Seasonal and Daily Movement Patterns by Wels Catfish (Silurus glanis) at the Northern Fringe of Its Distribution Range
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Fishing and Tagging
2.3. Estimates of Daylength
2.4. Data Handling and Statistical Analysis
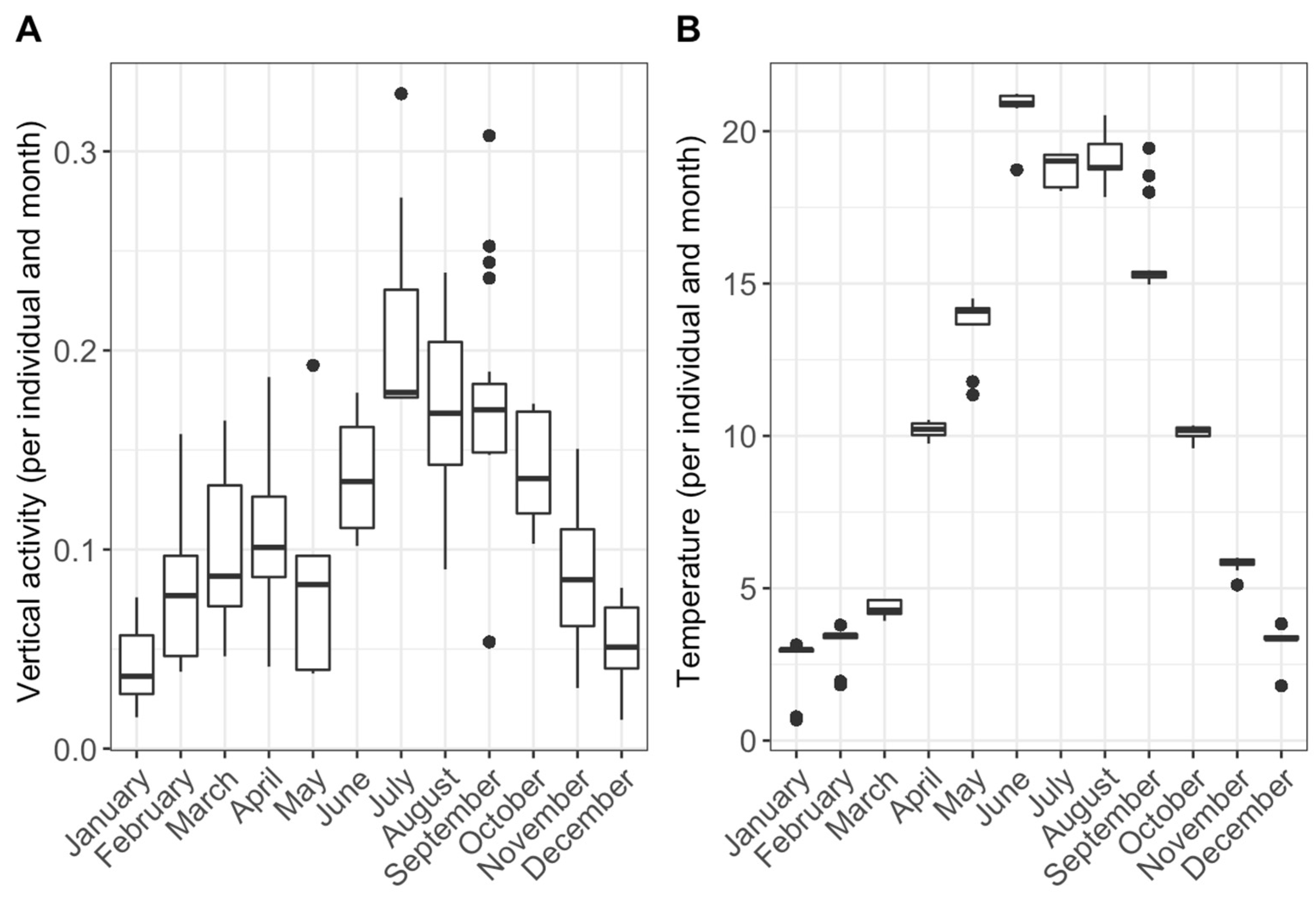
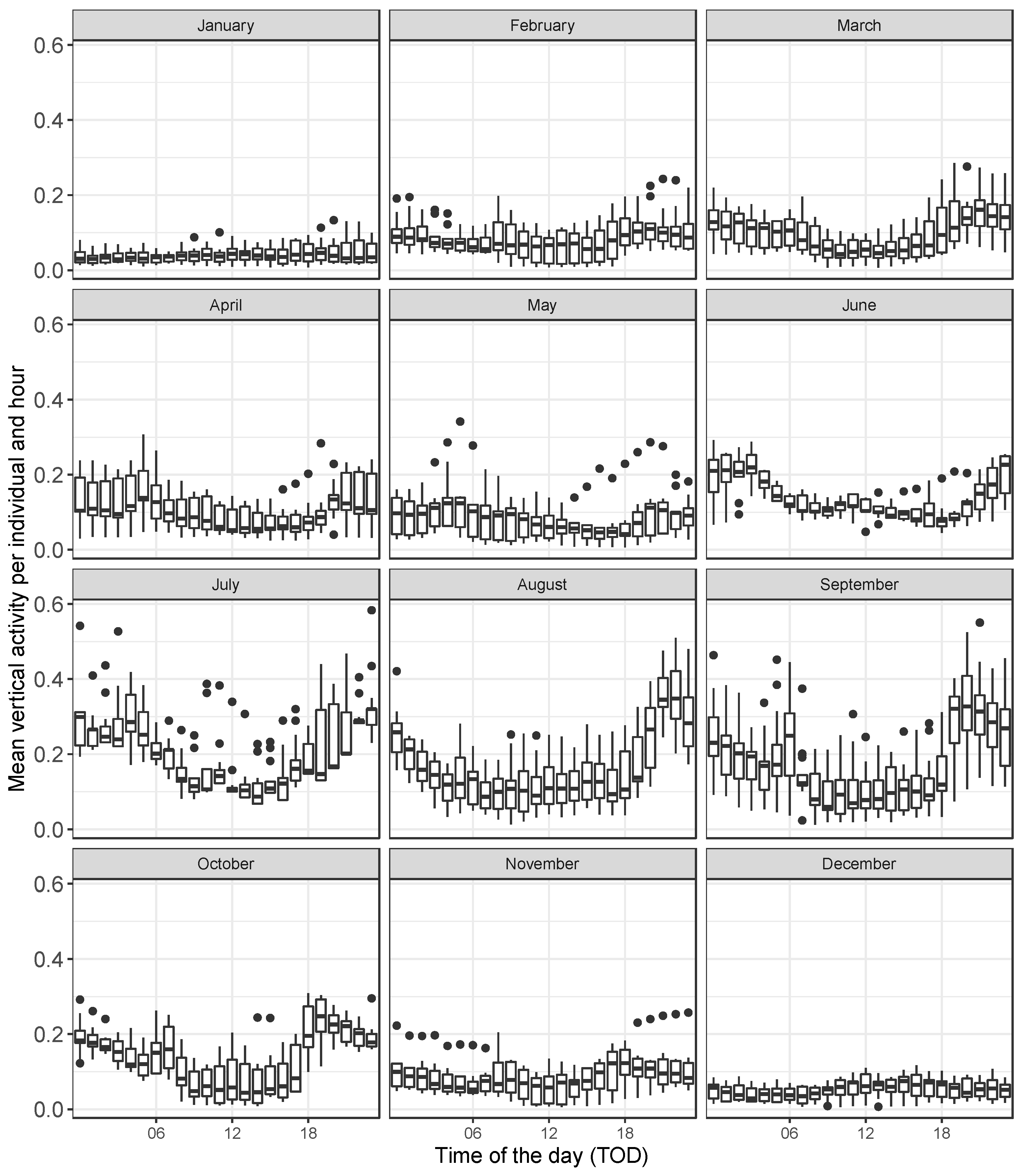
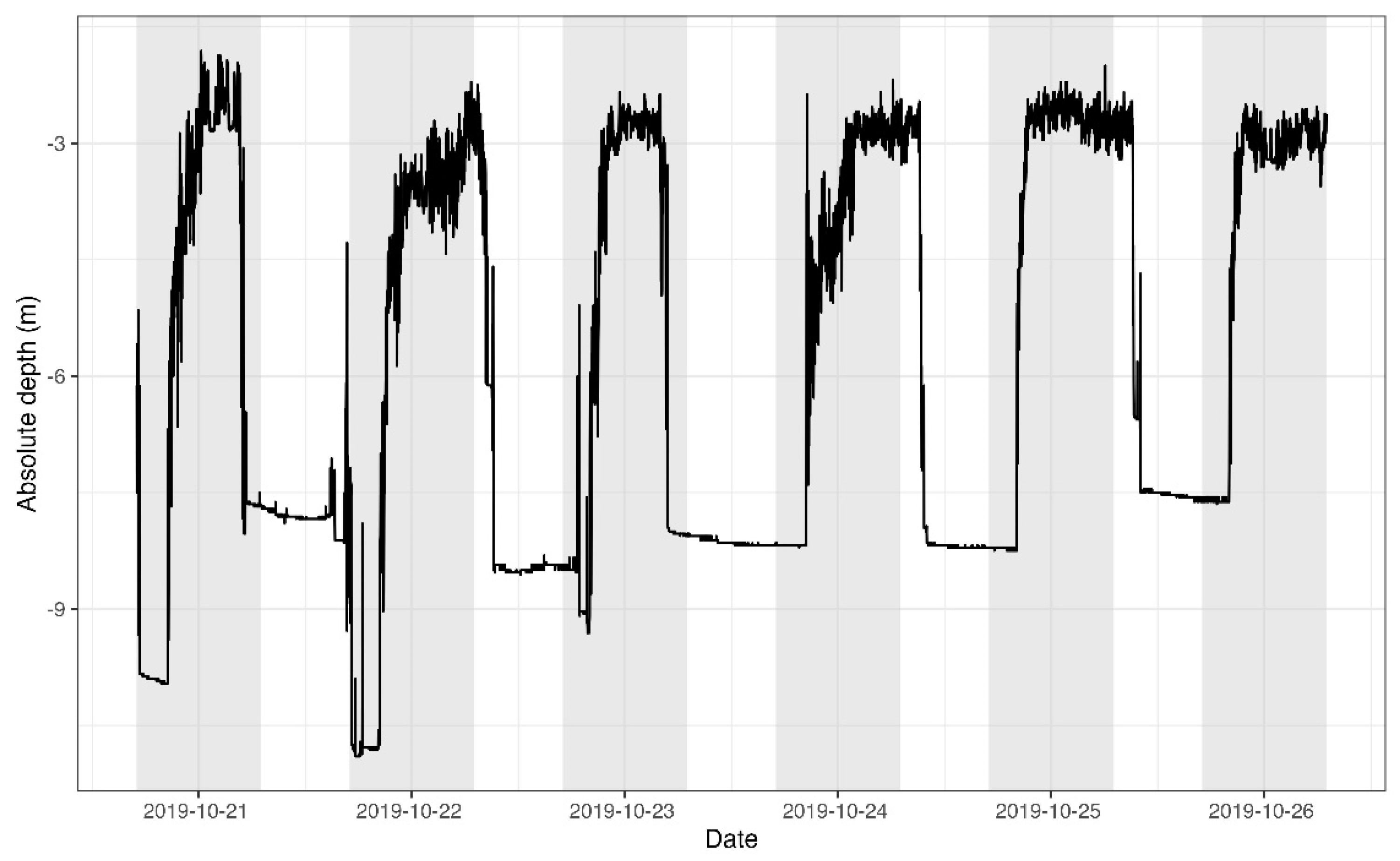
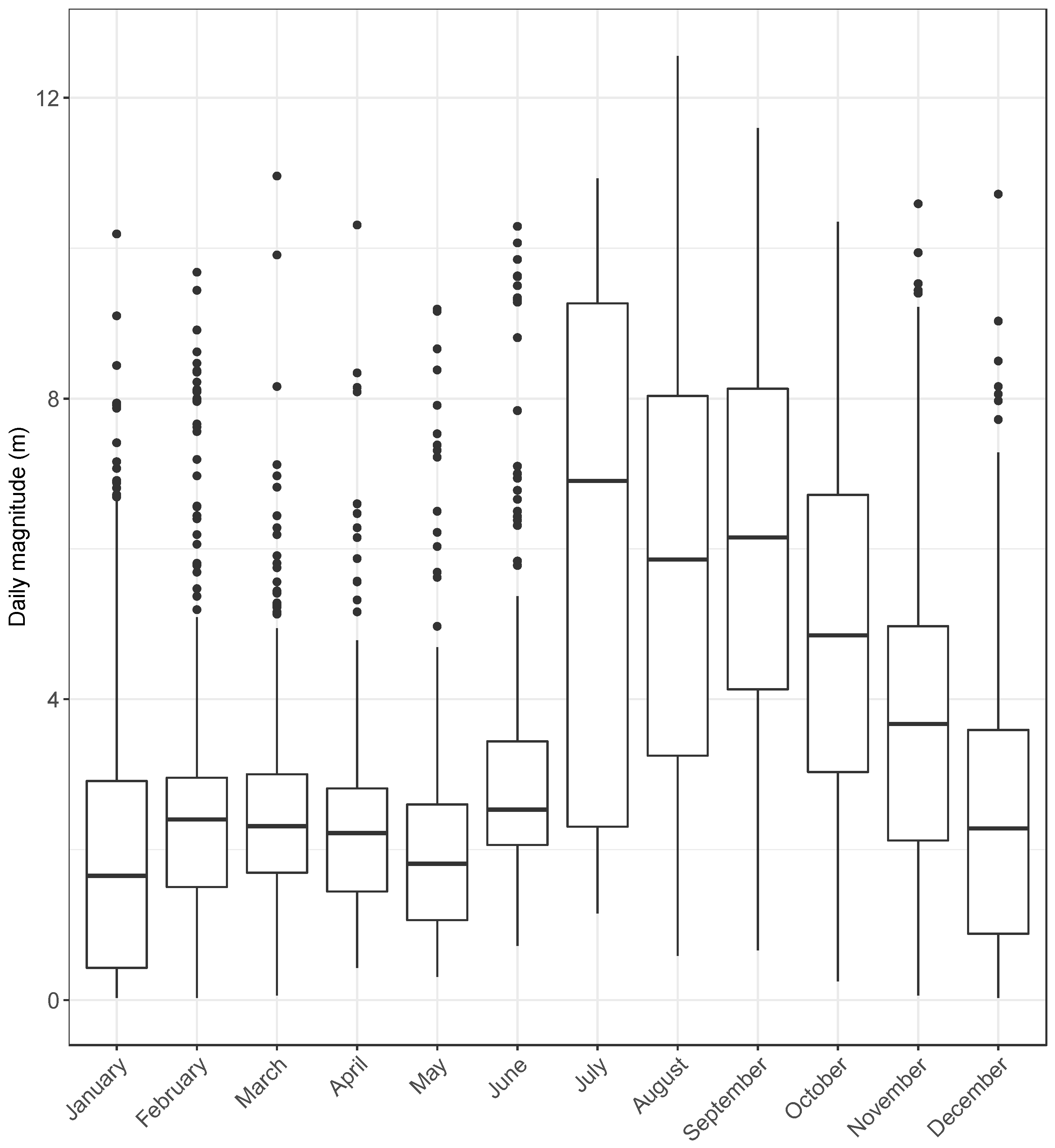
3. Results
4. Discussion
4.1. Daily Activity
4.2. Seasonal Activity
4.3. Do Early Summer Movement Patterns Coincide with Pre-Spawning Behavior?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergström, K.; Nordahl, O.; Söderling, P.; Koch-Schmidt, P.; Borger, T.; Tibblin, P.; Larsson, P. Exceptional longevity in northern peripheral populations of Wels catfish (Silurus glanis). Sci. Rep. 2022, 12, 8070. [Google Scholar] [CrossRef] [PubMed]
- Copp, G.H.; Britton, J.R.; Cucherousset, J.; García-Berthou, E.; Kirk, R.; Peeler, E.; Stakėnas, S. Voracious invader or benign feline? A review of the environmental biology of European catfish Silurus glanis in its native and introduced ranges. Fish Fish. 2009, 10, 252–282. [Google Scholar] [CrossRef]
- Vinterstare, J.; Nathanson, J.E. Åtgardsprogram för mal. Hav. Och. Vattenmyndigheten 2017, 33, 2017. [Google Scholar]
- Flink, H.; Tibblin, P.; Hall, M.; Hellström, G.; Nordahl, O. Variation among bays in spatiotemporal aggregation of Baltic Sea pike highlights management complexity. Fish. Res. 2023, 259, 106579. [Google Scholar] [CrossRef]
- Nordahl, O.; Tibblin, P.; Koch-Schmidt, P.; Berggren, H.; Larsson, P.; Forsman, A. Sun-basking fish benefit from body temperatures that are higher than ambient water. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180639. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.; Monk, C.T.; Arlinghaus, R.; Adam, T.; Alós, J.; Assaf, M.; Baktoft, H.; Beardsworth, C.E.; Bertram, M.G.; Bijleveld, A.I.; et al. Big-data approaches lead to an increased understanding of the ecology of animal movement. Science 2022, 375, eabg1780. [Google Scholar] [CrossRef] [PubMed]
- Benito, J.; Benejam, L.; Zamora, L.; García-Berthou, E. Diel cycle and effects of water flow on activity and use of depth by Common carp. Trans. Am. Fish. Soc. 2015, 144, 491–501. [Google Scholar] [CrossRef]
- Reebs, S.G. Plasticity of diel and circadian activity rhythms in fishes. Rev. Fish Biol. Fish. 2002, 12, 349–371. [Google Scholar] [CrossRef]
- Alanärä, A.; Burns, M.D.; Metcalfe, N.B. Intraspecific resource partitioning in brown trout: The temporal distribution of foraging is determined by social rank. J. Anim. Ecol. 2001, 70, 980–986. [Google Scholar] [CrossRef]
- Brännäs, E. Temporal resource partitioning varies with individual competitive ability: A test with Arctic charr Salvelinus alpinus visiting a feeding site from a refuge. J. Fish Biol. 2008, 73, 524–535. [Google Scholar] [CrossRef]
- Froland Steindal, I.A.; Whitmore, D. Circadian clocks in fish-what have we learned so far? Biology 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Volpato, G.L.; Trajano, E. Biological rhythms. Fish Physiol. 2005, 21, 101–153. [Google Scholar]
- Bolliet, V.; Aranda, A.; Boujard, T. Demand-feeding rhythm in rainbow trout and European catfish. Synchronisation by photoperiod and food availability. Physiol. Behav. 2001, 73, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Boujard, T.; Leatherland, J. Circadian rhythms and feeding time in fishes. Environ. Biol. Fishes 1992, 35, 109–131. [Google Scholar] [CrossRef]
- Mistlberger, R.E. Circadian food-anticipatory activity: Formal models and physiological mechanisms. Neurosci. Biobehav. Rev. 1994, 18, 171–195. [Google Scholar] [CrossRef]
- Valdimarsson, S.K.; Metcalfe, N.B.; Thorpe, J.E.; Huntingford, F.A. Seasonal changes in sheltering: Effect of light and temperature on diel activity in juvenile salmon. Anim. Behav. 1997, 54, 1405–1412. [Google Scholar] [CrossRef][Green Version]
- Boulêtreau, S.; Santoul, F. The end of the mythical giant catfish. Ecosphere 2016, 7, e01606. [Google Scholar] [CrossRef]
- Bouletreau, S.; Gaillagot, A.; Carry, L.; Tetard, S.; De Oliveira, E.; Santoul, F. Adult Atlantic salmon have a new freshwater predator. PLoS ONE 2018, 13, e0196046. [Google Scholar] [CrossRef]
- Carol, J.; Benejam, L.B.; García-Berthou, E. Growth and diet of European catfish (Silurus glanis) in early and late invasion stages. Fundam. Appl. Limnol. 2009, 174, 317–328. [Google Scholar] [CrossRef]
- Cucherousset, J.; Bouletreau, S.; Azemar, F.; Compin, A.; Guillaume, M.; Santoul, F. “Freshwater killer whales”: Beaching behavior of an alien fish to hunt land birds. PLoS ONE 2012, 7, e50840. [Google Scholar] [CrossRef]
- Syväranta, J.; Cucherousset, J.; Kopp, D.; Martino, A.; Cereghino, R.; Santoul, F. Contribution of anadromous fish to the diet of European catfish in a large river system. Naturwissenschaften 2009, 96, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Syväranta, J.; Cucherousset, J.; Kopp, D.; Crivelli, A.; Céréghino, R.; Santoul, F. Dietary breadth and trophic position of introduced European catfish Silurus glanis in the River Tarn (Garonne River basin), southwest France. Aquat. Biol. 2010, 8, 137–144. [Google Scholar] [CrossRef]
- Boujard, T. Diel rhythms of feeding activity in the European catfish, Silurus glanis. Physiol. Behav. 1995, 58, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Daněk, T.; Horký, P.; Kalous, L.; Filinger, K.; Břicháček, V.; Slavík, O. Seasonal changes in diel activity of juvenile European catfish Silurus glanis (Linnaeus, 1758) in Byšická Lake, Central Bohemia. J. Appl. Ichthyol. 2016, 32, 1093–1098. [Google Scholar] [CrossRef]
- Slavík, O.; Horký, P.; Bartoš, L.; Kolářová, J.; Randák, T. Diurnal and seasonal behaviour of adult and juvenile European catfish as determined by radio-telemetry in the River Berounka, Czech Republic. J. Fish Biol. 2007, 71, 101–114. [Google Scholar] [CrossRef]
- Nyqvist, D.; Calles, O.; Forneris, G.; Comoglio, C. Movement and activity patterns of non-native Wels catfish (Silurus glanis Linnaeus, 1758) at the confluence of a large river and its colder tributary. Fishes 2022, 7, 325. [Google Scholar] [CrossRef]
- Naughton, G.P.; Hogan, Z.S.; Campbell, T.; Graf, P.J.; Farwell, C.; Sukumasavin, N. Acoustic telemetry monitors movements of wild adult catfishes in the Mekong River, Thailand and Laos. Water 2021, 13, 641. [Google Scholar] [CrossRef]
- Říha, M.; Rabaneda-Bueno, R.; Jarić, I.; Souza, A.T.; Vejřík, L.; Draštík, V.; Blabolil, P.; Holubová, M.; Jůza, T.; Gjelland, K.; et al. Seasonal habitat use of three predatory fishes in a freshwater ecosystem. Hydrobiologia 2022, 849, 3351–3371. [Google Scholar] [CrossRef]
- Slavik, O.; Horky, P. Diel dualism in the energy consumption of the European catfish Silurus glanis. J. Fish Biol. 2012, 81, 2223–2234. [Google Scholar] [CrossRef]
- Capra, H.; Pella, H.; Ovidio, M. Movements of endemic and exotic fish in a large river ecosystem (Rhône, France). In Proceedings of the 10th International Conference on Ecohydraulics, Trondheim, Norway, 22–26 June 2014. [Google Scholar]
- Palm, S.; Vinterstare, J.; Nathanson, J.E.; Triantafyllidis, A.; Petersson, E. Reduced genetic diversity and low effective size in peripheral northern European catfish Silurus glanis populations. J. Fish Biol. 2019, 95, 1407–1421. [Google Scholar] [CrossRef]
- Jensen, A.; Lillie, M.; Bergstrom, K.; Larsson, P.; Hoglund, J. Whole genome sequencing reveals high differentiation, low levels of genetic diversity and short runs of homozygosity among Swedish wels catfish. Heredity 2021, 127, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Nordahl, O.; Bergström, K.; Koch-Schmidt, P.; Söderling, P.; Sunde, J.; Skov, C.; Tibblin, P.; Larsson, P. Wels catfish (Silurus glanis)—An apex predator in northern peripheral habitats. 2023; Unpublished material intended for publication. [Google Scholar]
- Miljödata, M.V.M. Swedish University of Agricultural Sciences (SLU). National Data Host Lakes and Watercourses, and National Data Host Agricultural Land. 2021. Available online: https://miljodata.slu.se/mvm/ (accessed on 16 May 2021).
- Vejřík, L.; Vejříková, I.; Kočvara, L.; Blabolil, P.; Peterka, J.; Sajdlová, Z.; Jůza, T.; Šmejkal, M.; Kolařík, T.; Bartoň, D.; et al. The pros and cons of the invasive freshwater apex predator, European catfish Silurus glanis, and powerful angling technique for its population control. J. Environ. Manag. 2019, 241, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Maechler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lecomte, J.; Benoît, H.P.; Ancelet, S.; Etienne, M.; Bel, L.; Parent, E. Compound Poisson-gamma vs. delta-gamma to handle zero-inflated continuous data under a variable sampling volume. Methods Ecol. Evol. 2013, 4, 1159–1166. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013.
- RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2019.
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 2018, 2. [Google Scholar]
- Wickham, H. Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- dplyr: A Grammar of Data Manipulation. Available online: https://dplyr.tidyverse.org/ (accessed on 14 October 2022).
- Grolemund, G.; Wickham, H. Dates and times made easy with lubridate. J. Stat. Softw. 2011, 40, 1–25. [Google Scholar] [CrossRef]
- Carol, J.; Zamora, L.; García-Berthou, E. Preliminary telemetry data on the movement patterns and habitat use of European catfish (Silurus glanis) in a reservoir of the River Ebro, Spain. Ecol. Freshw. Fish 2007, 16, 450–456. [Google Scholar] [CrossRef]
- Pohlmann, K.; Atema, J.; Breithaupt, T. The importance of the lateral line in nocturnal predation of piscivorous catfish. J. Exp. Biol. 2004, 207, 2971–2978. [Google Scholar] [CrossRef]
- Pohlmann, K.; Grasso, F.W.; Breithaupt, T. Tracking wakes: The nocturnal predatory strategy of piscivorous catfish. Proc. Natl. Acad. Sci. USA 2001, 98, 7371–7374. [Google Scholar] [CrossRef]
- Ciolac, A. Migration of fishes in Romanian Danube River (N° 1). Appl. Ecol. Environ. Res. 2004, 2, 143–163. [Google Scholar] [CrossRef]
- Mehner, T. Diel vertical migration of freshwater fishes—Proximate triggers, ultimate causes and research perspectives. Freshw. Biol. 2012, 57, 1342–1359. [Google Scholar] [CrossRef]
- Zaret, T.M. Predation and Freshwater Communities; Yale University Press: New Haven, CT, USA, 1980; 187p. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergström, K.; Berggren, H.; Nordahl, O.; Koch-Schmidt, P.; Tibblin, P.; Larsson, P. Seasonal and Daily Movement Patterns by Wels Catfish (Silurus glanis) at the Northern Fringe of Its Distribution Range. Fishes 2024, 9, 280. https://doi.org/10.3390/fishes9070280
Bergström K, Berggren H, Nordahl O, Koch-Schmidt P, Tibblin P, Larsson P. Seasonal and Daily Movement Patterns by Wels Catfish (Silurus glanis) at the Northern Fringe of Its Distribution Range. Fishes. 2024; 9(7):280. https://doi.org/10.3390/fishes9070280
Chicago/Turabian StyleBergström, Kristofer, Hanna Berggren, Oscar Nordahl, Per Koch-Schmidt, Petter Tibblin, and Per Larsson. 2024. "Seasonal and Daily Movement Patterns by Wels Catfish (Silurus glanis) at the Northern Fringe of Its Distribution Range" Fishes 9, no. 7: 280. https://doi.org/10.3390/fishes9070280
APA StyleBergström, K., Berggren, H., Nordahl, O., Koch-Schmidt, P., Tibblin, P., & Larsson, P. (2024). Seasonal and Daily Movement Patterns by Wels Catfish (Silurus glanis) at the Northern Fringe of Its Distribution Range. Fishes, 9(7), 280. https://doi.org/10.3390/fishes9070280




