Contemporary Trends in the Spatial Extent of Common Riverine Fish Species in Australia’s Murray–Darling Basin
Abstract
1. Introduction
Aims of this Study
2. Materials and Methods
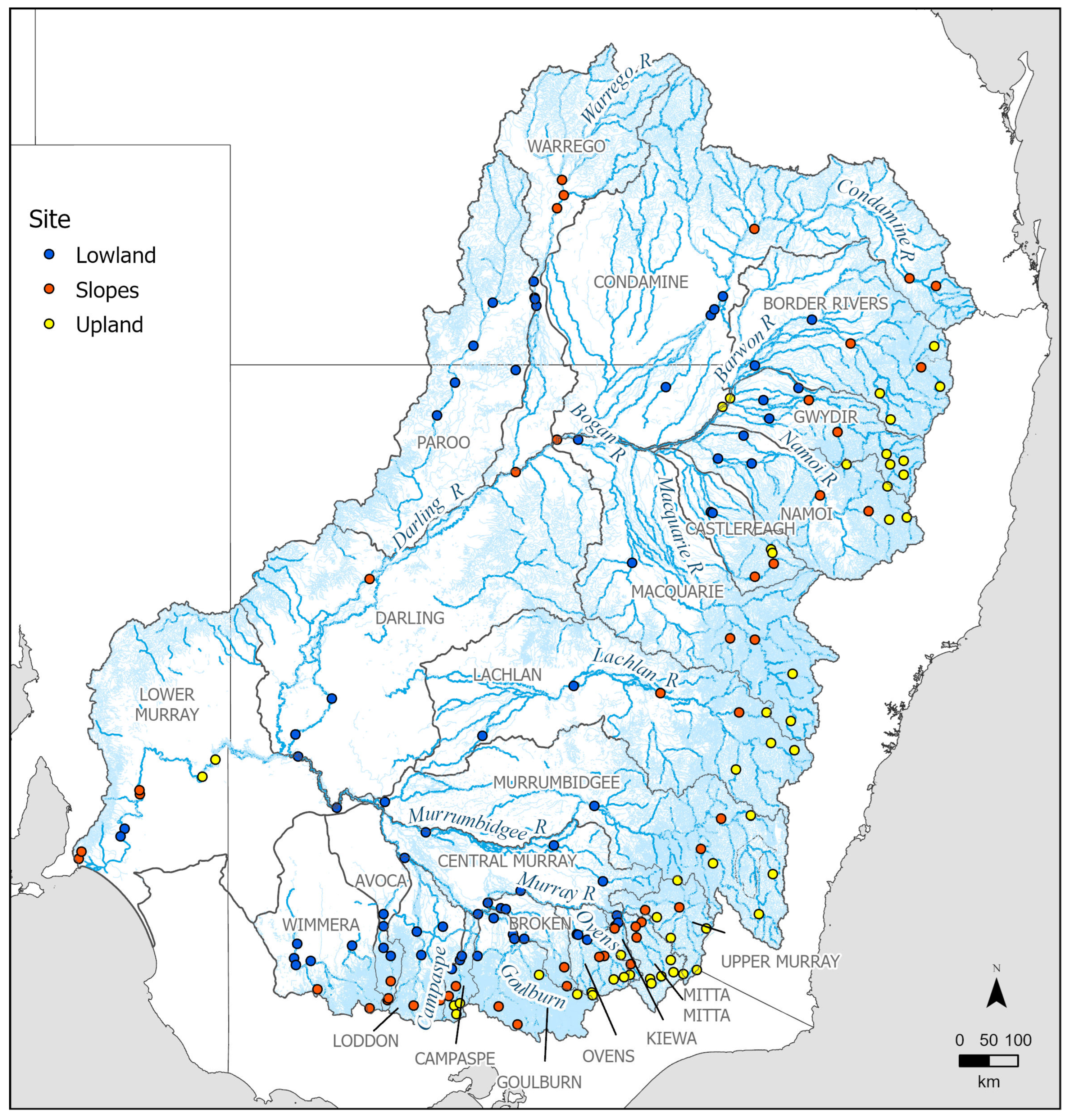
2.1. Study Area
2.2. Data Sets
- SRA (2004 to 2013) = every MDB valley and its sub-catchment was sampled once every three years with 14 to 28 sites per valley. Approximately 450 sites sampled per year;
- MDBFS (2014 to 2022) = every MDB valley and its sub-catchment was sampled once every year except 2019 and 2020, during which half of the MDB was sampled. Approximately 145 sites were sampled per year (except 2019 and 2020), with 4–8 sites per valley, and all sites were previously sampled SRA sites.
2.3. Spatial Extent of Common Fish in the MDB (2004 to 2022)
2.4. Statistical Analyses
3. Results
3.1. Common Fish Trends in Extent in the MDB (2004 to 2022)
3.2. Short-Lived Native Fish Species
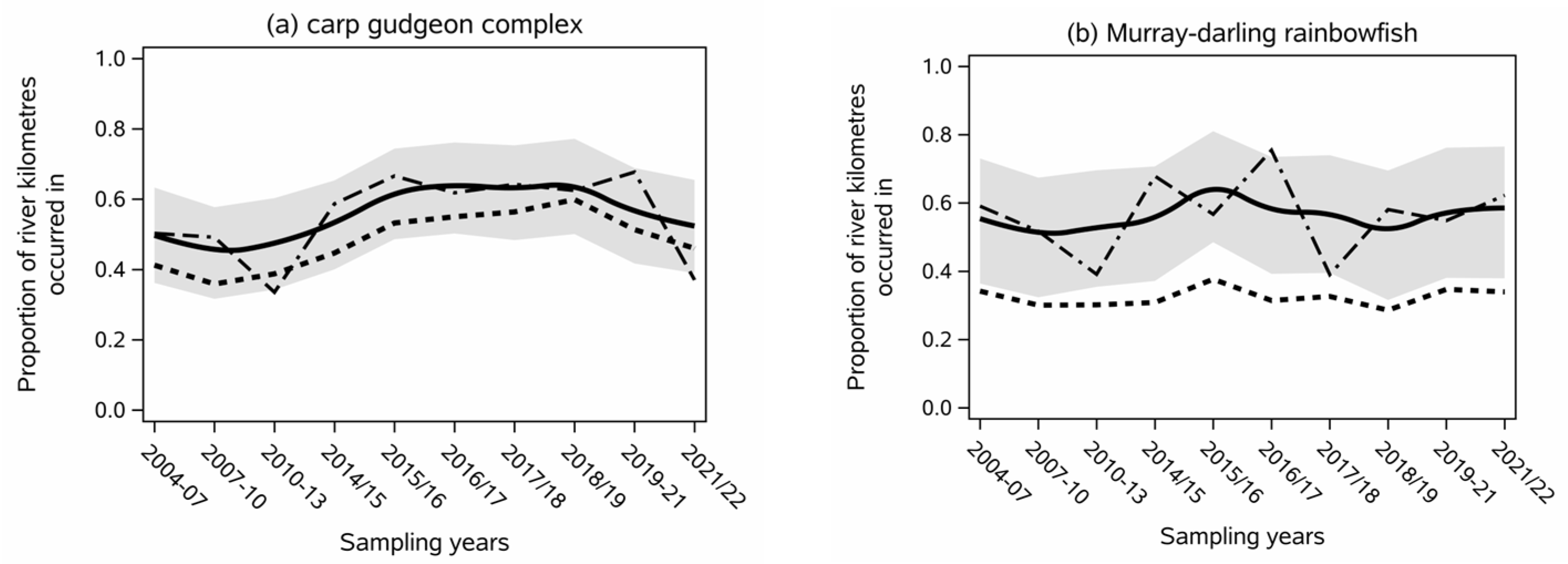
- Short-lived species all showed high inter-annual variability in their index of contemporary spatial extent (Figure 2).
- The carp gudgeon complex (Hypseleotris spp.) was estimated to occur in about 55% of its current distribution throughout the study, but the year-to-year variability in extent was high (range from 34% to 66%) (Figure 2a). The contemporary extent of carp gudgeons in 2010–2013 and 2020/21 was significantly lower compared to all sampling rounds from 2014 to 2020 (p < 0.01).
- Murray–Darling rainbowfish’s (Melanotaenia fluviatilis) contemporary spatial extent was also highly variable between rounds (39% to 75%), and no persistent trends were found. The largest contemporary extent for the species was 75% in 2016/17, which was significantly higher than the smallest extents of 2010–2013 and 2016/17 (p < 0.01) (Figure 2b).
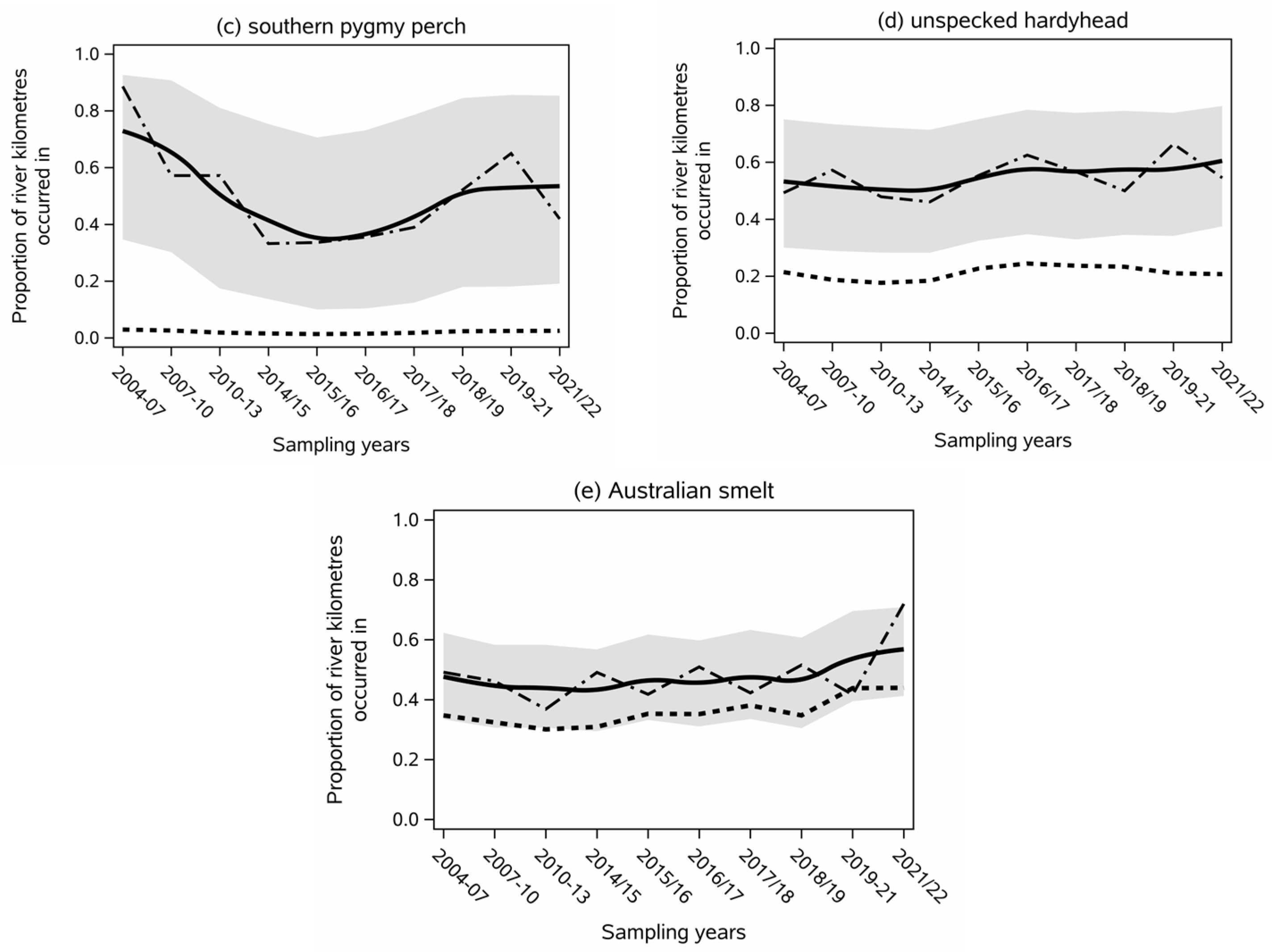
- Southern pygmy perch (Nannoperca australis) decreased from a three-round average spatial occurrence of 68% at the start of the data set to an average of 53% over the final three rounds (Figure 2c), but the trend was not statistically significant (p > 0.05). The lack of significance and low effect size for this species can be partially attributed to wider confidence intervals because of the low number of sub-catchments that it was collected in (Table 1).
- Unspecked hardyhead (Craterocephalus stercusmuscarum fulvus) was generally less variable than the other short-lived species between years (46% to 66%) and averaged a contemporary extent of 55% for the study period. The effect size was non-estimable.
- Australian smelt’s (Retropinna semoni) contemporary spatial extent was between 37% and 52% during the first nine sampling rounds but increased to 72% in 2021/22, which was significantly (p < 0.01) greater than in 2007–2013, 2017/18, or 2019–21 (Figure 2e).
3.3. Intermediate-Lived Native Fish Species
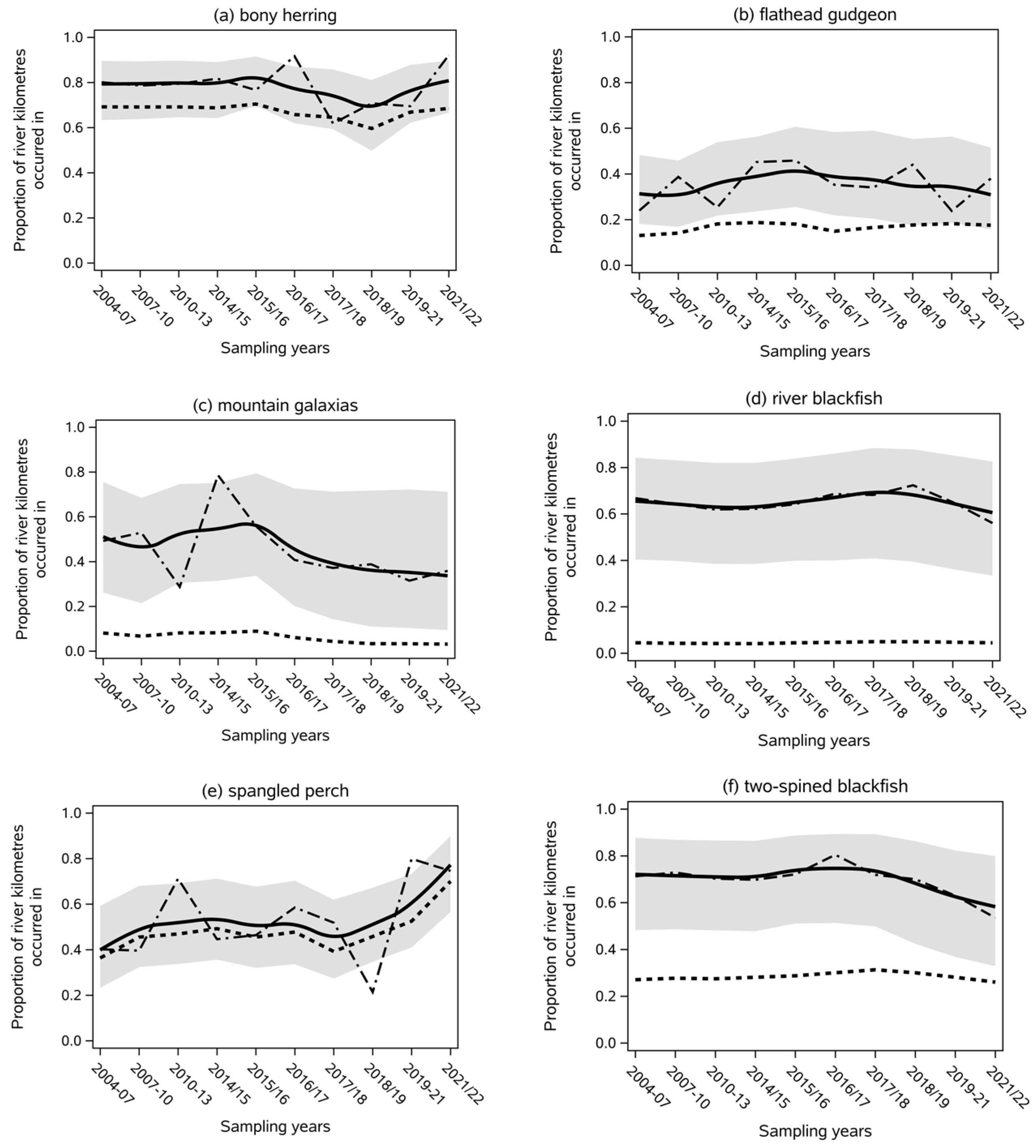
- Bony herring (Nematalosa erebi) had the highest contemporary spatial extent index of all native species and varied between 62 and 92% between sampling rounds (Figure 3a). The index achieved 92% in 2016/17 and 2021/22, which was significantly higher (p < 0.01) than the lower scores in 2017/18 to 2020/21 (Figure 3a).
- Flathead gudgeon (Philypnodon grandiceps) was very consistent in estimated contemporary spatial extent, averaging 35% throughout time (Figure 3b).
- Mountain galaxias averaged 45% and its peak extent of 78% in 2014/15 was significantly (p < 0.01) greater than that from 2010 to 2013 and in 2021/22 (Figure 3c).
- Northern river blackfish (Gadopsis marmarata) (average 65%) underwent slight but non-significant declines in their current extent in the last few years of the data (Figure 3d).
- Spangled perch (Leiopotherapon unicolor) was highly variable, occurring in between 21 and 80% of contemporary river kilometres in the study (Figure 3e). It had a significantly lower extent in 2004–2010 and 2018/19 than in 2019–2020 (p < 0.01) or 2010–2013 and 2021/2022 (p < 0.02).
- Two-spined blackfish (Gadopsis bispinosa) was also relatively consistent in contemporary extent, estimated to occur in ~70% of contemporary river kilometres throughout the study (Figure 3d). It also underwent slight but non-significant declines in its current extent in the last four rounds of the study (Figure 3d).
3.4. Long-Lived Native Fish Species
- Freshwater catfish (Tandanus tandanus) averaged 29% for the extent index throughout the study, with occasional dips to 17% and highs up to 51% (Figure 4a). The high variability between year-to-year estimates of spatial extent was high and the long-term average was low; consequently, the effect size for this species is more than 100% of the mean (Table 1).
- The contemporary spatial extent for golden perch averaged 68% and there was a visible but not significant overall increase in extent throughout the study (Figure 4b). The extent in 2016/17 (80%) and 2021/22 (84%) was significantly higher (p < 0.01) than in 2004–2007 (50%), 2007–2010 (61%), and 2017/18 (57%) (Figure 4b).
- Silver perch (Bidyanus bidyanus) was consistently low in river kilometres in which it was collected (average 19%) and had significantly low contemporary spatial extent from 2019 to 2021 (1%) compared to 2010–2013 (34%) and 2021/22 (32%) (Figure 4d).
3.5. Non-Native Species
- All of the non-native species had consistent extent distribution throughout the study, as indicated by narrow confidence intervals and smooth trend lines (Figure 5).
- Common carp was the most collected non-native species in the data set and was estimated to occur in between 74% and 90% of river kilometres throughout the study period (Figure 5b). Carp showed a consistent but non-significant (p > 0.05) increasing trend in extent throughout the monitoring period. Nevertheless, the final sampling round in 2021/2022 (90%) was significantly higher (p < 0.01) than the sampling rounds in 2004–2007, 2007–2010, and 2014/15 (Figure 5b).
- Eastern gambusia (Gambusia holbrooki) was detected in 60 of the 68 sub-catchments (Table 1), but it was rarely detected in more than 50% of its river kilometres in any sampling round and always between 35% and 62% of total river kilometres (Figure 5c). It was significantly higher (p < 0.01) in 2015/16 and 2016/17 than from 2004 to 2013 and in 2018/19 (Figure 5c).
- Redfin perch (Perca fluviatilis) (max 19%) and both brown and rainbow trout (Salmo trutta and Oncorhynchus mykiss) (<9%) generally occurred in low MDB river kilometres throughout the study period (Figure 5a,e,f). Redfin perch had a significantly lower spatial extent in 2014/15 than in the first and third sampling rounds (Figure 5f).
3.6. Species Summary of Baseline Assessments and Comparisons
4. Discussion
4.1. Summary
4.2. Species Trends
4.3. Surveillance Monitoring Returns Coarse Assessments
4.4. Factors That Can Influence Population Extent at a Larger Scale
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grill, G.; Lehner, B.; Thieme, M.; Geenen, B.; Tickner, D.; Antonelli, F.; Babu, S.; Borrelli, P.; Cheng, L.; Crochetiere, H.; et al. Mapping the world’s free-flowing rivers. Nature 2019, 569, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Reidy, C.A.; Dynesius, M.; Revenga, C. Fragmentation and flow regulation of the world’s large river systems. Science 2005, 308, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, P.C.; Brown, P.; Schiller, C.B.; Moffatt, D.B.; Bruce, A.M. River regulation and fish communities in the Murray-Darling River system, Australia. Regul. Rivers Res. Manag. 1995, 11, 363–375. [Google Scholar] [CrossRef]
- Davies, P.E.; Harris, J.H.; Hillman, T.; Walker, K.F. The Sustainable Rivers Audit: Assessing river ecosystem health in the Murray-Darling Basin, Australia. Mar. Freshw. Res. 2010, 61, 764–777. [Google Scholar] [CrossRef]
- Davies, P.E.; Harris, J.H.; Hillman, T.J.; Walker, K.F. SRA Report 1: A Report on the Ecological Health of Rivers in the Murray–Darling Basin, 2004–2007; Prepared by the Independent Sustainable Rivers Audit Group for the Murray–Darling Basin Ministerial Council; Murray–Darling Basin Commission: Canberra, Australian, 2008.
- Walker, K.F.; Sheldon, F.; Puckridge, J.T. A perspective on dryland river ecosystems. Regul. Rivers Res. Manag. 1995, 11, 85–104. [Google Scholar] [CrossRef]
- Kingsford, R.T. Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia. Austral Ecol. 2000, 25, 109–127. [Google Scholar] [CrossRef]
- Lester, R.E.; Webster, I.T.; Fairweather, P.G.; Young, W.J. Linking water-resource models to ecosystem-response models to guide water-resource planning—An example from the Murray–Darling Basin, Australia. Mar. Freshw. Res. 2011, 62, 279–289. [Google Scholar] [CrossRef]
- CSIRO. Water Availability in the Murray-Darling Basin: A Report from CSIRO to the Australian Government; CSIRO: Canberra, Australian, 2008.
- Whetton, P.; Chiew, F. Chapter 12—Climate change in the Murray–Darling Basin. In Ecohydrology from Catchment to Coast, Murray-Darling Basin, Australia; Hart, B.T., Bond, N.R., Byron, N., Pollino, C.A., Stewardson, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 1, pp. 253–274. [Google Scholar]
- Prosser, I.P.; Chiew, F.H.S.; Stafford Smith, M. Adapting water management to climate change in the Murray–Darling Basin, Australia. Water 2021, 13, 2504. [Google Scholar] [CrossRef]
- Sheldon, F.; Barma, D.; Baumgartner, L.J.; Bond, N.; Mitrovic, S.M.; Vertessy, R. Assessment of the causes and solutions to the significant 2018–19 fish deaths in the Lower Darling River, New South Wales, Australia. Mar. Freshw. Res. 2022, 73, 147–158. [Google Scholar] [CrossRef]
- Legge, S.; Woinarski, J.C.Z.; Scheele, B.C.; Garnett, S.T.; Lintermans, M.; Nimmo, D.G.; Whiterod, N.S.; Southwell, D.M.; Ehmke, G.; Buchan, A.; et al. Rapid assessment of the biodiversity impacts of the 2019–2020 Australian megafires to guide urgent management intervention and recovery and lessons for other regions. Divers. Distrib. 2022, 28, 571–591. [Google Scholar] [CrossRef]
- Rumpff, L.; Legge, S.M.; van Leeuwen, S.; Wintle, B.A.; Woinarski, J.C.Z. Australia’s Megafires: Biodiversity Impacts and Lessons from 2019–2020; CSIRO Publishing: Clayton, Australia, 2023; p. 512. [Google Scholar]
- Koehn, J.D.; Lintermans, M.; Copeland, C. Laying the foundations for fish recovery: The first 10 years of the Native Fish Strategy for the Murray-Darling Basin, Australia. Ecol. Manag. Restor. 2014, 15, 3–12. [Google Scholar] [CrossRef]
- Koehn, J.D.; Lintermans, M. A strategy to rehabilitate fishes of the Murray-Darling Basin, south-eastern Australia. Endanger. Species Res. 2012, 16, 165–181. [Google Scholar] [CrossRef]
- Murray-Darling Basin Authority. The Water Act 2007—Basin Plan 2012; Murray-Darling Basin Authority: Canberra, Australia, 2012.
- Barrett, J.; Lintermans, M.; Broadhurst, B. Key Outcomes of Native Fish Strategy Research: Technical Report; Institute for Applied Ecology, University of Canberra: Canberra, Australia, 2013. [Google Scholar]
- Robinson, W.A.; Lintermans, M.; Harris, J.H.; Guarino, F. A Landscape-Scale Electrofishing Monitoring Program Can Evaluate Fish Responses to Climatic Conditions in the Murray–Darling River System, Australia; American Fisheries Society Symposium; American Fisheries Society: Bethesda, MD, USA, 2019; Volume 90, pp. 179–201. [Google Scholar]
- Crook, D.A.; Schilling, H.T.; Gilligan, D.M.; Asmus, M.; Boys, C.A.; Butler, G.L.; Cameron, L.M.; Hohnberg, D.; Michie, L.E.; Miles, N.G.; et al. Multi-decadal trends in large-bodied fish populations in the New South Wales Murray–Darling Basin, Australia. Mar. Freshw. Res. 2023, 74, 899–916. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List Categories and Criteria, 2nd ed.; IUCN: Cambridge, UK, 2012; p. 32. [Google Scholar]
- Lintermans, M. Fishes of the Murray–Darling Basin: An Introductory Guide, 2nd ed.; Australian River Restoration Centre, Canberra: Canberra, Australia, 2023. [Google Scholar]
- Kaminskas, S. Alien pathogens and parasites impacting native freshwater fish of southern Australia: A scientific and historical review. Aust. Zool. 2021, 41, 696–730. [Google Scholar] [CrossRef]
- Britton, J.R. Contemporary perspectives on the ecological impacts of invasive freshwater fishes. J. Fish Biol. 2023, 103, 752–764. [Google Scholar] [CrossRef] [PubMed]
- Feio, M.J.; Silva, J.P.; Hughes, R.M.; Aguiar, F.C.; Alves, C.B.M.; Birk, S.; Callisto, M.; Linares, M.; Macedo, D.R.; Pompeu, P.S.; et al. The impacts of non-native species on river bioassessment. In Proceedings of the XXII Congress of the Iberian Association of Limnology, Vigo, Spain, 23–28 June 2024. [Google Scholar]
- MDBA. Delivering a Healthy Working Basin; About the Draft Basin Plan; Murray–Darling Basin Authority: Canberra, Australia, 2011.
- MDBA. Guide to the Proposed Basin Plan: Overview; Murray-Darling Basin Authority: Canberra, Australia, 2010; p. 223.
- Koehn, J.D. Managing people, water, food and fish in the Murray–Darling Basin, south-eastern Australia. Fish. Manag. Ecol. 2015, 22, 25–32. [Google Scholar] [CrossRef]
- Lesser, V.M.; Kalsbreek, W.D. A comparison of periodic survey designs employing multi-stage sampling. Environ. Ecol. Stat. 1997, 4, 117–130. [Google Scholar] [CrossRef]
- Lintermans, M.; Robinson, W.; Harris, J.H. Identifying the health of fish communities in the Murray-Darling Basin: The Sustainable Rivers Audit. In Proceedings of the 4th Australian Stream Management Conference: Linking Rivers to Landscapes, Launceston, Australia, 19–22 October 2004; p. 6. [Google Scholar]
- Lyon, J.P.; Bird, T.; Nicol, S.; Kearns, J.; O’Mahony, J.; Todd, C.R.; Cowx, I.G.; Bradshaw, C.J.A. Efficiency of electrofishing in turbid lowland rivers: Implications for measuring temporal change in fish populations. Can. J. Fish. Aquat. Sci. 2014, 71, 878–886. [Google Scholar] [CrossRef]
- Lyon, J.; Stuart, I.; Ramsey, D.; O’Mahony, J. The effect of water level on lateral movements of fish between river and off-channel habitats and implications for management. Mar. Freshw. Res. 2010, 61, 271–278. [Google Scholar] [CrossRef]
- Lintermans, M. Finding the needle in the haystack: Comparing sampling methods for detecting an endangered freshwater fish. Mar. Freshw. Res. 2016, 67, 1740–1749. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT®14.1, v.14.1; SAS Institute: Cary, NC, USA, 2015. [Google Scholar]
- Schmidt, D.J.; Bond, N.R.; Adams, M.; Hughes, J.M. Cytonuclear evidence for hybridogenetic reproduction in natural populations of the Australian carp gudgeon (Hypseleotris: Eleotridae). Mol. Ecol. 2011, 20, 3367–3380. [Google Scholar] [CrossRef] [PubMed]
- Thacker, C.E.; Geiger, D.L.; Unmack, P.J. Species delineation and systematics of a hemiclonal hybrid complex in Australian freshwaters (Gobiiformes: Gobioidei: Eleotridae: Hypseleotris). R. Soc. Open Sci. 2022, 9, 220201. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.H.; Gehrke, P.C. (Eds.) Fish and Rivers in Stress: The NSW Rivers Survey; NSW Fisheries Office of Conservation and CRC for freshwater Ecology: Sydney, Australia, 1997; p. 298.
- Dunn, C.G.; Paukert, C.P. A flexible survey design for monitoring spatiotemporal fish richness in nonwadeable rivers: Optimizing efficiency by integrating gears. Can. J. Fish. Aquat. Sci. 2020, 77, 978–990. [Google Scholar] [CrossRef]
- Lintermans, M.; Geyle, H.M.; Beatty, S.; Brown, C.; Ebner, B.C.; Freeman, R.; Hammer, M.P.; Humphreys, W.F.; Kennard, M.J.; Kern, P.; et al. Big trouble for little fish: Identifying Australian freshwater fishes in imminent risk of extinction. Pac. Conserv. Biol. 2020, 26, 365–377. [Google Scholar] [CrossRef]
- Lintermans, M. Recolonization by the mountain galaxias Galaxias olidus of a montane stream after the eradication of rainbow trout Oncorhynchus mykiss. Mar. Freshw. Res. 2000, 51, 799–804. [Google Scholar] [CrossRef]
- Balcombe, S.R.; Sheldon, F.; Capon, S.J.; Bond, N.R.; Hadwen, W.L.; Marsh, N.; Bernays, S.J. Climate-change threats to native fish in degraded rivers and floodplains of the Murray–Darling Basin, Australia. Mar. Freshw. Res. 2011, 62, 1099–1114. [Google Scholar] [CrossRef]
- Ellis, I.; Whiterod, N.; Linklater, D.; Bogenhuber, D.; Brown, P.; Gilligan, D. Spangled perch (Leiopotherapon unicolor) in the southern Murray-Darling Basin: Flood dispersal and short-term persistence outside its core range. Austral Ecol. 2015, 40, 591–600. [Google Scholar] [CrossRef]
- Sanger, A. Description of a new species of Gadopsis (Pisces: Gadopsidae) from Victoria. Proc. R. Soc. Vic. 1984, 96, 93–97. [Google Scholar]
- Unmack, P.J.; Sandoval-Castillo, J.; Hammer, M.P.; Adams, M.; Raadik, T.A.; Beheregaray, L.B. Genome-wide SNPs resolve a key conflict between sequence and allozyme data to confirm another threatened candidate species of river blackfishes (Teleostei: Percichthyidae: Gadopsis). Mol. Phylogenetics Evol. 2017, 109, 415–420. [Google Scholar] [CrossRef]
- Hammer, M.P.; Unmack, P.J.; Adams, M.; Raadik, T.A.; Johnson, J.B. A multigene molecular assessment of cryptic biodiversity in the iconic freshwater blackfishes (Teleostei: Percichthyidae: Gadopsis) of south-eastern Australia. Biol. J. Linn. Soc. 2014, 111, 521–540. [Google Scholar] [CrossRef]
- Forbes, J.; Watts, R.J.; Robinson, W.; Baumgartner, L.J.; McGuffie, P.; Cameron, L.M.; Crook, D. Assessment of stocking effectiveness for Murray cod (Maccullochella peelii) and golden perch (Macquaria ambigua) in rivers and impoundments of south eastern Australia. Mar. Freshw. Res. 2015, 67, 1410–1419. [Google Scholar] [CrossRef]
- Koehn, J.D.; Raymond, S.M.; Stuart, I.; Todd, C.R.; Balcombe, S.R.; Zampatti, B.P.; Bamford, H.; Ingram, B.A.; Bice, C.M.; Burndred, K.; et al. A compendium of ecological knowledge for restoration of freshwater fishes in Australia’s Murray–Darling Basin. Mar. Freshw. Res. 2020, 71, 1391–1463. [Google Scholar] [CrossRef]
- Yen, J.D.L.; Thomson, J.R.; Lyon, J.P.; Koster, W.M.; Kitchingman, A.; Raymond, S.; Stamation, K.; Tonkin, Z. Underlying trends confound estimates of fish population responses to river discharge. Freshw. Biol. 2021, 99, 1799–1812. [Google Scholar] [CrossRef]
- Poomchaivej, T.; Robinson, W.; Ning, N.; Baumgartner, L.J.; Huang, X. Suitability of tropical river fishes for PIT tagging: Results for four Lower Mekong species. Fish. Res. 2024, 272, 106930. [Google Scholar] [CrossRef]
- Tonkin, Z.; King, A.; Mahoney, J.; Morrongiello, J. Diel and spatial drifting patterns of silver perch Bidyanus bidyanus eggs in an Australian lowland river. J. Fish Biol. 2007, 70, 313–317. [Google Scholar] [CrossRef]
- TSSC. Conservation Advice Bidyanus bidyanus (silver perch). Threatened Species Scientific Committee, Environment Australia. 2013. Available online: https://www.environment.gov.au/biodiversity/threatened/species/pubs/76155-conservation-advice.pdf (accessed on 6 May 2024).
- Koehn, J.D. Carp (Cyprinus carpio) as a powerful invader in Australian waterways. Freshw. Biol. 2004, 49, 882–894. [Google Scholar] [CrossRef]
- Todd, C.R.; Koehn, J.D.; Stuart, I.G.; Wootton, H.F.; Zampatti, B.P.; Thwaites, L.; Conallin, A.; Ye, Q.; Stamation, K.; Bice, C. Balancing beneficial environmental flows in rivers with controlling invasive common carp: A modelling approach to assessing flow-release strategies. Biol. Invasions 2024, 26, 1437–1456. [Google Scholar] [CrossRef]
- Chervinski, J. Salinity tolerance of the mosquito fish, Gambusia affinis (Baird and Girard). J. Fish Biol. 2006, 22, 9–11. [Google Scholar] [CrossRef]
- El-Boray, K. Tolerance of mosquitofish Gambusia holbrooki (Girard, 1859) to temperature, salinity and pH. Aljouf Sci. Eng. J. 2014, 1, 10–14. [Google Scholar] [CrossRef]
- Pyke, G.H. Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 171–191. [Google Scholar] [CrossRef]
- Arthington, A.H.; Balcombe, S.R. Extreme flow variability and the ‘boom and bust’ ecology of fish in arid-zone floodplain rivers: A case history with implications for environmental flows, conservation and management. Ecohydrology 2011, 4, 708–720. [Google Scholar] [CrossRef]
- O’Connor, J.P.; O’Mahony, D.J.; O’Mahony, J.M. Movements of Macquaria ambigua, in the Murray River, south-eastern Australia. J. Fish Biol. 2005, 66, 392–403. [Google Scholar] [CrossRef]
- van Dijk, A.I.J.M.; Beck, H.E.; Crosbie, R.S.; de Jeu, R.A.M.; Liu, Y.Y.; Podger, G.M.; Timbal, B.; Viney, N.R. The millennium drought in southeast Australia (2001–2009): Natural and human causes and implications for water resources, ecosystems, economy, and society. Water Resour. Res. 2013, 49, 1040–1057. [Google Scholar] [CrossRef]
- Lennox, R.J.; Paukert, C.P.; Aarestrup, K.; Auger-Méthé, M.; Baumgartner, L.; Birnie-Gauvin, K.; Bøe, K.; Brink, K.; Brownscombe, J.W.; Chen, Y.; et al. One hundred pressing questions on the future of global fish migration science, conservation, and policy. Front. Ecol. Evol. 2019, 7, 286. [Google Scholar] [CrossRef]
- Morrongiello, J.R.; Beatty, S.J.; Bennett, J.C.; Crook, D.A.; Ikedife, D.N.E.N.; Kennard, M.J.; Kerezsy, A.; Lintermans, M.; McNeil, D.G.; Pusey, B.J.; et al. Climate change and its implications for Australia’s freshwater fish. Mar. Freshw. Res. 2011, 62, 1082–1098. [Google Scholar] [CrossRef]
- Koehn, J.D.; Lintermans, M.; Lyon, J.P.; Ingram, B.A.; Gilligan, D.M.; Todd, C.R.; Douglas, J.W. Recovery of the endangered trout cod, Maccullochella macquariensis: What have we achieved in more than 25 years? Mar. Freshw. Res. 2013, 64, 822–837. [Google Scholar] [CrossRef]
- Baumgartner, L.; Zampatti, B.; Jones, M.; Stuart, I.; Mallen-Cooper, M. Fish passage in the Murray-Darling Basin, Australia: Not just an upstream battle. Ecol. Manag. Restor. 2014, 15, 28–39. [Google Scholar] [CrossRef]
- Ebner, B.C.; Thiem, J.D.; Gilligan, D.M.; Lintermans, M.; Wooden, I.J.; Linke, S. Estimating species richness and catch per unit effort from boat electro-fishing in a lowland river in temperate Australia. Austral Ecol. 2008, 33, 891–901. [Google Scholar] [CrossRef]
- Crook, D.A.; O’Mahony, D.J.; Gillanders, B.M.; Munro, A.R.; Sanger, A.C.; Thurstan, S.; Baumgartner, L.J. Contribution of stocked fish to riverine populations of golden perch (Macquaria ambigua) in the Murray–Darling Basin, Australia. Mar. Freshw. Res. 2016, 67, 1401–1409. [Google Scholar] [CrossRef]
- Zampatti, B.; Leigh, S. Effects of flooding on recruitment and abundance of golden perch (Macquaria ambigua ambigua) in the lower River Murray. Ecol. Manag. Restor. 2013, 14, 135–143. [Google Scholar] [CrossRef]
- MDBC. Native Fish Strategy. 2006–2007 Annual Implementation Report; Murray-Darling Basin Commission: Canberra, Australia, 2008; p. 52.
- Barrett, J. Introducing the Murray-Darling Basin Native Fish Strategy and initial steps towards demonstration reaches. Ecol. Manag. Restor. 2004, 5, 15–23. [Google Scholar] [CrossRef]
- Manchester, S.J.; Bullock, J.M. The impacts of non-native species on UK biodiversity and the effectiveness of control. J. Appl. Ecol. 2000, 37, 845–864. [Google Scholar] [CrossRef]
- Zalewski, M.; Cowx, I.G. Factors affecting the efficiency of electrofishing. In Fishing with Electricity; Cowx, I.G., Lamarque, P., Eds.; Blackwell Science: London, UK, 1990; pp. 89–111. [Google Scholar]
- Penczak, T.; Głowacki, Ł. Evaluation of electrofishing efficiency in a stream under natural and regulated conditions. Aquat. Living Resour. 2008, 21, 329–337. [Google Scholar] [CrossRef]
- Reid, D.; Harris, J.H. New South Wales Inland Fisheries Data Analysis; Fisheries Research and Development Corporation: Canberra, Australia, 1997; p. 64. [Google Scholar]
- Dakin, W.J.; Kesteven, G.L. The Murray Cod (Maccullochella Macquariensis (Cuv. et Val.)) Bulletin of New South Wales State Fisheries; Department of Fisheries: Nelson Bay, Australia, 1938; pp. 1–18.
- Humphries, P.; Winemiller, K.O. Historical impacts on river fauna, shifting baselines, and challenges for restoration. BioScience 2009, 59, 673–684. [Google Scholar] [CrossRef]
- Stein, E.D.; Bernstein, B. Integrating probabalistic and targeted compliance monitoring for comprehensive watershed assessment. Environ. Monit. Assess. 2008, 144, 117–129. [Google Scholar] [CrossRef]
- Todd, C.; Koehn, J.; Pearce, L.; Dodd, L.; Humphries, P.; Morrongiello, J. Forgotten fishes: What is the future for small threatened freshwater fish? Population risk assessment for southern pygmy perch, Nannoperca australis. Aquat. Conserv. Mar. Freshw. Ecosyst. 2017, 27, 1290–1300. [Google Scholar] [CrossRef]
- Arthington, A.; McKay, R.; Russell, D.; Milton, D. Occurence of the introduced cichlid Oreochromis mossambicus (Peters) in Queensland. Mar. Freshw. Res. 1984, 35, 267–272. [Google Scholar] [CrossRef]


| Species | Common Name | Origin | Life Guild | Num. Sub-Catchments Collected | Num. Sub-Catchments Expected | Index Effect Size | Index % Change |
|---|---|---|---|---|---|---|---|
| Gambusia holbrooki | eastern gambusia | Non-native | Short-lived | 60 | 0 | 0.17 | 36% |
| Cyprinus carpio | common carp | Non-native | Long-lived | 54 | 0 | 0.09 | 11% |
| Retropinna semoni | Australian smelt | Native | Short-lived | 51 | 66 | 0.23 | 48% |
| Carassius auratus | goldfish | Non-native | Long-lived | 52 | 0 | 0.17 | 45% |
| Hypseleotris spp. | carp gudgeon complex | Native | Short-lived | 47 | 56 | 0.17 | 31% |
| Macquaria ambigua | golden perch | Native | Long-lived | 49 | 66 | 0.16 | 24% |
| Perca fluviatilis | redfin perch | Non-native | Long-lived | 44 | 0 | 0.08 | 61% |
| Maccullochella peelii | Murray cod | Native | Long-lived | 43 | 63 | 0.19 | 39% |
| Nematalosa erebi | bony herring | Native | Intermediate-lived | 29 | 49 | 0.15 | 19% |
| Philypnodon grandiceps | flathead gudgeon | Native | Intermediate-lived | 22 | 30 | 0.24 | 68% |
| Tandanus tandanus | freshwater catfish | Native | Long-lived | 23 | 50 | 0.32 | >100% |
| Galaxias olidus | mountain galaxias | Native | Intermediate-lived | 26 | 51 | 0.4 | 89% |
| Salmo trutta | brown trout | Non-native | Long-lived | 27 | 0 | 0.05 | 75% |
| Oncorhynchus mykiss | rainbow trout | Non-native | Intermediate-lived | 26 | 0 | 0.05 | 94% |
| Gadopsis marmarata | northern river blackfish | Native | Intermediate-lived | 20 | 53 | 0.18 | 28% |
| Melanotaenia fluviatilis | Murray–Darling rainbowfish | Native | Short-lived | 23 | 46 | 0.26 | 46% |
| Leiopotherapon unicolor | spangled perch | Native | Intermediate-lived | 23 | 33 | 0.28 | 53% |
| Bidyanus bidyanus | silver perch | Native | Long-lived | 21 | 51 | 0.26 | >100% |
| Craterocephalus stercusmuscarum fulvus | unspecked hardyhead | Native | Short-lived | 20 | 40 | na | na |
| Galaxias oliros * | obscure galaxias | Native | Intermediate-lived | 21 | 41 | na | na |
| Gadopsis bispinosa | two-spined blackfish | Native | Intermediate-lived | 18 | 22 | 0.3 | 43% |
| Nannoperca australis | southern pygmy perch | Native | Short-lived | 14 | 36 | na | na |
| Species | Confidence in Estimate | Average Extent (%) | Interpretation of Extent |
|---|---|---|---|
| Native Species—relative to pre-1980 distribution | |||
| Short-lived | |||
| unspecked hardyhead | Medium | 21 | Substantially declined in riverine habitats. |
| carp gudgeon complex | Medium | 48 | Widespread and abundant. |
| Murray–Darling rainbowfish | High | 33 | Has declined and is now patchily distributed. |
| southern pygmy perch | Low | 2 | Rare in main channel riverine habitats when using electrofishing. |
| Australian smelt | Medium | 36 | Widespread and abundant in lowland habitats. |
| Intermediate-lived | |||
| two-spined blackfish | High | 28 | Declined and fragmented distribution. |
| northern river blackfish | Low | 5 | Declined significantly in larger streams but assessment confounded by historic taxonomy. |
| mountain galaxias | Medium | 6 | Greatly reduced, especially in lowland streams or where trout are present. |
| spangled perch | Medium | 48 | Widespread in Northern Basin but penetrate Southern Basin rarely. |
| bony herring | High | 68 | Widespread and abundant. |
| flathead gudgeon | Low | 17 | Poorly sampled in riverine habitats by using electrofishing. |
| Long-lived | |||
| Murray cod | High | 35 | Has declined but widely distributed. Stocked |
| golden perch | High | 56 | Remain widespread in lowlands. Declined in uplands. Stocked. |
| freshwater catfish | Medium | 10 | Substantially declined in riverine habitats. |
| silver perch | Medium | 5 | Substantially declined in riverine habitats. Stocked |
| Non-Native Species—relative to all MDB riverine habitats | |||
| Short-lived | |||
| eastern gambusia | Medium | 48 | Successful invader. Widely distributed. |
| Intermediate-lived | |||
| goldfish | High | 38 | Successful invader in lowland rivers and some slope regions. |
| rainbow trout | High | 5 | Distributed widely in cool upland streams. Stocked. |
| Long-lived | |||
| common carp | High | 81 | Highly successful and widely distributed. |
| redfin perch | High | 13 | Absent from warmer waters in Northern MDB. |
| brown trout | High | 7 | Widely distributed but restricted to cool upland streams. Stocked. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, W.; Koehn, J.; Lintermans, M. Contemporary Trends in the Spatial Extent of Common Riverine Fish Species in Australia’s Murray–Darling Basin. Fishes 2024, 9, 221. https://doi.org/10.3390/fishes9060221
Robinson W, Koehn J, Lintermans M. Contemporary Trends in the Spatial Extent of Common Riverine Fish Species in Australia’s Murray–Darling Basin. Fishes. 2024; 9(6):221. https://doi.org/10.3390/fishes9060221
Chicago/Turabian StyleRobinson, Wayne, John Koehn, and Mark Lintermans. 2024. "Contemporary Trends in the Spatial Extent of Common Riverine Fish Species in Australia’s Murray–Darling Basin" Fishes 9, no. 6: 221. https://doi.org/10.3390/fishes9060221
APA StyleRobinson, W., Koehn, J., & Lintermans, M. (2024). Contemporary Trends in the Spatial Extent of Common Riverine Fish Species in Australia’s Murray–Darling Basin. Fishes, 9(6), 221. https://doi.org/10.3390/fishes9060221







