Evaluation of Immune Protection of a Bivalent Inactivated Vaccine against Aeromonas salmonicida and Vibrio vulnificus in Turbot
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Rearing
2.2. Preparation of Inactivated Vaccine
2.3. Fish Immunization and Challenge
2.4. Analysis of Serum Enzyme Activity
2.4.1. ACP Activity
2.4.2. LZM Activity
2.5. Specific Antibody Levels in Serum
2.6. Quantitative Real-Time Reverse Transcription-PCR (qRT-PCR) Analysis of Immune-Related Genes
2.7. Statistical Analyses
3. Results
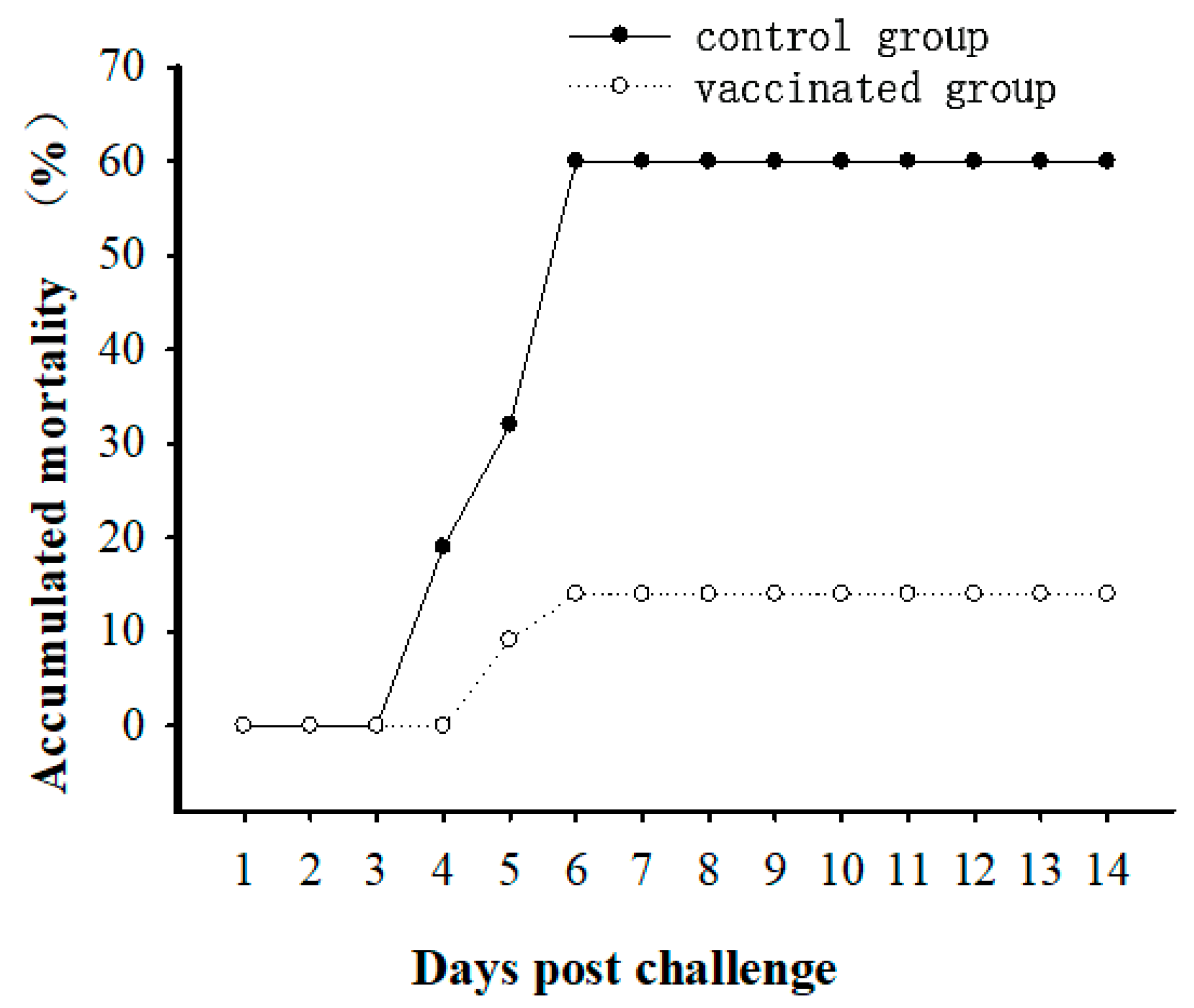
3.1. Immune Protective Ability of Vaccine
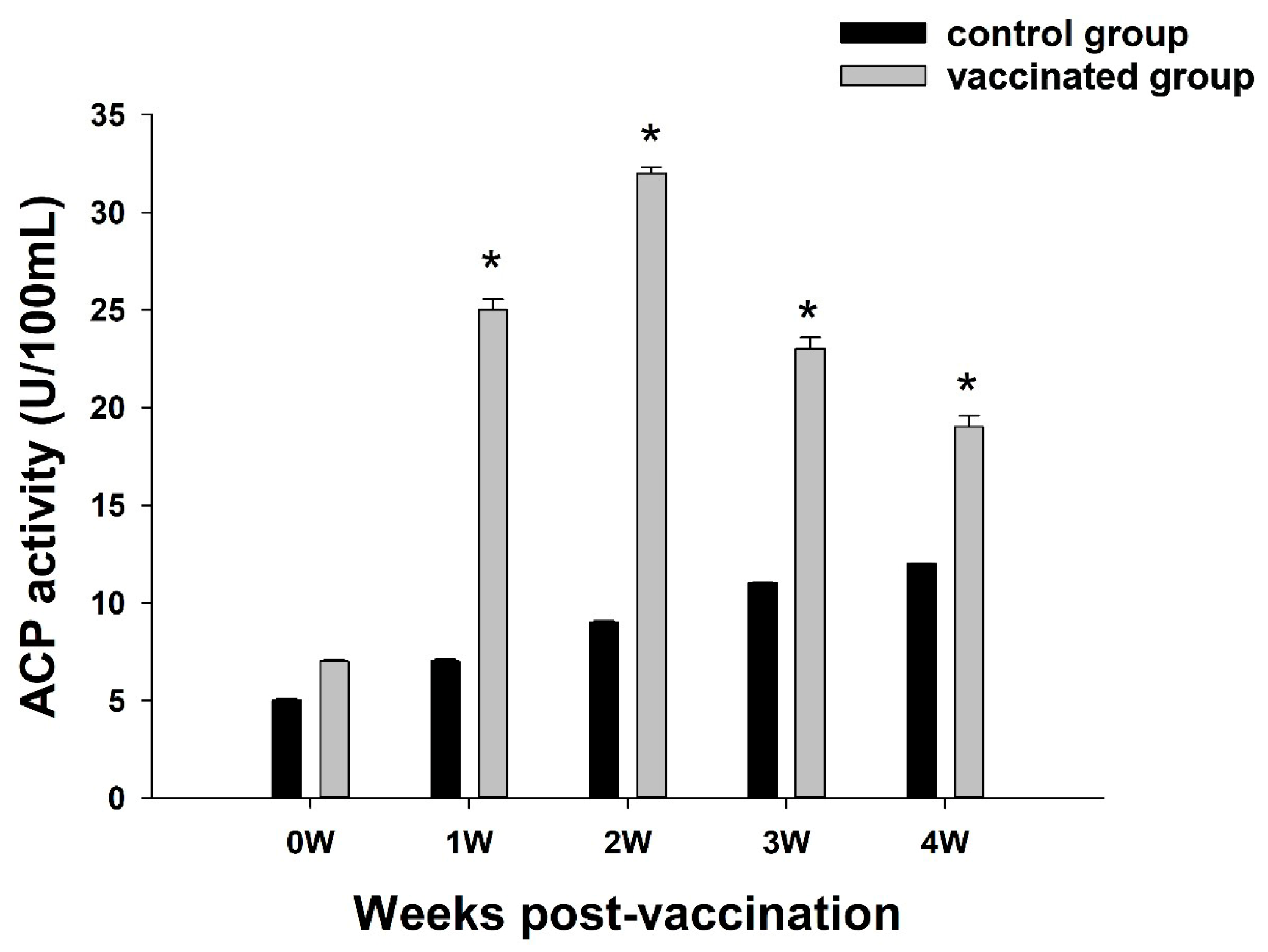
3.2. Analysis of ACP Activity
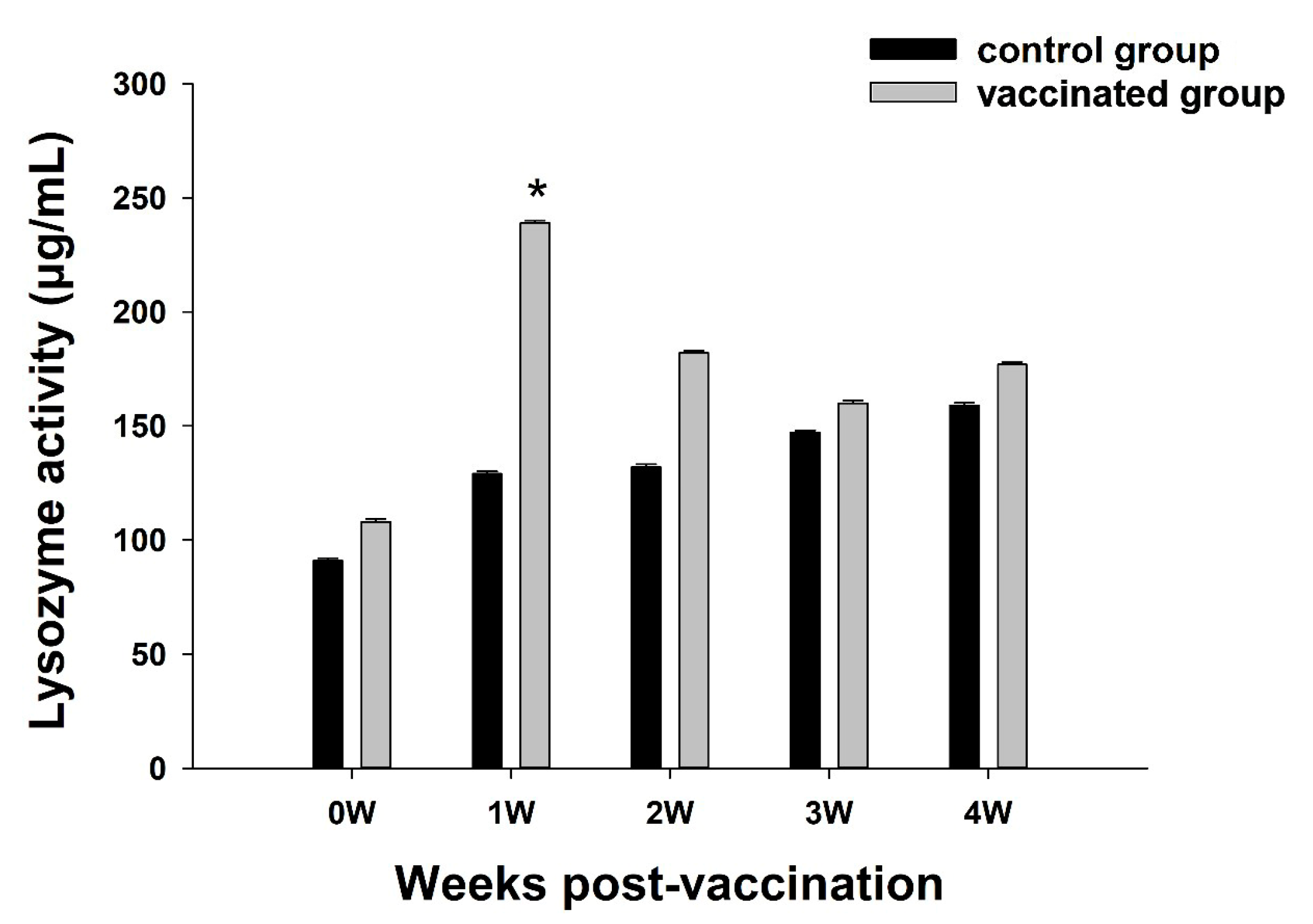
3.3. Analysis of LZM Activity
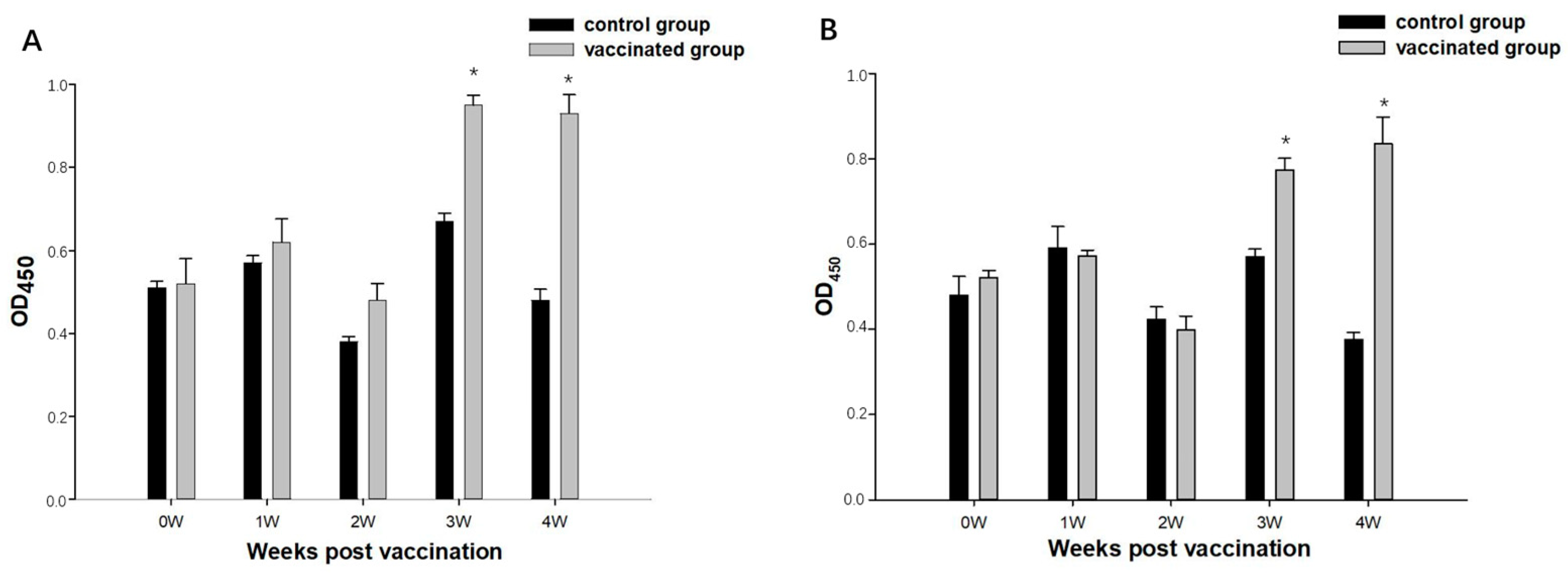
3.4. The Analysis of Antibody Titers
3.5. Expression Pattern of Immune-Related Genes during Immunization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Horne, M.T.; Richards, R.H.; Roberts, R.J.; Smith, P.C. Peracute vibriosis in juvenile turbot Scophthalmus maximus. J. Fish Biol. 2010, 11, 355–361. [Google Scholar] [CrossRef]
- Qin, L.; Xu, J.; Wang, Y.G. Edwardsiellosis in farmed turbot, Scophthalmus maximus (L.), associated with an unusual variant of Edwardsiella tarda: A clinical, aetiological and histopathological study. J. Fish Dis. 2014, 37, 103–111. [Google Scholar] [CrossRef]
- Lago, E.P.; Nieto, T.P.; Farto, R. Virulence factors of Aeromonas salmonicida subsp. salmonicida strains associated with infections in turbot Psetta maxima. Dis. Aquat. Org. 2012, 99, 145–151. [Google Scholar] [CrossRef]
- Menanteau-Ledouble, S.; Kumar, G.; Saleh, M.; El-Matbouli, M. Aeromonas salmonicida: Updates on an old acquaintance. Dis. Aquat. Org. 2016, 120, 49–68. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.R.; Shadvar, S.; Sadeghian, M.; Doomanlou, M.; Abdollahi, A.; Manshadi, S.A.D.; Sardari, A.; Rahdar, H.A.; Feizabadi, M.M. Endocarditis with Aeromonas salmonicida. IDCases 2019, 18, e00625. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.T.; Fernández-Bravo, A.; Sanchis, M.; Mayayo, E.; Figueras, M.J.; Charette, S.J. Investigation of the virulence and genomics of Aeromonas salmonicida strains isolated from human patients. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2019, 68, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.; Dudeja, M.; Nandy, S.; Das, A.K. Isolation of Aeromonas salmonicida from Human Blood Sample: A Case Report. J. Clin. Diagn. Res. JCDR 2014, 8, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Björnsdóttir, B.; Gudmundsdóttir, S.; Bambir, S.H.; Gudmundsdóttir, B.K. Experimental infection of turbot, Scophthalmus maximus (L.), by Aeromonas salmonicida subsp. achromogenes and evaluation of cross protection induced by a furunculosis vaccine. J. Fish Dis. 2005, 28, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Frans, I.; Michiels, C.W.; Bossier, P.; Willems, K.A.; Lievens, B.; Rediers, H. Vibrio anguillarum as a fish pathogen: Virulence factors, diagnosis and prevention. J. Fish Dis. 2011, 34, 643–661. [Google Scholar] [CrossRef]
- Givens, C.E.; Bowers, J.C.; DePaola, A.; Hollibaugh, J.T.; Jones, J.L. Occurrence and distribution of Vibrio vulnificus and Vibrio parahaemolyticus--potential roles for fish, oyster, sediment and water. Lett. Appl. Microbiol. 2014, 58, 503–510. [Google Scholar] [CrossRef]
- Liu, R.; Qiu, L.; Yu, Z.; Zi, J.; Yue, F.; Wang, L.; Zhang, H.; Teng, W.; Liu, X.; Song, L. Identification and characterisation of pathogenic Vibrio splendidus from Yesso scallop (Patinopecten yessoensis) cultured in a low temperature environment. J. Invertebr. Pathol. 2013, 114, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Janda, J.M.; Newton, A.E.; Bopp, C.A. Vibriosis. Clin. Lab. Med. 2015, 35, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Sadat, A.; El-Sherbiny, H.; Zakaria, A.; Ramadan, H. Prevalence, antibiogram and virulence characterization of Vibrio isolates from fish and shellfish in Egypt: A possible zoonotic hazard to humans. J. Appl. Microbiol. 2021, 131, 485–498. [Google Scholar] [CrossRef]
- Hoihuan, A.; Soonson, P.; Bunlipatanon, P.; Thawonsuwan, J.; Tanasomwang, V.; Areechon, N.; Unajak, S. Molecular genotyping and phenotyping of Vibrio vulnificus isolated from diseased, brown-marbled grouper (Epinephelus fuscoguttatus) in Thailand with preliminary vaccine efficacy analysis. Aquaculture 2021, 545, 737188. [Google Scholar] [CrossRef]
- Sanjuán, E.; Amaro, C. Protocol for Specific Isolation of Virulent Strains of Vibrio vulnificus Serovar E (Biotype 2) from Environmental Samples. Appl. Environ. Microbiol. 2004, 70, 7024–7032. [Google Scholar] [CrossRef][Green Version]
- Zhu, L.; Yang, Q.; Huang, L.; Wang, K.; Wang, X.; Chen, D.; Geng, Y.; Huang, X.; Ouyang, P.; Lai, W. Effectivity of oral recombinant DNA vaccine against Streptococcus agalactiae in Nile tilapia. Dev. Comp. Immunol. 2017, 77, 77–87. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, W.; Xu, Z. Current use and development of fish vaccines in China. Fish Shellfish Immunol. 2020, 96, 223–234. [Google Scholar] [CrossRef]
- Adams, A. Progress, challenges and opportunities in fish vaccine development. Fish Shellfish Immunol. 2019, 90, 210–214. [Google Scholar] [CrossRef]
- Irshath, A.A.; Rajan, A.P. Bacterial Pathogenesis in Various Fish Diseases: Recent Advances and Specific Challenges in Vaccine Development. Vaccines 2023, 11, 470. [Google Scholar] [CrossRef]
- Torres-Corral, Y.; Girons, A.; González-Barreiro, O.; Seoane, R. Effect of Bivalent Vaccines against Vibrio anguillarum and Aeromonas salmonicida Subspecie achromogenes on Health and Survival of Turbot. Vaccines 2021, 9, 906. [Google Scholar] [CrossRef]
- Zhou, S.; Li, Y.; Yi, J.; Zheng, X.; Huang, Q.; Su, L.; Guo, B.; Yang, Z.; Xiu, Y. Immune responses to Vibrio vulnificus formalin-killed vaccine and ghost vaccine in Scophthalmus maximus. J. Fish Dis. 2022, 45, 1511–1527. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Guo, H.; Guo, B.; Yi, J.; Yang, Z.; Zhou, S.; Xiu, Y. Efficacy of bivalent vaccine against Aeromonas salmonicida and Edwardsiella tarda infections in turbot. Fish Shellfish Immunol. 2023, 139, 108837. [Google Scholar] [CrossRef]
- Whyte, S.K. The innate immune response of finfish—A review of current knowledge. Fish Shellfish Immunol. 2007, 23, 1127–1151. [Google Scholar] [CrossRef]
- Yin, F.; Gong, H.; Ke, Q.; Li, A. Stress, antioxidant defence and mucosal immune responses of the large yellow croaker Pseudosciaena crocea challenged with Cryptocaryon irritans. Fish Shellfish Immunol. 2015, 47, 344–351. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Magnadottir, B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Romstad, A.B.; Reitan, L.J.; Midtlyng, P.; Gravningen, K.; Emilsen, V.; Evensen, O. Comparision of a serological potency assay for furunculosis vaccines (Aeromonas salmonicida subsp. salmonicida) to intraperitoneal challenge in Atlantic salmon (Salmo salar L.). Biol. J. Int. Assoc. Biol. Stand. 2014, 42, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Dijkstra, J.M. Major Histocompatibility Complex (MHC) Genes and Disease Resistance in Fish. Cells 2019, 8, 378. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]

| Gene | Primer Name | Nucleotide Sequence of Primer (5′–3′) |
|---|---|---|
| β-actin | β-actin-F | AATGAGCTGAGAGTTGCCCC |
| β-actin-R | AGCTTGGATGGCAACGTACA | |
| TLR 5 | TLR 5-F | GATCCCGGGCTTTAACACCA |
| TLR 5-R | GGGGAGGCTAGGAAGTTGTT | |
| CD 4 | CD 4-F | ACATACCAATCCGTGGCGAG |
| CD 4-R | GAAATCGCGTCGGACGATCA | |
| MHC I | MHC I-F | TGCTGAGAAAGCTCGACTCAC |
| MHC I-R | CTCGCCCCAAAGTTCACGTA | |
| MHC II | MHC II-F | ACTGGACTTCACCCCACAGT |
| MHC II-R | CATCAACCAATCAGCTGCACTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiu, Y.; Yi, J.; Feng, R.; Song, J.; Pang, Y.; Liu, P.; Zhou, S. Evaluation of Immune Protection of a Bivalent Inactivated Vaccine against Aeromonas salmonicida and Vibrio vulnificus in Turbot. Fishes 2024, 9, 131. https://doi.org/10.3390/fishes9040131
Xiu Y, Yi J, Feng R, Song J, Pang Y, Liu P, Zhou S. Evaluation of Immune Protection of a Bivalent Inactivated Vaccine against Aeromonas salmonicida and Vibrio vulnificus in Turbot. Fishes. 2024; 9(4):131. https://doi.org/10.3390/fishes9040131
Chicago/Turabian StyleXiu, Yunji, Jingyuan Yi, Ruixin Feng, Jiaxue Song, Yunfei Pang, Peng Liu, and Shun Zhou. 2024. "Evaluation of Immune Protection of a Bivalent Inactivated Vaccine against Aeromonas salmonicida and Vibrio vulnificus in Turbot" Fishes 9, no. 4: 131. https://doi.org/10.3390/fishes9040131
APA StyleXiu, Y., Yi, J., Feng, R., Song, J., Pang, Y., Liu, P., & Zhou, S. (2024). Evaluation of Immune Protection of a Bivalent Inactivated Vaccine against Aeromonas salmonicida and Vibrio vulnificus in Turbot. Fishes, 9(4), 131. https://doi.org/10.3390/fishes9040131






