The Relationship between Mean Length at Maturity and Maximum Length in Coral Reef Fish
Abstract
1. Introduction

2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| No. | Family | Species | Location | Sex | Lmax (cm) | Lm (cm) | Wmax (g) | D | LmaxD | LmD | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Acanthuridae | Acanthurus chirurgus | Pedro Bank, Jamaica | M | 35.0 | 17.0 | 978.3 | 0.66 | 10.33 | 6.43 | [41] |
| 2 | Acanthuridae | Acanthurus lineatus | Tutuila Isl. Amer. | U | 28.9 | 18.0 | 670.2 | 0.67 | 9.66 | 7.02 | [42] |
| 3 | Acanthuridae | Acanthurus lineatus | Pohnpei State, Micronesia | F | 20.5 | 16.8 | 250.3 | 0.72 | 8.81 | 7.63 | [43] |
| 4 | Acanthuridae | Acanthurus nigricauda | Pohnpei State, Micronesia | F | 22.6 | 18.4 | 311.2 | 0.71 | 9.15 | 7.91 | [43] |
| 5 | Acanthuridae | Acanthurus nigrofuscus | Coil reef, Northern Queensland | U | 15.5 | 10.5 | 85.7 | 0.77 | 8.26 | 6.12 | [44] |
| 6 | Acanthuridae | Acanthurus nigrofuscus | Yankee reef, N. Queensland | U | 17.9 | 10.5 | 108.7 | 0.76 | 8.95 | 5.96 | [44] |
| 7 | Acanthuridae | Acanthurus triostegus | Lakshadweep lagoons, India | U | 17.5 | 7.3 | 154.3 | 0.74 | 8.36 | 4.38 | [45] |
| 8 | Acanthuridae | Naso lituratus | Terengganu, Malaysia | U | 38.1 | 19.9 | 1331.2 | 0.64 | 10.36 | 6.82 | [46] |
| 9 | Albulidae | Albula vulpes | Florida Keys, US | F | 70.0 | 48.8 | 5534.2 | 0.58 | 11.56 | 9.39 | [47] |
| 10 | Albulidae | Albula vulpes | Florida Keys, US | M | 70.2 | 41.8 | 5584.8 | 0.58 | 11.56 | 8.57 | [47] |
| 11 | Apogonidae | Cheilodipterus artus | Terengganu, Malaysia | U | 17.7 | 11.2 | 112.6 | 0.76 | 8.80 | 6.21 | [46] |
| 12 | Apogonidae | Cheilodipterus macrodon | Terengganu, Malaysia | U | 23.6 | 15.1 | 306.8 | 0.71 | 9.46 | 6.89 | [46] |
| 13 | Apogonidae | Cheilodipterus quinquelineatus | Terengganu, Malaysia | U | 11.9 | 8.2 | 26.8 | 0.82 | 7.69 | 5.68 | [46] |
| 14 | Apogonidae | Ostorhinchus compressus | Terengganu, Malaysia | U | 11.1 | 7.4 | 24.2 | 0.83 | 7.34 | 5.24 | [46] |
| 15 | Apogonidae | Pterapogon kauderni | Banggai Archipelago | U | 7.6 | 4.9 | 10.4 | 0.87 | 5.79 | 3.99 | [48] |
| 16 | Balistidae | Balistapus undulatus | Kavieng, PNG | F | 20.2 | 15.7 | 217.6 | 0.73 | 8.89 | 7.40 | [49] |
| 17 | Balistidae | Balistes capriscus | Ghana | F | 34.0 | 14.5 | 679.5 | 0.67 | 10.76 | 6.06 | [50,51] |
| 18 | Balistidae | Balistes vetula | Pedro Bank, Jamaica | F | 39.0 | 23.5 | 1936.2 | 0.62 | 9.87 | 7.19 | [52] |
| 19 | Balistidae | Balistes vetula | Pedro Bank, Jamaica | M | 44.0 | 26.5 | 2738.9 | 0.61 | 10.01 | 7.35 | [52] |
| 20 | Belonidae | Tylosurus acus | Suez Canal, Egypt | M | 74.5 | 45.9 | 727.3 | 0.67 | 18.00 | 13.02 | [53] |
| 21 | Belonidae | Tylosurus acus | Suez Canal, Egypt | F | 74.5 | 45.3 | 727.3 | 0.67 | 18.00 | 12.91 | [53] |
| 22 | Belonidae | Tylosurus crocodilus | Suez Canal, Egypt | M | 94.4 | 50.1 | 1759.7 | 0.63 | 17.51 | 11.75 | [53] |
| 23 | Belonidae | Tylosurus crocodilus | Suez Canal, Egypt | F | 94.4 | 49.5 | 1759.7 | 0.63 | 17.51 | 11.66 | [53] |
| 24 | Carangidae | Alepes djedaba | Kerala, India | U | (26.2) | 16.0 | 339.0 | 0.71 | 10.03 | 7.08 | [54] |
| 25 | Carangidae | Alepes kleinii | SW Coast, India | U | (14.2) | 11.3 | 58.9 | 0.79 | 8.09 | 6.75 | [54] |
| 26 | Carangidae | Atule mate | Kerala, India | U | (29.0) | 15.4 | 360.9 | 0.70 | 10.68 | 6.85 | [54] |
| 27 | Carangidae | Carangoides bajad | Shathleen, Egypt | U | 56.4 | 34.8 | 2970.5 | 0.61 | 11.47 | 8.56 | [55] |
| 28 | Carangidae | Carangoides bajad | Coast of Abu Dhabi, UAE | U | (38.4) | 24.7 | 697.7 | 0.67 | 11.62 | 8.64 | [56] |
| 29 | Carangidae | Carangoides equula | Northern South China Sea | U | (28.1) | 18.7 | 513.0 | 0.69 | 9.88 | 7.48 | [57] |
| 30 | Carangidae | Caranx heberi | South Africa | U | 100.0 | 50.0 | 19,887.8 | 0.52 | 10.79 | 7.54 | [58,59] |
| 31 | Carangidae | Caranx ignobilis | Northwestern Islands, Hawaii | U | 162.6 | 56.0 | 83,360.9 | 0.45 | 9.87 | 6.12 | [60] |
| 32 | Carangidae | Caranx melampygus | Northwestern Islands, Hawaii | U | 70.8 | 32.7 | 10,551.0 | 0.55 | 10.23 | 6.71 | [60] |
| 33 | Carangidae | Caranx melampygus | Shathleen, Egypt | U | 73.9 | 44.3 | 652.8 | 0.68 | 18.30 | 12.96 | [55] |
| 34 | Carangidae | Caranx sexfasciatus | South Africa | U | 80.0 | 50.0 | 9456.4 | 0.55 | 11.19 | 8.64 | [58,59] |
| 35 | Carangidae | Decapterus macrosoma | Java Sea, Indonesia | M | 20.1 | 13.7 | 75.6 | 0.78 | 10.27 | 7.61 | [61,62] |
| 36 | Carangidae | Decapterus macrosoma | Java Sea, Indonesia | F | 20.1 | 14.3 | 75.6 | 0.78 | 10.27 | 7.88 | [61,62] |
| 37 | Carangidae | Decapterus maruadsi | East China Sea | U | 20.8 | 17.5 | 133.1 | 0.75 | 9.74 | 8.55 | [63,64] |
| 38 | Carangidae | Decapterus maruadsi | Gulf of Tonkin/Beibu Gulf | U | 24.2 | 17.1 | 100.1 | 0.76 | 11.38 | 8.74 | [65] |
| 39 | Carangidae | Decapterus punctatus | South Atlantic Bight | U | 21.0 | 11.0 | 125.3 | 0.75 | 9.88 | 6.08 | [66,67] |
| 40 | Carangidae | Elagatis bipinnulata | Pernambuco, Brazil | F | 97.0 | 64.6 | 7563.3 | 0.56 | 13.05 | 10.39 | [68] |
| 41 | Carangidae | Megalaspis cordyla | SW coast, India | U | (33.6) | 22.5 | 502.2 | 0.69 | 11.22 | 8.50 | [54] |
| 42 | Carangidae | Megalaspis cordyla | East Coast, India | U | (35.0) | 22.5 | 517.0 | 0.69 | 11.47 | 8.46 | [54] |
| 43 | Carangidae | Megalaspis cordyla | NW Coast India | U | (44.8) | 22.5 | 837.5 | 0.66 | 12.48 | 7.89 | [54] |
| 44 | Carangidae | Parastromateus niger | Taiwan Strait, Taiwan | U | 30.5 | 19.1 | 1131.1 | 0.65 | 9.22 | 6.80 | [69] |
| 45 | Carangidae | Scomberoides commersonnianus | Weipa region, Queensland, Australia | M | (108.3) | 38.5 | 11,888.7 | 0.54 | 12.58 | 7.19 | [70] |
| 46 | Carangidae | Scomberoides commersonnianus | Weipa region, Queensland, Australia | F | (122.6) | 63.5 | 16,788.9 | 0.52 | 12.45 | 8.82 | [70] |
| 47 | Carangidae | Selar crumenophthalmus | Caribbean coast, Colombia | U | (27.8) | 19.6 | 342.3 | 0.71 | 10.45 | 8.18 | [71] |
| 48 | Carangidae | Selaroides leptolepis | Tamil Nadu/Pondicherry, India | U | (17.0) | 8.9 | 69.0 | 0.78 | 9.12 | 5.53 | [54] |
| 49 | Carangidae | Selaroides leptolepis | Inner Gulf of Thailand | U | (16.8) | 8.9 | 80.4 | 0.77 | 8.87 | 5.44 | [72] |
| 50 | Carangidae | Seriola dumerili | Pelagie Islands, Italy | F | 157.2 | 114.3 | 43,955.9 | 0.48 | 11.31 | 9.70 | [73] |
| 51 | Carangidae | Seriola dumerili | Pelagie Islands, Italy | M | 157.2 | 118.4 | 43,009.6 | 0.48 | 11.37 | 9.92 | [73] |
| 52 | Carangidae | Trachinotus falcatus | Florida Keys/Tampa Bay, US | M | 85.5 | 48.6 | 13,816.4 | 0.53 | 10.73 | 7.94 | [74] |
| 53 | Carangidae | Trachinotus falcatus | Florida Keys/Tampa Bay, US | F | 91.6 | 54.7 | 16,760.5 | 0.52 | 10.69 | 8.16 | [74] |
| 54 | Carangidae | Trachurus lathami | Southern region, Brazil | U | 21.4 | 11.8 | 118.8 | 0.75 | 10.10 | 6.43 | [75] |
| 55 | Centriscidae | Centriscus scutatus | Terengganu, Malaysia | U | 15.0 | 10.0 | 4.2 | 0.91 | 11.78 | 8.14 | [46] |
| 56 | Chaenopsidae | Acanthemblemaria paula | Carrie Bow Cay, Belize | U | 2.0 | 1.3 | 0.0 | 1.12 | 2.17 | 1.31 | [76] |
| 57 | Chaetodontidae | Chaetodon auriga | Lakshadweep lagoons, India | U | 14.9 | 13.0 | 86.3 | 0.77 | 8.00 | 7.20 | [45] |
| 58 | Dorosomatidae | Amblygaster sirm | Lagoons, New Caledonia | U | 21.0 | 14.6 | 71.3 | 0.78 | 10.72 | 8.06 | [77] |
| 59 | Dorosomatidae | Herklotsichthys quadrimaculatus | Seychelles | U | 12.8 | 10.1 | 31.2 | 0.82 | 8.03 | 6.62 | [78] |
| 60 | Dorosomatidae | Opisthonema oglinum | Ceará, Brazil | M | 17.0 | 11.0 | 69.3 | 0.78 | 9.12 | 6.49 | [79,80] |
| 61 | Dorosomatidae | Opisthonema oglinum | Ceará, Brazil | F | 17.0 | 11.5 | 69.3 | 0.78 | 9.12 | 6.72 | [79,80] |
| 62 | Dorosomatidae | Opisthonema oglinum | Pernambuco, Brazil | U | 22.4 | 12.5 | 126.4 | 0.75 | 10.35 | 6.69 | [81] |
| 63 | Dorosomatidae | Sardinella albella | Mandapam, India | U | (10.9) | 7.8 | 16.7 | 0.85 | 7.56 | 5.67 | [82] |
| 64 | Engraulidae | Encrasicholina devisi | Ysabel Passage, PNG | U | (6.2) | 3.6 | 1.9 | 0.95 | 5.67 | 3.40 | [83] |
| 65 | Engraulidae | Encrasicholina devisi | Karnataka, India | U | 9.6 | 6.0 | 5.3 | 0.90 | 7.64 | 5.04 | [84] |
| 66 | Engraulidae | Encrasicholina heteroloba | Singapore Strait | U | (8.9) | 5.3 | 7.4 | 0.88 | 6.92 | 4.36 | [85] |
| 67 | Engraulidae | Stolephorus insularis | Singapore Strait | U | (10.0) | 5.3 | 8.1 | 0.88 | 7.56 | 4.33 | [85] |
| 68 | Fistulariidae | Fistularia commersonii | Mediterranean Sea, Lebanon | F | 113.0 | 65.4 | 1969.1 | 0.62 | 19.12 | 13.59 | [86] |
| 69 | Fistulariidae | Fistularia commersonii | Mediterranean Sea, Lebanon | M | 100.0 | 54.7 | 1368.0 | 0.64 | 19.16 | 13.01 | [86] |
| 70 | Gerreidae | Gerres filamentosus | Manila Bay, Philippines | M | 14.3 | 8.4 | 50.9 | 0.79 | 8.25 | 5.43 | [87] |
| 71 | Gerreidae | Gerres filamentosus | Manila Bay, Philippines | F | 12.7 | 7.9 | 35.6 | 0.81 | 7.84 | 5.35 | [87] |
| 72 | Gerreidae | Gerres longirostris | Southern Arabian Gulf | M | (17.9) | 16.3 | 1680.6 | 0.63 | 6.18 | 5.83 | [88] |
| 73 | Gerreidae | Gerres longirostris | Southern Arabian Gulf | F | (20.1) | 20.6 | 2404.1 | 0.61 | 6.34 | 6.43 | [88] |
| 74 | Gobiidae | Eviota melasma | Lizard Island, Australia | M | 2.7 | 1.1 | 0.1 | 1.07 | 2.91 | 1.10 | [89] |
| 75 | Gobiidae | Eviota melasma | Lizard Island, Australia | F | 2.7 | 1.2 | 0.1 | 1.07 | 2.91 | 1.16 | [89] |
| 76 | Gobiidae | Eviota queenslandica | Lizard Island, Australia | M | 2.6 | 1.3 | 0.1 | 1.08 | 2.77 | 1.34 | [89] |
| 77 | Gobiidae | Eviota queenslandica | Lizard Island, Australia | F | 2.6 | 1.4 | 0.1 | 1.08 | 2.77 | 1.43 | [89] |
| 78 | Gobiidae | Eviota sigillata | Lizard Island, Australia | M | 1.8 | 1.1 | 0.0036 | 1.13 | 1.94 | 1.13 | [89] |
| 79 | Gobiidae | Eviota sigillata | Lizard Island, Australia | F | 1.8 | 1.1 | 0.0036 | 1.13 | 1.94 | 1.14 | [89] |
| 80 | Gobiidae | Exyrias belissimus | Terengganu, Malaysia | U | 15.0 | 10.0 | 31.8 | 0.82 | 9.12 | 6.55 | [46] |
| 81 | Gobiidae | Istigobius decoratus | Terengganu, Malaysia | U | 13.0 | 9.0 | 22.4 | 0.83 | 8.46 | 6.23 | [46] |
| 82 | Gobiidae | Istigobius goldmanni | Terengganu, Malaysia | U | 6.0 | 5.0 | 2.3 | 0.94 | 5.37 | 4.53 | [46] |
| 83 | Haemulidae | Diagramma pictum | Southern Arabian Gulf | M | (57.6) | 30.7 | 1832.3 | 0.63 | 12.72 | 8.58 | [90] |
| 84 | Haemulidae | Diagramma pictum | Southern Arabian Gulf | F | (60.6) | 31.8 | 2137.0 | 0.62 | 12.76 | 8.55 | [90] |
| 85 | Haemulidae | Diagramma pictum | Arabian Gulf, Kuwait | U | (69.1) | 52.3 | 4963.3 | 0.58 | 11.72 | 9.97 | [91] |
| 86 | Haemulidae | Haemulon aurolineatum | Pernambuco, Brazil | M | 23.5 | 15.3 | 178.1 | 0.74 | 10.21 | 7.45 | [92] |
| 87 | Haemulidae | Haemulon aurolineatum | Pernambuco, Brazil | F | 23.5 | 15.0 | 178.1 | 0.74 | 10.21 | 7.34 | [92] |
| 88 | Haemulidae | Haemulon plumierii | Ceará State, Bazil | F | 34.3 | 16.9 | 843.6 | 0.66 | 10.45 | 6.53 | [93] |
| 89 | Haemulidae | Haemulon plumierii | Ceará State, Brazil | M | 27.7 | 18.6 | 446.9 | 0.69 | 10.00 | 7.59 | [93] |
| 90 | Haemulidae | Pomadasys stridens | Gulf of Suez | F | 18.3 | 10.3 | 104.9 | 0.76 | 9.13 | 5.90 | [94] |
| 91 | Haemulidae | Pomadasys stridens | Gulf of Suez | M | 18.3 | 9.1 | 104.9 | 0.76 | 9.13 | 5.36 | [94] |
| 92 | Hemiramphidae | Hemiramphus brasiliensis | Pernambuco, Brazil | M | 29.9 | 18.6 | 229.7 | 0.72 | 11.71 | 8.31 | [95] |
| 93 | Hemiramphidae | Hemiramphus brasiliensis | Pernambuco, Brazil | F | 29.9 | 19.3 | 229.7 | 0.72 | 11.71 | 8.53 | [95] |
| 94 | Hemiramphidae | Hemiramphus far | Bardawil lagoon, Egypt | M | 27.6 | 21.1 | 128.3 | 0.75 | 12.10 | 9.87 | [96] |
| 95 | Hemiramphidae | Hemiramphus far | Bardawil lagoon, Egypt | F | 28.1 | 21.3 | 127.9 | 0.75 | 12.25 | 9.94 | [96] |
| 96 | Holocentridae | Holocentrus adscensionis | Pernambuco, Brazil | F | 17.8 | 12.1 | 211.0 | 0.73 | 8.13 | 6.13 | [97,98] |
| 97 | Holocentridae | Holocentrus rufus | Jamaica | F | 23.0 | 13.5 | 206.8 | 0.73 | 9.84 | 6.67 | [99] |
| 98 | Holocentridae | Myripristis murdjan | Lakshadweep lagoons, India | U | 19.2 | 15.6 | 212.9 | 0.73 | 8.59 | 7.39 | [45] |
| 99 | Holocentridae | Sargocentron rubrum | Terengganu, Malaysia | U | 29.1 | 18.2 | 571.9 | 0.68 | 9.94 | 7.22 | [46] |
| 100 | Kyphosidae | Kyphosus bigibbus | Northwest Kyushu, Japan | F | 57.4 | 36.0 | 3327.5 | 0.60 | 11.35 | 8.58 | [100] |
| 101 | Kyphosidae | Kyphosus bigibbus | Northwest Kyushu, Japan | M | 50.6 | 28.4 | 2320.0 | 0.62 | 11.24 | 7.87 | [100] |
| 102 | Kyphosidae | Kyphosus cinerascens | Kavieng, Papua New Guinea | F | 34.0 | 22.6 | 935.2 | 0.66 | 10.21 | 7.80 | [49] |
| 103 | Kyphosidae | Kyphosus cinerascens | Kavieng, Papua New Guinea | M | 30.0 | 20.1 | 647.3 | 0.68 | 9.97 | 7.60 | [49] |
| 104 | Labridae | Halichoeres hortulanus | Lakshadweep lagoons, India | U | 28.9 | 12.8 | 356.2 | 0.70 | 10.67 | 6.02 | [45] |
| 105 | Labridae | Halichoeres marginatus | Lakshadweep lagoons, India | U | 17.9 | 7.0 | 99.6 | 0.76 | 9.04 | 4.42 | [45] |
| 106 | Lethrinidae | Lethrinus borbonicus | Southern Arabian Gulf | M | 28.7 | 22.1 | 366.8 | 0.70 | 10.57 | 8.80 | [101] |
| 107 | Lethrinidae | Lethrinus borbonicus | Southern Arabian Gulf | F | 28.7 | 21.3 | 366.8 | 0.70 | 10.57 | 8.57 | [101] |
| 108 | Lethrinidae | Lethrinus borbonicus | Gulf of Suez, South Sinai coast | U | 27.6 | 19.4 | 426.8 | 0.70 | 10.05 | 7.88 | [102] |
| 109 | Lethrinidae | Lethrinus borbonicus | Foul Bay, Egypt, Red Sea | U | 28.9 | 19.3 | 501.9 | 0.69 | 10.11 | 7.65 | [103] |
| 110 | Lethrinidae | Lethrinus lentjan | Southern Arabian Gulf | M | (29.2) | 24.6 | 446.9 | 0.69 | 10.36 | 9.21 | [104] |
| 111 | Lethrinidae | Lethrinus lentjan | Southern Arabian Gulf | F | (32.4) | 27.7 | 604.7 | 0.68 | 10.61 | 9.54 | [104] |
| 112 | Lethrinidae | Lethrinus microdon | Southern Arabian Gulf | M | (32.6) | 27.4 | 512.8 | 0.69 | 10.94 | 9.72 | [101] |
| 113 | Lethrinidae | Lethrinus microdon | Southern Arabian Gulf | F | (32.0) | 29.1 | 487.2 | 0.69 | 10.90 | 10.21 | [101] |
| 114 | Lethrinidae | Lethrinus nebulosus | Southern Arabian Gulf | M | 54.1 | 28.6 | 2230.2 | 0.62 | 11.80 | 7.95 | [90] |
| 115 | Lethrinidae | Lethrinus nebulosus | Southern Arabian Gulf | F | 55.7 | 27.6 | 2423.5 | 0.61 | 11.82 | 7.68 | [90] |
| 116 | Lethrinidae | Monotaxis grandoculis | Pohnpei state, Micronesia | F | 33.0 | 27.5 | 858.7 | 0.66 | 10.15 | 9.00 | [43] |
| 117 | Lutjanidae | Aphareus rutilans | South China Sea | U | (67.2) | 41.7 | 5356.0 | 0.58 | 11.36 | 8.62 | [105] |
| 118 | Lutjanidae | Aprion virescens | Hawaii, US | F | 102.8 | 44.9 | 15,361.5 | 0.53 | 11.57 | 7.47 | [106] |
| 119 | Lutjanidae | Apsilus dentatus | Jamaica | F | 54.0 | 40.0 | 2346.2 | 0.62 | 11.67 | 9.70 | [107,108] |
| 120 | Lutjanidae | Apsilus dentatus | Jamaica | M | 56.0 | 44.0 | 2634.2 | 0.61 | 11.68 | 10.08 | [107,108] |
| 121 | Lutjanidae | Etelis coruscans | Hawaii, US | F | 96.9 | 66.3 | 13,830.8 | 0.53 | 11.47 | 9.37 | [106] |
| 122 | Lutjanidae | Lutjanus apodus | Great Barrier Reef, Australia | M | 92.8 | 34.3 | 11,905.6 | 0.54 | 11.57 | 6.76 | [109] |
| 123 | Lutjanidae | Lutjanus apodus | Jamaica | F | 57.0 | 25.0 | 3764.4 | 0.59 | 11.04 | 6.77 | [107] |
| 124 | Lutjanidae | Lutjanus bohar | Great Barrier Reef, Australia | F | 67.5 | 42.9 | 5932.5 | 0.57 | 11.17 | 8.61 | [110] |
| 125 | Lutjanidae | Lutjanus buccanella | Jamaica | F | 49.0 | 24.0 | 1741.2 | 0.63 | 11.61 | 7.40 | [107,108] |
| 126 | Lutjanidae | Lutjanus buccanella | Jamaica | M | 49.0 | 26.0 | 1494.1 | 0.64 | 11.93 | 7.97 | [107,108] |
| 127 | Lutjanidae | Lutjanus carponotatus | Palm Island, GBR, Australia | F | 33.7 | 19.0 | 558.5 | 0.68 | 11.05 | 7.47 | [111] |
| 128 | Lutjanidae | Lutjanus carponotatus | Lizard Island, Australia | F | 35.4 | 19.0 | 646.4 | 0.68 | 11.15 | 7.32 | [111] |
| 129 | Lutjanidae | Lutjanus ehrenbergii | Southern Arabian Gulf | U | (23.0) | 20.4 | 199.1 | 0.73 | 9.89 | 9.06 | [104] |
| 130 | Lutjanidae | Lutjanus ehrenbergii | Southern Arabian Gulf | M | (20.8) | 19.9 | 148.0 | 0.74 | 9.59 | 9.27 | [104] |
| 131 | Lutjanidae | Lutjanus erythropterus | Great Barrier Reef, Australia | U | 62.4 | 48.5 | 34,639.3 | 0.49 | 7.60 | 6.72 | [112] |
| 132 | Lutjanidae | Lutjanus fulviflamma | Southern Arabian Gulf | M | (21.2) | 16.7 | 254.6 | 0.72 | 8.99 | 7.58 | [113] |
| 133 | Lutjanidae | Lutjanus fulviflamma | Southern Arabian Gulf | F | (22.4) | 18.7 | 301.0 | 0.71 | 9.14 | 8.04 | [113] |
| 134 | Lutjanidae | Lutjanus fulviflamma | Okinawa island | F | 34.2 | 19.6 | 931.8 | 0.66 | 10.25 | 7.11 | [114] |
| 135 | Lutjanidae | Lutjanus fulvus | Yaeyama Isl., Okinawa, Japan | M | 31.4 | 20.7 | 495.9 | 0.69 | 10.73 | 8.05 | [115] |
| 136 | Lutjanidae | Lutjanus fulvus | Yaeyama Isl., Okinawa, Japan | F | 33.2 | 22.5 | 585.6 | 0.68 | 10.85 | 8.32 | [115] |
| 137 | Lutjanidae | Lutjanus gibbus | Pohnpei State, Micronesia | F | 33.5 | 21.5 | 756.8 | 0.67 | 10.47 | 7.78 | [43] |
| 138 | Lutjanidae | Lutjanus lutjanus | Persian Gulf and Sea of Oman | U | 25.5 | 17.2 | 31.5 | 0.82 | 14.08 | 10.19 | [116] |
| 139 | Lutjanidae | Lutjanus malabaricus | Great Barrier Reef, Australia | F | 81.0 | 59.5 | 6923.8 | 0.57 | 12.01 | 10.09 | [117] |
| 140 | Lutjanidae | Lutjanus sebae | Great Barrier Reef, Australia | F | 72.0 | 54.8 | 7956.9 | 0.56 | 10.93 | 9.38 | [117] |
| 141 | Lutjanidae | Lutjanus synagris | Jamaica | F | 43.0 | 26.8 | 1288.8 | 0.64 | 11.27 | 8.31 | [118] |
| 142 | Lutjanidae | Lutjanus griseus | Florida, US | F | 72.4 | 23.0 | 6463.8 | 0.57 | 11.43 | 5.95 | [119,120] |
| 143 | Megalopidae | Megalops atlanticus | Santa Fe, Ceará State, Brazil | M | 153.6 | 120.0 | 23,369.2 | 0.51 | 12.97 | 11.44 | [121,122] |
| 144 | Megalopidae | Megalops atlanticus | Santa Fe, Ceará State, Brazil | F | 181.6 | 160.0 | 30,615.0 | 0.50 | 13.23 | 12.42 | [121,122] |
| 145 | Menidae | Mene maculata | Taiwan | U | 23.0 | 15.3 | 263.8 | 0.72 | 9.49 | 7.10 | [123] |
| 146 | Monacanthidae | Aluterus monoceros | Veraval, India | U | 58.9 | 48.5 | 2031.2 | 0.62 | 12.66 | 11.22 | [124] |
| 147 | Mugilidae | Mugil curema | Sergipe State, Brazil | M | 29.6 | 25.1 | 317.9 | 0.71 | 11.04 | 9.82 | [125] |
| 148 | Mugilidae | Mugil curema | Sergipe State, Brazil | F | 34.3 | 22.5 | 496.6 | 0.69 | 11.39 | 8.52 | [125] |
| 149 | Mullidae | Mulloidichthys flavolineatus | Lakshadweep lagoons, India | U | 24.2 | 16.0 | 200.7 | 0.73 | 10.27 | 7.58 | [45] |
| 150 | Mullidae | Mulloidichthys martinicus | Jamaica | F | 28.0 | 18.0 | 410.8 | 0.70 | 10.21 | 7.50 | [126] |
| 151 | Mullidae | Mulloidichthys martinicus | Jamaica | M | 28.0 | 19.0 | 332.3 | 0.71 | 10.55 | 8.02 | [126] |
| 152 | Mullidae | Pseudupeneus maculatus | Pernambuco, Brazil | U | 29.2 | 20.0 | 634.3 | 0.68 | 9.82 | 7.60 | [127] |
| 153 | Mullidae | Pseudupeneus maculatus | Jamaica | F | 24.9 | 18.0 | 232.5 | 0.72 | 9.97 | 8.10 | [126,128] |
| 154 | Mullidae | Pseudupeneus maculatus | Jamaica | M | 26.4 | 18.5 | 344.9 | 0.71 | 9.95 | 7.83 | [126,128] |
| 155 | Muraenidae | Muraena augusti | Canary Islands | U | 90.0 | 55.8 | 1750.1 | 0.63 | 17.00 | 12.58 | [129] |
| 156 | Muraenidae | Muraena helena | Adriatic Sea, Croatia | M | 121.0 | 79.0 | 3541.7 | 0.60 | 17.50 | 13.57 | [130] |
| 157 | Muraenidae | Muraena helena | Adriatic Sea, Croatia | F | 113.1 | 76.0 | 2679.8 | 0.61 | 17.88 | 14.03 | [130] |
| 158 | Muraenidae | Muraena helena | Canary Island | U | 134.0 | 75.1 | 5714.9 | 0.57 | 16.68 | 11.96 | [129] |
| 159 | Nemipteridae | Nemipterus japonicus | Manila Bay, Philippines | F | 16.2 | 9.2 | 69.2 | 0.78 | 8.79 | 5.66 | [87,131] |
| 160 | Platycephalidae | Platycephalus indicus | Hong Kong, China | M | 44.2 | 23.5 | 624.1 | 0.68 | 13.03 | 8.50 | [132] |
| 161 | Platycephalidae | Platycephalus indicus | Hong Kong, China | F | 62.2 | 45.7 | 1862.0 | 0.63 | 13.31 | 10.98 | [132] |
| 162 | Pomacanthidae | Pomacanthus maculosus | Southern Arabian Gulf | F | 33.3 | 21.6 | 1070.9 | 0.65 | 9.85 | 7.43 | [101] |
| 163 | Pomacentridae | Abudefduf vaigiensis | Lakshadweep lagoons, India | U | 16.8 | 10.7 | 146.4 | 0.75 | 8.18 | 5.83 | [45] |
| 164 | Pomacentridae | Chromis viridis | Lakshadweep lagoons, India | U | 9.7 | 4.9 | 21.4 | 0.83 | 6.65 | 3.78 | [45] |
| 165 | Pomacentridae | Dascyllus trimaculatus | Terengganu, Malaysia | U | 13.1 | 8.4 | 79.3 | 0.77 | 7.33 | 5.21 | [46] |
| 166 | Pomacentridae | Pomacentrus coelestis | Terengganu, Malaysia | U | 8.3 | 5.6 | 11.5 | 0.86 | 6.24 | 4.39 | [46] |
| 167 | Priacanthidae | Priacanthus hamrur | Saurashtra, India | F | 29.2 | 18.5 | 409.8 | 0.70 | 10.51 | 7.65 | [133] |
| 168 | Rachycentridae | Rachycentron canadum | Northwest Coast, India | U | (176.0) | 66.9 | 53,225.4 | 0.47 | 11.40 | 7.23 | [134] |
| 169 | Sciaenidae | Pennahia aneus | Manila Bay, Philippines | M | 21.1 | 13.1 | 128.9 | 0.75 | 9.89 | 6.89 | [87,135] |
| 170 | Sciaenidae | Pennahia aneus | Manila Bay, Philippines | F | 20.0 | 12.6 | 112.7 | 0.76 | 9.67 | 6.81 | [87,135] |
| 171 | Scombridae | Scomberomorus brasiliensis | Maranhâo | F | 79.5 | 41.1 | 3804.2 | 0.59 | 13.42 | 9.08 | [136] |
| 172 | Scombridae | Scomberomorus brasiliensis | Maranhâo, Brazil | M | 76.5 | 44.3 | 3405.3 | 0.60 | 13.42 | 9.68 | [136] |
| 173 | Scombridae | Scomberomorus brasiliensis | Rio Grande do Norte, Brazil | M | 72.7 | 31.2 | 3943.7 | 0.59 | 12.64 | 7.66 | [137] |
| 174 | Scombridae | Scomberomorus brasiliensis | Rio Grande do Norte, Brazil | F | 54.0 | 25.3 | 1675.4 | 0.63 | 12.43 | 7.70 | [137] |
| 175 | Scombridae | Scomberomorus cavalla | Ceará State, Brazil | F | 100.5 | 63.0 | 7535.8 | 0.56 | 13.32 | 10.25 | [138] |
| 176 | Scombridae | Scomberomorus cavalla | Ceará State, Brazil | F | 113.6 | 77.0 | 10,910.2 | 0.54 | 13.15 | 10.64 | [139] |
| 177 | Scombridae | Scomberomorus maculatus | Ceará, State, Brazil | F | 65.5 | 41.0 | 2304.0 | 0.62 | 13.19 | 9.88 | [138] |
| 178 | Scombridae | Scomberomorus maculatus | Ceará State, Brazil | F | 78.0 | 46.0 | 3878.6 | 0.59 | 13.22 | 9.67 | [140] |
| 179 | Scorpaenidae | Pterois russelii | Terengganu, Malaysia | U | 30.0 | 19.0 | 249.1 | 0.72 | 11.59 | 8.34 | [46] |
| 180 | Siganidae | Siganus canaliculatus | Southern Arabian Gulf | M | 33.2 | 21.5 | 731.9 | 0.67 | 10.46 | 7.82 | [141] |
| 181 | Siganidae | Siganus canaliculatus | Southern Arabian Gulf | F | 36.9 | 25.7 | 1004.9 | 0.66 | 10.65 | 8.40 | [141] |
| 182 | Sillaginidae | Sillago sihama | Gulf of Mannar, India | U | (26.2) | 12.8 | 137.7 | 0.75 | 11.52 | 6.73 | [142] |
| 183 | Sillaginidae | Sillago sihama | Pulicat Lake, India | U | (38.0) | 22.1 | 327.3 | 0.71 | 13.13 | 8.95 | [143] |
| 184 | Sparidae | Archosargus rhomboidalis | Terminos Lagoon, Mexico | U | 24.6 | 8.5 | 491.0 | 0.69 | 9.07 | 4.38 | [144] |
| 185 | Sparidae | Rhabdosargus sarba | Southern Arabian Gulf | M | 29.3 | 23.5 | 513.5 | 0.69 | 10.17 | 8.74 | [104] |
| 186 | Sparidae | Rhabdosargus sarba | Southern Arabian Gulf | F | 29.3 | 23.7 | 513.5 | 0.69 | 10.17 | 8.79 | [104] |
| 187 | Sparidae | Rhabdosargus sarba | South-eastern Australia | U | (25.1) | 19.4 | 325.1 | 0.71 | 9.79 | 8.17 | [145] |
| 188 | Sparidae | Sparus aurata | North Island, New Zealand | U | (55.9) | 24.0 | 3388.1 | 0.60 | 11.13 | 6.71 | [146] |
| 189 | Sparidae | Sparus aurata | Western North Island, N.Z. | U | (63.4) | 24.0 | 4818.1 | 0.58 | 11.21 | 6.37 | [146] |
| 190 | Sparidae | Sparus aurata | Western South Island, N.Z. | U | (66.1) | 24.0 | 5426.2 | 0.58 | 11.23 | 6.26 | [146] |
| 191 | Sphyraenidae | Sphyraena barracuda | Florida, USA | F | 141.8 | 65.6 | 60,587.5 | 0.46 | 9.99 | 6.98 | [147] |
| 192 | Synanceiidae | Inimicus didactylus | Terengganu, Malaysia | U | 25.0 | 16.0 | 231.4 | 0.72 | 10.28 | 7.44 | [46] |
| 193 | Synodontidae | Saurida tumbil | East China Sea | U | (54.7) | 25.7 | 2664.8 | 0.61 | 11.50 | 7.25 | [148] |
| 194 | Synodontidae | Saurida tumbil | Manila Bay, Philippines | M | 28.0 | 23.9 | 192.5 | 0.73 | 11.48 | 10.22 | [87] |
| 195 | Synodontidae | Saurida tumbil | Manila Bay, Philippines | F | 29.2 | 24.7 | 218.9 | 0.73 | 11.59 | 10.27 | [87] |
| 196 | Synodontidae | Saurida undosquamis | off Visakhapatnam, India | U | (34.1) | 20.9 | 364.2 | 0.70 | 11.96 | 8.48 | [149] |
| 197 | Synodontidae | Synodus variegatus | Terengganu, Malaysia | U | 36.8 | 15.6 | 659.8 | 0.68 | 11.40 | 6.40 | [46] |
| 198 | Synodontidae | Trachinocephalus myops | Minnan-Taiwan Bank | U | (41.0) | 16.5 | 804.7 | 0.67 | 11.86 | 6.47 | [150] |
| 199 | Tetraodontidae | Canthigaster valentini | Lizard Island, Australia | F | 8.8 | 5.8 | 22.3 | 0.83 | 6.14 | 4.33 | [151] |
| 200 | Tetraodontidae | Canthigaster valentini | Lizard Island, Australia | M | 10.7 | 6.7 | 39.5 | 0.81 | 6.77 | 4.63 | [151] |
| 201 | Tetraodontidae | Lagocephalus sceleratus | Suez Canal, Egypt | F | 76.5 | 42.2 | 5076.6 | 0.58 | 12.38 | 8.77 | [152] |
| 202 | Tetraodontidae | Lagocephalus sceleratus | Suez Canal, Egypt | M | 76.5 | 41.0 | 5076.6 | 0.58 | 12.38 | 8.63 | [152] |
| 203 | Tetraodontidae | Lagocephalus sceleratus | Rhodes, Greece | U | 61.5 | 35.1 | 2646.6 | 0.61 | 12.36 | 8.77 | [153] |
| 204 | Tetraodontidae | Lagocephalus sceleratus | Lebanon | U | 71.6 | 39.0 | 5439.2 | 0.58 | 11.75 | 8.27 | [154] |
| 205 | Tetraodontidae | Lagocephalus sceleratus | Southwest Cyprus | UI | 71.2 | 40.8 | 4454.7 | 0.59 | 12.18 | 8.80 | [155] |
| 206 | Tetraodontidae | Lagocephalus sceleratus | Southeast Cyprus | U | 78.0 | 47.6 | 5872.6 | 0.57 | 12.15 | 9.15 | [155] |
| 207 | Tetraodontidae | Lagocephalus sceleratus | Cyprus | F | 75.0 | 19.4 | 5355.1 | 0.58 | 12.11 | 5.54 | [156] |
References
- Trippel, E.A. Age at Maturity as a Stress Indicator in Fisheries: Biological Processes Related to Reproduction in Northwest Atlantic Groundfish Populations That Have Undergone Declines. BioScience 1995, 45, 759–771. [Google Scholar] [CrossRef]
- Prince, J.D.; Wilcox, C.; Hall, N. How to Estimate Life History Ratios to Simplify Data-Poor Fisheries Assessment. ICES J. Mar. Sci. 2023, 80, 2619–2629. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. Comment on “Metabolic Scaling Is the Product of Life-History Optimization”. Science 2023, 380, eade6084. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D.; Liang, C. A Reconceptualization of the Interactions between Spawning and Growth in Bony Fish. Sci. Mar. 2022, 86, e044. [Google Scholar] [CrossRef]
- van Oosten, J. The Whitefishes (Coregonus Clupeaformis). A Study of the Scales of Whitefishes of Known Ages. Zoologica 1923, 2, 380–412. [Google Scholar] [CrossRef]
- Lagler, K.F.; Bardach, J.E.; Miller, R.R.; Passino, D.R.M. Ichthyology, 2nd ed.; Wiley: New York, 1977; ISBN 978-0-471-51166-3. [Google Scholar]
- Quince, C.; Abrams, P.A.; Shuter, B.J.; Lester, N.P. Biphasic Growth in Fish I: Theoretical Foundations. J. Theor. Biol. 2008, 254, 197–206. [Google Scholar] [CrossRef]
- Pauly, D.; Cheung, W.W.L. Sound Physiological Knowledge and Principles in Modeling Shrinking of Fishes under Climate Change. Glob. Change Biol. 2017, 24, e15–e26. [Google Scholar] [CrossRef]
- Lester, N.P.; Shuter, B.J.; Abrams, P.A. Interpreting the von Bertalanffy Model of Somatic Growth in Fishes: The Cost of Reproduction. Proc. Biol. Sci. 2004, 271, 1625–1631. [Google Scholar] [CrossRef]
- Li, Z.; Lu, H.; Gan, X.; Jin, X. Growth and mortality of bottom threadfin bream Nemipterus bathybius in the mouth of Beibu Gulf, South China Sea. Fish. Sci. 2009, 28, 556–562. [Google Scholar]
- Pauly, D. A Mechanism for the Juvenile-to-Adult Transition in Fishes. ICES J. Mar. Sci. 1984, 41, 280–284. [Google Scholar] [CrossRef]
- Meyer, K.A.; Schill, D.J. The Gill-Oxygen Limitation Theory and Size at Maturity/Maximum Size Relationships for Salmonid Populations Occupying Flowing Waters. J. Fish Biol. 2021, 98, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Amarasinghe, U.S.; Pauly, D. The Relationship Between Size at Maturity and Maximum Size in Cichlid Populations Corroborates the Gill-Oxygen Limitation Theory (GOLT). AFS 2021, 34, 14–22. [Google Scholar] [CrossRef]
- Chen, Z.; Bigman, J.; Xian, W.; Liang, C.; Chu, E.; Pauly, D. The Ratio of Length at First Maturity to Maximum Length across Marine and Freshwater Fishes. J. Fish Biol. 2022, 101, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Chu, E.; Pauly, D. Key Information on 25 Species of Sturgeon; Family Acipenseridae. In Marine and Freshwater Miscellanea IV; Fisheries Centre Research Reports; Institute for the Oceans and Fisheries: Vancouver, BC, Canada, 2022; pp. 57–68. [Google Scholar]
- Keskiṅ, Ç.; Pauly, D. Testing Predictions of Length at First Maturity of Teleostean Fishes, given Their Maximum Length. Cybium 2023, 47, 249–257. [Google Scholar] [CrossRef]
- Pütter, A. Studien über physiologische Ähnlichkeit VI. Wachstumsähnlichkeiten. Pflügers Arch. 1920, 180, 298–340. [Google Scholar] [CrossRef]
- Pauly, D. The Gill-Oxygen Limitation Theory (GOLT) and Its Critics. Sci. Adv. 2021, 7, eabc6050. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D.; Lam, M.E. Too Hot or Too Cold: The Biochemical Basis of Temperature-Size Rules for Fish and Other Ectotherms. Environ. Biol. Fishes 2023, 106, 1519–1527. [Google Scholar] [CrossRef]
- De Jager, S.; Dekkers, W.J. Relations Between Gill Structure and Activity in Fish. Neth. J. Zool. 1974, 25, 276–308. [Google Scholar] [CrossRef]
- Palzenberger, M.; Pohla, H. Gill Surface Area of Water-Breathing Freshwater Fish. Rev. Fish Biol. Fish. 1992, 2, 187–216. [Google Scholar] [CrossRef]
- Pauly, D. Gill Size and Temperature as Governing Factors in Fish Growth: A Generalization of von Bertalanffy’s Growth Formula. Ph.D. Thesis, Christian-Albrechts-Universität Kiel, Kiel, Germany, 1979. [Google Scholar]
- Pauly, D. Why Do Fish Reach First Maturity When They Do? J. Fish Biol. 2022, 101, 333–341. [Google Scholar] [CrossRef]
- Pankhurst, N.W. Development. In Fish Physiology; Schreck, C.B., Tort, L., Farrell, A.P., Brauner, C.J., Eds.; Biology of Stress in Fish; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 295–331. [Google Scholar]
- Taylor, C.C. Cod Growth and Temperature. ICES J. Mar. Sci. 1958, 23, 366–370. [Google Scholar] [CrossRef]
- Froese, R.; Thorson, J.T.; Reyes Jr, R.B. A Bayesian Approach for Estimating Length-Weight Relationships in Fishes. J. Appl. Ichthyol. 2014, 30, 78–85. [Google Scholar] [CrossRef]
- Pauly, D. The Relationships between Gill Surface Area and Growth Performance in Fish: A Generalization of von Bertalanffy’s Theory of Growth. Berichte Dtsch. Wiss. Komm. Für Meeresforsch. 1981, 28, 251–282. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 2 November 2022).
- Bürkner, P.-C. Brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 2017, 80, 1–28. [Google Scholar] [CrossRef]
- Chang, J.; Rabosky, D.L.; Smith, S.A.; Alfaro, M.E. An r Package and Online Resource for Macroevolutionary Studies Using the Ray-Finned Fish Tree of Life. Methods Ecol. Evol. 2019, 10, 1118–1124. [Google Scholar] [CrossRef]
- Warren, M.; Pauly, D. The likely role of urea in delaying the size at first maturity of ureosmotic Chondrichthyes. Environ. Biol. Fishes, 2024; in press. [Google Scholar]
- Ricklefs, R.E.; Starck, J.M. Applications of Phylogenetically Independent Contrasts: A Mixed Progress Report. Oikos 1996, 77, 167–172. [Google Scholar] [CrossRef]
- Rohle, F.J. A comment on phylogenetic correction. Evolution 2006, 60, 1509–1515. [Google Scholar] [CrossRef]
- Revell, L.J. Phylogenetic Signal and Linear Regression on Species Data. Methods Ecol. Evol. 2010, 1, 319–329. [Google Scholar] [CrossRef]
- Budaev, S.; Jørgensen, C.; Mangel, M.; Eliassen, S.; Giske, J. Decision-Making From the Animal Perspective: Bridging Ecology and Subjective Cognition. Front. Ecol. Evol. 2019, 7, 164. [Google Scholar] [CrossRef]
- Nelson, H.R.; Altieri, A.H. Oxygen: The Universal Currency on Coral Reefs. Coral Reefs 2019, 38, 177–198. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Ostlund-Nilsson, S. Hypoxia in Paradise: Widespread Hypoxia Tolerance in Coral Reef Fishes. Proc. R. Soc. London Ser. B Biol. Sci. 2004, 271, S30–S33. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.E.; Hobbs, J.-P.A.; Östlund-Nilsson, S.; Munday, P.L. Hypoxia Tolerance and Air-Breathing Ability Correlate with Habitat Preference in Coral-Dwelling Fishes. Coral Reefs 2007, 26, 241–248. [Google Scholar] [CrossRef]
- Pauly, D.; Dimarchopoulou, D. Introduction: Fishes in a Warming and Deoxygenating World. Environ. Biol. Fishes 2022, 105, 1261–1267. [Google Scholar] [CrossRef]
- Heath, D.D.; Blouw, D.M. Are Maternal Effects in Fish Adaptive or Merely Physiological Side Effects? In Maternal Effects as Adaptations; Mousseau, T.A., Fox, C.W., Eds.; Oxford University Press: Oxford, UK, 1998; ISBN 978-0-19-511163-7. [Google Scholar]
- Reeson, P.H. The Biology, Ecology and Bionomics of the Surgeonfishes, Acanthuridae. In Caribbean Coral Reef Fishery Resources; Munro, J.L., Ed.; ICLARM Stud. Rev.; ICLARM: Manila, Philippines, 1983; pp. 178–190. [Google Scholar]
- Craig, P.; Choat, J.; Axe, L.M.; Saucerman, S. Population Biology and Harvest of a Coral Reef Surgeonfish (Acanthurus Lineatus) in American Samoa. Fish. Bull. 1997, 95, 680–693. [Google Scholar]
- Longnecker, K.; Langston, R. Rapid Reproductive Analysis of Four Heavily Exploited Reef Fishes from Pohnpei State, Federated States of Micronesia; Bishop Museum Press: Honolulu, HI, USA, 2016; p. 41. [Google Scholar]
- Hart, A.M.; Russ, G.R. Response of Herbivorous Fishes to Crown-of-Thorns Starfish Acanthaster Planci Outbreaks. III. Age, Growth, Mortality and Maturity Indices of Acanthurus Nigrofuscus. Mar. Ecol. Prog. Ser. 1996, 136, 25–35. [Google Scholar] [CrossRef]
- Murty, V.S. Marine Ornamental Fish Resources of Lakshadweep; CMFRI Special Publication; CMFRI: Kochi, India, 2002. [Google Scholar]
- Du, J.; Loh, K.-H.; Hu, E.; Zheng, X. Common Reef Fishes of Terengganu, Malaysia; Science Press: Beijing, China, 2019. [Google Scholar]
- Crabtree, R.E.; Harnden, C.W.; Snodgrass, D.; Stevens, C. Age, Growth, and Mortality of Bonefish, Albula vulpes, from the Waters of the Florida Keys. Fish. Bull. 1996, 94, 442–451. [Google Scholar]
- Ndobe, S.; Soemarno; Herawati, E.Y.; Setyohadi, D.; Moore, A.; Palomares, M.L.D.; Pauly, D. Life History of Banggai Cardinalfish, Pterapogon Kauderni (Actinopterygii: Perciformes: Apogonidae), in Banggai Islands and Palu Bay, Sulawesi, Indonesia. Acta Ichthyol. Piscat. 2013, 43, 237–250. [Google Scholar] [CrossRef]
- Longnecker, K.; Langston, R.; Bolick, H.; Crane, M.; Donaldson, T.J.; Franklin, E.C.; Kelokelo, M.; Kondio, U.; Potuku, T. Rapid Reproductive Analysis and Length-Weight Relations for Five Species of Coral-Reef Fishes (Actinopterygii) from Papua New Guinea: Nemipterus isacanthus, Parupeneus barberinus, Kyphosus cinerascens, Ctenochaetus striatus (Perciformes), and Balistapus undulatus (Tetraodontiformes). Acta Ichthyol. Piscat. 2017, 47, 107–124. [Google Scholar] [CrossRef][Green Version]
- Ofori-Danson, P.K. Growth of Grey Triggerfish Balistes Capriscus, Based on Growth Checks of the Dorsal Spine. Fishbyte 1989, 7, 11–12. [Google Scholar]
- Aggrey-Fynn, J. The Fishery of Balistes Capriscus (Balistidae) in Ghana and Possible Reasons for Its Collapse. Ph.D. Dissertation, University of Bremen, Bremen, Germany, 2007. [Google Scholar]
- Aiken, K.A. The Biology, Ecology and Bionomics of the Triggerfishes, Balistidae. In Caribbean Coral Reef Fishery Resources; Munro, J.L., Ed.; ICLARM Stud. Rev.; ICLARM: Manila, Philippines, 1983; pp. 191–205. [Google Scholar]
- Sabrah, M.M.; Amin, A.M.; Attia, A.O. Family Belonidae from the Suez Canal, Egypt: Age, Growth, Mortality, Exploitation Rate and Reproductive Biology. Egypt. J. Aquat. Biol. Fish. 2018, 44, 29–35. [Google Scholar] [CrossRef]
- Reuben, S.; Mohamad Kasim, H.; Sivakami, S.; Nair, P.N.R.; Kurup, K.N.; Sivadas, M.; Noble, A.; Nair, K.V.S.; Raje, S.G. Fishery, Biology and Stock Assessment of Carangid Resources from the Indian Seas. Indian J. Fish. 1992, 39, 195–234. [Google Scholar]
- Mehanna, S.; Mohammad, A.; Mahmoud, U. Fishery Status of Carangoides bajad and Caranx melampygus (Family Carangidae) from Shalateen Fishing Area, Red Sea, Egypt, Based on Yield per Recruit (Y/R) Analysis. Egypt. J. Aquat. Biol. Fish. 2016, 20, 21–68. [Google Scholar] [CrossRef]
- Grandcourt, E.M.; Al Abdessalaam, T.Z.; Francis, F.; Al Shamsi, A. Population Biology and Assessment of Representatives of the Family Carangidae Carangoides bajad and Gnathanodon speciosus (Forsskål, 1775), in the Southern Arabian Gulf. Fish. Res. 2004, 69, 331–341. [Google Scholar]
- Chen, P. Optimum First Capture Standards of Major Capture Species of the Northern South China Sea. J. Fish. China 2004, 28, 393–400. [Google Scholar]
- van der Elst, R.P.; Adkin, F. (Eds.) Marine Linefish: Priority Species and Research Objectives in Southern Africa; Oceanography Research Institute Special Publication; Oceanography Research Institute: Durban, South Africa, 1991. [Google Scholar]
- Torres, F. Tabular Data on Marine Fishes from Southern Africa. Part 1: Length-Weight Relationships. Fishbyte 1991, 9, 50–53. [Google Scholar]
- Sudekum, A.E.; Parrish, J.D.; Radtke, R.L.; Ralston, S. Life History and Ecology of Large Jacks in Undisturbed, Shallow, Oceanic Communities. Fish. Bull. 1991, 89, 493–513. [Google Scholar]
- Widodo, J. Maturity and Spawning of Shortfin Scad (Decapterus macrosoma) (Carangidae) of the Java Sea. Asian Fish. Sci. 1991, 4, 245–252. [Google Scholar] [CrossRef]
- Atmadja, S.B. Estimation of Growth and Mortality of Round Scad (Decapterus macrosoma) in the Java Sea, Indonesia. FAO Fish. Rep. 1988, 389, 324–345. [Google Scholar]
- Lin, L.; Zheng, Y.J.; Cheng, J.H.; Liu, Y.; Ling, J.Z. A preliminary study on fishery biology of main commercial fishes surveyed from the bottom trawl fisheries in the East China Sea. Mar. Sci. 2006, 30, 21–25. [Google Scholar]
- Lin, L.; Cheng, J.; Ling, J.; Zhang, H. First Capture Sizes of Major Commercial Fishes in the East China Sea Region. J. Fish. Sci. China 2006, 13, 250–256. [Google Scholar]
- Geng, P. A Study of Inter-Annual Changes in Growth, Mortality and Exploitation Rate of Representative Fish Stocks in Beibu Gulf; Shanghai Ocean University: Shanghai, China, 2019. [Google Scholar]
- Hales, L.S.J. Distribution, Abundance, Reproduction, Food Habits, Age, and Growth of Round Scad, Decapterus punctatus, in the South Atlantic Bight. Fish. Bull. 1987, 85, 251–268. [Google Scholar]
- Wilk, S.J.; Morse, W.W.; Ralph, D.E. Length-Weight Relationships of Fishes Collected in the New York Bight. Bull. New Jersey Acad. Sci. 1978, 23, 58–64. [Google Scholar]
- Pinheiro, P.B.; Hazin, F.H.V.; Travassos, P.; Oliveira, P.G.V.; Carvalho, F.; Rêgo, M.G. The Reproductive Biology of the Rainbow Runner, Elagatis Bipinnulata (Quoy & Gaimard, 1825) Caught in the São Pedro and São Paulo Archipelago. Braz. J. Biol. 2011, 71, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Chen, M.; Du, J.; Zhenbin, L.; Shengyun, Y. Age and Growth Changes and Population Dynamics of the Black Pomfret (Parastromateus niger) and the Frigate Tuna (Auxis Thazard Thazard), in the Taiwan Strait. Lat. Am. J. Aquat. Res. 2012, 40, 649–656. [Google Scholar] [CrossRef]
- Griffiths, S.; Fry, G.; van der Velde, T. Age, Growth and Reproductive Dynamics of the Talang Queenfish (Scomberoides Commersonnianus) in Northern Australia; CSIRO Cleveland: Celevland, Australia, 2005; ISBN 978-1-921061-08-0. [Google Scholar]
- Escobar, F.D.; Polo, C.J.; Alonso, J.C.; Puentes, V. Estados de Los Principales Recursos Pesqueros de Colombia 2014; Autoridad Nacional de Acuicultura y Pesca—AUNAP: Bogotá, Colombia, 2014; ISBN 978-958-58993-3-9. [Google Scholar]
- Morsuwan, P. On the Biology of Slender Trevally, Caranx leptolepis, in the Gulf of Thailand. Marine Fish Laboratory: Bangkok Thailand, 1970.
- Marino, G.; Mandich, A.; Massari, A.; Andaloro, F.; Porrello, S.; Finoia, M.G.; Cevasco, F. Aspects of Reproductive Biology of the Mediterranean Amberjack (Seriola dumerilii Risso) during the Spawning Period. J. Appl. Ichthyol. 1995, 11, 9–24. [Google Scholar] [CrossRef]
- Crabtree, R.E.; Hood, P.; Snodgrass, D. Age, Growth, and Reproduction of Permit (Trachinotus falcatus) in Florida Waters. Fish. Bull. 2002, 100, 26–34. [Google Scholar]
- Saccardo, S.A.; Cergole, M.C.; Masumoto, C. Trachurus Lathami Nichols, 1920. In Análise das Principais Pescarias Commerciais da Região Sudeste-Sul do Brasil: Dinâmica Populacional das Espécies em Explotaçao; Cergole, M.C., Ávila-da-Silva, A.O., Rossi-Wongtchowski, C.L.D.B., Eds.; Instituto Oceanográfico: São Paulo, Brazil, 2005; pp. 156–161. [Google Scholar]
- Johnson, G.D.; Brothers, E.B. Acanthemblemaria Paula, a New Diminutive Chaenopsid (Pisces: Blennioidei) from Belize with Comments on Life History. Proc. Biol. Soc. Wash. 1989, 120, 1018–1030. [Google Scholar]
- Conand, F. Biology and Phenology of Amblygaster sirm (Clupeidae) in New Caledonia, a Sardine of the Coral Environment. Bull. Mar. Sci. 1991, 48, 137–149. [Google Scholar]
- de Moussac, G.; Poupon, J.C. Croissance et Ovogénèsis d’Herklotsichthys punctatus (Pisces, Clupeidae) Ruppel, 1837 Aux Seychelles. Cybium 1986, 10, 31–45. [Google Scholar]
- Alves, M.I.M.; Sawaya, P. Sobre a reprodução da sardinha-bandeira, Opisthonema oglinum (Le sueur), na costa do estado do Ceará (Brasil). Arq. Ciências Mar 1975, 15, 19–28. [Google Scholar]
- Bezerra, R.C. Relacâo Comprimento-Peso Da Sardinha-Bandeira, Opisthonema Oglinum (LeSueur), No Estado Do Ceará. Arq. Est. Mar. Univ. Fed. Ceará 1968, 8, 225–227. [Google Scholar]
- LINO; da Silva, M.A. Estudo Biológico-Pesqueiro da Manjuba Opisthonema Oglinum (Lesueur, 1818) da Região de Itapissuma, Pernambuco. Master’s Thesis, Universidade Federal Rural de Pernambuco, Recife, Brazil, 2003. Available online: https://bdtd.ibict.br/vufind/Record/URPE_cf1178c45b99e5eba3f960e083317969 (accessed on 2 November 2022).
- Sekharan, K.V. Observations on the Choodai Fishery of Mandapam Area. Indian J. Fish. 1955, 2, 113–131. [Google Scholar]
- Dalzell, P.J. The Population Biology and Management of Bait-Fish in Papua New Guinea Waters; Report; Fisheries Research and Surveys Branch, Department of Primary Industry: Port Moresby, Independent State of Papua New Guinea, 1984. [Google Scholar]
- CMFRI. Annual Report 2016-17; Central Marine Fisheries Research Institute: Kochi, India, 2016; p. 345. [Google Scholar]
- Tham, A.K. A Contribution to the Study of the Growth of Members of the Genus Stolephorus Lacépède in Singapore Strait. Proc. Indo-Pac. Fish. Comm. 1967, 12, 1–25. [Google Scholar]
- Bariche, M.; Kajajian, A.; Azzurro, E. Reproduction of the Invasive Bluespotted Cornetfish Fistularia Commersonii (Teleostei, Fistulariidae) in the Mediterranean Sea. Mar. Biol. Res. 2013, 9, 169–180. [Google Scholar] [CrossRef]
- Manacop, P.R. The Sexual Maturity of Some Commercial Fishes Caught in Manila Bay. Philipp. J. Sci. 1936, 59, 383–391. [Google Scholar]
- Grandcourt, E.M.; Al Abdessalaam, T.Z.; Francis, F.; Al Shamsi, A.T. Fisheries Biology of a Short-Lived Tropical Species: Gerres Longirostris (Lacépède, 1801) in the Arabian Gulf. ICES J. Mar. Sci. 2006, 63, 452–459. [Google Scholar] [CrossRef]
- Depczynski, M.; Bellwood, D.R. Shortest Recorded Vertebrate Lifespan Found in a Coral Reef Fish. Curr. Biol. 2005, 15, R288–R289. [Google Scholar] [CrossRef]
- Grandcourt, E.; Al Abdessalaam, T.Z.; Al Shamsi, A.T.; Francis, F. Biology and Assessment of the Painted Sweetlips (Diagramma pictum (Thunberg, 1792)) and the Spangled Emperor (Lethrinus nebulosus (Forsskål, 1775)) in the Southern Arabian Gulf. Fish. Bull. 2006, 104, 75–88. [Google Scholar]
- Lee, J.U.; Al-Baz, A. Assessment of Fish Stocks Exploited by Fish Traps in the Arabian Gulf Area. Asian Fish. Sci. 1989, 2, 213–231. [Google Scholar] [CrossRef]
- Lima, M.M.; Lessa, R.P.; Duarte-Neto, P.J. Haemulon Aurolineatum. In Dinâmica de Populações e Avaliação de Estoques dos Recursos Pesqueiros da Região Nordeste; Lessa, R.P., Nóbrega, M.F., Júnior, J.L.B., Eds.; Departamento de Pesca—Universidade Federal Rural de Pernambuco: Recife, Brazil, 2004; Volume 2, pp. 142–150. [Google Scholar]
- Shinozaki-Mendes, R.A.; Santander-Neto, J.; Silva, J.R.F.; Hazin, F.H.V. Reproductive Biology of Haemulon Plumieri (Teleostei: Haemulidae) in Ceará State, Northeastern Brazil. Braz. J. Biol. 2013, 73, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Osman, H.M.; Saber, M.A.; El Ganainy, A.A. Population Structure of the Striped Piggy Pomadasys stridens in the Gulf of Suez. Egypt. J. Aquat. Res. 2019, 45, 53–58. [Google Scholar] [CrossRef]
- Nóbrega, M.F.; Monteiro, A.; Lessa, R.P. Hemiramphus Brasiliensis. In Dinâmica de Populações e Avaliação de Estoques dos Recursos Pesqueiros da Região Nordeste; Lessa, R.P., Nóbrega, M.F., Júnior, J.L.B., Eds.; Departamento de Pesca—Universidade Federal Rural de Pernambuco: Recife, Brazil, 2004; Volume 2, pp. 151–161. [Google Scholar]
- Mehanna, S.F.; Salem, M.; Mahmoud, H.S. Some Biological Aspects and Reproductive Dynamic of the Black-Barred Halfbeak Hemiramphus far (Family: Hemiramphidae) in Bardawil Lagoon, Egypt. Egypt. J. Aquat. Biol. Fish. 2019, 23, 127–137. [Google Scholar] [CrossRef]
- Shinozaki-Mendes, R.A.; Hazin, F.H.V.; De Oliveira, P.G.; de Carvalho, F.C. Reproductive Biology of the Squirrelfish, Holocentrus adscensionis (Osbeck, 1765), Caught off the Coast of Pernambuco, Brazil. Sci. Mar. 2007, 71, 715–722. [Google Scholar] [CrossRef]
- Nomura, H. Length-Weight Tables of Some Fish Species from Northeastern Brazil. Arq. Est. Biol. Mar. Univ. Fed. Ceará 1965, 5, 103–105. [Google Scholar]
- Wyatt, J.R. The Biology, Ecology and Bionomics of the Squirrelfishes, Holocentridae. In Caribbean Coral Reef Fishery Resources; Munro, J.L., Ed.; ICLARM Stud. Rev.; ICLARM: Manila, Philippines, 1983; pp. 50–58. [Google Scholar]
- Yamaguchi, A.; Kume, G.; Yoshimura, Y.; Kiriyama, T.; Yoshimura, Y. Spawning Season and Size at Sexual Maturity of Kyphosus bigibbus (Kyphosidae) from Northwest Kyushu, Japan. Ichthyol. Res. 2011, 58, 283–287. [Google Scholar] [CrossRef]
- Grandcourt, E.; Al Abdessalaam, T.Z.; Francis, F.; Al Shamsi, A.T. Age-Based Life History Parameters and Status Assessments of by-Catch Species (Lethrinus borbonicus, Lethrinus microdon, Pomacanthus maculosus and Scolopsis taeniatus) in the Southern Arabian Gulf. J. Appl. Ichthyol. 2010, 26, 381–389. [Google Scholar] [CrossRef]
- El-Ganainy, A.; Amin, A. Age, Growth, Mortality Rates and Corresponding Yield Estimates of the Snubnose Emperor Lethrinus Borbonicus from South Sinai Coast, Gulf of Suez, Egypt. Egypt. J. Aquat. Biol. Fish. 2012, 16, 27–34. [Google Scholar] [CrossRef]
- Mehanna, S. Population Dynamics and Management of Snubnose Emperor Lethrinus bungus (L. borbonicus) from the Foul Bay, Red Sea; INOC-XI International Symposium: Bogor, Indonesia, 2011; pp. 121–129. [Google Scholar]
- Grandcourt, E.; Al Abdessalaam, T.Z.; Francis, F.; Al Shamsi, A.T. Demographic Parameters and Status Assessments of Lutjanus ehrenbergii, Lethrinus lentjan, Plectorhinchus sordidus and Rhabdosargus sarba in the Southern Arabian Gulf. J. Appl. Ichthyol. 2011, 27, 1203–1211. [Google Scholar] [CrossRef]
- Li, Y. Species Diversity and Biology of Fish in Coral Reef Waters of Xisha, Zhongsha and Nansha Islands, South China Sea. PhD Thesis, Ocean University of China, Qingdao, China, 2010. [Google Scholar]
- Everson, A.R.; Williams, H.A.; Ito, B.M. Maturation and Reproduction in Two Hawaiian Eteline Snappers, Uku, Aprion virescens, and Onaga, Etelis coruscans. Fish. Bull. 1989, 87, 877–888. [Google Scholar]
- Thompson, R.; Munro, J.L. The Biology, Ecology and Bionomics of Caribbean Reef Fishes: Lutjanidae (Snappers). In Caribbean Coral Reef Fishery Resources; Munro, J.L., Ed.; ICLARM Stud. Rev.; ICLARM: Manila, Philippines, 1983; pp. 94–109. [Google Scholar]
- Pauly, D. On the Interrelationships between Natural Mortality, Growth Parameters, and Mean Environmental Temperature in 175 Fish Stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Williams, D.M.; Russ, G.R. Review of Data on Fishes of Commercial and Recreational Fishing Interest in the Great Barrier Reef; Great Barrier Reef Marine Park Authority Res. Publ; Great Barrier Reef Marine Park Authority: South Townsville, Australia, 1994; p. 103. [Google Scholar]
- Marriott, R.J.; Mapstone, B.D.; Begg, G.A. Age-Specific Demographic Parameters, and Their Implications for Management of the Red Bass, Lutjanus bohar (Forsskal 1775): A Large, Long-Lived Reef Fish. Fish. Res. 2007, 83, 204–215. [Google Scholar] [CrossRef]
- Kritzer, J. Sex-Specific Growth and Mortality, Spawning Season, and Female Maturation of the Stripey Bass (Lutjanus carponotatus) on the Great Barrier Reef. Fish. Bull. 2004, 102, 94–107. [Google Scholar]
- Newman, S.J.; Cappo, M.; Williams, D.M. Age, Growth, Mortality Rates and Corresponding Yield Estimates Using Otoliths of the Tropical Red Snappers, Lutjanus erythropterus, L. malabaricus and L. sebae, from the Central Great Barrier Reef. Fish. Res. 2000, 48, 1–14. [Google Scholar] [CrossRef]
- Grandcourt, E.; Al Abdessalaam, T.Z.; Francis, F. Age, Growth, Mortality and Reproduction of the Blackspot Snapper, Lutjanus fulviflamma (Forsskål, 1775), in the Southern Arabian Gulf. Fish. Res. 2006, 78, 203–210. [Google Scholar] [CrossRef]
- Shimose, T.; Tachihara, K. Age, Growth and Maturation of the Blackspot Snapper Lutjanus fulviflammus around Okinawa Island, Japan. Fish. Sci. 2005, 71, 48–55. [Google Scholar] [CrossRef]
- Shimose, T.; Nanami, A. Age, Growth, and Reproductive Biology of Blacktail Snapper, Lutjanus fulvus, around the Yaeyama Islands, Okinawa, Japan. Ichthyol. Res. 2014, 61, 322–331. [Google Scholar] [CrossRef]
- Kamali, S.; Ramzani, M.; Kamali, I. Study of Big Eye Snapper (Lutjanus lutjanus) Reproduction in Persian Gulf Waters and Oman Sea. J. Anim. Environ. 2017, 9, 269–274. [Google Scholar]
- Mcpherson, G.R.; Squire, L. Age and Growth of Three Dominant Lutjanus Species of the Great Barrier Reef Inter-Reef Fishery. Asian Fish. Sci. 1992, 5, 25–36. [Google Scholar] [CrossRef]
- Aiken, K.A. Aspects of Reproduction, Age and Growth of the Lane Snapper, Lutjanus synagris (Linnaeus, 1758) in Jamaican Coastal Water. In Proceedings of the 52nd Gulf and Caribbean Fisheries Institute, Key West, FL, USA, 1–5 November 1999; LeRoy Creswell, R., Ed.; Gulf and Caribbean Fisheries Institute: Fort Pierce, FL, USA, 2001; pp. 116–134. [Google Scholar]
- Starck, W.A.I. The Biology of the Grey Snapper, Lutjanus griseus (Linnaeus), in the Florida Keys. In Investigations on the Gray Snapper, Lutjanus Griseus; Starck, W.A.I., Schroeder, R.E., Eds.; Studies in Tropical Oceanography; Rosenstiel School of Marine and Atmospheric Sciences, University of Miami Press: Miami, USA, 1971; pp. 11–150. [Google Scholar]
- Burton, M. Age, Growth, and Mortality of Gray Snapper, Lutjanus griseus, from the East Coast of Florida. Fish. Bull. 2001, 99, 254–265. [Google Scholar]
- de Menezes, M.F.; Paiva, M.P. Notes on the Biology of Tarpon, Tarpon atlanticus (Cuvier and Valenciennes), from Coastal Waters of Ceará, Santa Fé, Brazil. Arq. Ciências Mar 1966, 6, 83–98. [Google Scholar]
- Menezes, M.F. Relacão Peso-Comprimento Do Camurupim, Tarpon atlanticus (Valenciennes) No Nordeste Brasileiro. Arq. De Ciências Do Mar 1967, 7, 101–102. [Google Scholar]
- Hwang, S.-Y.; Chen, C.-T.; Liu, K.-M. Age and Growth of the Moon Fish, Mene maculata, before and after Heavy Exploitation in Southwestern Taiwan Waters. J. Fish. Soc. Taiwan 2002, 29, 299–311. [Google Scholar]
- Ghosh, S.; Thangavelu, R.; Mohammed, G.; Dhokia, H.; Zala, M.; Savaria, Y.; Polara, J.; Ladani, A. Sudden Emergence of Fishery and Some Aspects of Biology and Population Dynamics of Aluterus monoceros (Linnaeus, 1758) at Veraval. Indian J. Fish. 2011, 58, 31–34. [Google Scholar]
- Araújo, A.R.; Silva, F.D. Aspectos Da Pesca e Biologia Da Tainha, Mugil curema (Osteichthyes: Mugilidae), No Estuario Do Rio Vaza Barris, Sergipe, Brasil. Arq. Cienc. Mar 2013, 46, 29–38. [Google Scholar]
- Munro, J.L. The Biology, Ecology and Bionomics of the Goatfishes, Mullidae. In Caribbean Coral Reef Fishery Resources; Munro, J.L., Ed.; ICLARM Stud. Rev.; ICLARM: Manila, Philippines, 1983; pp. 142–154. [Google Scholar]
- Santana, F.M.; Morize, E.; Lessa, R. Age and Growth of the Spotted Goatfish, Pseudupeneus maculatus (Bloch, 1793) in Brazil, Validated through Marginal Increment and Oxytetracycline Dyes in the Sagittae. J. Appl. Ichthyol. 2006, 22, 132–137. [Google Scholar] [CrossRef]
- Munro, J.L. Aspects of the Biology and Ecology of Caribbean Reef Fishes: Mullidae (Goat Fishes). J. Fish Biol. 1976, 9, 79–97. [Google Scholar] [CrossRef]
- Jiménez, S.; Schönhuth, S.; Lozano, I.J.; González, J.A.; Sevilla, R.G.; Diez, A.; Bautista, J.M. Morphological, Ecological, and Molecular Analyses Separate Muraena Augusti from Muraena Helena as a Valid Species. Cope 2007, 2007, 101–113. [Google Scholar] [CrossRef]
- Matić-Skoko, S.; Tutman, P.; Petrić, M.; Skaramuca, D.; Đikić, D.; Lisičić, D.; Skaramuca, B. Mediterranean Moray Eel Muraena helena (Pisces: Muraenidae): Biological Indices for Life History. Aquat. Biol. 2011, 13, 275–284. [Google Scholar] [CrossRef]
- Wang, X.H.; Qiu, Y.S.; Zhu, G.P.; Du, F.Y.; Sun, D.R.; Huang, S.L. Length-Weight Relationships of 69 Fish Species in the Beibu Gulf, Northern South China Sea. J. Appl. Ichthyol. 2011, 27, 959–961. [Google Scholar] [CrossRef]
- Ho, C.M. Biology and Fishery of the Bartail Flathead, Platycephalus Indicus (Linn., 1758), in the Northern South China Sea. PhD Dissertation, The University of Hong Kong, Hong Kong, China, 2005. [Google Scholar]
- Kizhakudan, S.; Zala, M. Dynamics of Priacanthus hamrur (Forsskal) Exploited off Saurashtra Coast. Indian J. Fish. 2006, 53, 409–416. [Google Scholar]
- Moosamikandy, S.; Kurup, M. Stock Assessment of Cobia Rachycentron canadum (Linnaeus, 1766) Occurring in the North West Coast of India. Indian J. Geo-Mar. Sci. 2016, 45, 378–387. [Google Scholar]
- Tuuli, C.D.; Sadovy de Mitcheson, Y.; Liu, M. Reproductive Biology of the Greyfin Croaker Pennahia Anea in the Northern South China Sea. Ichthyol. Res. 2011, 58, 302–309. [Google Scholar] [CrossRef]
- Lima, P.R.; Lessa, R.; de Castro, A.C.; de Jesus Azevedo, J.W. Tamanho E idade de primeira maturação do serra, Scomberomorus brasiliensis (Osteichthyes; Scombridae-Collette Russo & Zavalla-Camin, 1978) no litoral ocidental do Maranhão-Brasil. Bol. Laboratório Hidrobiol. 2009, 22, 39–44. [Google Scholar]
- Lima, J.T.; Fonteles Filho, A.A.; Chellappa, S. Biologia reprodutiva da serra, Scomberomorus brasiliensis (Osteichthyes: Scombridae), em águas costeiras do Rio Grande do Norte. Arq. Ciências Mar 2007, 40, 24–30. [Google Scholar]
- Gesteira, T.C.V.; Lôbo de, A.L. Época de Reprodução, Tamanho e Idade na Primeira Desova da Cavala e da Serra, na Costa Do Estado do Ceará (Brasil). 1976. Available online: https://repositorio.ufc.br/handle/riufc/1654 (accessed on 16 February 2023).
- Ivo, C.T.C. Época de Desova e Idade na Primeira Maturação Sexual da Cavala, Scomberomorus Cavalla (Cuvier), no Estado do Ceará. 1972. Available online: https://repositorio.ufc.br/handle/riufc/1715 (accessed on 16 February 2023).
- Gesteira, T.C.V. Sobre a reprodução e fecundidade da serra, scomberomorus maculatus (mitchill), no estado do ceará. Arq. Ciências Mar 1972, 12, 117–122. [Google Scholar]
- Grandcourt, E.; Al Abdessalaam, T.; Francis, F.; Al Shamsi, A. Population Biology and Assessment of the White-Spotted Spinefoot, Siganus canaliculatus (Park, 1797), in the Southern Arabian Gulf. J. Appl. Ichthyol. 2007, 23, 53–59. [Google Scholar] [CrossRef]
- Radhakrishnan, N. A Contribution to the Biology of Indian Sand Whiting Sillago Sihama (Forskal). Indian J. Fish. 1957, 4, 254–283. [Google Scholar]
- Krishnamurthy, K.N.; Kaliyamurthy, M. Studies on the age and growth of sandwhiting sillago sihama (forskal) from pulicat lake with observations on its biology and fishery. Indian J. Fish. 1978, 25, 84–97. [Google Scholar]
- Chavance, P.; Yáñez-Arancibia, A.; Flores-Hernandez, D.; Lara, A.; Amezcua, F. Ecology, Biology and Population Dynamics of Archosargus rhomboidalis (Pisces, Sparidae) in a Tropical Coastal Lagoon System, Southern Gulf of Mexico. An. Inst. Cienc. Mar Limnol. Univ. Nac. Auton. Mex. 1986, 13, 11–30. [Google Scholar]
- Hughes, J.M.; Stewart, J.; Kendall, B.W.; Gray, C.A. Growth and Reproductive Biology of Tarwhine Rhabdosargus Sarba (Sparidae) in Eastern Australia. Mar. Freshw. Res. 2008, 59, 1111–1123. [Google Scholar] [CrossRef]
- Annala, J.H. Report from the Fishery Assessment Plenary, May 1994: Stock Assessments and Yield Estimates; Unpublished Report Held in Maf Fisheries Greta Point Library; Fisheries Science Group, Ministry for Primary Industries: Wellington, New Zealand, 1994. [Google Scholar]
- De Sylva, D.P. Systematics and Life History of the Great Barracuda, Sphyraena Barracuda (Walbaum); Studies in Tropical Oceanography; University of Miami Press: Coral Gables, FL, USA, 1963. [Google Scholar]
- Shindo, S. Note on the Study on the Stock of Lizard Fish, Saurida tumbil in the East China Sea. Proc. Indo-Pac. Fish. Comm. 1972, 13, 298–305. [Google Scholar]
- Rajkumar, U.; Sivakami, S.; Rao, K.N.; Kingsly, H.J. Lizardfish Fishery, Biology and Population Dynamics of Saurida Undosquamis (Richardson) off Visakhapatnam. Indian J. Fish. 2003, 50, 149–156. [Google Scholar]
- Du, J.; Lu, Z.; Yang, S.; Chen, M. Studies on Ecological Characteristics Variation and Population Dynamics of Four Lizardfishes in the Southern Taiwan Straits. Acta Oceanol. Sin. 2011, 30, 72–81. [Google Scholar] [CrossRef]
- Gladstone, W.; Westoby, M. Growth and Reproduction in Canthigaster valentini (Pisces, Tetraodontidae): A Comparison of a Toxic Reef Fish with Other Reef Fishes. Environ. Biol. Fishes 1988, 21, 207–221. [Google Scholar] [CrossRef]
- Sabrah, M.; El-Ganainy, A.; Zaky, M.A. Biology and Toxicity of the Pufferfish Lagocephalus sceleratus (GMELIN, 1789) from the Gulf of Suez. Egypt. J. Aquat. Res. 2006, 32, 283–297. [Google Scholar]
- Kalogirou, S. Ecological Characteristics of the Invasive Pufferfish Lagocephalus Sceleratus (Gmelin, 1789) in Rhodes, Eastern Mediterranean Sea. A Case Study. Mediterr. Mar. Sci. 2013, 14, 251–260. [Google Scholar] [CrossRef]
- Boustany, L.; El Indary, S.; Nader, M. Biological Characteristics of the Lessepsian Pufferfish Lagocephalus Sceleratus off Lebanon. Cah. De Biol. Mar. 2015, 56, 137–142. [Google Scholar] [CrossRef]
- Rousou, M.; Ganias, K.; Kletou, D.; Loucaides, A.; Tsinganis, M. Maturity of the Pufferfish Lagocephalus Sceleratus in the Southeastern Mediterranean Sea. Sex. Early Dev. Aquat. Org. 2014, 1, 35–44. [Google Scholar] [CrossRef]
- Michailidis, N. Study on the Lessepian Migrant Lagocephalus sceleratus in Cyprus. In Proceedings of the Report of the Technical Meeting on the Lessepsian Migration and Its Impact on Eastern Mediterranean Fishery, Nicosia, Cyprus, 7–9 December 2010; FAO: Nicosia, Cyprus, 2010; pp. 74–87. [Google Scholar]
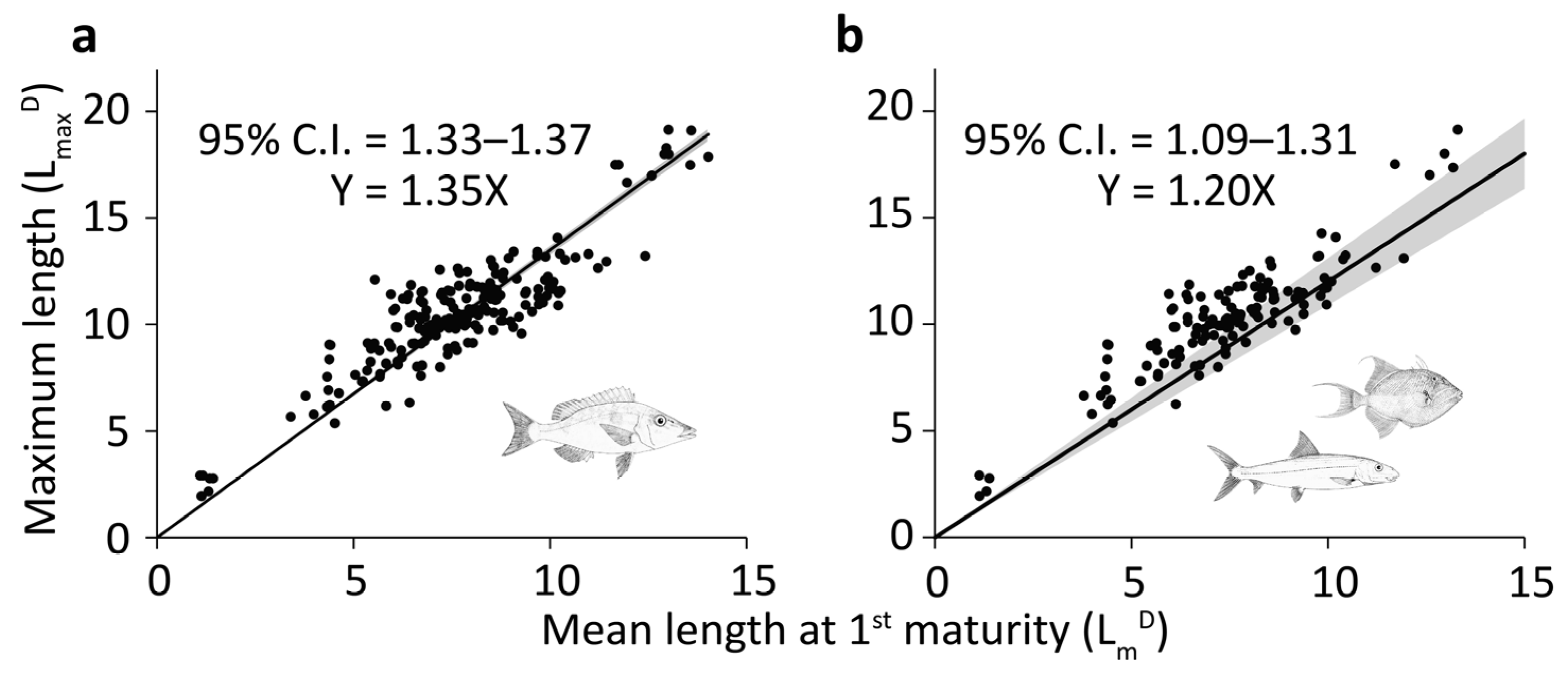
| Dataset | N | Slope (95% C.I.) |
|---|---|---|
| All cases | 207 | 1.35 (1.33–1.37) |
| Species on FishTree with phylogeny considered | 120 | 1.20 (1.09–1.31) |
| Species on FishTree without phylogeny considered | 120 | 1.34 (1.31–1.37) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, E.; Pauly, D. The Relationship between Mean Length at Maturity and Maximum Length in Coral Reef Fish. Fishes 2024, 9, 130. https://doi.org/10.3390/fishes9040130
Chu E, Pauly D. The Relationship between Mean Length at Maturity and Maximum Length in Coral Reef Fish. Fishes. 2024; 9(4):130. https://doi.org/10.3390/fishes9040130
Chicago/Turabian StyleChu, Elaine, and Daniel Pauly. 2024. "The Relationship between Mean Length at Maturity and Maximum Length in Coral Reef Fish" Fishes 9, no. 4: 130. https://doi.org/10.3390/fishes9040130
APA StyleChu, E., & Pauly, D. (2024). The Relationship between Mean Length at Maturity and Maximum Length in Coral Reef Fish. Fishes, 9(4), 130. https://doi.org/10.3390/fishes9040130






