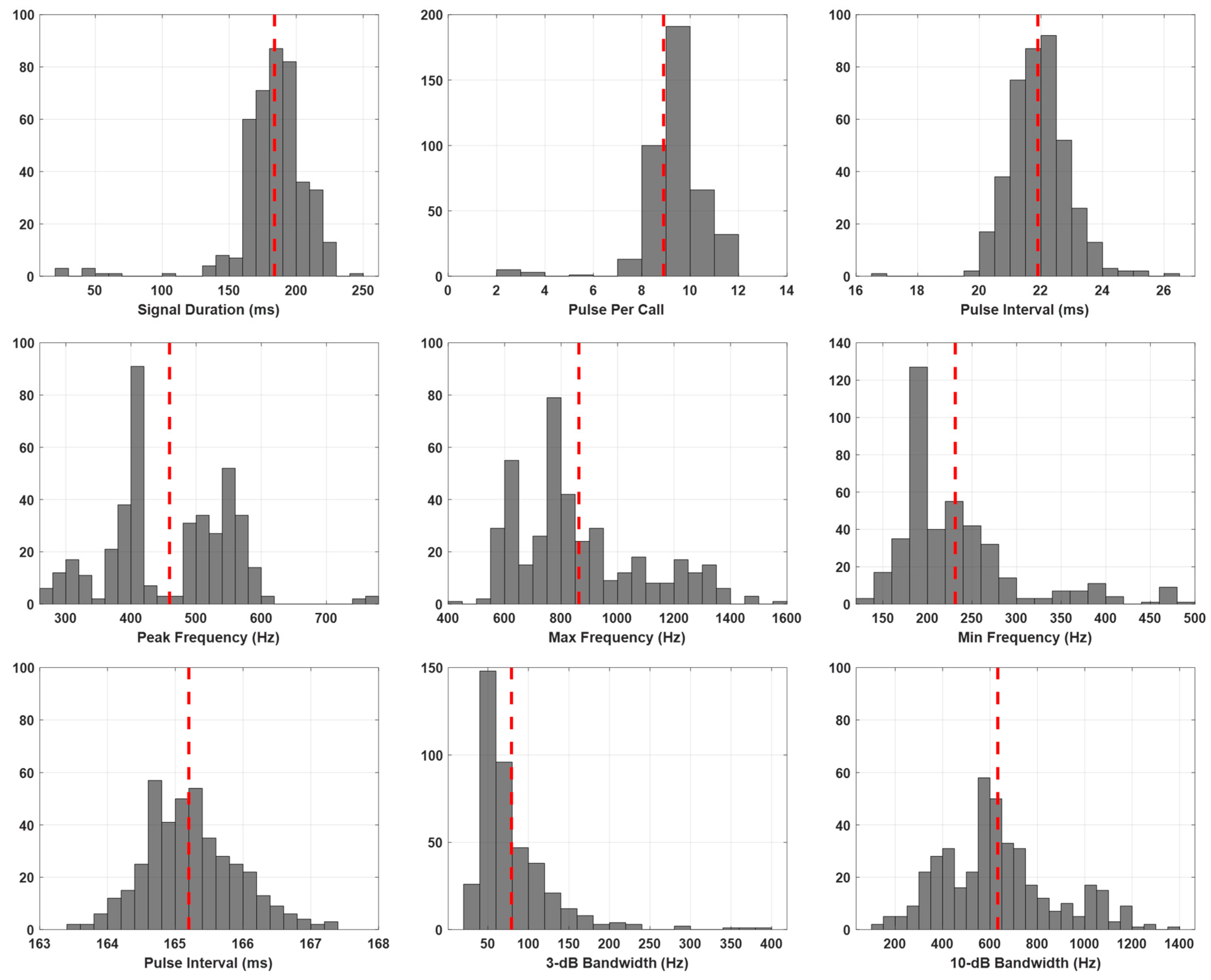

| Acoustic Parameters | n | Mean ± S.D | Minimum | Maximum |
|---|---|---|---|---|
| Pulse duration (ms) | 411 | 183.8 ± 27.4 | 23.2 | 240.4 |
| Pulse interval (ms) | 21.9 ± 1.0 | 16.6 | 26.1 | |
| Pulse per call | 8.9 ± 1.36 | 2 | 12 | |
| Sound pressure level (0-peak) (dB re 1 µPa) | 165.2 ± 0.7 | 163.4 | 167.4 | |
| Peak frequency (Hz) | 459.2 ± 93.8 | 265 | 768 | |
| Max frequency (Hz) | 863.0 ± 225.9 | 447 | 1589 | |
| Min frequency (Hz) | 231.2 ± 67.9 | 129 | 475 | |
| 3 dB bandwidth (Hz) | 79.1 ± 47.4 | 30 | 381 | |
| 10 dB bandwidth (Hz) | 632.8 ± 237.6 | 108 | 1370 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Yoon, Y.G.; Cho, S.; Kim, S.; Kim, M.; Kang, D. Acoustic Characteristics of Spawning Biological Sounds of Brown Croaker (Miichthys miiuy). Fishes 2024, 9, 251. https://doi.org/10.3390/fishes9070251
Kim H, Yoon YG, Cho S, Kim S, Kim M, Kang D. Acoustic Characteristics of Spawning Biological Sounds of Brown Croaker (Miichthys miiuy). Fishes. 2024; 9(7):251. https://doi.org/10.3390/fishes9070251
Chicago/Turabian StyleKim, Hansoo, Young Geul Yoon, Sungho Cho, Sunhyo Kim, Mira Kim, and Donhyug Kang. 2024. "Acoustic Characteristics of Spawning Biological Sounds of Brown Croaker (Miichthys miiuy)" Fishes 9, no. 7: 251. https://doi.org/10.3390/fishes9070251
APA StyleKim, H., Yoon, Y. G., Cho, S., Kim, S., Kim, M., & Kang, D. (2024). Acoustic Characteristics of Spawning Biological Sounds of Brown Croaker (Miichthys miiuy). Fishes, 9(7), 251. https://doi.org/10.3390/fishes9070251








