Abstract
Successful aquacultural production of juvenile fish requires that the optimal rearing conditions be applied. However, for many fish species, there is a significant knowledge gap concerning these conditions. In this study, we evaluated the effects of stocking density on the survival and growth of brown meagre, Sciaena umbra (L.), during rearing trials in an experimental hatchery. This research forms part of a feasibility project to improve the aquacultural production of brown meagre. Four initial larval density treatments (5, 10, 30, and 70 larvae per L) were established. At 0, 9, 17, 22, and 25 days post-hatching, we measured the total length, coefficient of variation in length, and specific growth rate. The physicochemical water parameters remained at satisfactory levels for fish culture throughout the experiment. Lower densities promoted better growth in terms of total length, specific growth rate, and survival. We observed a significant negative correlation between larval density and length growth/survival. Thus, the low larval density treatment yielded the highest survival (48.5% ± 3.46%), growth in final total length (11.9 ± 1.09 mm), and specific growth rate (5.13% ± 0.39% per day). Increased stocking density therefore negatively affects growth and survival, reduces homogeneity, but increases the production of S. umbra larvae. This study helps identify optimal aquaculture conditions for maximizing the production of juvenile S. umbra for ecological restoration.
Key Contribution:
We describe for the first time the stocking density effect on the survival and growth of Sciaena umbra larvae. The results indicate a negative correlation between stocking density and survival/growth as well as density reducing the homogeneity, but increasing the production.
1. Introduction
Brown meagre (Sciaena umbra, L. 1758) is a teleost fish species and a member of the Sciaenidae family (croakers and drums), which includes 70 genera and 270 marine, brackish, and freshwater species distributed worldwide [1]. It is a coastal, nektobenthic, sedentary, and gregarious species that inhabits shelters on rocky bottoms or hides within seagrass meadows [2]. This species is found in the eastern Atlantic Ocean, the Mediterranean Basin, the Black Sea, and the Sea of Azov [3]. It is a gonochoristic fish that reaches sexual maturity at the age of 3 to 4 years, equivalent to 20–30 cm total length [2,4], with a spawning period in summer, i.e., from May to August [2,5]. The brown meagre is of significant commercial and ecological interest. It is a patrimonial species targeted by small-scale artisanal fisheries in the Mediterranean Sea and the northeast Atlantic [6]. However, S. umbra has been largely impacted by overfishing for decades (i.e., recreational and commercial fishing) and habitat degradation, and it is currently classified as a “near threatened” species by the International Union for Conservation of Nature [7] and listed in Annex III (protected faunal species) of the Barcelona Convention. Environmental degradation and inappropriate fisheries management are common causes of the decline and even the collapse of fisheries and also affect S. umbra populations [8].
Restocking is one option to help depleted stocks recover; methods and conditions must be identified that favor the successful mass production of environmentally fit juveniles and their release to sustain natural populations. Sciaenids are considered efficient aquaculture species given their relatively simple needs to ensure successful rearing [9]. Recently, S. umbra has been proposed as a candidate for marine finfish diversification in commercial aquaculture and for restocking programs in the Mediterranean Sea because this species (i) has a fast growth rate; (ii) has shown successful spontaneous reproduction of wild fish in captivity; (iii) is of patrimonial interest; and (iv) is sought for its high-quality flesh [10,11]. Indeed, there is increased interest worldwide in fast-growing species in aquaculture, such as the greater amberjack (Seriola dumerili) and the cobia (Rachycentron canadum), where more efforts have been made [12]. Therefore, determining the optimal environmental conditions and possible limiting factors for attaining elevated survival and growth rates in the juvenile phase of S. umbra is imperative.
A substantial amount of prior knowledge is necessary for producing large numbers of individuals for commercial and stock enhancement. Successful larval production relies on creating optimal rearing practices, environmental conditions, and feeding strategies [13,14]. For intensively cultured fish species, improvements in survival can be achieved using zootechnical improvements in the early production stages [15,16]. In aquaculture, one of the most critical management decisions is determining the stocking density for each culture cycle [17,18]. The effects of stocking density have been studied for several aquaculture species [19,20,21] and several impacts were identified, including effects on water quality, growth and survival, welfare, physiological state parameters, and technical parameters such as productivity and yield [22,23]. Increased stocking density generally leads to heightened aggressiveness [24], hierarchical phenomena [25], cannibalism [26], and stress levels [20] among the fish in rearing enclosures. These negative consequences can significantly affect size heterogeneity, survival, and growth performance in aquacultural fish populations [27]. However, the impact of stocking density on survival and growth can vary or even produce contradictory results, depending on the species, rearing conditions, and fish age [20,28,29]. These variations among the studies emphasize the importance of understanding how stocking density influences the survival and growth of S. umbra larvae. Moreover, to date, no studies have examined the influence of stocking density on the survival and growth of S. umbra larvae.
In this context, several research programs have been developed at the Stella Mare research unit, Corsica, France, focusing particularly on patrimonial species such as Maja squinado [30], Palinurus elephas [31], Dentex dentex, and S. umbra [32] for restoration purposes. These programs aim to increase the production of juveniles to eventually supply sufficient quantities for ecological restoration experiments around the island. The objective of this study was to assess the zootechnical performance by assessing the survival and growth of brown meagre larvae reared in a recirculating aquaculture system at four stocking densities (5, 10, 30, and 70 larvae per L).
2. Materials and Methods
2.1. Brood Stock Maintenance and Embryo Production
The experiment was conducted at the Stella Mare Research Center hatchery of the University of Corsica, located in Biguglia, France. Fertilized eggs of brown meagre were obtained through the spontaneous spawning of a captive brood stock. This brood stock consisted of 24 wild breeders—12 males (mean weight 815 ± 205 g) and 12 females (mean weight 995 ± 346 g; mean ± SD)—captured between 2018 and 2021 by professional Corsican fishermen. The breeders were reared under natural photoperiod and temperature conditions in a 10 m³ cylindroconical tank connected to a recirculating aquaculture system unit. The brood stock was fed three times per week with a diet comprising Vitalis CAL dry food (Skretting, Fontaine les Vervins, France), frozen shrimp (Penaeus notialis), frozen mussels (Mytilus chilensis), and frozen clams (Ruditapes philippinarum). The fertilized eggs, i.e., buoyant eggs, were collected using a 100 μm net collector. They were then separated from the non-viable sinking eggs and distributed into two 80 L cylindroconical tanks with gentle aeration. The tanks were maintained at a density of 1300 eggs per L under controlled conditions: water exchange at 10% to 15% per hour, temperature between 18.5 and 20 °C, salinity at 38 ppt, pH 7.6 ± 0.2, and ammonia, nitrate, nitrite levels maintained at >0.05 mg/L.
2.2. Experimental Design and Larval Rearing
The 25-day experiment commenced at 0-days post-hatching (DPH). Newly hatched larvae were measured (n = 30, mean total length (TL): 3.3 ± 0.1 mm) and were counted volumetrically and randomly assigned to 220 L black cylindroconical tanks connected to a flow-through circulation system. Larvae were stocked at four different densities: 5 (very low: V-L), 10 (low: L), 30 (medium: M), and 70 larvae per L (high: H). All treatments were conducted in triplicate. Gas equilibrium and oxygen saturation were monitored using an Oxyguard Handy Polaris 2 and TGP probes (Oxyguard International, Farum, Denmark). Water temperature was maintained at 20.5 ± 1 °C using a heat pump, pH at 7.6 ± 0.2, ammonia/nitrite/nitrate levels were kept above 0.02 mg/L using the flow-through system. Oxygen saturation was maintained between 65% and 95%. Lighting was provided by a 230 W halogen source located 80 cm above the surface, delivering 200 lux of illumination, with a photoperiod of 10 L:14 D. Water quality parameters remained stable throughout the experiment.
Larvae were fed ad libitum from day 4 to day 10 with Gemma Micro 75 artificial diet (Skretting, France), followed by AF Artemia nauplii (INVE Aquaculture, Salt Lake City, USA) from days 7 to 21. From days 18 to 25, larvae were fed enriched EG48 Artemia metanauplii (INVE Aquaculture, Salt Lake City, USA) enriched with DHA Prot Selco (INVE Aquaculture, Salt Lake City, USA). Moreover, a live prey supplement was manually added to each tank, depending on larval activity. Feeding rate and food size were gradually adjusted according to consumption and fish size, respectively, in order to ensure that food quantity and size were not a limiting factor in the experiment.
2.3. Experimental Procedure and Sample Collection
Larvae from the experimental treatments were randomly sampled at 0, 9, 17, 22, and 25 DPH during the experiment (n = 30 per replicate) (see Millot et al. [11] for the pictures of ontogenetic development). The sample size and sampling frequency were selected on the basis of the technical constraints of the sampling procedure and to minimize stress for the larvae caused by handling. Larvae were anesthetized in benzocaine propylene glycol (50 mL.m−3), photographed under a VisiScope SZT360-6 stereomicroscope (VWR International, Rosny-sous-Bois, France) connected to an Axiocam ERc 5s camera (ZEISS, Rueil Malmaison, France), and their total body length (TL, mm) was measured using a Labscope (ZEISS, Rueil Malmaison, France). Before returning the fish to their respective tanks, the bottom drain was checked to remove any dead individuals. The same procedure was repeated 4 h after the fish were returned to the tanks. Thus, no mortality was observed as a result of the subsequent sampling. At 25 DPH, all tanks were emptied, and living larvae were manually counted to calculate survival. The number of deceased fish was counted from 10 to 25 DPH, as larvae present in the samples collected before 10 DPH rapidly degraded and were impossible to count.
2.4. Performance Metrics
The effects of stocking density on zootechnical performance were determined by calculating the following indices for each experimental treatment:
where Ni and Nf are the initial and final number of larvae in the experiment, respectively; TLi and TLf are the average total length at the beginning and end of the experiment (±0.1 mm), respectively; t is the duration of the experiment in days (t = 25), SD is the standard deviation, and TL is the average total length (mm).
2.5. Statistical Analysis
All statistical analyses were performed using R software version 4.2.0 [33], with results presented as mean ± standard deviation (SD) across three replicates per treatment. Normality and homogeneity of variance were assessed by examining residuals using Shapiro–Wilk and Levene’s tests, respectively. Data that were non-normal and had heterogeneous variance were normalized by arcsine or logarithmic transformations to reduce variability [34]. Differences between treatments based on stocking density were determined using one-way ANOVA, followed by Tukey’s honestly significant difference (HSD) post hoc test. Survival rates over time for each treatment density were examined using Kaplan–Meier survival analysis with a Weibull error distribution, performed using the “survival” package in R [35,36]. Kaplan–Meier plots to display the results of Mantel–Cox tests were generated using the “survminer” package in R [37]. The Mantel–Cox test, also known as the log-rank test, is a nonparametric hypothesis-based test to compare survival distribution curves across at least two samples. When multiple pairwise comparisons of survival curves by treatment were performed, the resulting p values were adjusted using Tukey’s method. A significance threshold of α = 0.05 was used for all statistical analyses. It should be noted that for replicate 3, a mortality peak occurred at 19 DPH for the 10 larvae per L (low: L) stocking density treatment. This was due to a technical error made by the manipulator so this sample was removed from the data set to avoid statistical bias.
3. Results
At the end of the experiment (25 DPH), we observed a noticeable increase in total length in all treatments compared to the initial lengths, with increases of 8.64 ± 1.09 mm (V-L), 8.36 ± 1.22 mm (L), 7.00 ± 1.46 mm (M), and 6.06 ± 1.88 mm (H) (Figure 1). Consequently, the average final total length (TLf) of larvae ranged from 9.36 ± 1.88 in treatment H to 11.94 ± 1.09 in treatment V-L (Figure 1, Table 1). Growth significantly decreased with increased stocking density (F = 11.86, df = 3, p ˂ 0.001), with the lowest growth observed for larvae reared at the highest density. This trend was quantified by the specific growth rate (SGRTL), which significantly decreased as stocking densities increased (F = 10.7, df = 3, p ˂ 0.001), with values ranging from 4.09% ± 0.86% per day (treatment H) to 5.13% ± 0.39% per day (treatment V-L). Thus, we observed a direct relationship in total length with respect to stocking density (H ˂ M ˂ L ˂ V-L), from the beginning (0 DPH) to the end of the experiment (25 DPH) (Table 1).
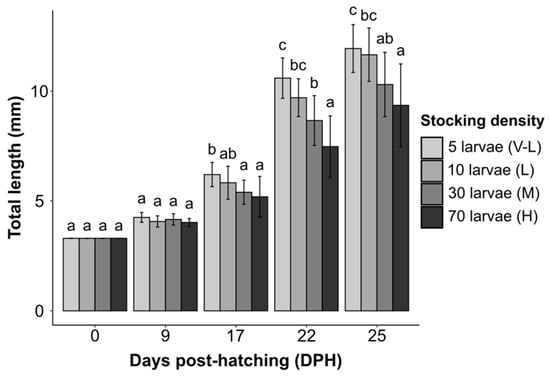
Figure 1.
Total length (TL ± SD) of Sciaena umbra larvae during rearing under laboratory conditions at different stocking densities. Different letters indicate statistically significant differences from each other (ANOVA, Tukey’s post hoc test, HSD, p < 0.05).

Table 1.
Effects of stocking density on growth and survival of Sciaena umbra larvae reared under controlled conditions. Values are means ± SD.
A summary of the experimental results after 25 days’ rearing is presented in Table 1. Survival (SR), measured at the end of the experiment, differed significantly among the four stocking densities (F = 10.69, df = 3, p ˂ 0.01). A significantly higher survival was observed for the very low (V-L) stocking density treatment than for the M and H treatments (Tukey’s test; p ˂ 0.05). However, no significant difference was observed between V-L and L (Table 1). The survival curves over time for all treatments differed significantly (log-rank: χ² = 1580, df = 3, p ˂ 0.001). Moreover, survival differed significantly among the treatments over time (Tukey’s test: p ˂ 0.05) (Figure 2). The Kaplan–Meier survival curves revealed that 50% of larval S. umbra mortality occurred between 0 and 10 DPH for all treatments, except for the V-L treatment (Figure 2).
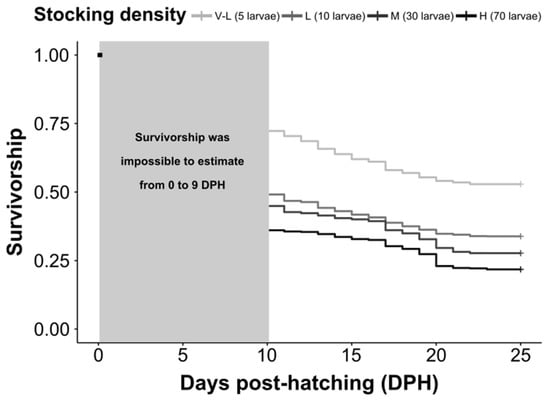
Figure 2.
Estimated survival, calculated using a log-rank (Mantel–Cox) test, for Sciaena umbra larval development at various stocking densities. Black dot on the top left indicates the beginning of the experiment for all treatments (100% survival).
The coefficient of variation for total length (CVTL) provided information on size heterogeneity. At 25 DPH, CVTL ranged from 6.54% ± 3.29% to 12.57% ± 7.89%. The CVTL among treatments differed significantly (F = 4.355, df = 3, p ˂ 0.01). The CVTL in treatment H was significantly higher than in treatments V-L and L (Tukey’s test; p ˂ 0.05). The CVTL for treatment H did not differ from that of treatment M (Table 1). After DPH 17, the coefficient in treatment H was significantly higher than in all other treatments (Tukey’s test; p ˂ 0.05) and remained higher than in treatments V-L and L at 22 DPH. Over time, the coefficient of treatment M increased similarly to treatment H, but we observed no significant difference with treatments V-L and L. Finally, we found a positive correlation between growth parameters and survival, regardless of the stocking density (Figure 3).
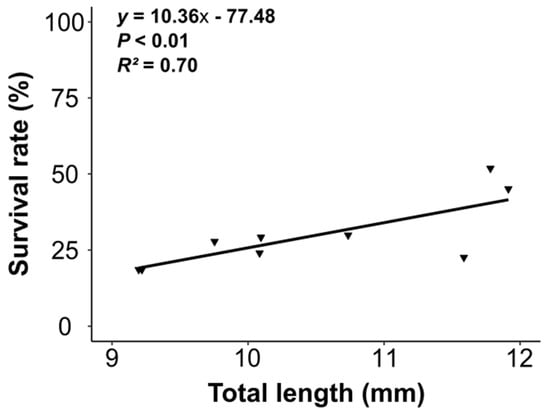
Figure 3.
Correlation between growth and survival of Sciaena umbra larvae at 25 DPH. Black triangles represent the values of the replicates.
4. Discussion
Density is a crucial parameter in the aquaculture industry. When aiming for maximized production, increasing stocking density optimizes costs and space, which is necessary to increase profits [38]. Generally, high stocking density is regarded as a stressor that can negatively influence fish growth parameters and survival. Most studies on fish have shown that relatively high stocking densities usually produce low species welfare [28,39]. However, it is notable that several species can tolerate high stocking densities without harmful effects on survival and growth [40,41]. Thus, the impact of high-density conditions on fish is inconsistent, indicating that the effects of stocking density vary [41], depending on the species, rearing conditions, and age of the fish [29].
The growth of S. umbra in this study is higher than that reported by Millot et al. [11], who documented 9.09 ± 0.88 mm TL at 25 DPH. Compared to other larviculture species in the literature, it is also higher than that observed for various sparids, such as snapper (Pagrus auratus) (TL: 8.6 ± 0.5 mm) [42], sharp snout seabream (Diplodus puntazzo) (TL ranging from 5.0 to 5.6 mm at 25 DPH) [43], and pink dentex (Dentex gibbosus) (TL: 9.02 ± 0.37 mm at 31 DPH) [44]. However, these results were lower than those of faster-growing species, such as bluefin tuna (Thunnus orientalis) (TL: 18 mm at 19 DPH) [45] and greater amberjack (Seriola dumerili) (TL: 35.63 ± 6.52 mm at 40 DPH) [46].
We found a negative correlation between larval stocking density and larval size at 25 DPH. Specifically, larval density significantly improves larval growth in length (i.e., final total length and specific growth rate), being higher when larvae are reared at very low stocking densities (5 larvae per L). Numerous studies of commercial aquaculture species have reported a similar pattern, showing that lower larval stocking densities are associated with higher growth rates in species such as the gilthead sea bream (Sparus aurata), red porgy (Pagrus pagrus), cobia (Rachycentron canadum), spotted sand bass (Paralabrax maculatofasciatus), yellowfin porgy (Acanthopagrus latus), African catfish (Clarias gariepinus), meagre (Argyrosomus regius), rainbow trout (Oncorhynchus mykiss), Atlantic cod (Gadus morhua), and Nile tilapia (Oreochromis niloticus) [19,21,23,28,47,48,49,50,51,52,53]. Several factors related to the rearing parameters can explain the higher growth at lower densities, including greater (1) food availability and (2) vital space. Conversely, lower growth occurs at higher densities because of (1) reduced appetites [54], (2) more aggressive behaviors [55], (3) poorer water quality [56], (4) increased food competition [57], (5) stress associated with reduced reservoir space [21], and (6) an increased susceptibility to disease [58,59]. Nevertheless, Wallace et al. [60], Jørgensen et al. [61], and Papoutsoglou et al. [62] reported increased growth at high larval densities for Arctic char (Salvelinus alpinus) and European sea bass (Dicentrarchus labrax).
In general, survival follows a similar trend to growth, i.e., lower larval density is associated with greater survival, as reported in most reared species, such as Atlantic cod and silver hake (Merluccius bilinearis) [63], common sea bream [48,52,53], gilthead seabream [52,64], common dentex [65], yellowtail flounder (Pleuronectes ferrugineus) [66], and cobia [47]. However, Roo et al. [21] reported a positive correlation between initial larval density and survival for meagre larvae at 30 DPH, with higher survival observed in larvae reared at high densities. Imorou Toko et al. [67] found a similar pattern for vundu (Heterobranchus longifilis) at 24 DPH, as did Hatziathanasiou et al. [27] for European sea bass. Indeed, several studies suggest that high larval density can influence the social behavior of some fish species by reducing social dominance and antagonistic individual behavior, thereby improving survival and reducing growth [16], such as in largemouth bass (Micropterus salmoides) [68] and Arctic char [69].
In this study, the highest recorded survival reached 52.88% ± 3.78%, which is closer to that reported by Abreu [70] at 61.5% but lower than the survival reported by Hamzaçebi and Can [10] at 72.5% ± 0.70%. The differences among the studies could be attributed to dissimilar experimental conditions. Indeed, Hamzaçebi and Can [10] reared the larvae in a green-water system. Nevertheless, compared to other studies on fast-growing species, our observed survival rate was close to that for meagre (Argyrosomus regius; 53.24% ± 12.03%) [21], and sharp snout seabream (Puntazzo puntazzo; 60.6%) [70], although higher than for the greater amberjack (Seriola dumerili; 3.5%) [46].
As observed for many reared species, stocking density in our study likely had a significant impact on fish size heterogeneity. Generally, size heterogeneity is both the cause as well as the consequence of cannibalistic behavior [14]. However, we observed no cannibalistic behavior during the experiment, which is consistent with the previous observations of Millot et al. [11] and Hamzaçebi and Can [10]. Cannibalistic behavior tends to emerge at 30 DPH [11] and between 35 and 40 DPH [10]. Cannibalism is a common problem in most intensively cultured sciaenids [21,26] and other species such as the yellowtail kingfish (Seriola lalandi) [71]. In this study, the heterogeneous larval growth is because the high population density reduces the space available for each larva [72], leading to food competition and stress from social interactions between larvae [73]. This social hierarchy increases with rising population densities [74].
With all the elements developed above, we can see that comparing research results can be challenging because growth processes in teleost fish are notably influenced by stocking density but this depends not only on the specimen’s age but also on the species. For example, some fish are cannibalistic in the larval phase [75] but may or may not exhibit other agonistic behaviors in adulthood, whereas other fish are nonaggressive as juveniles but become territorial with age [76]. Elevated stocking densities typically result in increased stress levels (cortisol) across various fish species [54,77]. Nevertheless, the opposite has been reported for gregarious species at the juvenile stage, which prefer to group together to avoid stressful situations, such as the Japanese meagre (Argyrosomus japonicus) [78] and meagre (A. regius) [20].
Production yield estimates, derived from the growth and survival of aquaculture animals, are used to estimate the economic return of culture operations [79]. The final net yield of fish was directly related to stocking density, up to 3,353 ± 806 individuals in a 220 L tank for high stocking density (H), whereas only 582 ± 42 individuals were obtained for the very low stocking density (V-L). Yengkokpam et al. [38] found a positive correlation between stocking density and production yield for fingerlings (Labeo bata), similar to our findings. They reported that for a stocking density of 75 fingerlings·m−3, the net yield was greater than at lower stocking densities of 25 and 50 fingerlings·m−3, but no further increase was observed beyond 75 fingerlings·m−3 because the threshold or carrying capacity had been reached. Hence, to find the optimal stocking density, there is a trade-off between survival percentage and end production. Nevertheless, the production yield per unit at similar stocking densities may depend on the aquacultural conditions. Moreover, economic performance is an important factor to consider in selecting culture conditions. It is crucial to evaluate the full cost–benefit when estimating net profit [79].
Thanks to all the points presented above, we can see that the effects of stocking density can be multifactorial and vary according to the species, rearing conditions and age. In this experiment, feeding was ad libitum, water quality was constant, and no disease was observed. Thus, low survival, reduced growth and size heterogeneity (for the high stocking density treatment) could be explained by an increase in intra-specific competition and/or an increase in stress caused by the reduction in available space. Thus, further investigations are necessary to fully understand the effects of stocking density on Sciaena umbra. This includes the following: (1) examining the cortisol level in the blood, (2) studying the behavior, (3) studying the impact of stocking densities to ascertain if there is an inversion of the trend—namely, a positive correlation between stocking density and larval growth/survival for older individuals—as demonstrated in gregarious species by Millán-Cubillo et al. [20] for meagre (Argyrosomus regius) and Saoud et al. [29] for rabbitfish (Siganus rivulatus); (4) incorporating additional performance metrics, such as food intake, body weight, biomass gain, feed conversion ratio, and condition factors to better understand the consequences of stocking density on brown meagre; and (5) examining the skeletal structure to observe possible impacts of stocking density, as high stocking densities have been shown to affect skeletal growth in common dentex (Dentex dentex) [65] and red porgy (Pagrus pagrus) [53]. The ultimate goal is to identify the optimum stocking density that ensures good survival, production efficiency, and larval health.
5. Conclusions
This study highlights the varying rearing responses of brown meagre larvae under different stocking densities. Stocking density negatively affected S. umbra larval growth and survival at higher stocking densities. Lower rearing densities promoted better growth in terms of total length, specific growth rate, and survival. Negative correlation between larval density and length growth/survival was observed. This research enhances our knowledge regarding the rearing of S. umbra and may be beneficial for commercial applications and for developing management strategies and restoration programs for endangered S. umbra populations in the wild. Further investigations are also necessary to fully understand the effects of stocking density for S. umbra such as cortisol levels, behavior monitoring or other larval rearing performance metrics. A thorough cost–benefit analysis is essential to recommend the most suitable initial stocking density.
Author Contributions
Conceptualization, M.D., S.P. and R.M.; methodology, J.-J.F., M.D., R.M., S.P., A.D. and A.B.; formal analysis, R.M.; investigation, J.-J.F., M.D., R.M., S.P., A.D. and A.B.; resources, M.D., S.P., L.A., A.S., E.P., J.-F.L., R.H., L.G., A.D. and A.B.; data curation, M.D., R.M. and S.P.; writing—original draft preparation, M.D., R.M. and S.P.; writing—review and editing, J.-J.F., R.M. and A.V.; visualization, M.D., R.M. and S.P.; supervision, J.-J.F. and A.A.; project administration, A.A., J.-J.F. and R.B.; funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the European Regional Development Fund FEDER 2017-2020 under the research program HAL2 and the French State–Region planning contract CPER 2020-2022 under the project DHAVID, both coordinated by the UAR 3514 STELLA MARE (University of Corsica Pasquale Paoli-CNRS).
Institutional Review Board Statement
The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The European Directive 2010/63/EU guidelines for the Care and Use of Laboratory Animals were followed. Reference of ethics committee file: APAFIS23731 and UAR 3514 Stella Mare-UCPP-CNRS animal health approval number: A.2B.001. Approval date: 29 November 2021.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Thanks are given to the professional fishermen Christophe Gena, Jean-Toussaint Lucchini, and Alexandre Lucchini for brood stock captures. Thanks also to all the staff members of the Stella Mare marine research center. The authors are also thankful to Luana Gil for her help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nelson, J.S.; Grande, T.C.; Wilson, M.V. Fishes of the World, 5th ed.; John Wiley & Sons: London, UK, 2016. [Google Scholar]
- Grau, A.; Linde, M.; Grau, A.M. Reproductive Biology of the Vulnerable Species Sciaena umbra Linnaeus, 1758 (Pisces: Sciaenidae). Sci. Mar. 2009, 73, 67–81. [Google Scholar] [CrossRef]
- Chao, L.N. Sciaenidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, J.C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; pp. 865–874. [Google Scholar]
- Harmelin-Vivien, M.; Cottalorda, J.M.; Dominici, J.M.; Harmelin, J.G.; Le Diréach, L.; Ruitton, S. Effects of Reserve Protection Level on the Vulnerable Fish Species Sciaena Umbra and Implications for Fishing Management and Policy. Glob. Ecol. Conserv. 2015, 3, 279–287. [Google Scholar] [CrossRef]
- Ragonese, S.; Camilleri, M.; Gancitano, S.; Rizzo, P.; Bono, G.; Fiorentino, F. Evaluating Age at Sexual Maturity in Sciaena Umbra Linnaeus, 1758 (Osteichthyes, Sciaenidae) on the Basis of Otolith Micro-Structure. Biol. Mar. Mediterr. 2002, 9, 789–791. [Google Scholar]
- Engin, S.; Seyhan, K. Age, Growth, Sexual Maturity and Food Composition of Sciaena umbra in the South-eastern Black Sea, Turkey. J. Appl. Ichthyol. 2009, 25, 96–99. [Google Scholar] [CrossRef]
- Chao, L.; Sciaena umbra. The IUCN Red List of Threatened Species 2020. e.T198707A130230194. Available online: https://www.iucnredlist.org/species/198707/130230194 (accessed on 23 February 2022).
- Born, A.F.; Immink, A.J.; Bartley, D.M. Marine and Coastal Stocking: Global Status and Information Needs. Marine Ranching; FAO Fisheries Technical Paper; FAO: Rome, Italy, 2004. [Google Scholar]
- Silberschneider, V.; Gray, C.A. Synopsis of Biological, Fisheries and Aquaculture-Related Information on Mulloway Argyrosomus japonicus (Pisces: Sciaenidae), with Particular Reference to Australia. J. Appl. Ichthyol. 2008, 24, 7–17. [Google Scholar] [CrossRef]
- Hamzaçebi, S.; Can, E. First Results on Spawning and Larval Rearing of the Brown Meagre Sciaena umbra. Nat. Sci. 2021, 2, 22–28. [Google Scholar] [CrossRef]
- Millot, R.; Demolliens, M.; Ducos, S.; Pugliese, S.; Vanalderweireldt, L.; Delmas, A.; Boussard, A.; Aiello, A.; Durieux, E.D.H. Embryonic and Larval Development of Corsican Brown Meagre, Sciaena umbra (Linnaeus 1758), Rearing in Captivity from the Mediterranean Sea. Aquacult. Int. 2023, 31, 117–140. [Google Scholar] [CrossRef]
- Ottolenghi, F.; Silvestri, C.; Giordano, P.; Lovatelli, A.; New, M.B. Capture-Based Aquaculture. The Fattening of Eels, Groupers, Tunas and Yellowtails; FAO: Rome, Italy, 2004. [Google Scholar]
- Cowan, J.H.; Rose, K.A.; DeVries, D.R. Is Density-Dependent Growth in Young-of-the-Year Fishes a Question of Critical Weight? Rev. Fish Biol. Fish. 2000, 10, 61–89. [Google Scholar] [CrossRef]
- Kestemont, P.; Jourdan, S.; Houbart, M.; Mélard, C.; Paspatis, M.; Fontaine, P.; Cuvier, A.; Kentouri, M.; Baras, E. Size Heterogeneity, Cannibalism and Competition in Clutured Predatory Fish Larvae: Iotic and Abiotic Influences. Aquaculture 2003, 227, 333–356. [Google Scholar] [CrossRef]
- Baras, E. Sibling Cannibalism among Juvenile Vundu under Controlled Conditions: I. Cannibalistic Behaviour, Prey Selection and Prey Size Selectivity. J. Fish Biol. 1999, 54, 82–105. [Google Scholar] [CrossRef]
- Baskerville-Bridges, B.; Kling, L.J. Larval Culture of Atlantic Cod (Gadus morhua) at High Stocking Densities. Aquaculture 2000, 181, 61–69. [Google Scholar] [CrossRef]
- Seginer, I. Are Restricted Periods of Over-Stocking of Recirculating Aquaculture Systems Advisable? A Simulation Study. Aquac. Eng. 2009, 41, 194–206. [Google Scholar] [CrossRef]
- Villanueva, R.R.; Araneda, M.E.; Vela, M.; Seijo, J.C. Selecting Stocking Density in Different Climatic Seasons: A Decision Theory Approach to Intensive Aquaculture. Aquaculture 2013, 384–387, 25–34. [Google Scholar] [CrossRef]
- Holm, J.C.; Refstie, T.; Bø, S. The Effect of Fish Density and Feeding Regimes on Individual Growth Rate and Mortality in Rainbow Trout (Oncorhynchus mykiss). Aquaculture 1990, 89, 225–232. [Google Scholar] [CrossRef]
- Millán-Cubillo, A.F.; Martos-Sitcha, J.A.; Ruiz-Jarabo, I.; Cárdenas, S.; Mancera, J.M. Low Stocking Density Negatively Affects Growth, Metabolism and Stress Pathways in Juvenile Specimens of Meagre (Argyrosomus Regius, Asso 1801). Aquaculture 2016, 451, 87–92. [Google Scholar] [CrossRef]
- Roo, J.; Hernandez-Cruz, C.M.; Borrero, C.; Schuchardt, D.; Fernández-Palacios, H. Effect of Larval Density and Feeding Sequence on Meagre (Argyrosomus regius; Asso, 1801) Larval Rearing. Aquaculture 2010, 302, 82–88. [Google Scholar] [CrossRef]
- de Oliveira, E.G.; Pinheiro, A.B.; de Oliveira, V.Q.; da Silva Júnior, A.R.M.; de Moraes, M.G.; Rocha, Í.R.C.B.; Costa, F.H.F. Effects of Stocking Density on the Performance of Juvenile Pirarucu (Arapaima gigas) in Cages. Aquaculture 2012, 370–371, 96–101. [Google Scholar] [CrossRef]
- El-Sayed, A. Effects of Stocking Density and Feeding Levels on Growth and Feed Efficiency of Nile Tilapia (Oreochromis niloticus L.) Fry. Aquacult. Res. 2002, 33, 621–626. [Google Scholar] [CrossRef]
- Sakakura, Y.; Tsukamoto, K. Ontogeny of Aggressive Behaviour in Schools of Yellowtail, Seriola quinqueradiata. Environ. Biol. Fishes 1999, 56, 231–242. [Google Scholar] [CrossRef]
- Schreck, C.B. Stress and Compensation in Teleostean Fishes; Response to Social and Physical Factors. In Stress and Fish; Pickering, A.D., Ed.; Academic Press: Cambridge, MA, USA, 1981; pp. 295–321. [Google Scholar]
- Battaglene, S.C.; Talbot, R.B. Hormone Induction and Larval Rearing of Mulloway, Argyrosomus hololepidotus (Pisces: Sciaenidae). Aquaculture 1994, 126, 73–81. [Google Scholar] [CrossRef]
- Hatziathanasiou, A.; Paspatis, M.; Houbart, M.; Kestemont, P.; Stefanakis, S.; Kentouri, M. Survival, Growth and Feeding in Early Life Stages of European Sea Bass (Dicentrarchus labrax) Intensively Cultured under Different Stocking Densities. Aquaculture 2002, 205, 89–102. [Google Scholar] [CrossRef]
- Alvarez-González, C.A.; Ortiz-Galindo, J.L.; Dumas, S.; Martínez-Díaz, S.F.; Hernández-Ceballos, D.E.; Alamo, T.G.-D.; Moreno-Legorreta, M.; Penta-Martínez, R.; Civera-Cerecedo, R. Effect of Stocking Density on the Growth and Survival of Spotted Sand Bass Paralabrax maculatofasciatus Larvae in a Closed Recirculating System. J. World Aquacult. Soc. 2001, 32, 130–137. [Google Scholar] [CrossRef]
- Saoud, I.P.; Ghanawi, J.; Lebbos, N. Effects of Stocking Density on the Survival, Growth, Size Variation and Condition Index of Juvenile Rabbitfish Siganus rivulatus. Aquacult. Int. 2008, 16, 109–116. [Google Scholar] [CrossRef]
- Millot, R.; Debattice, C.; Filippi, J.J.; Bracconi, J.; Gattacceca, N.; Crescioni, A.; Ronchi-Perfetti, J.B.; Vela, A.; Bastien, R.; Aiello, A. Effect of Stocking Density on Survival and Growth of Post-Settlement Juveniles of Aquaculture Reared Mediterranean Spider Crab Maja squinado (Herbst, 1788). J. World Aquacult. Soc. 2024; Submitted. [Google Scholar]
- Filippi, J.J.; Millot, R.; Bracconi, J.; Ligorini, V.; Gattacceca, N.; Crescioni, A.; Ronchi-Perfetti, J.B.; Demolliens, M.; Pugliese, S.; Delmas, A.; et al. Effect of Light Intensity on the Survival of European Spiny Lobster (Palinurus elephas) Larvae Reared in Aquaculture System. Aquacult. Rep. 2024, 36, 102083. [Google Scholar] [CrossRef]
- Ducos, S.; Pugliese, S.; Demolliens, M.; Beraud, L.; Boussard, A.; Delmas, A.; Agostini, S.; Garcia, J.; Aiello, A.; Durieux, E.D.H. Ontogeny of Swimming Performance of Hatchery-Reared Post-Larvae and Juvenile Fish: A Case of Two Threatened Mediterranean Species. J. Fish Biol. 2022, 101, 846–856. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org (accessed on 12 April 2023).
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; The Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- Therneau, T.M. A Package for Survival Analysis in S. Available online: https://CRAN.R-project.org/package=survival (accessed on 2 July 2015).
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2000. [Google Scholar]
- Kassambara, A.; Kosinski, M.; Biecek, P. Survminer: Drawing Survival Curves Using “Ggplot2”. Available online: https://rpkgs.datanovia.com/survminer/index.html (accessed on 9 March 2021).
- Yengkokpam, S.; Das, B.K.; Debnath, D.; Das, P.; Yadav, A.K.; Sharma, N.; Bhattacharjya, B.K. Effect of Stocking Density on Growth and Yield of Labeo Bata Fingerlings Reared in Cages. Aquacult. Rep. 2020, 18, 100506. [Google Scholar] [CrossRef]
- Keer, N.R.; Datta, M.K.; Patel, A.B.; Priyanka, R.; Rathor, M.K.; Das, S. Effect of Stocking Density on Growth and Survival of Cirrhinus reba (Hamilton, 1822) during Spawn to Fry Nursing (Outdoor). J. Entomol. Zool. Stud. 2018, 6, 640–643. [Google Scholar]
- Kaiser, H.; Weyl, O.; Hecht, T. The Effect of Stocking Density on Growth, Survival and Agonistic Behaviour of African Catfish. Aquacult. Int. 1995, 3, 217–225. [Google Scholar] [CrossRef]
- Niazie, E.H.N.; Imanpoor, M.; Taghizade, V.; Zadmajid, V. Effects of Density Stress on Growth Indices and Survival Rate of Goldfish. Glob. Vet. 2013, 10, 365–371. [Google Scholar]
- Battaglene, S.C.; Talbot, R.B. Induced Spawning and Larval Rearing of Snapper, Pagrus auratus (Pisces: Sparidae), from Australian Waters. N. Z. J. Mar. Freshw. Res. 1992, 26, 179–185. [Google Scholar] [CrossRef]
- Boglione, C.; Giganti, M.; Selmo, C.; Cataudella, S. Morphoecology in Larval Fin-Fish: A New Candidate Species for Aquaculture, Diplodus puntazzo (Sparidae). Aquacult. Int. 2003, 11, 17–41. [Google Scholar] [CrossRef]
- Fernández-Palacios, H.; Montero, D.; Socorro, J.; Izquierdo, M.S.; Vergara, J.M. First Studies on Spawning, Embryonic and Larval Development of Dentex Gibbosus (Rafinesque, 1810) (Osteichthyes, Sparidae) under Controlled Conditions. Aquaculture 1994, 122, 63–73. [Google Scholar] [CrossRef]
- Kawamura, G.; Masuma, S.; Tezuka, N.; Koiso, M.; Jinbo, T.; Namba, K. Morphogenesis of Sense Organs in the Bluefin Tuna Thunnus Orientalis; Browman, H.I., Skiftesvik, A.B., Eds.; University of Bergen: Bergen, Norway, 2003; pp. 123–135. [Google Scholar]
- Papandroulakis, N.; Mylonas, C.C.; Maingot, E.; Divanach, P. First Results of Greater Amberjack (Seriola dumerili) Larval Rearing in Mesocosm. Aquaculture 2005, 250, 155–161. [Google Scholar] [CrossRef]
- Faulk, C.K.; Kaiser, J.B.; Holt, G.J. Growth and Survival of Larval and Juvenile Cobia Rachycentron canadum in a Recirculating Raceway System. Aquaculture 2007, 270, 149–157. [Google Scholar] [CrossRef]
- Hernández-Cruz, C.M.; Salhi, M.; Bessonart, M.; Izquierdo, M.S.; González, M.M.; Fernández-Palacios, H. Rearing Techniques for Red Porgy (Pagrus pagrus) during Larval Development. Aquaculture 1999, 179, 489–497. [Google Scholar] [CrossRef]
- Lambert, Y.; Dutil, J.D. Food Intake and Growth of Adult Atlantic Cod (Gadus morhua L.) Reared under Different Conditions of Stocking Density, Feeding Frequency and Size-Grading. Aquaculture 2001, 192, 233–247. [Google Scholar] [CrossRef]
- Leu, M.Y.; Chou, Y. Induced Spawning and Larval Rearing of Captive Yellowfin Porgy Acanthopagrus Latus (Houttuyn). Aquaculture 1996, 143, 155–166. [Google Scholar] [CrossRef]
- Papoutsoglou, S.E.; Papaparaskeva-Papoutsoglou, E.; Alexis, M.N. Effect of Density on Growth Rate and Production of Rainbow Trout (Salmo gairdneri Rich.) over a Full Rearing Period. Aquaculture 1987, 66, 9–17. [Google Scholar] [CrossRef]
- Roo, J.; Hernández-Cruz, C.M.; Fernández-Palacios, H.Y.; Izquierdo, M.S. Development of skeletal deformities in gilthead sea bream (Sparus aurata) reared under different larval culture and dietary conditions. Eur. Aquac. Soc. Spec. Publ. 2005, 36, 441–444. [Google Scholar]
- Roo, J.; Hernández-Cruz, C.M.; Socorro, J.A.; Fernández-Palacios, H.; Izquierdo, M.S. Effect of rearing techniques over, survival growth and skeletal abnormalities development in red porgy (Pagrus pagrus) larvae. In Proceedings of the 29th Annual Larval Fish Conference, Barcelona, Spain, 11–14 July 2005. [Google Scholar]
- Wendelaar Bonga, S.E. The Stress Response in Fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Hecht, T.; Battaglene, S.; Talbot, B. Effect of Larval Density and Food Availability on the Behaviour of Pre-Metomorphosis Snapper, Pagrus auratus (Sparidae). Mar. Freshw. Res. 1996, 47, 223–231. [Google Scholar]
- Yu, M.L.; Perlmutter, A. Growth Inhibiting Factors in the Zebrafish, Brachydanio Rerio and the Blue Gourami, Trichogaster trichopterus. Growth 1970, 34, 153–175. [Google Scholar] [PubMed]
- Hagen, N. Effect of Different Prey and Larval Densities on the Gut Content of Plaice (Pleuronectes Platessa L.) at Initial Feeding. In Physiological and Biochemical Aspects of Fish Development; Walther, B.T., Fynn, H.J., Eds.; University of Bergen: Bergen, Norway, 1993; pp. 180–182. [Google Scholar]
- Liu, H.W.; Stickney, R.R.; Dickhoff, W.W.; McCaughran, D.A. Early Larval Growth of Pacific Halibut Hippoglossus stenolepis. J. World Aquacult. Soc. 1993, 24, 482–485. [Google Scholar] [CrossRef]
- Wedemeyer, G.A.; McLeay, D.J. Methods for Determining the Tolerance of Fishes to Environmental Stressors. In Stress and Fish; Pickering, D., Ed.; Academic Press: Cambridge, MA, USA, 1981; pp. 51–78. [Google Scholar]
- Wallace, J.C.; Kolbeinshavn, A.; Reinsnes, T.G. The Effects of Stocking Density on Early Growth in Arctic Charr, Salveelinus alpinus (L.). Aquaculture 1988, 73, 101–110. [Google Scholar] [CrossRef]
- Jørgensen, E.H.; Christiansen, J.S.; Jobling, M. Effects of Stocking Density on Food Intake, Growth Performance and Oxygen Consumption in Arctic Charr (Salvelinus alpinus). Aquaculture 1993, 110, 191–204. [Google Scholar] [CrossRef]
- Papoutsoglou, S.E.; Tziha, G.; Vrettos, X.; Athanasiou, A. Effects of Stocking Density on Behavior and Growth Rate of European Sea Bass (Dicentrarchus Labrax) Juveniles Reared in a Closed Circulated System. Aquacult. Eng. 1998, 18, 135–144. [Google Scholar] [CrossRef]
- Buckley, L.J.; Smigielski, A.S.; Halavik, T.A.; Burns, B.R.; Laurence, G.C. Growth and Survival of the Larvae of Three Temperate Marine Fish Species at Discrete Prey Densities. II. Cod (Gadus morhua), Winter Flounder (Pseudopleuronectes Americanus), and Silver Hake (Merluccius bilinearis). In Physiological and Biochemical Aspects of Fish Development; Walther, B.T., Fyhn, H.J., Eds.; University of Bergen: Bergen, Norway, 1993; pp. 183–195. [Google Scholar]
- Parra, G.; Yufera, M. Feeding, Physiology and Growth Responses in First-Feeding Gilthead Sea Bream (Sparus Aurata L.) Larvae in Relation to Prey Density. J. Exp. Mar. Biol. Ecol. 2000, 243, 1–15. [Google Scholar] [CrossRef]
- Giménez, G.; Estévez, A. Effects of Two Culturing Techniques on the Growth, Survival and Larval Quality of Dentex Dentex Linnaeus, 1758. Aquacult. Res. 2008, 39, 354–361. [Google Scholar] [CrossRef]
- Rabe, J.; Brown, J.A. A Pulse Feeding Strategy for Rearing Larval Fish: An Experiment with Yellowtail Flounder. Aquaculture 2000, 191, 289–302. [Google Scholar] [CrossRef]
- Imorou Toko, I.; Fiogbé, E.D.; Kestemont, P. Determination of Appropriate Age and Stocking Density of Vundu Larvae, Heterobranchus Longifilis (Valenciennes 1840), at the Weaning Time. Aquacult. Res. 2008, 39, 24–32. [Google Scholar] [CrossRef]
- Fleming, I.A.; Johansen, P.H. Density and Agonistic Behavior in Young-of-the-Year Largemouth Bass Micropterus salmoides. Can. J. Zool. 1984, 62, 1454–1455. [Google Scholar] [CrossRef]
- Brown, G.E.; Brown, J.A.; Srivastava, R.K. The Effect of Stocking Density on the Behavior of Arctic Charr Salvelinus alpinus L. J. Fish Biol. 1992, 41, 955–963. [Google Scholar] [CrossRef]
- Abreu, E.T. Cultivo Larvario de Corvina Negra (Sciaena Umbra) y Sargo Picudo (Puntazzo Puntazzo) Mediante La Tecnología Semi-Intensiva de Mesocosmos: Aspectos Biológicos y Tecnológicos; Universidad de Las Palmas de Gran Canaria: Las Palmas de Gran Canaria, Spain, 2004. [Google Scholar]
- Moran, D. Size Heterogeneity, Growth Potential and Aggression in Juvenile Yellowtail Kingfish (Seriola lalandi, Valenciennes). Aquacult. Res. 2007, 38, 1254–1264. [Google Scholar] [CrossRef]
- Hepher, B.; Prugnin, Y. Commercial Fish Farming: With Special Reference to Fish Culture in Israel; Wiley: London, UK, 1981. [Google Scholar]
- Koebele, B.P. Growth and Size Hierarchy Effect: An Experimental Assessment of Three Proposed Mechanisms of Activity Differences, Disproportional Food Acquisition Physiological Stress. Environ. Biol. Fishes 1985, 12, 181–188. [Google Scholar] [CrossRef]
- Huntingford, F.A.; Metcalfe, N.B.; Thorpe, J.E.; Graham, W.D.; Adams, C.E. Social Dominance and Body Size in Atlantic Salmon Parr, Salmo salar L. J. Fish Biol. 1990, 36, 877–881. [Google Scholar] [CrossRef]
- Hecht, T.; Pienaar, A.G. A Review of Cannibalism and Its Implications in Fish Larviculture. J. World Aquacult. Soc. 1993, 24, 246–261. [Google Scholar] [CrossRef]
- Goldan, O.; Popper, D.; Karplus, I. Management of Size Variation in Juvenile Gilthead Sea Bream (Sparus aurata). Particle Size and Frequency of Feeding Dry and Live Food. Aquaculture 1997, 152, 181–190. [Google Scholar] [CrossRef]
- Ashley, P.J. Fish Welfare: Current Issues in Aquaculture. Appl. Anim. Behav. Sci. 2007, 104, 199–235. [Google Scholar] [CrossRef]
- Pirozzi, I.; Booth, M.; Pankhurst, P. The Effect of Stocking Density and Repeated Handling on the Growth of Juvenile Mulloway, Argyrosomus japonicus (Temminck & Schlegel 1843). Aquacult. Int. 2009, 17, 199–205. [Google Scholar] [CrossRef]
- Abou, Y.; Fiogbé, E.D.; Micha, J.C. Effects of Stocking Density on Growth, Yield and Profitability of Farming Nile Tilapia, Oreochromis niloticus L., Fed Azolla Diet, in Earthen Ponds. Aquacult. Res. 2007, 38, 595–604. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).