Abstract
Aquafarms should reduce the use of fishmeal and fish oil in nutritional feed. One such accessible and relatively inexpensive food component that could successfully meet the challenge posed by aquaculture is algae. The objective of the present study was to evaluate the algae meal inclusion of Chlorella and Spirulina sp. in a diet for rainbow trout, evaluating its effects on fish growth, histological parameters and fillet quality. Experiments were carried out to replace 50% and 100% of fishmeal with Spirulina sp. and Chlorella vulgaris in feed for rainbow trout (Oncorhynchus mykiss W.) cultured in a recirculation system. At the end of the experimental period, the highest mean live weight was measured in rainbow trout fed a feed containing 50% algal meal in the feed. The absorptive vacuolization of cells was increased the most and lamina propria was average thickened when fed 50% algae diets. Fat droplets in the hepatocytes were larger in the 50% algae meal fed group, and their nuclei were replaced in the peripheral zone of the cells. Substitution of fishmeal with 50% algal meal in fish feed resulted in a 36.44% reduction in the lipid content of rainbow trout fillets compared to control fish.
Key Contribution:
This article concerns feeding rainbow trout in a recirculating system with 50 and 100% replacement of fish meal with algae.
1. Introduction
Aquaculture is one of the world’s fastest growing agricultural sectors. A prerequisite for this is a continuous increase in the Earth’s population and the related difficulties in feeding it, as well as the ever-increasing demand for healthy foods in the world. The industry, whose main activities are related to the reproduction and cultivation of various types of aquatic organisms, must also respond to this challenge. Fish meat is especially valuable and useful, which in terms of its dietary value and high quality competes stronglyand even surpasses with its consumption benefits those of other meats from domestically raised animals [1,2].
Fishmeal is considered a desirable animal product in compound feed formulation in aquaculture. The reasons for this lie in its high protein content, balanced amino acid composition, and high digestibility [3]. Fishmeal and fish oil prices more than doubled in 2000 and remain consistently higher than plant-based alternatives. Aquafarms should reduce the use of fishmeal and fish oil in nutritional feed, and these obligations are reinforced by sustainability targets throughout the supply chain [4]. Nevertheless, the share of global fishmeal consumption used by the aquaculture sector (compared to livestock and non-food products) increased from 33% in 2000 to 69% in 2016, while the share of global fish oil used by aquaculture has grown over the same period from 55% to 75% [5].
A major goal of scientists involved in the nutrition of hydrobionts is to discover and implement cheaper sources of protein in this industry [6]. One such accessible and relatively inexpensive food component that could successfully meet the challenge posed by aquaculture is algae.
An increasingly popular solution to this problem is feeding farmed fish with microalgae meal instead of the traditionally used fishmeal [7]. Food made from protein-rich algae does not require aquaculture centers to purchase or catch wild fish, so they do not contribute to overfishing. Feeding microalgae meal benefits the aquaculture business and the environment.
The studies of a number of scientists [8,9,10,11,12,13,14,15,16,17] demonstrated the possibility of partial or complete replacement of fishmeal in the ration of rainbow trout with sources of proteins of vegetable origin such as soybean meal, sunflower meal, flour, cottonseed meal, rapeseed meal, peanut meal, etc.
Regarding the use of algae meal as a substitute for part of the fishmeal in fish rations, the following species have been worked on: fish from the river Oreochromis [18,19,20], in Pagrus major [21], in Dicentrarchuslabrax [22], in Siganuscanaliculatus [23], and in Penaeusmonodon [24]. Research in this area in rainbow trout (Oncorhynchus mykiss) is limited up to now.
Microalgae are widely used as model plants for research because they are small in size, multiply rapidly, are easy to handle, and have high sensitivity to organic and inorganic substances [25]. These aquatic plants are cultivated due to their high content of proteins, fatty acids and biologically active substances. Freshwater algae from the genera Spirulina and Chlorella are called superfoods because they have a positive effect on every system in the body, exhibitantioxidant properties and strengthen the immune system [26].
Chlorella vulgaris is an attractive food source due to its high protein content of up to 45%, 20% fat, 20% carbohydrate, 5% fiber and 10% minerals and vitamins [27]. Chlorella vulgaris is one of the best microalgae used widely in global production for food products, pharmaceutical products, as biofuel, etc. [28,29].
Spirulina is recognized as one of the greatest potential sources of protein and other vital nutrients among plants. This cyanobacterium is the most cultivated photosynthetic prokaryote, as its biomass is widely used as a health food, feed additive and as a source of the blue pigment cyanophycin, which is used in cosmetics and foods [30]. Spirulina has the highest usable protein value compared to egg powder, milk, fish, chicken and beef [31].
The supplied algae supplemented diets on Nile tilapia did not provoke histopathological changes in the intestines and liver samples. Algae is beneficial to diets for Nile tilapia up to 5%. It does not affect the fish tissues [32].
Supplementing Shrimp hydrolysate improves the health status of the intestinal histostructure in the juvenile giant trevally, while also improving growth performance and feed utilization [33].
An investigation on the algae protein replacement of plant protein shows that the algae protein improves the feed consumption and fish growth when the quantity of the same protein is up to 50%. When the value of this protein is higher than 50%, the growth performance is not affected [34].
A literature review revealed a lack of histological studies on feeding rainbow trout with algae meal.
Apart from representing rich sources of protein and lipids, Spirulina and Chlorella do not contain lignin, contain little hemicellulose in the fibers, and suggest better digestibility [35]. The two microalgae are complimentary as when the fish were fed with spirulina, better food conversion, and weight gain were shown. When fed with chlorella, the samples exhibited the best protein efficiency ratio [36].We chose the two types of microalgae, Spirulina platensis and Chlorella vulgaris, because one species contains larger amounts of certain essential amino acids and fatty acids, while the other contains different ones. By combining the two types, our hypothesis was that they would more closely approximate the digestibility and assimilability of the nutritional components found in fish meal, and that this would beneficially affect the growth, intestinal morphology, and meat quality of the experimental fish.
Most studies have sought to determine and compare the advantages of one type of microalgae over another, and to establish the optimal level that could positively replace fishmeal without harm [37,38]. There is a lack of research on the joint replacement of fishmeal from these two species and for optimum dietary addition levels of C. vulgaris and S. platensis for the growth performance of most fish species.
The objective of the present study was to evaluate the algae meal inclusion of Chlorela vulgaris and Spirulina platensis in the diets of rainbow trout, evaluating its effects on fish growth, histological parameters and fillet quality.
2. Materials and Methods
2.1. Recirculation System and Experimental Fish
During the experiments with rainbow trout, a closed recirculation system was used in the “Educational-Experimental Aquaculture Base” at the Trakia University, Stara Zagora. The recirculation aquaculture system (RAS) consists of 14 concrete tanks included in a general recirculation water supply (Figure 1). Each tank has a total volume of 1 m3, and auseful volume of 0.750 m3. A total of 9 tanks were used. The experiment was carried out in 3 replicates. Water purification in the recirculation system takes place thanks to two mechanical filters, a biofilter. To compensate for water losses from the tanks, we added daily up to 10–15% fresh water from the total volume of the recirculation system. Throughout the experiments, the water in the recirculation system was heated by a heating system. During the experiments, additional aeration of the water was provided by an aerator system.Feeding was carried out manually at 4 different time periods (8, 11, 14, 17 h). The photoperiod was 16:8.
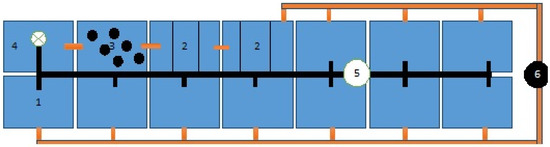
Figure 1.
Recirculation aquaculture system: 1. Tanks; 2. Mechanical filter; 3. Biological filter; 4. Tank with pump; 5. Incoming water; 6. Outgoing water.
The trout used in the experiments were purchased from Fish Farm—Peshtera. Healthy fish with no visible injuries were selected and transported to the Aquaculture Experimental Base, Trakia University, Stara Zagora. Experimental fish were 3 months old, not sexually mature. The start of the experiments was carried out after a seven-day adaptation period of the transported fish in the experimental base. The duration of the trials was 60 days. When betting on trial options, all fish were weighed individually (g).
The mean weight of the experimental fish (225 in number) was 24.30 ± 7.3 g (control), 24.16 ± 6.1 g (50%) and 24.14 ± 6.8 g (100%), respectively, with no statistically significant difference in weights (p > 0.05) between different individuals in experimental variants. They were placed in each tank and fed 2 mm pelleted feed and a daily ration of 2.2% of their body weight.
2.2. Extrusion Treatment
A software program (Alaska, Windows 2015, Version 3) of the company “Foodservice Engineering AD” was used for the composition of the experimental feeds with the addition of Spirulina and Chlorella algae. Experiments were carried out to replace 50% and 100% of fishmeal with Spirulina sp. and Chlorella vulgaris in feed for rainbow trout (Oncorhynchus mykiss W.) cultured in a recirculation system.The composition of the combined feeds involved in the experiment can be seen in Table 1. The algal meal was purchasedfinely ground (48.62% crude protein, 8.85% fat) from ZoyaBG® (Sofia, Bulgaria). The diets used in the present study were isocaloric and isoprotein, which can be seen from the Table 1.

Table 1.
Composition and nutritional value of the feeds used (control, 50% and 100% spirulina and chlorella content) for feeding trout.
All experimental feeds were carried out on a 20-mm single screw extruder—BRABENDER 20 DN—(Berlin, Germany) with a screw diameter of 20 mm, equipped with a measuring device for torque Mn, N.m., die temperature, °C and die pressure MPa (Figure 2).
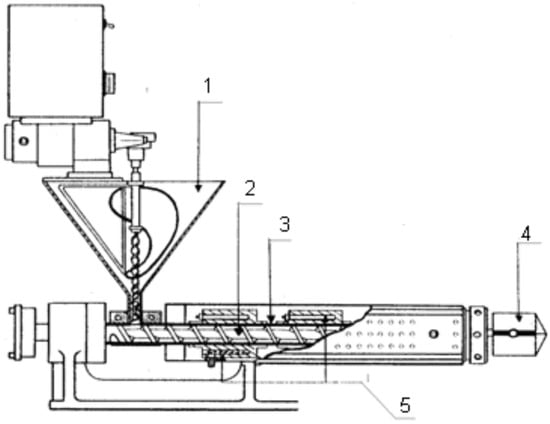
Figure 2.
Single screw laboratory extruder—BRABENDER 20 DN: 1—feeding device; 2—working screw; 3—cylinder; 4—die with nozzle and heater; 5—heating devices; by Cherpokov et al. [39].
The extruder was cooled with water and air. It has a cylinder diameter of 20.05 mm and a length of 406.5 mm and is provided with a cutting mechanism. During the experiments, the following modes of operation were used:
- -
- Temperatures in the three zones of the extruder: t1 = 120 °C; t2 = 140 °C; t3 = 160 °C;
- -
- Working screw with a compression ratio K = 2:1;
- -
- Nozzle with internal diameter Do = 2 mm;
- -
- Feeding screw speed Nf = 40 min−1;
- -
- Working screw speed Nw = 140 min−1;
The inlet humidity of the mixtures was fixed at 28%. This is one of the most important parameters that influences the properties the extruded feeds produced.
2.3. Hydrochemical Indicators
During the experimental period, the water temperature (°C), dissolved oxygen (mg·L−1), pH, electrical conductivity (µS·cm−1) in tanks were measured daily by multimeter HQ40D (Hach Lange®) (Hach Lange GmbH, Headquarter, Düsseldorf, Germany). The Nitrogen nitrate and Nitrate nitrogen, mg.L−1 (BDS EN ISO 10304-1:2009) were examined in laboratory weekly. The hydrochemical parameters of the tanks in which fish from different experimental variants were grown during the experimental period correspond to the cultivation of this species (Table 2).

Table 2.
Mean values of hydrochemical parameters during the trial.
2.4. Growth Parameters of Experimental Fish
The average weight of individual fish (g) was calculated at the beginning and end of the experiment to determine the effect of the feed additives used. At the end of the experiments, the specific fish growth rate (%/day), gain (g), fish survival rate (%) and feed conversion ratio were calculated:
Specific growth rate (SGR) [40]:
where, SGR—the specific growth rate of fish (%/day); Wi—average initial weight, g; Wf—average final weight, g; n—number of days
Feed conversion ratio (FCR)
Survival (%) = (final number of fish/initial number of fish) × 100
2.5. Histological Analyses
A total of six tissue specimens (three from the posterior intestine and three from the liver) from the corresponding experimental group were used to prepare histological slides. The specimens were fixed in 10% aqueous formaldehyde solution (Merck KGaA, Darmastadt, Germany) for 48 h. Afterwards, they were washed under running tap water, dehydrated in ascending ethanol sequence, clarified in xylene and embedded in paraffin-wax. Histological cuts with a thickness of 5 μm were made using a rotary microtome YD-335A (J. Y. M. A. Ltd., Jinhua, China). The preparations were stained with hematoxylin (Erlich)—eosin.
The obtained preparations were studied via light microscope Leica DM 1000 (Leica Microsystems CMS GmbH, Wetzlar, Germany), and the results were recorded using a digital camera Leica DPC290 (Leica Microsystems CMS GmbH, Wetzlar, Germany). The results from the morphometric analysis were obtained via software LAS V 410.0 2016.
We explored the following parameters of the structures which comprise the walls of the posterior intestine: thickness of Tunica mucosa, Tunica submucosa, Tunica muscularis, Tunica serosa, height of the epithelial cells, villus height and crypt depth.
2.6. Chemical Composition of Fish Meat
For the purpose of research, muscle samples were taken from 6 psc. per experimental group. The determination of the chemical composition—moisture, protein, lipids, dry matter and ash content (%)—of meat of rainbow trout was performed in Central Scientific Laboratory at Trakia University, Stara Zagora. The meat samples for the relevant analysiswere taken ferom the dorsal fish muscles and prepared according to AOAC (2006, method 983.18) to determine the following parameters:
Water content, % (BDS11374-86); Protein content, % (BDS-ISO 5983, Kjeldahl method, using Kjeltec 8400 apparatus, FOOS, Sweden); Lipid content, % (BDS-ISO 6492, Soxlet method, using a Soxtec 2050 apparatus, FOOS, Hoganas, Sweden); Crude ash, % (BDS11374-86).
2.7. Data Analysis
The results were statistically processed by one-way ANOVA, and in the presence of a reliable influence of the factor, the differences between the mean values in the groups were evaluated by the Fisher’s Least Significant Difference (LSD) method at a confidence level of p < 0.05. The software product employed was Statistica 10 (Statistica for Windows, StatSoft. Inc., Tulsa, OK, USA, 2010).
3. Results and Discussion
3.1. GrowthParameters
Fish survival during the experimental period was 97.3% in the control, and in experimental variants B and C 98.7% and 97.3%, respectively (Table 3).

Table 3.
Growth parameters of rainbow trout during the trial.
The mean initial live weight of rainbow trout from the control and experimental options B and C was 24.30 ± 7.3 g, 24.16 ± 6.1 g and 24.14 ± 6.8 g, respectively, and the differences were minimal and not statistically significant (p > 0.05) (Table 3).
At the end of the experimental period, the highest mean live weight was measured in rainbow trout fed a feed containing 50% algal meal in the feed. This parameter in the fish of option B was 14.57% and 5.08% higher compared to the final weight of variants A and C, respectively, and the differences were statistically significant (p < 0.01) (Table 3).
The average individual weight of rainbow trout in group B fed with feed containing 50% algal meal was 33.81 ± 2.1 g, which was 25.46% and 8.66% higher than that found in control group A (p < 0.01) and the experimental group of fish C (Table 3). The specific growth rate (SGR) of rainbow trout in group B fed a ration containing 50% algae meal was 18.62% higher than that found in control group A (p < 0.01) (Table 3). An analysis of the daily amounts of feed fed in the control and experimental groups was performed. At the end of the experiment, the analysis of the feed consumption data showed that the feed conversion ratio of the trout of the experimental group B fed with 50% algae meal was 1.07, while that of the control fish was 1.48, and 1.23 was for the fish from variant C fed with 100% algal meal (Table 3). The better utilization of the feed, in which the fishmeal in the ration was replaced at 50% and 100% by the algae Spirulina and Chlorella reflected positively on the growth of the trout of the experimental groups cultivated in the recirculation system. Growth parameters of rainbow trout from groups fed 50% and 100% replacement of fishmeal with algae were better. Aquatic plants have been found to be able to positively affect growth in fish by supporting the activity of digestive enzymes, which in turn lead to increased growth of hydrobionts [41]. The presence of proteins, amino acids, minerals, vitamins and flavonoids in microalgae from the genera Spirulina and Chlorella are important for the growth of hydrobionts.
According to various scientists, partial replacement of fishmeal with Spirulina and Chlorella improves growth parameters, feed conversion, immune status and antioxidant response in fish [42,43,44]. Raji et al. [45] using Chlorella and Spirulina algal meal found almost identical growth rates that were better than fish fed fish meal. They found 68.5% and 69.4% optimum replacement of fishmeal with Spirulina and Chlorella, respectively, in the feed of Clarias gariepinus. Radhakrishnan et al. [46] established a significantly increased level of growth of Macrobrachium rosenbergii fed with C. vulgaris meal up to 50%. At higher Chlorella supplementations (75% and 100%) a slight decrease in growth and feed digestibility was observed. Fadl et al. [47] including 15% Chlorella in a Nile tilapia (Oreochromis niloticus) diet found a 30% reduction in FCR compared to the control feed. Nandeesha et al. [48] conducted a 120-day trial with common carp replacing fishmeal with 25%, 50%, 75% and 100% Spirulina. They found that spirulina improved specific growth rate as well as feed conversion ratio. During a 65-day trial of replacing 0%, 50%, 75% and 100% of fishmeal with Spirulina in hybrid red tilapia fries (Oreochromis niloticus × Oreochromis mossambicus) diets, El-Sheekh et al. [49] found best growth performance when fed 75% Spirulina feed.
3.2. Histological and Micrometric Analyses
In the histological samples of the liver and posterior intestine, there were no alterations observed. The intestinal mucosa of the fishes fed with algae diets were normal and demonstrated the same morphological features, found in the control group, i.e., the mucosal folds of the intestine had normal structure. The absorptive vacuolization of cells was increased the most and lamina propria was average thickened when fed 50% algae diets. Lamina propria of the intestinal mucosa was intact, without leucocytes infiltration (Figure 3, Table 4).
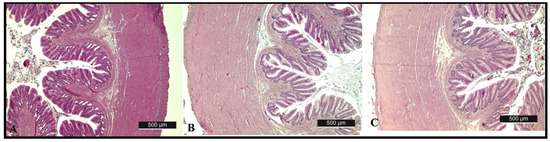
Figure 3.
Microphotograph of the intestine of rainbow trout (Oncorhynchus mykiss) fed with control diet (A), 50% algae meal (B), 100% algae meal (C).

Table 4.
Histometric parameters of some structures in the posterior intestine.
Lipid droplets were found in the hepatocytes when fed with both algae supplemented diets. Fat droplets in the hepatocytes were larger in the 50% algae meal fed group, and their nuclei were replaced in the peripheral zone of the cells.
A 50% algae meal fed diet is enough to improve the values of the studied histological structures in the intestines of the rainbow trout. This is a prerequisite for the improvement in growth parameters (Figure 4).
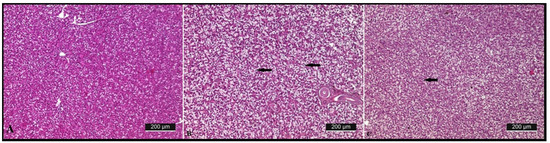
Figure 4.
Microphotograph of the liver of rainbow trout (Oncorhynchus mykiss) fed with control diet (A), 50% algae meal (B), 100% algae meal (C).
Our results regarding the replacement of fish meal with algae meal corresponded to the published data [34]. According to our findings, 50% algae meal fed diet is enough to improve the values of the studied histological structures in the intestines of the fishes.
Our thesis is that an algae supplemented diet does not provoke histopathological changes in the liver and intestines in rainbow trout. Thus, the given data are similar to the previously published results, regarding the algae supplemented diets on Nile tilapia [32].
The addition of Spirulina to the fish diet is recommended because it improves the intestinal flora and breaks down the ingested food components. Thus, more nutrients are extracted and enzymes are produced that transport fats for metabolism [50]. The inclusion of Chlorella in a Koi carp diet increases the digestive enzymes in the intestines and pancreas and increases the digestibility of food [51].
3.3. Chemical Composition of Meat
Feeding the fish with 50% algal meal in the feed resulted in 2.54% higher moisture content in the rainbow trout fillets of group B compared to that measured in the control fish (p < 0.001) (Table 5). The highest amount of protein was recorded in group B, but the difference in protein content between fish from group C and the control group was insignificant (p > 0.05). Substitution of fishmeal with 50% algal meal in fish feed resulted in a 36.44% reduction in the lipid content of rainbow trout fillets compared to control fish and 6.84% in group trout C, the difference being statistically significant (p < 0.001). This result shows that fish fed with algal meal is more useful and dietary in its quality as human food. The ash content in the trout fillets of the control was higher by 12.57% in group B and by 7.42% in experimental group C (Table 5).

Table 5.
Chemical composition of rainbow trout fillets during the experiment.
Promya and Chitmanat [52] established a 21% higher moisture content in catfish fillets fed a Spirulina supplement compared to the control. Inclusion of Chlorella and Spirulina in fish diets was associated with a decrease in lipids and an increase in moisture [53].
Rainbow trout is considered a medium-fatty fish, with a muscle fat content between 2 and 7% [54], and our results were in this range of 3.40–5.35% and confirm this. Raji et al. [36] conducted an experiment with the African catfish (Clarias gariepinus) fed with fodder containing Spirulina or Chlorella replacing fishmeal portion. They found that the Chlorella diet significantly reduced lipid content by 27% compared to the control. In studies conducted by other scientists with plant supplements to the feed of rainbow trout, they also found lower values for the fat content in the fillets of the experimental fish [55].
4. Conclusions
Substitution of fish meal with 50% and 100% algae meal from Spirulina and Chlorella in feed for rainbow trout cultured in a recirculation system in the present study positively affected hydrochemical parameters. Substitution of fishmeal with 50% algae meal in compound feed for rainbow trout cultured in a recirculation system significantly increased final weight, average individual weight and growth by 14.57%, 25.46% and 18.62%, respectively, compared to the control. A 50% algae meal fed diet is sufficient to improve the values of the studied histological structures in the intestines of the fishes. Substitution of fishmeal with 50% algal meal in fish feed resulted in a 36.44% reduction in the lipid content of rainbow trout fillets compared to control fish.
Author Contributions
Conceptualization, K.V. and I.S.; Methodology, K.V.; Software, I.S.; Validation, K.V., I.S. and S.S.; Investigation, A.S.; Resources, K.V.; Data curation, K.S.-Y.; Writing—original draft, K.V.; Writing—review and editing, I.S.; Visualization, D.Y.; Supervision, K.V.; Project administration, I.S.; Funding acquisition, I.S. All authors have read and agreed to the published version of the manuscript.
Funding
Funded by Bulgarian Ministry of Education and Science (MES) in the frames of Bulgarian National Recovery and Resilience Plan, Component “Innovative Bulgaria”, the Project № BG-RRP-2.004-0006-C02 “Development of research and innovation at Trakia University in service of health and sustainable well-being”.
Institutional Review Board Statement
The study protocol was approved by the Ethical Commission of Trakia University (№3336/2014).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data arecontained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Olaganathan, R.; Kar Mun, A. Impact of aquaculture on the livelihoods and food security of rural communities. Int. J. Fish. Aquat. Sci. 2017, 5, 278. [Google Scholar]
- Ocran, J.N. Feed resources and policy options on feed for aquaculture production in Africa: A review. Int. J. Fish. Aquat. Sci. 2020, 8, 19–23. [Google Scholar]
- Macusi, E.; Cayacay, M.; Borazon, E.; Sales, A.; Habib, A.; Fadli, N.; Santos, M. Protein Fishmeal Replacement in Aquaculture: A Systematic Review and Implications on Growth and Adoption Viability. Sustainability 2023, 15, 12500. [Google Scholar] [CrossRef]
- Oliva-Teles, A.; Enes, P.; Couto, A.; Peres, H. Replacing fish meal and fish oil in industrial fish feeds. Feed. Feed. Pract. Aquac. 2022, 231–268. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-Year Retrospective Review of Global Aquaculture. Nature 2021, 591, 551. [Google Scholar] [CrossRef] [PubMed]
- Hodar, A.; Vasava, R.; Mahavadiya, D.; Joshi, N. Fish meal and fish oil replacement for aqua feed formulation by using alternative sources: A review. J. Exp. Zool. India 2020, 23, 13–21. [Google Scholar]
- Hua, K.; Cobcroft, J.; Cole, A.; Condon, K.; Jerry, D.; Mangott, A.; Praeger, C.; Vucko, M.; Zeng, C.; Zenger, K.; et al. The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Tacon, A.; Webster, J.; Martinez, C. Use of solvent extracted sunflower seed meal in complete diets for fingerling rainbow trout (Salmo gairdneri Richardson). Aquaculture 1984, 43, 381–389. [Google Scholar] [CrossRef]
- Hughes, S.G. Use of lupin flour as a replacement for full-fat soy in diets for rainbow trout (Oncorhynchus mykiss). Aquaculture 1991, 93, 57–62. [Google Scholar] [CrossRef]
- Moyano, F.; Gardenete, G.; Higuera, M. Nutritive Value of Diets Containing a High Percentage of Vegetable Proteins for Trout, Oncorhynchus mykiss. Aquat. Living Resour. 1992, 5, 23–29. [Google Scholar] [CrossRef]
- Pongmaneerat, J.; Watanabe, T. Utilization of soybean meal as protein source in diets for rainbow trout. Agric. Food Sci. 1992, 58, 1983–1990. [Google Scholar] [CrossRef]
- Sanz, A.; Morales, A.; Higuera, M.; Cardenete, G. Sunflower meal compared with soybean meal as substitutes for fish meal in rainbow trout (Oncorhynchus mykiss) diets: Protein and energy utilization. Aquaculture 1994, 128, 287–300. [Google Scholar] [CrossRef]
- Morales, A.; Gardenete, G.; Higuera, M.; Sanz, A. Effects of dietary protein source on growth, feed conversation and energy utilization in rainbow trout, Oncorhynchus mykiss. Aquaculture 1994, 124, 117–126. [Google Scholar] [CrossRef]
- Gomes, E.; Rema, P.; Kaushik, S. Replacament of fish meal by plant proteins in the diet of rainbow trout (Oncorhynchus mykiss): Digestibility and growth performance. Aquaculture 1995, 130, 177–186. [Google Scholar] [CrossRef]
- Cheng, Z.; Hardy, R. Apparent digestibility coefficients and nutritional value of cottonseed meal for rainbow trout (Oncorhynchus mykiss). Aquaculture 2002, 212, 361–372. [Google Scholar] [CrossRef]
- Thiessen, D.; Campbell, G.; Tyler, R. Utilization of thin distillers’ solubles as a palatability enhancer in rainbow trout (Oncorhynchus mykiss) diets containing canola meal or air-classified pea protein. Aquac. Nutr. 2003, 9, 1–10. [Google Scholar] [CrossRef]
- Luo, L.; Xue, M.; Wu, X.; Cai, X.; Cao, H.; Liang, Y. Partial or total replacement of fishmeal by solvent-extracted cottonseed meal in diets for juvenile rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2006, 12, 418–424. [Google Scholar] [CrossRef]
- Appler, H.; Jauncey, K. The utilization of a filamentous green alga (Cladophora glomerata (L) Kutzin) as a protein source in pelleted feeds for Sarotherodon (Tilapia) niloticus fingerlings. Aquaculture 1983, 30, 21–30. [Google Scholar] [CrossRef]
- Appler, H.N. Evaluation of Hydrodictyon reticulatum as protein source in feeds for Oreochromis (Tilapia) niloticus and Tilapia zillii. J. Fish Biol. 1985, 27, 327–334. [Google Scholar] [CrossRef]
- Chow, C.; Woo, N. Bioenergetic studies on an omnivorous fish, Oreochromis mossambicus: Evaluation of utilization of Spirulina algae in feed. In The Second Asian Fisheries Forum; Hirano, R., Hanyu, I., Eds.; Asian Fisheries Society: Manila, Philippines, 1990; pp. 291–294. [Google Scholar]
- Kalla, A.; Yoshimatsu, T.; Araki, T.; Zhang, D.; Yamamoto, T.; Sakamoto, S. Use of Porphyra spheroplasts as feed additive for red sea bream. Fish. Sci. 2008, 74, 104–108. [Google Scholar] [CrossRef]
- Valente, L.; Gouveia, A.; Rema, P.; Matos, J.; Gomes, E.; Pinto, I. Evaluation of three seaweeds Gracilaria bursa-pastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 2006, 252, 85–91. [Google Scholar] [CrossRef]
- Tacon, A.; Rausin, N.; Kadari, M.; Cornelis, P. The food and feeding of marine finfish in floating net cages at the National Seafarming Development Centre, Lampung, Indonesia: Rabbitfish, Siganus canaliculatus (Park). Aquac. Fish. Manag. 1990, 21, 375–390. [Google Scholar] [CrossRef]
- Briggs, M.; Funge-Smith, S. The potential use of Gracilaria sp. meal in diets for juvenile Penaeus monodon Fabricius. Aquac. Res. 1996, 27, 345–354. [Google Scholar]
- Ansa, E.; Allotey, G.; Lubberding, H.; Ampofo, J.; Gijzen, H. Performance of a hybrid algal and duckweed pond system treating raw domestic wastewater. Ghana J. Sci. 2012, 52, 3–16. [Google Scholar]
- El-Sheekh, M.; Mahmoud, Y.; Abo-Shady, A.; Hamza, W. Efficacy of Rhodotorula glutinis and Spirulina platensis carotenoids in immunopotentiation of mice infected with Candida albicans SC5314 and Pseudomonas aeruginosa 35. Folia Microbiol. 2010, 55, 61–67. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae: Biotechnology and Microbiology; Cambridge Studies in Biotechnology, 10; Cambridge University Press: Cambridge, UK, 2008; pp. 124–146. [Google Scholar]
- Mendes, R.; Nobre, B.; Cardoso, M.; Pereira, A. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorganica Chim. Acta 2003, 356, 328–334. [Google Scholar] [CrossRef]
- Ahmad, J.; Fathurrahman, L.; Siti Hajar, A. Bath phytoremediation of aquaculture wastewater of Silver Barramundi (Lates calcarifer) utilizing green microalgae Chlorella sp. J. Fish. Aquat. Sci. 2013, 8, 516–525. [Google Scholar]
- Borowitzka, M.A. Biology of microalgae. In Microalgae in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2018; pp. 23–72. [Google Scholar]
- Saharan, V.; Jood, S. Nutritional composition of Spirulina platensis powder and its acceptability in food products. Int. J. Adv. Res. 2017, 5, 2295–2300. [Google Scholar] [CrossRef]
- Hussein, E. Effect of seaweed supplemented diets on Nile tilapia, Oreochromis niloticus performance. Int. J. Fish. Aquat. Stud. 2017, 5, 205–210. [Google Scholar]
- Nguyen, M.; Fotedar, R.; Pham, H. Can shrimp hydrolysate improve the efficacy of meat and bone meal diet in juvenile giant trevally Caranx ignobilis? Aquac. Int. 2023, 32, 1909–1926. [Google Scholar] [CrossRef]
- Hussein, E.; Dabrowski, K.; El-Saidy, D.; Lee, B. Enhancing the growth of Nile tilapia larvae/juveniles by replacing plant (gluten) protein with algae protein. Aquacult. Res. 2013, 44, 937–949. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Raji, A.; Jimoh, W.; Bakar, N.; Taufek, N.; Muin, H.; Alias, Z.; Milow, P.; Razak, S. Dietary use of Spirulina (Arthrospira) and Chlorella instead of fish meal on growth and digestibility of nutrients, amino acids and fatty acids by African catfish. J. Appl. Phycol. 2020, 32, 1763–1770. [Google Scholar] [CrossRef]
- Badwy, T.; Ibrahim, E.; Zeinhom, M. Partial replacement of fish meal with dried microalga (Chlorella spp. and Scenedesmus spp.) in Nile tilapia (Oreochromis niloticus) diets. In From the Pharaohs to the Future: Proceedings of the 8th International Symposium on Tilapia in Aquaculture; Egypt Ministry of Agriculture: Cairo, Egypt, 2008; pp. 801–810. [Google Scholar]
- Abdulrahman, N.; Ameen, H. Replacement of fishmeal with microalgae Spirulina on common carp weight gain, meat and sensitive composition and survival. Pak. J. Nutr. 2014, 13, 93–98. [Google Scholar] [CrossRef]
- Cherpokov, Y.; Sirakov, I.; Stoyanova, S.; Velichkova, K.; Simitchiev, A.; Nenov, V.; Slavov, T. The influence of Nu Pro® as a substitution of fish meal on the growth performance of rainbow trout (Oncorhynchus mykiss W.) cultivated in recirculating system. Bulg. J. Agric. Sci. 2020, 26, 223–231. [Google Scholar]
- Zhou, C.; Wu, H.; Tan, P.; Chi, Y.; Yang, H. Optimal dietary methionine requirement for juvenile cobia (Rachycentron canadum). Aquaculture 2006, 258, 551–557. [Google Scholar] [CrossRef]
- Van Hai, N. The use of medicinal plants as immunostimulants in aquaculture: A review. Aquaculture 2015, 446, 88–96. [Google Scholar] [CrossRef]
- Flores, G.; Hernández, L.; Araiza, M.; López, O. Effects of total replacement of fishmeal with spirulina powder and soybean meal on juvenile rainbow trout (Oncorhynchus mykiss Walbaum). Isr. J. Aquacul. Bamidgeh 2012, 64, 790. [Google Scholar]
- Xu, W.; Gao, Z.; Qi, Z.; Qiu, M.; Peng, J.; Shao, R. Effect of dietary Chlorella on the growth performance and physiological parameters of Gibel carp, Carassius auratus gibelio. Turk. J. Fish. Aquat. Sc. 2014, 14, 53–57. [Google Scholar]
- Cengic-Džomba, C.; Džomba, E.; Hadžic, D. An Overview of Using Algae Meal in Feeding Freshwater Fish Species. In 32nd Scientific-Expert Conference of Agriculture and Food Industry; Springer: Cham, Switzerland, 2023; pp. 171–182. [Google Scholar] [CrossRef]
- Raji, A.A.; Junaid, O.Q.; Milow, P.; Taufek, N.M.; Fada, A.M.; Kolawole, A.A.; Alias, Z.; Razak, S.A. Partial replacement of fishmeal with Spirulina platensis and Chlorella vulgaris and its effect on growth and body composition of African catfish Clarias gariepinus (Burchell 1822). Indian J. Fish. 2019, 66, 100–111. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Bhavan, P.; Seenivasan, C.; Muralisankar, T. Effect of dietary replacement of fishmeal with Chlorella vulgaris on growth performance, energy utilization and digestive enzymes in Macrobrachium rosenbergii postlarvae. Int. J. Fish. Aquac. 2016, 7, 62–70. [Google Scholar]
- Fadl, S.; ElGohary, M.; Elsadany, A.; Gad, D.; Hanaa, F.; El-Habashi, N. Contribution of microalgae-enriched fodder for the Nile tilapia to growth and resistance to infection with Aeromonas hydrophila. Algal Res. 2017, 27, 82–88. [Google Scholar] [CrossRef]
- Nandeesha, M.; Gangadhar, B.; Varghese, T.; Keshavanath, P. Effect of feeding Spirulina platensis on the growth, proximate composition and organoleptic quality of common carp. Cyprinus carpio L. Aquac. Res. 1998, 29, 305–312. [Google Scholar] [CrossRef]
- El-Sheekh, M.; El-Shourbagy, I.; Shalaby, S.; Hosny, S. Effect of feeding Arthrospira platensis (Spirulina) on growth and carcass composition of hybrid red tilapia (Oreochromis niloticus × Oreochromis mossambicus). Turk. J. Fish. Aquat. Sci. 2014, 14, 471–478. [Google Scholar] [CrossRef] [PubMed]
- James, R.; Sampath, K.; Thangarathinam, R.; Vasudevan, I. Effect of dietary spirulina level on growth, fertility, coloration and leucocyte count in red swordtail, Xiphophorus helleri. Isr. J. Aquac. Bamidgeh. 2006, 58, 97–104. [Google Scholar] [CrossRef]
- Khani, M.; Soltani, M.; Shamsaie Mehrjan, M.; Foroudi, F.; Ghaeni, M. The effects of Chlorella vulgaris supplementation on growth performance, blood characteristics and digestive enzymes in koi (Cyprinus carpio). Iran. J. Fish. Sci. 2017, 16, 832–843. [Google Scholar]
- Promya, J.; Chitmanat, C. The effects of Spirulina platensis and Cladophora Algae on the Growth Performance, Meat Quality and Immunity Stimulating Capacity of the African Sharptooth Catfish (Clarias gariepinus). Int. J. Agric. Biol. 2011, 13, 77–82. [Google Scholar]
- Rema, P.; Jorge, S.; Leite, F.; Basto, S. Efficacy of microalgae (Chlorella sp. and Spirulina sp.) and insect (Tenebrio molitor) meals as fishmeal replacers in feed for juvenile tench (Tinca tinca). Rev. Port. Zoot. 2021, 6, 37–48. [Google Scholar]
- Jobling, M. Nutrient partitioning and the influence of feed composition on body composition. In Food Intake in Fish; Houlihan, D., Boujard, T., Jobling, M., Eds.; Blackwell Science Ltd.: Oxford, UK, 2001; p. 414. [Google Scholar]
- Yılmaz, E.; Akyurt, İ.; Günal, G. Use of duckweed, Lemna minor, as a protein feedstuff in practical diets for common carp, Cyprinus carpio, fry. Turk. J. Fish. Aquat. Sci. 2004, 4, 18–25. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).