Abstract
The combined production of three varieties of lettuce (romaine, iceberg, and red leaf) with flathead grey mullet (Mugil cephalus) was tested in triplicate in three independent coupled aquaponic units with no thermal control. For this purpose, a total of 114 fish (2.5 kg/m3) were stocked in each fish tank (2 m3), and 92 lettuces were planted in the hydroponic unit (6 m2). As no thermal control was included in the design of the aquaponic system, water temperatures declined from maximum values of 20.4 °C to minimum values of 5.0 °C, which directly affected fish growth. However, the conditions imposed by the aquaponic system were suitable for promoting lettuce’s growth and external appearance, as no pests or leaf discoloration were noticed. Lettuce survival was similar among the three tested varieties (98.5 ± 1.7%). The yields for the romaine and iceberg varieties were 384 ± 100 g/lettuce and 316 ± 70 g/lettuce, respectively, and that for the red leaf variety was lower, at 176 ± 75 g/lettuce. Yield values ranged between 3.6 and 4.4 kg/m2 depending on the replicate considered (4.0 ± 0.4 kg/m2). According to present results, each aquaponic unit required ca. 2.6–2.7 L of water per unit of lettuce produced.
Key Contribution:
This study shows that regardless of the low water temperatures that directly affect fish growth performance under the absence of thermal control, coupled aquaponic systems are a valuable tool for producing plants in a sustainable way.
1. Introduction
Sustainability is in the portfolio of all economic sectors, and the agrifood industry is not an exception. This is neither a final condition nor an achievable goal; sustainability is a way ahead. Under this scenario, sustainable strategies for improving blue food economies are essential to design a new approach for transitioning towards more responsible, comprehensive, exploitable, and positive impact–generating production and consumption models. Among the different aquatic food systems, aquaponics—which is defined as a farming method that combines fish farming and hydroponics (cultivating plants in water without soil) in a symbiotic environment—is considered as one of the most sustainable. In aquaponic systems, fish feces and uneaten pellets provide a nutrient-rich solution for plants, and the plants help filter and purify the water for the fish. This creates a closed-loop system where both fish and plants benefit from each other’s presence [1,2]. Aquaponics offers several advantages, including water conservation (as it uses significantly less water than traditional soil-based agriculture), efficient nutrient use, and the potential for high-yield and fast-growing crops. Additionally, it minimizes the need for synthetic fertilizers and provides a natural way to grow both fish and plants [1].
Classical aquaponic systems, commonly known as coupled or closed-loop aquaponic systems, were introduced over 30 years ago [3]. In these systems, the aquaculture unit and the hydroponic unit are integrated into a single loop, with process water flowing from the aquaculture unit to the hydroponic unit and back. This inevitably results in the same water quality for both fish and plants, creating a compromise in the optimal rearing conditions for each production line [4]. The second aquaponic technology commonly used at the industrial level involves decoupled systems organized in separate loops, where process water is primarily recirculated within the respective unit. This setup allows for the better control of species-specific requirements. The water circulates within the individual unit (either the recirculating aquaculture system, RAS, or hydroponics), and water loss due to plant evapotranspiration is replenished as needed. Process water is directed from the fish tanks to the hydroponic reservoir via a one-way valve, ensuring that water from the hydroponic unit does not return to the fish tanks. This separation enables the independent management of conditions within the hydroponic unit, if necessary [5].
Globally, the most common species raised in freshwater aquaponic systems include Nile tilapia (Oreochromis niloticus), common carp (Cyprinus carpio), silver carp (Hypophthalmichthys molitrix), grass carp (Ctenopharyngodon idella), bluegill (Lepomis macrochirus), catfish (Clarias gariepinus), pacu (Piaractus mesopotamicus), koi carp (Cyprinus rubrofuscus), and various ornamental fish species [6]. In Europe, aquaponic systems are focused on farming, by order of importance, tilapia, ornamental fish, rainbow trout (Oncorhynchus mykiss), and perch (Perca fluviatilis) [7]. However, aquaponics should not be restricted to a few fish species, and as a consequence, new species should be tested with regards to their suitability and adaptation to aquaponic systems. Among them, the flathead grey mullet (Mugil cephalus) has been postulated as a suitable species for these farming systems [8]. Furthermore, this euryhaline, omnivorous species stands out for having lower dietary protein requirements compared to the strictly carnivorous farmed fish species [9]. According to FAO aquaculture statistics [10], a total of 11,938.6 t of M. cephalus were produced in 2021, with Indonesia (46.3%), Israel (16.8%), and China (11.8%) being the main producers of this species in extensive and semi-intensive aquaculture conditions [11]. Furthermore, M. cephalus is recognized as an ideal species for addressing food needs in developing countries, showcasing its versatility; moreover, it is highly valued in developed countries for its ability to yield both flesh and valuable processed by-products such as salted mullet roe, also known as ‘bottarga’ [12].
The selection of plant species suitable for aquaponics is influenced by the stocking density of fish in tanks, the compound feed used to feed fish, and the resulting nutrient concentration in aquacultural effluent. Plants with low-to-medium mineral nutritional requirements, such as lettuce, herbs, and specialty greens—including spinach (Spinacia oleracea), chive (Allium spp.), basil (Ocimum basilicum), and watercress (Nasturtium officinale)—adapt well to aquaponic systems, whereas fruit-bearing plants, such as tomatoes, bell peppers, and cucumbers, which have higher nutritional demands, perform better in heavily-stocked and well-established aquaponic systems [3].
Although the functioning of coupled freshwater systems is well described in the literature for a wide number of fish and plant species [13,14,15,16], most of the available literature describes the operation of these systems under optimal environmental conditions. The functioning of these systems with no control on air and water temperatures remains less explored in terms of fish and plant production outputs. Consequently, the objective of the current study was to evaluate the functioning and production of a coupled freshwater aquaponic system devoted to the combined production of flathead grey mullet and three varieties of lettuce (iceberg, romaine, and red leaf) under suboptimal water temperatures.
2. Materials and Methods
2.1. The Coupled Aquaponic System
The experimental coupled aquaponic system was composed of three independent aquaponic units within a greenhouse (Figure 1). Each aquaponic unit (experimental replicate) consisted of a 2000 L tank for holding fish, followed by a sedimentation tank where suspended particles, such as uneaten pellets and fish feces, settled down and were removed. Then, water flowed into a 125 L biological filter tank filled with bio-balls, where nitrifying bacteria converted ammonia into nitrites and nitrates. Water flowed by gravity into the hydroponic unit where lettuces were supported on polystyrene rafts that covered the entire surface. Strong aeration was provided by a 0.3 kW air blower to ensure sufficient oxygen for the growth of plant roots and other compartments within the aquaponic system. Finally, water was pumped (2470 L/h; 0.1 kW) from the hydroponic unit back to the fish-rearing tank through a submerged water pump (SERA Pond Precision SP2000, SERA GmbH, Heinsberg, Germany). The total volume of each aquaponic unit was 7.1 m3, with a water turnover time for an entire circulation cycle of ca. 3 h. No water exchange occurred between the three aquaponic units since they were independent of each other and their hydraulic circuits were not interconnected. Water and air temperatures were ambient, and no thermal control was provided either for the aquaponic water nor the greenhouse air.
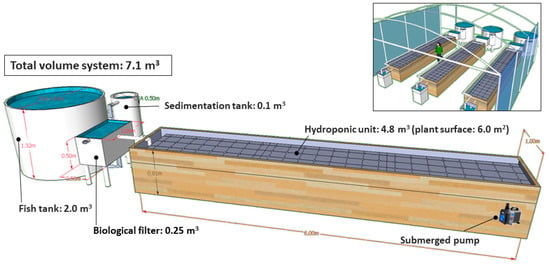
Figure 1.
Schematic view of a coupled aquaponic system and its different compartments devoted to the combined production of flathead grey mullet (Mugil cephalus) and three varieties of lettuce (Lactuca sativa). The inner image shows the experimental set-up composed of the three aquaponic units within the greenhouse, where each unit was considered as a replicate.
2.2. Experimental Design
A total of 342 flathead grey mullet juveniles (44.1 ± 10.1 g in body weight, BW and 13.1 ± 1.1 cm in standard length, SL; mean ± standard deviation) were used in the present study. Fish (n = 114) were stocked in each tank within each aquaponic unit at an initial stocking biomass of 2.5 kg/m3. Three varieties of lettuce (iceberg, romaine, and red leaf) were purchased at a local seedling store (stage of three to four leaves) and planted in each hydroponic unit (n = 92 lettuces per aquaponic unit; 30–31 lettuces per variety; Supplementary File S1). In all aquaponic units, romaine lettuces were planted proximal to the biological filter tank, the middle third was planted with red leaf lettuces, and the distal section of the hydroponic unit was planted with lettuces from the iceberg variety. Aeration was provided into each compartment of the aquaponic unit (fish tank, biological filter, and hydroponic tank) by a diffusing stone connected to an air blower. No inorganic fertilizer was applied into the system since no external signs of mineral deficiency (i.e., discoloration, leaf deformation, chlorosis, browning, dark green color, or die-off among others) were detected during the 99 days of the trial (14 October–21 January).
During the trial, flathead grey mullets were fed with a commercial diet containing 54% crude protein, 18% crude fat, and 19.5 MJ/kg digestible energy (T-Nutra 1.1 MP; 1.1 mm pellet size; Skretting, Cojobar, Burgos, Spain). As water temperature progressively decreased from October to January (Figure 2), the feeding ration was progressively adjusted from 2.5% to 0.5% of the stocked biomass to reduce the amount of uneaten feed due to reduced feed intake. Values of feed intake could not be determined since the recovery of uneaten feed pellets was not feasible.
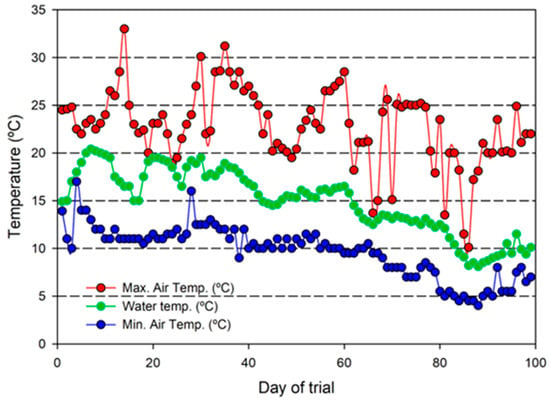
Figure 2.
Daily maximum and minimum air temperatures (°C) in the greenhouse housing the coupled aquaponic system farming flathead grey mullet (Mugil cephalus) and three varieties of lettuce (Lactuca sativa). Average water temperature values (°C) calculated from the three replicate aquaponic units are also provided for the entire trial (99 days).
During the study, water quality parameters (temperature, oxygen, and pH) were measured daily, whereas nitrogenous compounds in water (ammonia, nitrites, and nitrates) were measured once a week. Water temperature and dissolved oxygen were determined with an OXI330 (Crison Instruments, Alella, Spain), and pH was registered with a pH meter 507 (Crison Instruments, Spain). Water ammonia, nitrite, and nitrate levels were quantified with a Photometer System MD600/MaxiDirect (Lovibond, Dortmund, Germany) using specific reagent kits. The following reagents from Tintometer GmbH (Dortmund, Germany) were used for the detection of ammonia, nitrites, and nitrates, respectively: Vario Ammonia Cyanurate F10 (catalog number 531370) and Vario Ammonia Salicylate F10 (catalog number 531380), Vario Nitri 3 F10 (catalog number 530980), and Vario Nitrate Chomotropic Powder Pack (catalog number 535580). Air temperature was measured by a mercury thermometer. Photoperiod followed the normal regime of the season (October–January; latitude 40°37′41″ N). To support the reproducibility of the three aquaponic units (replicates) and potentially account for deviations in plant and fish growth within each system, we find it valuable to provide the average values for each aquaponic unit (n = 99 per unit; daily measurements during the trial). Additionally, we will present the evolution of the average values for water temperature calculated from the three replicates. Values from each aquaponic unit were provided for water ammonia, nitrites, and nitrates.
2.3. Evaluation of the Biological Performance of the Aquaponic System
At the beginning and the end of the trial, all fish from each tank were netted and gently anesthetized with buffered (pH = 7.2) tricaine methanesulfonate (100 mg/L; Sigma-Aldrich, Madrid, Spain) in order to measure their size in BW and SL. Specific growth rate (SGR) in terms of BW was calculated as SGR (% BW/day) = 100 × [(ln BWfinal − ln BWinitial)/days], whereas fish body condition was assessed using Fulton’s body condition factor (K = 100 × BW/SL3). At the end of the trial, the weight of the aerial part of all lettuces was individually measured. Furthermore, aquaponic plant yield (APY, kg/m2) was calculated considering the production of the aerial part of lettuces (in kg) per m2 in each hydroponic unit for the duration of the trial. Fish and lettuce survival was calculated by counting the number of fish and plants at the end of the study. The amount of water needed for producing a lettuce was calculated considering the yield of the hydroponic unit and the total volume of water needed for compensating the water losses from evapotranspiration of plants and regular maintenance operations of the coupled aquaponic system.
2.4. Statistical Analyses
Data are presented as mean ± standard deviation. Flathead grey mullet growth in terms of BW and SL between the onset and the end of the study were compared by a t-test once data were checked for normality and homoscedasticity and properly transformed (log10) when they did not follow a normal distribution (i.e., BW). The skewness values that measure the degree of asymmetry of the distribution and kurtosis that measures the degree of peakedness and flatness were computed for BWfinal and Kfinal distributions. Average lettuce weight (g) and yield values (kg/m2) from the three tested varieties (romaine, iceberg, and red leaf) were compared by a split-plot ANOVA as the distribution of the three varieties of lettuce was not randomized within the hydroponic unit (split-plot design). The level of significance for statistical analyses was set at 0.05 (5%). Statistical analysis and data plotting were done using SigmaPlot for Windows version 15 (INPIXON, Berlin, Germany), whereas the split-plot ANOVA was run using SPSS Statistics version 26.0.0 (IBM Corporation, Armonk, NY, USA).
3. Results and Discussion
This trial was run under late autumn and early winter conditions that resulted in a rapid and progressive decrease in air temperatures within the greenhouse that ranged from 24.9 °C at the beginning of the trial to 7.0 °C at the end of the study (Figure 2). Air temperature changes resulted in a decrease in water temperatures (from maximum values of 33.0 °C recorded in October to minimum values of 4.0 °C in January) as no thermal control was included in the design of the aquaponic system.
The water temperature average values in the fish tanks in each of the three aquaponic units was 15.0 ± 3.4 °C, 14.9 ± 4.1 °C, and 14.9 ± 3.6 °C (n = 99 per unit), respectively (the evolution of the average values from the three aquaponics units throughout the trial are displayed in Figure 2). Oxygen levels were higher than 95% saturation in all replicate units, with values ranging from 9.0 to 12.1 mg/L in the fish tank and 9.5 to 12.8 mg/L in the hydroponic unit. pH values slightly decreased throughout the trial, ranging from 7.51 to 7.11, 7.53 to 7.15, and 7.51 to 7.10 in each of the three replicate aquaponic units.
The three units behaved similarly with regard to the values of the nitrogenous compounds dissolved in water; thus, the average ammonia, nitrites, and nitrates levels were 0.6 ± 0.05 mg/L NH4+, 2.0 ± 0.03 mg/L NO2−, and 25.0 ± 5.1 mg/L NO3−, respectively (Figure 3). Regardless of the low temperatures at which the trial was conducted, the transformation of ammonia into nitrates was attributed to the maturation of the biological filter and the growth of nitrifying bacteria such as chemosynthetic autotrophic bacteria that obtain their energy from inorganic compounds and heterotrophic bacteria that obtain their energy from organic substances [17,18]. Bacterial ammonia transformation was recently described by Ruiz et al. [19] when studying the microbial composition of this aquaponic system by 16S rRNA gene amplicon sequencing of the V3–V4 region.
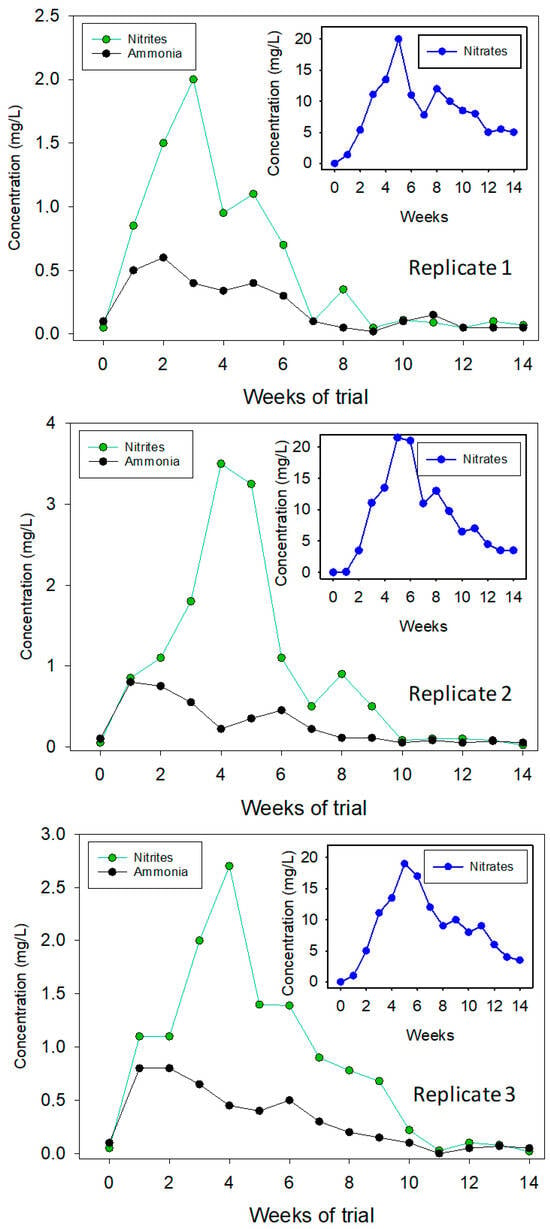
Figure 3.
Weekly average values of ammonia, nitrites, and nitrates in the coupled aquaponic system farming flathead grey mullet (Mugil cephalus) and three varieties of lettuce (Lactuca sativa).
The low water temperatures directly compromised fish growth performance in terms of the BW and SL of flathead grey mullet reared in a coupled aquaponic system with no thermal control (Table 1). No statistically significant differences in terms of SL were found between the beginning and the end of the trial (p > 0.05). However, there was a slight but statistically significant increase in BW (10%) during the trial (t-test; t = −13.9; d.f. = 4; p < 0.05). The values of the SGR in BW were really low, ranging from 0.105 to 0.114%BW/day depending on the aquaponic unit considered. At the end of the trial, the size distribution in BW was not normally distributed in any of the three replicate tanks (Kolmogorov–Smirnov test, p > 0.05), whereas differences in skewness and kurtosis values were found in the fish tanks (Figure 4).

Table 1.
Survival, growth performance in body weight (BW) and standard length (SL), and specific growth rate (SGR) of flathead grey mullet (Mugil cephalus) and three varieties of lettuce (Lactuca sativa) reared in freshwater aquaponic system during winter conditions. Data are presented for each of the coupled aquaponic units tested in this trial as well as the average and standard deviation calculated from them, as each unit is statistically independent of the others (replicates). Different letters within the same column indicate statistically significant differences between initial and final values (t-test, p < 0.05).
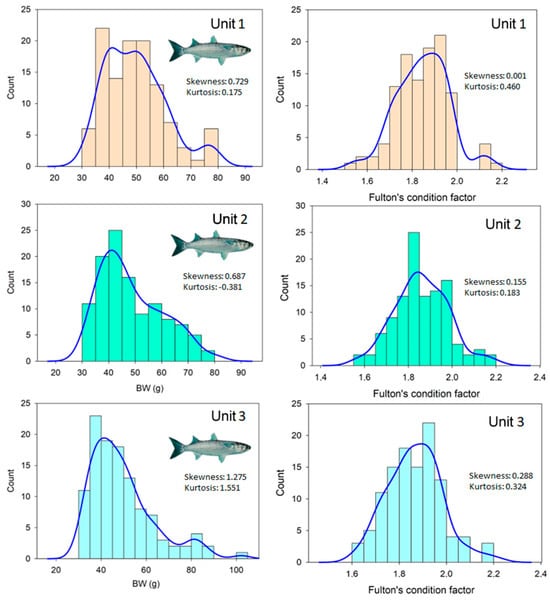
Figure 4.
Distribution in final body weight (g) and Fulton’s condition factor of flathead grey mullet (Mugil cephalus) reared in a coupled aquaponic system in combination with three varieties of lettuce (Lactuca sativa). The skewness and kurtosis values for each size distribution are also provided. The blue line represents the kernel density plot that displays the distribution of values in the dataset using one continuous curve without being affected by the number of bins used in the histogram.
There was a statistically significant decrease in the Fulton’s condition factor during the trial (t-test; t = 4.3; d.f. = 4; p < 0.05), with the Kfinal values being lower (5%) than those recorded at the beginning of the trial (1.86 ± 0.02 vs. 1.95 ± 0.03, respectively). The Fulton’s condition factors were normally distributed in all replicates, with differences in skewness and kurtosis values among replicate tanks (Figure 4). These results may be attributed to the absence of growth in body length and the small increase in BW that resulted in the slightly poorer condition of the fish at the end of the trial. According to its natural range of distribution, flathead grey mullet is considered an eurythermal species with a preference for warmer waters, being able to tolerate temperatures between 13 and 33 °C in natural water bodies [20]. However, the poorer growth and condition of fish under current experimental conditions may be attributed to the low water temperatures experienced during the trial, which ranged from 20.4 to 8.1 °C and led to a reduction in feeding to adjust its metabolic oxygen demand [21]. These results contrasted with other studies run at higher water temperatures where flathead grey mullets were successfully grown using biofloc technology [22] in polyculture conditions with Nile tilapia [23] or in water recirculation systems [9]. The optimal temperature range for this species in terms of growth performance is typically between 20 and 26 °C [24], whereas in natural environments flathead grey mullets avoid waters with lower temperatures than 18 °C [20]. Under current experimental conditions, flathead grey mullet juveniles were able to withstand a period of 61 days below 18 °C, water temperatures that are considered suboptimal [20], without any adverse signs on fish condition. A crucial aspect of aquaponics is ensuring precise water temperature control within a narrow range of changes in water temperature [25]. This high-level management of water temperature is essential for optimizing fish metabolism. When the water temperature is accurately maintained without significant fluctuations, fish exhibit efficient feeding and feed conversion, resulting in improved growth rates. Additionally, stable and predictable waste load releases under these conditions benefit plant culture in the aquaponic system [25]. Thus, current results indicated that flathead grey mullets under aquaponic conditions should be farmed in subtropical and warm waters, even though they tolerate colder waters than reported from field studies [20].
It is important to note that the focus of this study extended beyond evaluating the performance of flathead grey mullets in aquaponic conditions during the winter, since the study also aimed to assess the production of three varieties of lettuce within the coupled aquaponic units under these thermal winter conditions.
According to Rusu et al. [26], lettuces are plants without high heat requirements and with the capacity to resist cold weather conditions. They indicated that the best temperatures for lettuce growth ranged between 16 and 20 °C, even though temperature tolerance depended on the developmental stage and light intensity. Under current experimental conditions, the three varieties of lettuce adapted well to the coupled aquaponic system running during winter conditions that resulted in low air and water temperatures (Figure 2). In this sense, the lettuces were visually inspected to detect fungal or bacterial diseases growing in their leaves, as well as signs of mineral and nutrient deficiencies. The external examination of the lettuces indicated that their condition was optimal in terms of the absence of pests, leaf color, and appearance, which indicated that the conditions imposed by the aquaponic system were suitable for promoting lettuce’s growth and external appearance (Supplementary File S2). A total of 276 lettuces with a final biomass of ca. 76 kg were harvested at the end of the trial (lettuce survival = 98.5 ± 1.7%) (Table 1). Survival was similar among the three tested varieties (romaine, iceberg, and red leaf) (split-plot ANOVA; p > 0.05). In terms of growth, all varieties of lettuce (romaine, iceberg, and red leaf) adapted very well to aquaponic conditions, and their growth was within the normal values. In particular, the yield for romaine and iceberg varieties was 384 ± 100 g/lettuce and 316 ± 70 g/lettuce, respectively, whereas for red leaf it was significantly lower, at 176 ± 75 g/lettuce (split-plot ANOVA; F = 72.4, d.f. 2; p < 0.001; Figure 4). Such differences were not due to an aquaponic unit effect and variety-specific adaption to the aquaponic system in which they were grown (split-plot ANOVA; F = 0.27, d.f. 2; p > 0.05), but rather to the intrinsic characteristics of each variety, such as their shape, consistency, texture, and the number and size of their leaves. The average size for each variety of lettuce was similar to the values reported by conventional farming systems in terms of their number of leaves, color, and weight. Although data from different studies are barely comparable due to differences in farming systems, irrigation procedures, and the duration of trials, among other study-specific factors, lettuce weights produced in the coupled aquaponic system were within the range of values reported from other studies evaluating different lettuce-producing systems (i.e., ground, indoor controlled environments, and hydroponic systems, among others) [27,28,29,30,31].
The present lettuce yield values from coupled freshwater aquaponics ranged between 3.6 and 4.4 kg/m2 (4.0 ± 0.4 kg/m2) for the period between October and January (Figure 5), even though these values may be slightly underestimated because the trial was run with different varieties of lettuce (romaine, iceberg, and red leaf), and not all of them had similar yields (romaine and iceberg > red leaf). Lettuce yield values from the current study were similar to those obtained by the combined aquaponic production of Murray cod (Maccullochella peelii peelii) and lettuce (4.1 kg/m2) [32], but slightly lower than the APY values (5.0 ± 1.1 kg/m2) reported by Nozzi et al. [16] from an aquaponic system growing tilapia and romaine lettuce. However, in the current study, we evaluated simultaneously three varieties of lettuce (romaine, iceberg, and red leaf), whereas in the study from Nozzi et al. [16], only romaine was tested. As shown in the present study, romaine and iceberg lettuce have higher yield values than the red leaf variety, so this would explain why the yield values (kg/m2) observed in our trial (4.0 ± 0.4 kg/m2) were within the lower range of values (4.0–6.1 kg/m2) reported by the former authors. Another plausible explanation is the different temperatures at which both studies were run. In our trial, the water temperature dropped from 20.4 to 5.0 °C, whereas in the study of Nozzi et al. [16] romaine lettuce was grown in combination with tilapia at relatively constant water temperatures that ranged between 24.8 and 29.8 °C. Depending on the studies considered, the APY values are to some extent lower or similar than those reported when growing lettuce in hydroponic systems (5.5–6.0 kg/m2) [16,33,34], even though lettuce yields may be improved and reach yields similar to hydroponic systems by tailor-made solutions on the design and management of the aquaponic system [32], as well as guaranteeing dissolved nitrogen levels above 1.4 mmol/L [34].
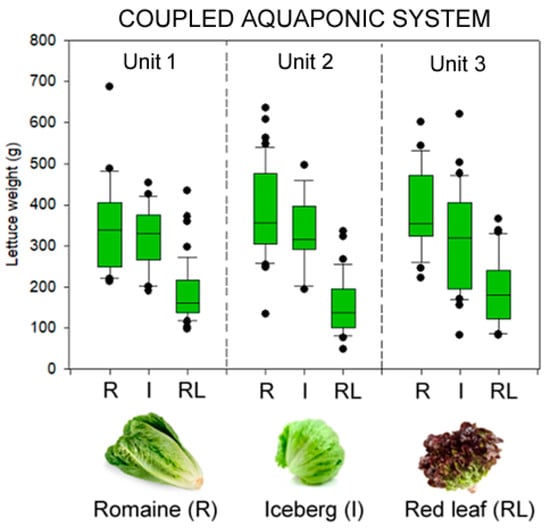
Figure 5.
Box plot of the aerial weight (g) of the three varieties of lettuce (romaine, iceberg, and red leaf) grown in a coupled aquaponic system in combination with flathead grey mullet (Mugil cephalus). Data are shown for each independent unit that composed the aquaponic system.
Aquaponics is often credited with efficient water usage. According to Lennard [30], an optimized lab-scale aquaponic system demonstrated water savings of 90% or more compared to standard recirculating aquaculture systems. Later studies have evidenced that these savings may be as high as 98.5–99% when aquaponic systems are designed to use water as efficiently as possible and replace only the water lost by plant evapotranspiration [3]. When considering the water needed per unit of lettuce produced is ca. 70 L in conventional ground systems [35], the tested coupled aquaponic system resulted in remarkable water savings of 96%, since each aquaponic unit required only ca. 2.6–2.7 L of water per unit of lettuce produced, even though the production time was longer than other similar studies [36]. However, these values may vary depending on the plant’s evapotranspiration rates, which depend on their growth rate, rearing temperatures, and nutrient availability, among other factors [37].
4. Conclusions
This study showed that coupled freshwater aquaponics dedicated to the production of lettuce and flathead grey mullet may be a valid strategy during winter conditions in aquaponic units devoid of a thermal control system that exclusively depends on air temperatures when water temperatures may be suboptimal in terms of fish and plant growth. Regardless of the low temperatures experienced during the trial and the lack of fish growth, the nutrients in the water were enough to support lettuce growth and quality, as production yields and the absence of signs of mineral deficits upon external examination of the lettuce leaves indicated. Among the three varieties of lettuce tested, romaine and iceberg resulted in higher production yields than the red leaf variety. Such differences in productivity were attributed to the intrinsic characteristics of the variety considered rather than its adaptation to the aquaponic system. The combined farming of lettuce with flathead grey mullet represented an important water saving of up to 96% (2.6–2.7 L/lettuce) when compared to conventional systems. Regardless of the lack of thermal control in the system, growing lettuces in combination with flathead grey mullet under low water temperatures (15.0 ± 3.4 °C) was a valid strategy in terms of lettuce production until water temperatures were not high enough to support fish growth and represents a feasible strategy for implementing these sustainable farming systems in areas with limited investment capacity in this technology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes9060189/s1.
Author Contributions
Conceptualization, E.G. and R.C.; methodology, E.G.; formal analysis, E.G. and A.R.; investigation, E.G.; system design, R.C.; data collection, S.M. and E.H.; data curation, E.G. and A.R.; writing—original draft preparation, E.G.; writing—review and editing, all authors; visualization, E.G.; supervision, E.G.; project administration, E.G. and R.C.; funding acquisition, E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was conducted within the NewTechAqua project. The project received funding from the European Union’s Horizon 2020 research and innovation program under Grant Agreement No. 862658.
Institutional Review Board Statement
All procedures involving fish and plant manipulation and tissue sampling from experimental animals complied with the Spanish (law 1078 32/2007 and Royal Decree 1201/2015) and ongoing European legislation (EU2010/63). The experimental protocol was authorized by the Ethical Committee of the Institute of Agrifood Research and Technology and the Generalitat of Catalunya, Direcció General de Polítiques Ambientals i Medi Natural (CEEA 11264/2021).
Data Availability Statement
Data are available on request to the corresponding author.
Acknowledgments
The authors are thankful to all the technical and maintenance staff from IRTA-La Ràpita who dedicated their time to building the tested aquaponic system and supported the running of the trial. We are thankful to C. Alcaraz for his statistical support with the split-plot ANOVA.
Conflicts of Interest
The authors declare no conflicts of interest. The funders (European Union’s Horizon 2020 research and innovation program) had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Baganz, G.F.; Junge, R.; Portella, M.C.; Goddek, S.; Keesman, K.J.; Baganz, D.; Kloas, W. The aquaponic principle—It is all about coupling. Rev. Aquac. 2022, 14, 252–264. [Google Scholar] [CrossRef]
- Love, D.C.; Fry, J.; Li, X.; Hill, E.S.; Genello, L.; Semmens, K.; Thompson, R.E. Commercial aquaponics production and profitability: Findings from an international survey. Aquaculture 2015, 435, 67–74. [Google Scholar] [CrossRef]
- Lennard, W.; Goddek, S. Aquaponics: The basics. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 113–144. [Google Scholar] [CrossRef]
- Palm, H.W.; Knaus, U.; Applebaum, S.; Strauch, S.M.; Kotzen, B. Coupled Aquaponic Sytems. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 163–200. [Google Scholar] [CrossRef]
- Goddeck, S.; Joyce, A.; Wuertz, S.; Körner, O.; Bläser, I.; Reuter, M.; Keesman, K.J. Decoupled Aquaponic Systems. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 201–230. [Google Scholar] [CrossRef]
- Farrell, L. Which Species to Raise in an Aquaponics System? Available online: https://revolutionized.com/species-to-raise-in-aquaponics-system/ (accessed on 4 March 2024).
- Villarroel, M.; Junge, R.; Komives, T.; König, B.; Plaza, I.; Bittsánszky, A.; Joly, A. Survey of aquaponics in Europe. Water 2016, 8, 468. [Google Scholar] [CrossRef]
- Rossi, L.; Bibbiani, C.; Fierro-Sañudo, J.F.; Maibam, C.; Incrocci, L.; Pardossi, A.; Fronte, B. Selection of marine fish for integrated multi-trophic aquaponic production in the Mediterranean area using DEXi multi-criteria analysis. Aquaculture 2021, 535, 736402. [Google Scholar] [CrossRef]
- Bertini, A.; Natale, S.; Gisbert, E.; Andrée, K.B.; Concu, D.; Dondi, F.; de Cesare, A.; Inidio, V.; Gatta, P.P.; Bonaldo, A.; et al. Exploring the application of Corynebacterium glutamicum single cell protein in the diet of flathead grey mullet (Mugil cephalus): Effects on growth performance, digestive enzymes activity and gut microbiota. Front. Mar. Sci. 2023, 10, 1172505. [Google Scholar] [CrossRef]
- FAO. Fishery and Aquaculture Statistics. Global production by production source 1950–2021 (FishStatJ). In FAO Fisheries and Aquaculture Division; FAO: Rome, Italy, 2023; Available online: https://www.fao.org/fishery/en/statistics/software/fishstatj (accessed on 2 April 2024).
- Crosetti, D. Current state of grey mullet fisheries and culture. In Biology, Ecology and Culture of Grey Mullet (Mugilidae); Crossetti, D., Blaber, S., Eds.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Abingdon, UK, 2016; pp. 388–450. [Google Scholar] [CrossRef]
- Khemis, I.B.; Hamza, N.; Sadok, S. Nutritional quality of the fresh and processed grey mullet (Mugilidae) products: A short review including data concerning fish from freshwater. Aquat. Living Resour. 2019, 32, 2. [Google Scholar] [CrossRef]
- Zappernick, N.; Nedunuri, K.V.; Islam, K.R.; Khanal, S.; Worley, T.; Laki, S.L.; Shah, A. Techno-economic analysis of a recirculating tilapia-lettuce aquaponics system. J. Clean. Prod. 2022, 365, 132753. [Google Scholar] [CrossRef]
- Ani, J.S.; Manyala, J.O.; Masese, F.O.; Fitzsimmons, K. Effect of stocking density on growth performance of monosex Nile Tilapia (Oreochromis niloticus) in the aquaponic system integrated with lettuce (Lactuca sativa). Aquac. Fish. 2022, 7, 328–335. [Google Scholar] [CrossRef]
- Calone, R.; Pennisi, G.; Morgenstern, R.; Sanyé-Mengual, E.; Lorleberg, W.; Dapprich, P.; Gianquinto, G. Improving water management in European catfish recirculating aquaculture systems through catfish-lettuce aquaponics. Sci. Tot. Environ. 2019, 687, 759–767. [Google Scholar] [CrossRef]
- Nozzi, V.; Graber, A.; Schmautz, Z.; Mathis, A.; Junge, R. Nutrient management in aquaponics: Comparison of three approaches for cultivating lettuce, mint and mushroom herb. Agronomy 2018, 8, 27. [Google Scholar] [CrossRef]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J.J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia–nitrogen in aquaculture systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef]
- Moschos, S.; Kormas, K.A.; Karayanni, H. Prokaryotic diversity in marine and freshwater recirculating aquaculture systems. Rev. Aquac. 2022, 14, 1861. [Google Scholar] [CrossRef]
- Ruiz, A.; Scicchitano, D.; Palladino, G.; Nanetti, E.; Candela, M.; Furones, D.; Sanahuja, I.; Carbó, R.; Gisbert, E.; Andree, K.B. Microbiome study of a coupled aquaponic system: Unveiling the independency of bacterial communities and their beneficial influences among different compartments. Sci. Rep. 2023, 13, 19704. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, A.K.; Panfili, J.; Durand, J.D. A global review of the cosmopolitan flathead mullet Mugil cephalus Linnaeus 1758 (Teleostei: Mugilidae), with emphasis on the biology, genetics, ecology and fisheries aspects of this apparent species complex. Rev. Fish Biol. Fisher. 2012, 22, 641–681. [Google Scholar] [CrossRef]
- Cech, J.J., Jr.; Wohlschlag, D.E. Seasonal patterns of respiration, gill ventilation, and hematological characteristics in the striped mullet, Mugil cephalus L. Bull. Mar. Sci. 1982, 32, 130–138. [Google Scholar]
- Garcés, S.; Lara, G. Applying biofloc technology in the culture of Mugil cephalus in subtropical conditions: Effects on water quality and growth parameters. Fishes 2023, 8, 420. [Google Scholar] [CrossRef]
- Mehrim, A.; Refaey, M.; Khalil, F.; Shaban, Z. Impact of mono- and polyculture systems on growth performance, feed utilization, and economic efficiency of Oreochromis niloticus, Mugil cephalus, and Mugil capito. J. Anim. Poult. Prod. 2018, 9, 393–400. [Google Scholar] [CrossRef][Green Version]
- Kibenge, F.S.B. Descriptions of major farmed aquatic animal species. In Aquaculture Pathophysiology; Frederick, S.B., Kibenge, B.B., Roger, S.-M.C., Eds.; Aquaculture Pathophysiology; Academic Press: New York, NY, USA, 2022; Volume 1, pp. 1–44. [Google Scholar] [CrossRef]
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.V.; Jijakli, H.; Thorarinsdottir, R. Challenges of Sustainable and Commercial Aquaponics. Sustainability 2015, 7, 4199–4224. [Google Scholar] [CrossRef]
- Rusu, T.; Moraru, P.I.; Mintas, O.S. Influence of environmental and nutritional factors on the development of lettuce (Lactuca sativa L.) microgreens grown in a hydroponic system: A review. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12427. [Google Scholar] [CrossRef]
- Barbosa, G.L.; Gadelha, F.D.A.; Kublik, N.; Proctor, A.; Reichelm, L.; Weissinger, E.; Halden, R.U. Comparison of land, water, and energy requirements of lettuce grown using hydroponic vs. conventional agricultural methods. Int. J. Environ. Res. Public Health 2015, 12, 6879–6891. [Google Scholar] [CrossRef]
- Matysiak, B.; Ropelewska, E.; Wrzodak, A.; Kowalski, A.; Kaniszewski, S. Yield and quality of romaine lettuce at different daily light integral in an indoor controlled environment. Agronomy 2022, 12, 1026. [Google Scholar] [CrossRef]
- Amin, A.R.; Nissa, N.S.; Permadi, M.G. Growth and production of several Romaine lettuce varieties (Lactuca sativa var. Romana) on various ratios of ammonium-nitrate in hydroponic nutrition formulations. IOP Conf. Ser. Earth Environ. Sci. 2019, 343, 012021. [Google Scholar] [CrossRef]
- Bozkurt, S.; Mansuroglu, G.S.; Kara, M.; Onder, S. Responses of lettuce to irrigation levels and nitrogen forms. Afr. J. Agric. Res. 2009, 4, 1171–1177. [Google Scholar] [CrossRef]
- Kurunc, A. Effects of water and salinity stresses on growth, yield, and water use of iceberg lettuce. J. Sci. Food Agric. 2021, 101, 5688–5696. [Google Scholar] [CrossRef] [PubMed]
- Lennard, W.A. Aquaponic Integration of Murray Cod (Maccullochella peelii peelii) Aquaculture and Lettuce (Lactuca sativa) Hydroponics. Ph.D. Thesis, RMIT University, Melbourne, VIC, Canada, 2005. Available online: https://library.deakin.edu.au/record=b2326562 (accessed on 4 March 2024).
- Pantanella, E.; Cardarelli, M.; Colla, G.; Rea, A.; Marcucci, A. Aquaponic vs. hydroponic: Production and quality of lettuce crop. Acta Hortic. 2010, 927, 887–893. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; van Os, E.; Anseeuw, D. Hydroponic Technologies. In Aquaponics Food Production Systems; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 77–112. [Google Scholar] [CrossRef]
- Viscon Group. Deep Water Cultivation of Lettuce. Available online: https://viscongroup.eu/news/deep-water-cultivation-of-lettuce/ (accessed on 6 March 2024).
- Colt, J.; Schuur, A.M.; Weaver, D.; Semmens, K. Engineering design of aquaponics systems. Rev. Fish. Sci. Aquac. 2022, 30, 33–80. [Google Scholar] [CrossRef]
- Vicente de Paulo, R.; Tavares, A.L.; de Sousa, I.F.; da Silva, T.G.; de Holanda, R.M.; de Souza, E.P.; da silva, B.B.; Braba, C.C.; Almeida, R.S. Evapotranspiration, water use efficiency and crop coefficient of three lettuce varieties grown in a tropical region. Rev. Ciências Agrárias 2018, 41, 798–805. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).