Abstract
While benthic characteristics of coral reef habitats are a major driver of the structure of coral reef fish assemblages, non-reef habitats adjacent to coral reefs (e.g., mangroves, seagrass beds, and macroalgal beds) can affect reef fish assemblages. Here, we investigate how reef fish assemblages respond to local-scale benthic habitats within a coral reef and larger-scale adjacent seascape features (habitats within 500 m of coral reefs) on Siquijor Island in the Philippines. We examined an abundance of species for the entire reef fish assemblage and within the assemblages of parrotfishes (subfamily Scarinae) and wrasses (family Labridae). Five distinct habitat types were identified in a cluster analysis, which incorporated benthic characteristics within coral reefs and habitats adjacent to coral reefs. We found that the diversity and structure of coral reef fish assemblages were affected by benthic characteristics within coral reefs and also by benthic habitat types adjacent to coral reefs. Individual species responses and juveniles of certain species demonstrated uniquely high abundances in habitat clusters characterized by the non-reef habitats surrounding coral reefs. Considering coral reef habitats and adjacent non-reef habitats as a holistic, interconnected seascape will provide better estimations of the drivers of the structures of coral reef fish assemblages.
Keywords:
adjacent habitats; seascape configuration; macroalgae; seagrass; mangrove; parrotfish; wrasse Key Contribution:
Coral reef fish assemblage structure and diversity are influenced by both the characteristics within the reef itself and the types of habitats present at 500 m (e.g., mangroves, seagrass beds, and macroalgae beds).
1. Introduction
Understanding the spatial scale at which organisms use habitats has been a fundamental component of ecology [1,2,3]. The spatial scale at which species use habitat affects population dynamics, species distribution, and community structure [4,5]. In demersal marine fishes, the importance of different habitats available at different spatial scales has long been recognized for many species [6,7,8,9]. Recently, this topic has gained renewed interest from marine ecologists, stimulated by improved methods of habitat mapping such as remote sensing and spatial analysis techniques [10,11]. Stemming from landscape ecology, seascape ecology takes an organism-based perspective to explore how the spatial distribution of habitats affects species patterns and processes [12,13]. A specific focus is understanding the extent to which density, biomass, diversity, and assemblage structure of coral reef fishes are influenced by within-coral reef habitats and the availability and spatial configuration of other benthic habitats adjacent to coral reefs [14,15,16,17,18]. While the characteristics of coral reef benthos are known to influence populations and assemblages of coral reef fish [19,20,21], the effect of other benthic habitats adjacent to coral reefs remains equivocal and highly variable, both among species and seascapes [22,23].
For benthic habitats across a seascape to impact coral reef fishes, the assumption is that individuals can travel large distances (hundreds to thousands of meters), move across habitat boundaries, and use a range of habitats for things such as food and shelter. Therefore, the proximity and total area of different habitats adjacent to coral reefs are spatial metrics that are expected to have the greatest influence on reef fish populations and assemblages of reef fishes [24]. However, the spatial scale at which species of reef fish respond to the seascape is still relatively unknown [25,26]. This scale may vary for different species [27,28], and it can be location- or region-specific [22]. Many reef fishes exhibit regular migrations between habitats, such as emperors (family Lethrinidae) and grunts (family Haemulidae), which travel diurnally from coral reef habitats to seagrass beds to feed [6,29,30]. Perhaps the best-documented link between habitats in a tropical seascape is ontogenetic migrations, where reef fishes use non-reef habitats as juveniles before migrating to coral reefs as adults [9,31,32,33,34]. The movement of reef fishes among benthic habitats is a major mechanism for connectivity that contributes to ecosystem processes such as nutrient transport [35], trophic transfers [36], and population replenishment [37].
In shallow tropical seascapes, the most conspicuous non-reef habitats available to fish are mangroves, seagrass beds, and macroalgal beds. Mangroves are well known to harbor high densities of juvenile reef fish and can contribute to an increased biomass of certain species of fishes on coral reefs [8,38,39,40]. The importance of mangroves as habitats for reef fish is most pronounced in regions like the Caribbean, where mangroves do not experience large tidal ranges, compared to the Indo-Pacific, where mangroves are often exposed at low tide [22,41], thus limiting the time that individuals can use this habitat. The connectivity of seagrass beds and coral reefs is well documented, affecting the presence, density, and diversity of fish species on coral reefs [42,43,44] and altering the feeding patterns of some coral reef fish [45,46]. Macroalgal beds have recently been highlighted as an important habitat for reef fish [47,48,49,50], and there is increasing evidence supporting the role of macroalgal beds as a reef fish nursery [51]. Many tropical seascape studies focus on coral reefs and one other non-reef benthic habitat. Some studies evaluate the ecological connections of both mangroves and seagrasses to coral reefs. But very few evaluate the combined effects of mangroves, seagrass beds, and macroalgal beds on coral reef fish assemblages [52]. Incorporating spatial characteristics of multiple benthic habitats can improve our understanding of the effects of marine reserves [53,54,55], ecological processes like herbivory [15,56,57,58], and patterns in fish assemblage structure [16,59].
This study investigated how different benthic habitats at multiple spatial scales affect the assemblage structure of coral reef fishes on an island in the Philippines. Spatial measures of different coral reef and non-reef habitats (mangroves, seagrass beds, macroalgal beds, coral reef, reef flat, and sand) combined with percent cover benthos within coral reefs (hard coral, soft coral, rubble, EAM (epilithic algal matrix), structural complexity, and depth) were incorporated into a multi-scale approach to explore the combined effects of within-coral reef metrics and adjacent habitat metrics at the seascape scale. We first investigated such effects on the entire assemblage of non-cryptic diurnally active coral reef fish, and then on the parrotfishes (family Labridae, subfamily Scarinae) and wrasses (family Labridae). Parrotfishes are well documented to use multiple benthic habitats and are present in tropical non-reef habitats as juveniles [43,49,60]. Wrasses have also demonstrated significant responses to the spatial arrangement of multiple habitats in tropical seascapes, such as altered assemblage structure [61,62], increased densities when non-reef habitats are near coral reefs [50], and utilization of non-reef habitats as juveniles [63]. Specifically, we aimed to achieve the following: 1. Understand how coral reef habitats and multiple adjacent benthic habitats affect coral reef fish assemblages; 2. identify which species are most strongly affected by the adjacent benthic habitats; and 3. explore whether fish diversity on coral reefs is influenced by adjacent benthic habitats.
2. Materials and Methods
2.1. Study Area and Coral Reef Surveys
Siquijor Island is a relatively small island in the Central Visayas region of the Philippines and is characterized by a range of submerged benthic marine habitats that vary in occurrence and total area (Figure 1). Some areas have expansive areas of seagrass, macroalgal beds, lagoons, and reef flats (e.g., the western corner of the island), whereas other areas around the island (e.g., the southeast portion of the island) have much more narrow strips of shallow non-reef habitats. Siquijor has a relatively low tidal range of 2.2 m (ArcGIS Global Tidal Range), where small areas of shallow seagrass and macroalgae areas are exposed at low tide, with limited submerged channels present during low tides. Here, a coral reef habitat is defined as coral reef crest (5–8 m) and coral reef slope (10–15) areas, which are dominated by hard coral cover. Non-reef habitats are defined as those that are not dominated by hard coral cover, and they include mangrove stands, lagoons, seagrass beds, macroalgae beds, reef flats, and sand areas.
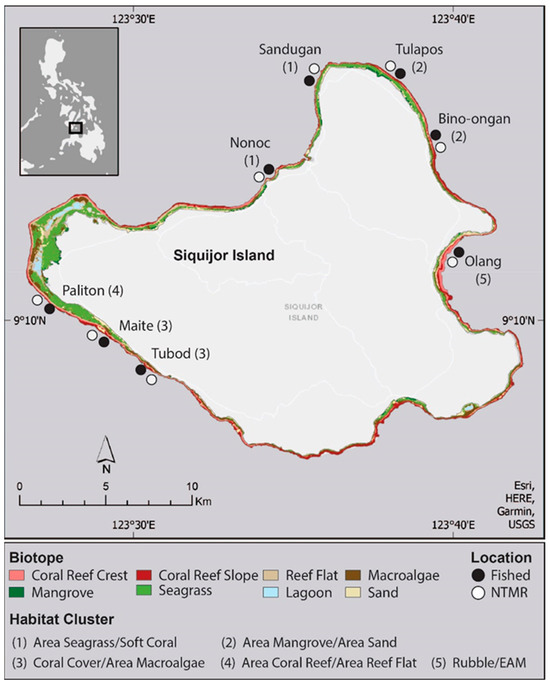
Figure 1.
Map of Siquijor Island with classified submerged habitats. Black dots indicate the locations of fish and benthic habitat surveys conducted on coral reefs. For each location, a fished and no take marine reserve (NTMR) site was surveyed. Numbers represent habitat cluster identification based on k-means clustering analysis. Cluster describes the habitats within 500 m of survey sites, which characterizes the area identified by hierarchical cluster analysis.
2.2. Coral Reef Surveys
Surveys of coral reef fishes and benthic habitat on coral reefs were conducted in April–July 2016. Locations for coral reef survey sites were selected with a diversity of non-reef habitats surrounding coral reefs. Each location had an established No-Take Marine Reserve (NTMR) and a fished control site with similar adjacent habitats to the NTMR. This allowed us to evaluate any potential fish assemblage differences driven by NTMR effects. At each of the eight locations, a fished and NTMR site were selected, resulting in a total of 16 survey sites on Siquijor Island (Figure 1). NTMR and fished sites within the eight pairs of locations were no more than 500 m from one another, and the benthic habitats between NTMR and fished sites were matched as closely as possible, resulting in relatively similar benthic cover composition between adjacent NTMR and fished sites (Supplementary Materials Table S1) [64]. Surveys of fish and benthic habitats were conducted only on coral reef habitats. At each site, three to four transects were laid following the in of the reef by an observer simultaneously surveying fish, first on the coral reef slope (10–15 m depth), then the coral reef crest (5–8 m depth), resulting in 6–8 transects per site. The number of transects was dictated by the size of the NTMR, as most NTMRs on Siquijor Island are small (less than 250 m wide). In total, there were 108 transects. Fish surveys were conducted during daytime hours between 9 a.m. and 3 p.m., where large mobile reef fishes were identified and counted on a 50-meter-long and 5-meter-wide transect. On the return swim, smaller reef fish (<10 cm TL) were identified and counted on a 2-meter-wide belt. When present, juveniles were recorded and identified by genus due to difficulties in identifying some juvenile fishes to the species level in situ (e.g., parrotfishes). To estimate the percent cover of the benthos, substrates were identified every 50 cm along the 50 m transect and classified based on benthic categories of rubble, crustose coralline algae, epilithic algal matrix (EAM), macroalgae, soft coral, hard coral, and others. Macroalgae and soft coral were identified by the genus when possible. Hard coral was identified by the genus and classified into a growth form (e.g., branching, tabulate, massive, and encrusting). The ‘other’ category included sponges, tunicates, and gorgonians. Depth and structural complexity were also measured for the transect, and structural complexity was estimated on a 0–5 scale, where 0 represented flat (i.e., no vertical relief) and 5 equated to a highly complex structure [65].
2.3. Seascape-Level Spatial Analysis
The availability of benthic habitats adjacent to coral reefs (total area of the habitat within 500 m radius of the coral reef) across the Siquijor seascape was calculated using satellite-derived habitat maps. The methodology of the habitat classification process is detailed in Sievers et al. 2020b [62]. Briefly, adjacent habitats were classified using the maximum likelihood classification method in ArcGIS v.10.0, informed by geo-referenced habitat data points collected in situ. Classified submerged benthic habitats were macroalgal beds, seagrass beds, mangroves, reef flats, sand, and coral reefs. Lagoons were not included in this analysis due to lagoon habitats being beyond the 500 m distance from coral reef survey sites. The total area (km2) of each habitat type was measured within a 500 m radius of each site for fish and benthic surveys on coral reef (Supplementary Materials Table S2). The 500 m scale was selected, as it was previously identified as the most relevant distance for describing fish abundance and biomass patterns in this location [50]. A rectangular polygon (50 m long × 5 m wide) was drawn around the perimeter of all survey transects at a coral reef site, and 500 m Euclidian distance buffers from the boundary of each site polygon were created. Buffer polygons were then clipped by deep water (approximately 20 m depth) and the shoreline to represent the total submerged shallow marine benthic habitat within a 500 m distance to coral reef sites. The proportional area of each adjacent habitat type was calculated at the site level as the total area of the habitat divided by the total area of the clipped 500 m buffer polygon, representing the availability of that habitat type within the seascape. Global Moran’s I was calculated for habitats within 500 m of each survey site to evaluate potential spatial autocorrelation among sites and locations.
2.4. Statistical Analysis
Sites around Siquijor were classified into habitat clusters to estimate how reef fish assemblages may vary based on the combination of local (within coral reef) and broad-scale (area of adjacent benthic habitats) seascape characteristics. A hierarchical clustering analysis using percent cover of benthic variables from coral reef transects and the proportion of adjacent areas from seascape variables at the location level was generated using the pvclust package v.2.2.0 [66] to group individual habitat metrics and identify habitat clusters [67]. Rather than group sites by environmental, geographic, or location effects, we sought to understand how the benthic habitats surrounding a coral reef may influence fish assemblage structures on that reef, and therefore, we classified each transect replicated by the coral reef benthic features as well as the adjacent benthic habitat characteristics. Clustering analysis enables such classification using statistical methods to group similar transects based on their habitat features. Pvclust uses scaled and centered standardized habitat values, assesses uncertainty in hierarchical cluster analysis, and allows for bootstrapping and calculated probability analyses to assign p-values to the clusters. p-values were calculated using 10,000 multi-scale bootstrapping, and clusters were considered significant when the approximately unbiased (AU) p-value was greater than 0.95. Each individual transect was assigned to a cluster irrespective of location by using a k-means partial clustering process using the k-means function in the cluster package v. 2.1.0 [68] All 108 transects were assigned to a cluster type to confirm clusters and visualize their relationships with specific habitat variables, and a correlation-based multivariate redundancy analysis (RDA) was performed using the vegan package v. 2.5.6 [69] in R. Predictor variables were square-root transformed if necessary to ensure normality and achieve heterogeneity in the data. Variables were centered and scaled for the RDA analysis (Figure 2B).
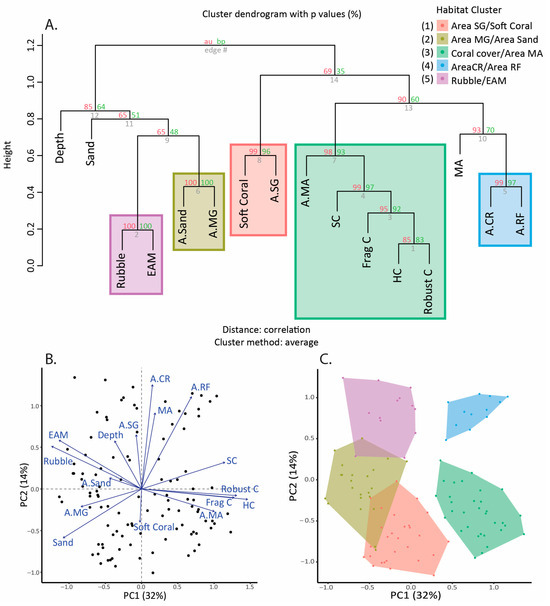
Figure 2.
(A) Hierarchical cluster dendrogram of within reef benthic habitats and seascape measures of multiple habitats. At each branch, the left value (red) is the approximately unbiased (AU) p-value, and the right value (green) is the bootstrap probability (BP) p-value. The AU value is used to define habitat clusters, where branches with AU > 95% are significant. The numbers on the habitat cluster legend are the same as in Figure 1. A.MG = area of mangrove; A.MA = area of macroalgae; A.SG = area of seagrass; A.CR = area of coral reef; and A.RF = area of reef flat. SC = structural complexity. Frag C = fragile coral. HC = hard coral. Robust C = robust coral. MA = macroalgae. (B) Redundancy analysis (RDA) visualizing the variation in habitat variables along two principal component axes. Dots are individual transects, and vectors are plotted variables. (C) RDA grouped by habitat cluster identified in the hierarchical cluster analysis. Points are individual transects.
To investigate how fish assemblages differed by habitat cluster, non-metric multidimensional scaling (nMDS) ordination analysis based on Bray–Curtis dissimilarity was performed to compare reef fish assemblages among clusters. nMDS analyses were run on fish density (count per 1000 m2) for 1. all fish species; 2. parrotfishes (family Labridae; subfamily Scarinae); and 3. wrasses (family Labridae). Parrotfishes and wrasses were selected as they demonstrated the strongest population responses to the presence of non-reef benthic habitats adjacent to coral reefs in our study area [50]. Fish species density (count per 1000 m2) was fourth-root-transformed and standardized using Wisconsin standardization to reduce the influence of highly abundant species. nMDS was also used to compare fish assemblages between fished and NTMR sites.
To identify which fish species and habitat variables were significantly driving multivariate patterns, the envfit function from the vegan package v.2.5.6 was run for scores of fish species and scores for coral reef benthos and adjacent benthic habitats. Vectors of scores were plotted to visualize the relationship between fish and habitat variables. Fish species and habitat variables with significant correlations (p < 0.05) were selected and plotted using the permutation feature in envfit to calculate a p-value. Differences in fish assemblages between habitat clusters and between fishing effects (NTMR versus fished areas) were tested using a one-way PERMANOVA for each fish group (permutations = 999) using the adonis function in the vegan package v.2.5.6, applied to fourth-root-transformed and Wisconsin standardized data. To assess significant differences in fish assemblages between habitat clusters, pairwise comparisons were conducted using the pairwise.adonis function in pairwiseAdonis v.0.0.1, which uses Bray–Curtis distance measures and Bonferroni corrections to compare clusters.
A similarity percentage analysis (SIMPER) was used to identify the dissimilarity among assemblages across habitat clusters. SIMPER was conducted in R with 999 permutations, which provide p-values to identify species significantly driving differences between assemblage groups. The permutation results are less influenced by species with a high variability in density or very high abundances, which might otherwise give them high contributions, even though they do not differ among groups. Species that were repeatedly observed at the top of the contribution list and had significant p-values were interpreted as fish species significantly driving differences between clusters. For parrotfishes, with only 19 species, all significant contrasts were considered. For wrasses, with 52 species, the top 20 from the contribution list were considered. And for the all-species analysis, with 248 species, the top 50 contributions were considered. We then explored if there were consistencies in habitat cluster comparisons, where certain fish species were repeatedly observed as significantly describing the dissimilarities in fish assemblages for each cluster compared to all other clusters.
Finally, to understand any effect of habitat clusters on the diversity of reef fishes, we calculated a Shannon–Wiener diversity index for each fish species group (all species, parrotfishes, and wrasses). We then explored the effect of habitat clusters on fish diversity using generalized linear models with glm in the MASS package v. 7.3.51.5 (Venables and Ripley 2002), using the habitat clusters as the predictors and the Shannon–Wiener index values as the response variables. Post hoc multiple comparison Tukey tests were used to identify the differences in fish species diversity between habitat clusters.
3. Results
A total of 5297 individual fish from 248 species were recorded in the coral reef habitat, with 52 wrasse species and 19 parrotfish species observed (Supplementary Materials Table S3). Hierarchical clustering identified five significant habitat clusters (Figure 2A). For some clusters, the classifications of the clusters were based solely on coral reef benthos variables or solely on benthic habitats adjacent to coral reefs. However, in two of the five cases, the classifications comprised a combination of both types of habitat data (Table 1, Figure 2).

Table 1.
Classification of habitat clusters from k-means clustering analysis results. Columns identify which coral reef habitat variables and which seascape variables are associated to each cluster, as well as the number of transects allocated to each cluster.
The first habitat cluster, “Area Seagrass/Soft Coral”, was characterized by a dominance of seagrass habitat within 500 m of transects as well as a high percent cover of soft coral on transects. The second cluster, “Area Mangrove/Area Sand”, was uniquely classified by a greater area of sand and mangroves within 500 m of coral reef survey transects compared to other clusters. Additionally, this cluster had a relatively high percent cover of rubble, EAM, and sand on coral reef transects (Figure 3). The third cluster, “Coral/Area Macroalgae”, was characterized by a higher percent live hard coral cover on transects, greater structural complexity, and the largest area of macroalgae adjacent to the coral reef than any other cluster. The fourth cluster, “Area Coral Reef/Area Reef Flat”, was represented as having large areas of coral reef surrounding coral reef transects as well as large areas of reef flat within 500 m of sites, representing areas with consolidated reef substratum. Although this final cluster also has large areas of seagrass (Figure 3), it was classified by its unique habitat cluster, which represents a greater area of coral reef and reef flat habitats compared to other clusters. It is also defined by its relatively even distribution of benthic habitat types on coral reefs, where no single benthic type dominates with more than 40% cover (Figure 3). The final cluster, “Rubble/EAM”, was characterized by a high percent cover of rubble and epilithic algal matrix (EAM) and a lack of hard coral on benthic transects from coral reef surveys (Figure 2 and Figure 3). Statistical analysis identified these clusters as unique, and we acknowledge that all habitat types are present within each cluster. As expected, these clusters are predominately grouped by location (Figure 1), indicating that the clustering analysis does have some location effect in addition to the influence of benthic and seascape habitat variation. However, spatial data were not significantly spatially autocorrelated (Moran’s I = 0.370, p = 0.24).
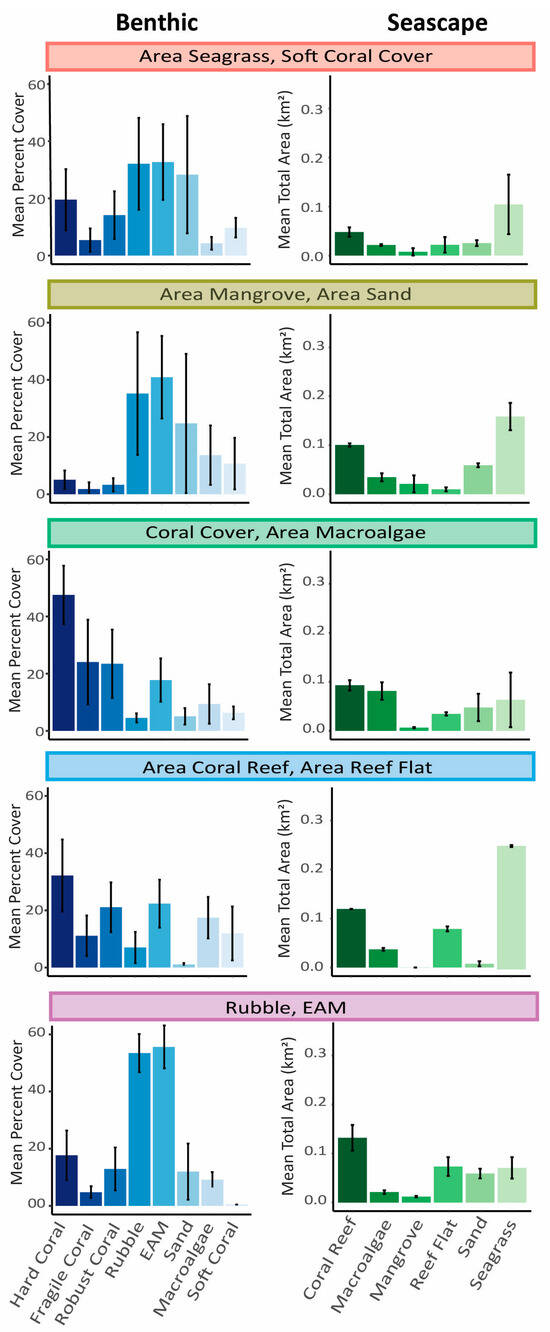
Figure 3.
Average (mean) and variation (standard deviation) of percent cover or area of seascape variables (km2) within each habitat cluster. The left column (blue) is the mean percent cover of benthic coral reef habitat variables observed on coral reef transects. The right column (green) is the mean total area of habitat types within 500 m of fish and benthic survey locations. Colored bars above each pair of graphs is to easily reference the habitat clusters when comparing with other figures.
When evaluating the potential effects of fishing, nMDS and SIMPER results indicated weakly significant differences in the assemblage structure of reef fish between fished and NTMR locations, explaining only 3–4% of the total variation. Furthermore, no effect of protection status (fished vs. NTMRs) was observed on fish diversity.
The primary factors separating habitat clusters and consequently assemblages of fishes seemed to be benthic cover characteristics such as live hard coral cover or lack thereof, resulting in higher cover of EAM and rubble (Figure 4, column C). The secondary drivers were the adjacent habitat types within 500 m of coral reef surveys.
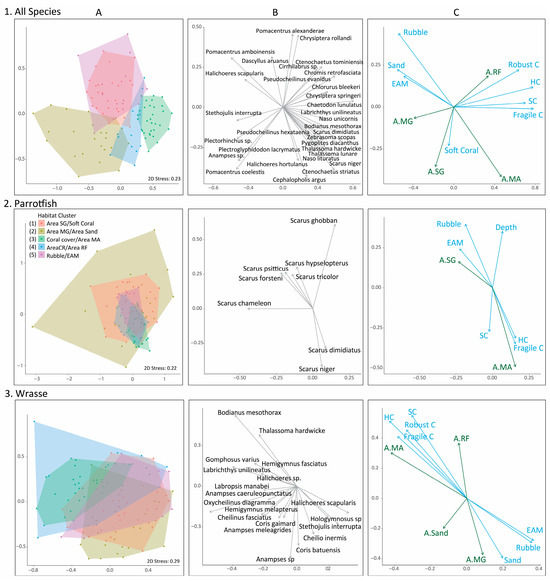
Figure 4.
Fish assemblage results for all species observed (row 1), parrotfish (row 2), and wrasses (row 3). Column (A) displays the nonmetric multidimensional scaling (nMDS) analysis depicting similarities in fish assemblage structure among habitat clusters. Colored polygons are the different habitat clusters. MA = macroalgae; MG = mangrove; SG = seagrass; CR = coral reef; RF = reef flat. EAM = epilithic algal matrix. Column (B) displays the vectors of individual fish species with significant (p < 0.05) correlations resulting from the envfit analysis. Column (C) displays habitat variables significantly (p < 0.05) correlated to fish assemblage structure. Blue vectors and variables are within-habitat benthic variables describing coral reef habitat, and the green vectors are seascape-level variables describing the area of different habitat types. Fragile C = fragile coral; robust C = robust coral. HC = hard coral; SC = structural complexity.
For all fish species observed (n = 248 species), there was clear separation in assemblage structure between most habitat cluster groups, with the only significant overlap between the “Rubble/EAM” and “Area Seagrass/Soft Coral” clusters (Figure 4(1A)). To further refine the species list, the species presented from the envfit analysis were those with a significance of p < 0.001 (Figure 4(1B)). When examined in conjunction with the habitat vectors (Figure 4(1C)), there was a clear separation of habitat vectors, which aligned with fish species.
The pairwise PERMANOVA comparisons also indicated that reef fish assemblages were significantly different among all five habitat clusters (Supplementary Materials Table S4). SIMPER analysis indicated that particular species were consistently driving significant differences between habitat cluster comparisons (Table 2, Supplementary Materials Table S5). “Area Seagrass/Soft Coral” had a few unique species characterizing that cluster. For the habitat cluster “Area Mangrove/Area Sand”, the species that were significantly driving the dissimilarity of this cluster were Ctenochaetus striatus, Acanthurus nigrofuscus, and adult and juvenile Plectorhinchus spp. Fish species that differentiated the habitat cluster “Coral/Area Macroalgae” were Naso lituratus, Zebrasoma scopas, and Naso unicornis. For the “Area Coral Reef/Area Reef Flat” cluster, the species driving the unique assemblage structure were Pterocaesio tile, Caesio caerulaurea, Caesio teres, and Naso vlamingii. The species described in the “Rubble/EAM” cluster were Ctenochaetus binotatus and Naso minor.

Table 2.
Fish species results from the percentage similarity analysis (SIMPER) identify species uniquely attributed to each habitat cluster. Species are selected from the entire SIMPER output, and the species listed here are only species that were significant in the SIMPER output and were repeatedly selected as describing that habitat cluster across all cluster comparisons. Full SIMPER comparisons are listed in Supplementary Materials Table S5.
For parrotfishes (subfamily Scarinae), the clusters had a very different structure in nMDS space compared to the analysis that considered all species (Figure 4). Most clusters were noticeably more constrained in nMDS space and had greater overlap compared to the all-species analysis. However, the cluster “Area Mangrove/Area Sand” was spread over a large area of the MDS space, encapsulating all other habitat clusters. These results were further confirmed by the pairwise PERMANOVA results, which revealed that four of the ten cluster comparisons were not statistically different from one another (Supplementary Materials Table S4). The clusters “Area Coral Reef/Area Reef Flat”, “Area Seagrass/Soft Coral”, and “Rubble/EAM” had fish assemblages that were not statistically different from one another.
When evaluating the wrasse assemblages, the clusters had greater separation across nMDS space compared to parrotfish (Figure 4(3A)). The PERMANOVA pairwise comparisons identified that the fish assemblage from the “Rubble/EAM” cluster was similar to most other fish assemblage clusters, sitting in the middle of MDS space (Figure 4(3A)). However, fish assemblages from “Rubble/EAM” and “Coral/Area Macroalgae” were significantly different from one another. There was a strong separation in the nMDS space between significant habitat variables (Figure 4(3C)). One habitat grouping was for live hard coral cover, structural complexity, area of macroalgae, and area of reef flat, whereas in the opposite direction, it was rubble, EAM, sand, and area of mangrove habitat. The SIMPER analysis again revealed distinct species driving assemblage differences among habitat clusters (Table 2).
The Shannon–Weiner diversity of reef fishes differed significantly between habitat clusters for all three species groups analyzed (Figure 5). When evaluating the entire fish assemblage, the “Rubble/EAM” cluster and the “Area Mangrove/Area Sand” cluster had significantly lower fish species diversity compared to the other three clusters. For parrotfish, there were more nuanced diversity results, but the highest diversity was observed on the sites characterized by “Rubble/EAM”, with the lowest on sites from the “Area Mangrove/Area Sand” cluster. Wrasses, interestingly, showed an opposite diversity response to parrotfishes, with the highest wrasse density in sites from the habitat cluster “Area Mangrove/Area Sand” and “Coral/Area Macroalgae”, with the lowest diversity for “Area Coral Reef/Area Reef Flat”.
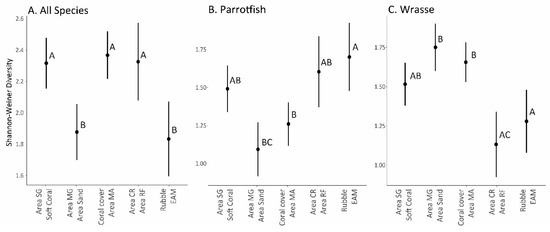
Figure 5.
Shannon–Weiner diversity estimates (fitted values with 95% confidence intervals) in the habitat cluster for (A) all species, (B) parrotfish, and (C) wrasse. Letters adjacent to points are significance groupings based on post hoc multiple comparisons Tukey’s tests.
4. Discussion
This study investigates the combined effect of coral reef benthos and the area of different habitats adjacent to the coral reefs on assemblages of coral reef fish. It differs from most previous studies that focused on the effect on reef fish assemblages of only one non-reef habitat adjacent to coral reefs [69,70]. While it is well known that within-coral reef benthic habitats drive species patterns and assemblages [19,20,21,71], the effects of multiple adjacent habitats at a seascape scale and the combined effects of benthic habitat across spatial scales (within reefs and adjacent to reefs) are understudied. Using a cluster analysis to identify habitat clusters allowed for an improved understanding of how the relative differences in benthic habitats on coral reefs and adjacent habitats surrounding those reefs may affect fish assemblage structure. Here, we demonstrate that non-reef habitats within 500 m of coral reef habitat can meaningfully describe differences in fish assemblage structures and species diversity of fishes on coral reefs. These results support the notion that benthic habitats in seascapes are inextricably linked.
For all fish species observed, adjacent habitat metrics occupied unique sections of the multivariate space in the nMDS plots and had a strong relationship with many of the species driving differences in fish assemblages among habitat clusters. For example, two conspicuous and abundant surgeonfish species observed on coral reefs, Ctenochaetus striatus and Acanthurus nigrofuscus, were attributed to the cluster described by the area of mangrove and area of sand habitat near coral reefs (Table 2). C. striatus is a common detritivore [72], and A. nigrofuscus is an herbivore that feeds on turf algae [73]. Mangrove habitats are soft sediment habitats that experience tidally driven changes that transport organic material and detritus from estuarine and intertidal systems out onto submerged reef habitats [74,75]. Potentially, here, coral reefs near mangroves have higher levels of detritus and nutrients, which support the growth of turf algae. Another detritivorous surgeonfish, Ctenochaetus binotatus, was distinct for the “Rubble/EAM” cluster, which has shown clear correlations with the cover of dead substrata over long periods [72]. Interestingly, Naso minor was associated with the “Rubble/EAM” cluster. Naso minor is a mid-water reef-associated, nominally planktivorous species that has been anecdotally recorded to feed on benthic algae and is more common on reef slopes, which have high rubble and sand cover compared to slopes with high coral cover in the central Philippines [76]. The cluster “Area Coral Reef/Area Reef Flat” identified a few fusilier species (Caesionidae) and Naso vlamingii as distinctive to this habitat group. The reef sites in this cluster had steep reef slopes suitable for these midwater, mobile, reef-associated planktivorous species. Fusiliers (Pterocaesio, Caesio) are highly mobile, but they require complex coral reef structures to sleep [77], and Naso vlamingii is a moderately mobile species, but it is highly territorial [78,79]. Perhaps Naso vlamingii needs large areas of reef to reduce density-dependent competition. For the cluster characterized by live coral cover and adjacent macroalgal habitat “Coral/Area Macroalgae”, three herbivorous fishes, Naso lituratus, Zebrasoma scopas, and Naso unicornis, were identified as distinct to this habitat cluster. These species are abundant grazers and browsers, with the two Naso species known to feed on fleshy brown macroalgae [80,81]. Naso unicornis has been shown to travel hundreds of meters to foraging areas to feed on fleshy macroaglae [82]. Across an entire coral reef fish assemblage, we see strong and distinct correlations between the abundance of coral reef fish and habitats beyond the coral reef, emphasizing the importance of including multiple habitats across a seascape in studies of the structure of reef fish assemblages.
For parrotfishes, assemblages between habitat clusters were quite similar to each other, which is unsurprising given that there were only 19 species of parrotfishes observed. However, the habitat cluster characterized by the area of mangrove and sand in the surrounding seascape had much greater assemblage space than the other clusters. Although mangroves can be important for parrotfish habitats in the Caribbean [83,84] and east Africa [43,85], mangroves seem to be less influential on reef fish assemblages in the Coral Triangle [22,49], especially assemblages of parrotfishes. The benthic composition of the reef in the mangrove and sand cluster was similar to other clusters (e.g., “Rubble/EAM”), suggesting that differences in the benthic structure between the clusters were important in driving differences in fish assemblage structures for parrotfishes. The remaining habitat clusters had relatively small nMDS space. Parrotfishes have highly specialized feeding modes, where they target microautotrophs [86,87], and are therefore tightly linked to the benthic composition of the reef [88]. The three most compact assemblage spaces were the habitat clusters describing benthic coral reef habitat (“Rubble/EAM”, “Coral/Area Macroalgae”, and “Area Coral Reef/Area Reef Flat”). Although the benthic composition varies substantially among these groups, we hypothesize that parrotfishes are so tightly linked to their benthic food resource needs that species in these clusters target the necessary food sources irrespective of their abundance, and thus, they maintain a similar assemblage structure. For example, adult Chlorurus microrhinos, a parrotfish species highly associated with coral reefs, will change their feeding behavior and home range depending on the availability of resources to maintain access to their specific resources [89]. Furthermore, some species change their feeding behaviors based on the structural connectivity of surrounding habitats (e.g., the distance to adjacent seagrass beds). Eggertsen et al. (2020) [90] observed increased bite rates for Scarus ghobban when near seagrass beds. We too found that Scarus ghobban contributed strongly to the assemblage structure of parrotfish in the seagrass habitat cluster (Table 2). Although parrotfish assemblages are tightly clustered within coral reef habitats, distinct species characterizing clusters are driven by both coral reef benthic composition and non-reef habitats adjacent to those coral reefs in a seascape. Perhaps non-reef habitats function as complimentary nurseries for the juveniles of a diversity of parrotfish species [60], and the degree to which they are utilized likely depends on their context, connectivity, and condition. The results here provide further evidence that parrotfish diversity is linked to the presence and diversity of specific non-reef habitats near coral reef habitats.
Wrasses had a much larger assemblage area in nMDS space compared to parrotfishes while still exhibiting differences in species composition among habitat clusters (Figure 4). Wrasses are a diverse group of fishes with varied feeding and swimming strategies [91,92]. While wrasses respond to changes in benthic composition [91,93,94], they seem to be less tightly coupled to the benthic habitat [94,95], potentially explaining the overlap in assemblage space among the habitat clusters. It is interesting to note that Thalassoma spp. did not appear in any of the SIMPER analyses. Thalassoma hardwicke was listed as significantly correlated to live coral and structural complexity, which corroborates relationships found on the Great Barrier Reef [94] but is contrary to the responses observed for Thalassoma spp. from other islands in the Philippines [93]. The lack of Thalassoma spp. in the SIMPER analysis is likely because this genus is abundant, highly mobile [92], and a habitat generalist [96], allowing them to be evenly distributed across most or all habitat clusters. Some wrasse species selected as distinct from a habitat cluster are clearly responding to within-coral reef benthic characteristics (Table 2). For example, Novaculichthys taeniourus, the rockmover wrasse, was associated with the “Rubble/EAM” cluster, and Labrichthys unilineatus, a corallivore [97], with the “Coral/Area Macroalgae” cluster. Yet, other species correlate with the area of non-reef habitats adjacent to coral reefs. Coris gaimard was correlated with the area of sand in the nMDS and characterized the habitat cluster “Area Coral Reef/Area Reef Flat”. C. gaimard has previously shown low associations with any coral reef benthic type [91,98], and therefore might be more strongly associated with the non-reef habitats adjacent to coral reefs, including reef flat habitats which are characterized by coral interspersed within sandy patches. Cheilio inermis was characteristic of the habitat cluster as “Area Seagrass/Soft Coral”. C. inermis has higher densities on reefs close to seagrass habitats [43,99], is a common predator in seagrass beds [100,101,102], and its juveniles use seagrass beds [103]. Cheilinus chlorurus characterized the habitat cluster “Area Mangrove/Area Sand”. This species has inconsistent responses to within-coral reef benthic characteristics [94] and has one of the largest foraging distances among wrasses [91], suggesting a high potential to use multiple benthic habitats across a connected seascape. The spatial arrangement of a habitat can alter the assemblage structure of wrasses in the western and eastern Indian Ocean [43,62,104], and we demonstrate that this may also be the case on the island of Siquijor in the Philippines. Further exploration of the extent with which coral reef wrasses are reliant on non-reef habitats adjacent to coral reefs is warranted. It is possible that wrasses maintain important ecological connections of benthic habitats within tropical seascapes.
Our findings suggest that incorporating a combination of within-coral reef benthic habitat and adjacent benthic habitat metrics is useful for describing coral reef fish assemblages in a diverse seascape. Some studies show that seascape-level metrics, like adjacent habitats, are highly influential in describing fish assemblage patterns [16], while others observe a secondary influence of seascape context [45,105,106]. Interestingly, we did not find a significant effect of NTMRs on the assemblage structure of reef fish. While positive NTMR effects on the abundance of species targeted by fishing and on the catch of reef fish have been shown in the area [107,108], NTMRs can have less influence on fish abundances compared to geographical and environmental influences [64] or the strong bottom-up influence of the benthos [109]. Perhaps the small size of reserves and the large diversity of species (target and non-target) also influenced the limited effects of NTMRs on the assemblage structure of reef fish in this study.
Species groups, as well as life stages, seem to alter the order in which habitat scales are prioritized, and a combination of fine- and broad-scale metrics are likely the best predictors [17,46]. For example, for juvenile wrasses in non-reef habitats such as macroalgal beds or seagrasses, within-patch metrics (e.g., canopy structure) were the primary factors affecting density and abundance, but adults were more influenced by seascape-level metrics such as patch area and isolation [62]. It is therefore critical to consider the focal habitat of interest with respect to the species and life stage evaluated. Here, we focused on adult fishes in coral reefs, but relationships with juvenile fishes emerged in two notable instances. Sweetlips (Plectorhinchus spp.) and parrotfish (Chlorurus spp. and Scarus spp.) juveniles were listed as distinct for habitat clusters, which were characterized by their surrounding habitats (Table 2). Plectorhinchus spp. had strong relationships with the area of mangrove habitat and sand within 500 m of coral reefs. Previous research has recorded mangrove use from a few species of this genus (P. gibbosus and P. albovittatus) [22,104] and occasionally in other non-reef habitats, such as seagrass beds [25,42,61]. Alternatively, it may be equally likely that the strong relationship is the result of this taxa’s tendency to associate with isolated coral patches (i.e., large coral bommies) within extensive areas of sand habitats. Other related species from the grunt family (Haemulidae) are well known to use non-reef habitats, predominately seagrass beds and sand, for daily foraging migrations and as nursery habitats [25,46,49,110,111]. Here, we find that reef sites with a greater proportion of mangroves and sand near coral reefs had higher numbers of juvenile Plectorhinchus. We hypothesize that ontogenetic migrations may create linkages between mangroves and coral reefs, and further exploration into specific fish movements and physical environmental dynamics (e.g., tidal movement and organic matter exchange) should be explored. For parrotfishes, macroalgal beds have previously been identified as important habitats, affecting density and biomass on coral reefs in this area [50], and they are a particularly valuable habitat for juveniles [60]. Macroalgal beds have recently been recognized as an important habitat in a tropical seascape and as potential nursery grounds for many coral reef species [48,101]. It is important to note that these surveys were conducted in April–July when there was a dense canopy structure in Sargassum beds. Seasonal variations in biomass per unit area of macroalgae will likely have some effect on the dynamics of fish that utilize this adjacent non-reef habitat. Further research on the seasonal variability of the abundance of benthic habitats adjacent to coral reefs is recommended. While some species and life stages might be more associated with fine-scale within-coral reef metrics, it is important to consider the context in which these relationships are explored and the potential connectivity with other benthic habitats occurring across a seascape.
Whether a species or assemblage responds to within-coral reef or adjacent benthic habitat variables depends heavily on the context of the seascape, which species are being evaluated, and which life stages are observed. Ultimately, incorporating multiple spatial scales provides better estimations of species patterns [12,112], ensuring that researchers are capturing the relevant spatial scale [26,113]. In our study system on Siquijor, all habitats within 500 m of coral reef sites should be considered potentially influential. Among the five habitat clusters, the coral reef benthic composition was similar between many of them (e.g., “Rubble/EAM” and “Area Mangrove/Area Sand”). If within-coral reef benthic characteristics were the only drivers of fish assemblages, one would expect fish assemblage structure and diversity to be similar in these instances. However, the differences between clusters were apparent and significant across the entire fish assemblage, including wrasses and parrotfishes, which implies the importance of the adjacent non-reef habitats. Additionally, if the effect of location on Siquijor was the sole influence for fish assemblages, the nMDS plots would have very little overlap with one another. The all-species analysis did exhibit the strongest location effect, with the most distinct assemblages among clusters (Figure 4). However, both parrotfishes and wrasses showed greater overlap in nMDS clusters, indicating a stronger habitat effect relative to the location. While certainly geomorphology and location are important in structuring species assemblage patterns, the location effect is weaker for parrotfishes and wrasses compared to the all-species analysis, confirming that variability in habitat is a driver of assemblage structure. Certainly, location effects do exist, but one of the largest causes of location effects on reef fish assemblages is the benthos, and therefore, these factors are difficult to tease apart. While environmental (e.g., temperature), biophysical (e.g., larval supply) and geomorphological (e.g., aspect) variation likely influence species assemblages on Siquijor, it does not negate the importance of evaluating the relative influence of benthic habitats across multiple spatial scales and was beyond the current scope of these exploratory results. We acknowledge the importance of these drivers and suggest further research to develop these datasets for incorporation into broad-scale seascape studies.
Here, the combination of coral reef benthos and adjacent habitats affected the structure of reef fish assemblages on coral reefs, suggesting the linked nature of many benthic habitats in this tropical seascape. It is also necessary to be cautious of interpretations of the relative importance of different habitat scales, as responses of species to spatial scales are usually the consequence of different ecological processes. For example, fish relationships to within-habitat benthic composition (e.g., coral cover) are probably a fish’s response to shelter or food. In contrast, the presence, extent, and configuration of adjacent non-reef habitats across a seascape likely influence processes like fish migrations (including foraging) and ontogenetic habitat shifts of fish, which operate at different temporal scales and life stages. Considering non-reef habitats adjacent to coral reefs and coral reef habitats as a holistic, interconnected seascape will provide better estimations of the drivers of reef fish assemblages.
5. Conclusions
This study investigated how reef fish assemblages on coral reefs are influenced by both the characteristics within the reef itself and the surrounding areas (e.g., mangroves, seagrass beds, and macroalgae beds) within 500 m. Five distinct habitat clusters identified habitat types that consisted of a combination of benthic cover and the area of different habitats adjacent to coral reefs. Within these clusters, coral reef fish assemblages varied significantly, indicating that a combination of within-reef benthic habitat and seascape-level measures of the surrounding habitats operate in conjunction to influence fish assemblages. Species significantly driving assemblage differences between habitat clusters reflected species potential relationships to adjacent non-reef habitats. For example, detritivorous surgeonfish species uniquely described the habitat clusters with high cover of rubble and EAM, but also for sites with large areas of adjacent mangrove habitat. Responses to adjacent habitat variables were less conspicuous for parrotfishes, perhaps due to their tight link to specific dietary needs (i.e., as microautotrophs). Wrasses demonstrated specific relationships that may be driven by the influence of adjacent non-reef habitats; for example, Cheilio inermis’ unique abundance level in the habitat cluster with large areas of adjacent seagrass habitat. Individual species responses and juveniles of certain species demonstrated uniquely high abundances in habitat clusters characterized by the non-reef habitats surrounding coral reefs. While we cannot fully eliminate the location effects driving these differences, there were nuanced habitat differences irrespective of location in our results. Including data on adjacent habitats alongside reef characteristics provides a more comprehensive understanding of factors shaping fish communities. Investigating the specific movements and ecological processes connecting fish populations across the seascape would provide valuable future insights. This study emphasizes the crucial role of considering coral reefs and adjacent areas as a unified seascape for effective conservation and management of these vital ecosystems.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fishes9040137/s1, Table S1: Average percent cover of benthic habitat at each site; Table S2: Total area (km2) of each habitat within 500 meters of coral reef fish and benthic survey sites.; Table S3: Full species list from survey transects of fishes on coral reef habitat.;Table S4: Pairwise post-hoc comparison tests from PERMANOVA analysis comparing the fish assemblage structure between habitat clusters for all species, parrotfishes, and wrasses; Table S5: Output for SIMPER analysis for all species (A), parrotfish (B), and wrasse (C).
Author Contributions
K.T.S. conceptualized, analyzed, and wrote the manuscript. E.C.M. contributed to data collection. E.C.M., R.A.A., and G.R.R. aided in project development and editorial assistance. All authors have read and agreed to the published version of the manuscript.
Funding
Funding provided by the PADI Foundation, James Cook University and the ARC Centre of Excellence for Coral Reef Studies. Partial funding was provided to GRR and ECM by Sea World Research and Rescue Foundation Inc. Grant number: SWR/3/2016.
Institutional Review Board Statement
Research was conducted with approval from the JCU Animal Ethic Committee under approval code A2253 from 4 December 2015 to 31 December 2018.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
This work would not have been possible without the invaluable contributions of the late Angel Alcala. His groundbreaking work paved the way for research in the Philippines, and his lifelong dedication to exploring and protecting the natural ecosystems of his country granted us access to crucial research sites. He also provided essential logistical and administrative support, significantly enhancing the feasibility of this project.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967. [Google Scholar]
- Moilanen, A.; Nieminen, M. Simple Connectivity Measures in Spatial Ecology. Ecology 2002, 83, 1131–1145. [Google Scholar] [CrossRef]
- Tischendorf, L.; Bender, D.J.; Fahrig, L. Evaluation of Patch Isolation Metrics in Mosaic Landscapes for Specialist vs. Generalist Dispersers. Landsc. Ecol. 2003, 18, 41–50. [Google Scholar] [CrossRef]
- Ault, T.R.; Johnson, C.R. Spatially and Temporally Predictable Fish Communities on Coral Reefs. Ecol. Monogr. 1998, 68, 25–50. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of Habitat Fragmentation on Biodiversity. Annu. Rev. Ecol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Ogden, J.C.; Buckman, N.S. Movements Foraging Groups and Diurnal Migrations of the Striped Parrot Fish Scarus-Croicensis Scaridae. Ecology 1973, 54, 589–596. [Google Scholar] [CrossRef]
- Shulman, M.J.; Ogden, J.C. What Controls Tropical Reef Fish Populations: Recruitment or Benthic Mortality? An Example in the Caribbean Reef Fish Haemulon flavolineatum. Mar. Ecol. Prog. Ser. 1987, 39, 233–242. [Google Scholar] [CrossRef]
- Nagelkerken, I.; van der Velde, G.; Gorissen, M.W.; Meijer, G.J.; van’t Hof, T.; den Hartog, C. Importance of Mangroves, Seagrass Beds and the Shallow Coral Reef as a Nursery for Important Coral Reef Fishes, Using a Visual Census Technique. Estuar. Coast. Shelf Sci. 2000, 51, 31–44. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Dorenbosch, M.; Verberk, W.C.E.P.; De Morinière, E.C.; van der Velde, G. Importance of Shallow-Water Biotopes of a Caribbean Bay for Juvenile Coral Reef Fishes: Patterns in Biotope Association, Community Structure and Spatial Distribution. Mar. Ecol. Prog. Ser. 2000, 202, 175–192. [Google Scholar] [CrossRef]
- Grober-Dunsmore, R.; Pittman, S.J.; Caldow, C.; Kendall, M.S.; Frazer, T.K. A Landscape Ecology Approach for the Study of Ecological Connectivity across Tropical Marine Seascape. In Ecological Connectivity among Tropical Coastal Ecosystems; Nagelkerken, I., Ed.; Springer Science: Berlin/Heidelberg, Germany, 2009; pp. 493–530. ISBN 978-90-481-2405-3. [Google Scholar]
- Wedding, L.M.; Lepczyk, C.A.; Pittman, S.J.; Friedlander, A.M.; Jorgensen, S. Quantifying Seascape Structure: Extending Terrestrial Spatial Pattern Metrics to the Marine Realm. Mar. Ecol. Prog. Ser. 2011, 427, 219–232. [Google Scholar] [CrossRef]
- Pittman, S.J.; McAlpine, C.A.; Pittman, K.M. Linking Fish and Prawns to Their Environment: A Hierarchical Landscape Approach. Mar. Ecol. Prog. Ser. 2004, 283, 233–254. [Google Scholar] [CrossRef]
- Boström, C.; Pittman, S.J.; Simenstad, C.; Kneib, R.T. Seascape Ecology of Coastal Biogenic Habitats: Advances, Gaps, and Challenges. Mar. Ecol. Prog. Ser. 2011, 427, 191–217. [Google Scholar] [CrossRef]
- Chittaro, P.M.; Usseglio, P.; Sale, P.F. Variation in Fish Density, Assemblage Composition and Relative Rates of Predation among Mangrove, Seagrass and Coral Reef Habitats. Environ. Biol. Fishes 2005, 72, 175–187. [Google Scholar] [CrossRef]
- Mumby, P.J.; Hastings, A. The Impact of Ecosystem Connectivity on Coral Reef Resilience. J. Appl. Ecol. 2008, 45, 854–862. [Google Scholar] [CrossRef]
- Olds, A.D.; Connolly, R.M.; Pitt, K.A.; Maxwell, P.S. Primacy of Seascape Connectivity Effects in Structuring Coral Reef Fish Assemblages. Mar. Ecol. Prog. Ser. 2012, 462, 191–203. [Google Scholar] [CrossRef]
- Berkström, C.; Jörgensen, T.L.; Hellström, M. Ecological Connectivity and Niche Differentiation between Two Closely Related Fish Species in the Mangrove-Seagrass-Coral Reef Continuum. Mar. Ecol. Prog. Ser. 2013, 477, 201–215. [Google Scholar] [CrossRef]
- Pittman, S.J.; Olds, A.D. Seascape Ecology of Fishes on Coral Reefs. In Ecology of Fishes on Coral Reefs; Mora, C., Ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 274–282. ISBN 9781316105412. [Google Scholar]
- Pratchett, M.S.; Hoey, A.S.; Wilson, S.K.; Messmer, V.; Graham, N.A.J. Changes in Biodiversity and Functioning of Reef Fish Assemblages Following Coral Bleaching and Coral Loss. Diversity 2011, 3, 424–452. [Google Scholar] [CrossRef]
- Coker, D.J.; Wilson, S.K.; Pratchett, M.S. Importance of Live Coral Habitat for Reef Fishes. Rev. Fish Biol. Fish. 2014, 24, 89–126. [Google Scholar] [CrossRef]
- Russ, G.R.; Miller, K.I.; Rizzari, J.R.; Alcala, A.C. Long-Term No-Take Marine Reserve and Benthic Habitat Effects on Coral Reef Fishes. Mar. Ecol. Prog. Ser. 2015, 529, 233–248. [Google Scholar] [CrossRef]
- Igulu, M.M.; Nagelkerken, I.; Dorenbosch, M.; Grol, M.G.G.; Harborne, A.R.; Kimirei, I.A.; Mumby, P.J.; Olds, A.D.; Mgaya, Y.D. Mangrove Habitat Use by Juvenile Reef Fish: Meta-Analysis Reveals That Tidal Regime Matters More than Biogeographic Region. PLoS ONE 2014, 9, e114715. [Google Scholar] [CrossRef] [PubMed]
- Hemingson, C.R.; Bellwood, D.R. Biogeographic Patterns in Major Marine Realms: Function Not Taxonomy Unites Fish Assemblages in Reef, Seagrass and Mangrove Systems. Ecography 2018, 41, 174–182. [Google Scholar] [CrossRef]
- Fahrig, L. Rethinking Patch Size and Isolation Effects: The Habitat Amount Hypothesis. J. Biogeogr. 2013, 40, 1649–1663. [Google Scholar] [CrossRef]
- Berkström, C.; Gullström, M.; Lindborg, R.; Mwandya, A.W.; Yahya, S.A.S.; Kautsky, N.; Nyström, M. Exploring ‘Knowns’ and ‘Unknowns’ in Tropical Seascape Connectivity with Insights from East African Coral Reefs. Estuar. Coast. Shelf Sci. 2012, 107, 1–21. [Google Scholar] [CrossRef]
- Jackson, H.B.; Fahrig, L. What Size Is a Biologically Relevant Landscape? Landsc. Ecol. 2012, 27, 929–941. [Google Scholar] [CrossRef]
- Pittman, S.J.; Brown, K.A. Multi-Scale Approach for Predicting Fish Species Distributions across Coral Reef Seascapes. PLoS ONE 2011, 6, e20583. [Google Scholar] [CrossRef]
- Johnson, A.F.; Jenkins, S.R.; Hiddink, J.G.; Hinz, H. Linking Temperate Demersal Fish Species to Habitat: Scales, Patterns and Future Directions. Fish Fish. 2013, 14, 256–280. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Dorenbosch, M.; Verberk, W.C.E.P.; Cocheret De La Morinière, E.; Van Der Velde, G. Day-Night Shifts of Fishes between Shallow-Water Biotopes of a Caribbean Bay, with Emphasis on the Nocturnal Feeding of Haemulidae and Lutjanidae. Mar. Ecol. Prog. Ser. 2000, 194, 55–64. [Google Scholar] [CrossRef]
- Appeldoorn, R.S.; Aguilar-Perera, A.; Bouwmeester, B.L.K.; Dennis, G.D.; Hill, R.L.; Merten, W.; Recksiek, C.W.; Williams, S.J. Movement of Fishes (Grunts: Haemulidae) across the Coral Reef Seascape: A Review of Scales, Patterns and Processes. Caribb. J. Sci. 2009, 45, 304–316. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Sheaves, M.; Baker, R.; Connolly, R.M. The Seascape Nursery: A Novel Spatial Approach to Identify and Manage Nurseries for Coastal Marine Fauna. Fish Fish. 2015, 16, 362–371. [Google Scholar] [CrossRef]
- Beck, M.W.; Heck, K.L.; Able, K.W.; Childers, D.L.; Eggleston, D.B.; Gillanders, B.M.; Halpern, B.; Hays, C.G.; Hoshino, K.; Minello, T.J.; et al. The Identification, Conservation, and Management of Estuarine and Marine Nurseries for Fish and Invertebrates. Bioscience 2001, 51, 633–641. [Google Scholar] [CrossRef]
- Adams, A.J.; Ebersole, J.P. Use of Back-Reef and Lagoon Habitats by Coral Reef Fishes. Mar. Ecol. Prog. Ser. 2002, 228, 213–226. [Google Scholar] [CrossRef]
- Ogden, J.C.; Quinn, T.P. Migration of Coral Reef Fishes: Ecological Significance and Orientation Mechanisms. In Mechanisms of Migration in Fishes; Springer: Boston, MA, USA, 1984; pp. 293–308. [Google Scholar]
- Meyer, J.L.; Schultz, E.T. Migrating Haemulid Fishes as a Source of Nutrients and Organic Matter on Coral Reefs. Limnol. Oceanogr. 1985, 30, 146–156. [Google Scholar] [CrossRef]
- Harborne, A.R.; Nagelkerken, I.; Wolff, N.H.; Bozec, Y.M.; Dorenbosch, M.; Grol, M.G.G.; Mumby, P.J. Direct and Indirect Effects of Nursery Habitats on Coral-Reef Fish Assemblages, Grazing Pressure and Benthic Dynamics. Oikos 2016, 125, 957–967. [Google Scholar] [CrossRef]
- Nakamura, Y.; Horinouchi, M.; Shibuno, T.; Tanaka, Y.; Miyajima, T.; Koike, I.; Kurokura, H.; Sano, M. Evidence of Ontogenetic Migration from Mangroves to Coral Reefs by Black-Tail Snapper Lutjanus Fulvus: Stable Isotope Approach. Mar. Ecol. Prog. Ser. 2008, 355, 257–266. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Grol, M.G.G.; Mumby, P.J. Effects of Marine Reserves versus Nursery Habitat Availability on Structure of Reef Fish Communities. PLoS ONE 2012, 7, e36906. [Google Scholar] [CrossRef]
- Lefcheck, J.S.; Hughes, B.B.; Johnson, A.J.; Pfirrman, B.W.; Rasher, D.B.; Smyth, A.R.; Williams, B.L.; Beck, M.W.; Orth, R.J. Are Coastal Habitats Important Nurseries? A Meta-Analysis. Conserv. Lett. 2019, 12, e12645. [Google Scholar] [CrossRef]
- Abu El-Regal, M.A.; Ibrahim, N.K. Role of Mangroves as a Nursery Ground for Juvenile Reef Fishes in the Southern Egyptian Red Sea. Egypt. J. Aquat. Res. 2014, 40, 71–78. [Google Scholar] [CrossRef]
- Kimirei, I.A.; Nagelkerken, I.; Slooter, N.; Gonzalez, E.T.; Huijbers, C.M.; Mgaya, Y.D.; Rypel, A.L. Demography of Fish Populations Reveals New Challenges in Appraising Juvenile Habitat Values. Mar. Ecol. Prog. Ser. 2015, 518, 225–237. [Google Scholar] [CrossRef]
- Nakamura, Y.; Horinouchi, M.; Nakai, T.; Sano, M. Food Habits of Fishes in a Seagrass Bed on a Fringing Coral Reef at Iriomote Island, Southern Japan. Ichthyol. Res. 2003, 50, 15–22. [Google Scholar] [CrossRef]
- Dorenbosch, M.; Grol, M.G.G.; Christianen, M.J.A.; Nagelkerken, I.; van Der Velde, G. Indo-Pacific Seagrass Beds and Mangroves Contribute to Fish Density and Diversity on Adjacent Coral Reefs. Mar. Ecol. Prog. Ser. 2005, 302, 63–76. [Google Scholar] [CrossRef]
- Grober-Dunsmore, R.; Frazer, T.K.; Lindberg, W.J.; Beets, J. Reef Fish and Habitat Relationships in a Caribbean Seascape: The Importance of Reef Context. Coral Reefs 2007, 26, 201–216. [Google Scholar] [CrossRef]
- Davis, J.P.; Pitt, K.A.; Fry, B.; Olds, A.D.; Connolly, R.M. Seascape-Scale Trophic Links for Fish on Inshore Coral Reefs. Coral Reefs 2014, 33, 897–907. [Google Scholar] [CrossRef]
- Berkström, C.; Eggertsen, L.; Goodell, W.; Cordeiro, C.A.M.M.; Lucena, M.B.; Gustafsson, R.; Bandeira, S.; Jiddawi, N.; Ferreira, C.E.L. Thresholds in Seascape Connectivity: The Spatial Arrangement of Nursery Habitats Structure Fish Communities on Nearby Reefs. Ecography 2020, 43, 882–896. [Google Scholar] [CrossRef]
- Fulton, C.J.; Abesamis, R.A.; Berkström, C.; Depczynski, M.; Graham, N.A.J.; Holmes, T.H.; Kulbicki, M.; Noble, M.M.; Radford, B.T.; Tano, S.; et al. Form and Function of Tropical Macroalgal Reefs in the Anthropocene. Funct. Ecol. 2019, 33, 989–999. [Google Scholar] [CrossRef]
- Fulton, C.J.; Berkström, C.; Wilson, S.K.; Abesamis, R.A.; Bradley, M.; Åkerlund, C.; Barrett, L.T.; Bucol, A.A.; Chacin, D.H.; Chong-Seng, K.M.; et al. Macroalgal Meadow Habitats Support Fish and Fisheries in Diverse Tropical Seascapes. Fish Fish. 2020, 21, 700–717. [Google Scholar] [CrossRef]
- Sambrook, K.; Hoey, A.S.; Andréfouët, S.; Cumming, G.S.; Duce, S.; Bonin, M.C. Beyond the Reef: The Widespread Use of Non-Reef Habitats by Coral Reef Fishes. Fish Fish. 2019, 20, 903–920. [Google Scholar] [CrossRef]
- Sievers, K.T.; McClure, E.C.; Abesamis, R.A.; Russ, G.R. Non-Reef Habitats in a Tropical Seascape Affect Density and Biomass of Fishes on Coral Reefs. Ecol. Evol. 2020, 10, 13673–13686. [Google Scholar] [CrossRef] [PubMed]
- Eggertsen, L.; Ferreira, C.E.L.; Fontoura, L.; Kautsky, N.; Gullström, M.; Berkström, C. Seaweed Beds Support More Juvenile Reef Fish than Seagrass Beds in a South-Western Atlantic Tropical Seascape. Estuar. Coast. Shelf Sci. 2017, 196, 97–108. [Google Scholar] [CrossRef]
- Sambrook, K.; Bonin, M.C.; Bradley, M.; Cumming, G.S.; Duce, S.; Andréfouët, S.; Hoey, A.S. Broadening Our Horizons: Seascape Use by Coral Reef-Associated Fishes in Kavieng, Papua New Guinea, Is Common and Diverse. Coral Reefs 2020, 39, 1187–1197. [Google Scholar] [CrossRef]
- Huntington, B.E.; Karnauskas, M.; Babcock, E.A.; Lirman, D. Untangling Natural Seascape Variation from Marine Reserve Effects Using a Landscape Approach. PLoS ONE 2010, 5, e12327. [Google Scholar] [CrossRef] [PubMed]
- Olds, A.D.; Connolly, R.M.; Pitt, K.A.; Maxwell, P.S. Habitat Connectivity Improves Reserve Performance. Conserv. Lett. 2012, 5, 56–63. [Google Scholar] [CrossRef]
- Olds, A.D.; Albert, S.; Maxwell, P.S.; Pitt, K.A.; Connolly, R.M. Mangrove-Reef Connectivity Promotes the Effectiveness of Marine Reserves across the Western Pacific. Glob. Ecol. Biogeogr. 2013, 22, 1040–1049. [Google Scholar] [CrossRef]
- Verweij, M.C.; Nagelkerken, I.; Wartenbergh, S.L.J.; Pen, I.R.; van Der Velde, G. Caribbean Mangroves and Seagrass Beds as Daytime Feeding Habitats for Juvenile French Grunts, Haemulon flavolineatum. Mar. Biol. 2006, 149, 1291–1299. [Google Scholar] [CrossRef]
- Yabsley, N.A.; Olds, A.D.; Connolly, R.M.; Martin, T.S.H.; Gilby, B.L.; Maxwell, P.S.; Huijbers, C.M.; Schoeman, D.S.; Schlacher, T.A. Resource Type Influences the Effects of Reserves and Connectivity on Ecological Functions. J. Anim. Ecol. 2016, 85, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.S.H.; Olds, A.D.; Olalde, A.B.H.; Berkström, C.; Gilby, B.L.; Schlacher, T.A.; Butler, I.R.; Yabsley, N.A.; Zann, M.; Connolly, R.M. Habitat Proximity Exerts Opposing Effects on Key Ecological Functions. Landsc. Ecol. 2018, 33, 1273–1286. [Google Scholar] [CrossRef]
- Henderson, C.J.; Gilby, B.L.; Lee, S.Y.; Stevens, T. Contrasting Effects of Habitat Complexity and Connectivity on Biodiversity in Seagrass Meadows. Mar. Biol. 2017, 164, 117. [Google Scholar] [CrossRef]
- Sievers, K.T.; Abesamis, R.A.; Bucol, A.A.; Russ, G.R. Unravelling Seascape Patterns of Cryptic Life Stages: Non-Reef Habitat Use in Juvenile Parrotfishes. Diversity 2020, 12, 376. [Google Scholar] [CrossRef]
- Vanderklift, M.A.; How, J.; Wernberg, T.; MacArthur, L.D.; Heck, K.L.; Valentine, J.F. Proximity to Reef Influences Density of Small Predatory Fishes, While Type of Seagrass Influences Intensity of Their Predation on Crabs. Mar. Ecol. Prog. Ser. 2007, 340, 235–243. [Google Scholar] [CrossRef]
- van Lier, J.R.; Wilson, S.K.; Depczynski, M.; Wenger, L.N.; Fulton, C.J. Habitat Connectivity and Complexity Underpin Fish Community Structure across a Seascape of Tropical Macroalgae Meadows. Landsc. Ecol. 2018, 33, 1287–1300. [Google Scholar] [CrossRef]
- Evans, R.D.; Wilson, S.K.; Field, S.N.; Moore, J.A.Y. Importance of Macroalgal Fields as Coral Reef Fish Nursery Habitat in North-West Australia. Mar. Biol. 2014, 161, 599–607. [Google Scholar] [CrossRef]
- McClure, E.C.; Sievers, K.T.; Abesamis, R.A.; Hoey, A.S.; Alcala, A.C.; Russ, G.R. Higher Fish Biomass inside than Outside Marine Protected Areas despite Typhoon Impacts in a Complex Reefscape. Biol. Conserv. 2020, 241, 108354. [Google Scholar] [CrossRef]
- Wilson, S.K.; Graham, N.A.J.; Polunin, N.V.C. Appraisal of Visual Assessments of Habitat Complexity and Benthic Composition on Coral Reefs. Mar. Biol. 2007, 151, 1069–1076. [Google Scholar] [CrossRef]
- Suzuki, R.; Shimodaira, H. R Package, version 1.2-2; Hierarchical Clustering with P-Values via Multiscale Bootstrap Resampling; RStudio: Boston, MA, USA, 2011. [Google Scholar]
- Jouffray, J.B.; Wedding, L.M.; Norström, A.V.; Donovan, M.K.; Williams, G.J.; Crowder, L.B.; Erickson, A.L.; Friedlander, A.M.; Graham, N.A.J.; Gove, J.M.; et al. Parsing Human and Biophysical Drivers of Coral Reef Regimes. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182544. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Singh, S. A Survey of Clustering Techniques. Int. J. Comput. Appl. 2010, 7, 1–5. [Google Scholar] [CrossRef]
- Gillanders, B.M.; Able, K.W.; Brown, J.A.; Eggleston, D.B.; Sheridan, P.F. Evidence of Connectivity between Juvenile and Adult Habitats for Mobile Marine Fauna: An Important Component of Nurseries. Mar. Ecol. Prog. Ser. 2003, 247, 281–295. [Google Scholar] [CrossRef]
- Kimirei, I.A.; Nagelkerken, I.; Griffioen, B.; Wagner, C.; Mgaya, Y.D. Ontogenetic Habitat Use by Mangrove/Seagrass-Associated Coral Reef Fishes Shows Flexibility in Time and Space. Estuar. Coast. Shelf Sci. 2011, 92, 47–58. [Google Scholar] [CrossRef]
- McClure, E.C.; Hoey, A.S.; Sievers, K.T.; Abesamis, R.A.; Russ, G.R. Relative Influence of Environmental Factors and Fishing on Coral Reef Fish Assemblages. Conserv. Biol. 2020, 35, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Russ, G.R.; Payne, C.S.; Bergseth, B.J.; Rizzari, J.R.; Abesamis, R.A.; Alcala, A.C. Decadal-Scale Response of Detritivorous Surgeonfishes (Family Acanthuridae) to No-Take Marine Reserve Protection and Changes in Benthic Habitat. J. Fish Biol. 2018, 93, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.M.; Russ, G.R. Response of Herbivorous Fishes to Crown-of-Thorns Starfish Acanthaster planci Outbreaks. III. Age, Growth, Mortality and Maturity Indices of Acanthurus nigrofuscus. Mar. Ecol. Prog. Ser. 1996, 136, 25–35. [Google Scholar] [CrossRef]
- Bouillon, S.; Connolly, R.M.; Lee, S.Y. Organic Matter Exchange and Cycling in Mangrove Ecosystems: Recent Insights from Stable Isotope Studies. J. Sea Res. 2008, 59, 44–58. [Google Scholar] [CrossRef]
- Lee, S.Y.; Primavera, J.H.; Dahdouh-Guebas, F.; Mckee, K.; Bosire, J.O.; Cannicci, S.; Diele, K.; Fromard, F.; Koedam, N.; Marchand, C.; et al. Ecological Role and Services of Tropical Mangrove Ecosystems: A Reassessment. Glob. Ecol. Biogeogr. 2014, 23, 726–743. [Google Scholar] [CrossRef]
- Randall, J.E. Acanthuridae. In Smith’s Sea Fishes; Smith, M.M., Heemstra, P.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 811–823. [Google Scholar]
- Russ, G.R.; Aller-Rojas, O.D.; Rizzari, J.R.; Alcala, A.C. Off-Reef Planktivorous Reef Fishes Respond Positively to Decadal-Scale No-Take Marine Reserve Protection and Negatively to Benthic Habitat Change. Mar. Ecol. 2017, 38, e12442. [Google Scholar] [CrossRef]
- Russ, G.R.; Alcala, A.C.; Maypa, A.P. Spillover from Marine Reserves: The Case of Naso vlamingii at Apo Island, the Philippines. Mar. Ecol. Prog. Ser. 2003, 264, 15–20. [Google Scholar] [CrossRef]
- Abesamis, R.A.; Russ, G.R. Density-Dependent Spillover from a Marine Reserve: Long-Term Evidence. Ecol. Appl. 2005, 15, 1798–1812. [Google Scholar] [CrossRef]
- Choat, J.H.; Clements, K.D.; Robbins, W.D. The Trophic Status of Herbivorous Fishes on Coral Reefs 1: Dietary Analyses. Mar. Biol. 2002, 140, 613–623. [Google Scholar] [CrossRef]
- Hoey, A.S.; Bellwood, D.R. Cross-Shelf Variation in Browsing Intensity on the Great Barrier Reef. Coral Reefs 2010, 29, 499–508. [Google Scholar] [CrossRef]
- Bierwagen, S.L.; Price, D.K.; Pack, A.A.; Meyer, C.G. Bluespine Unicornfish (Naso unicornis) Are Both Natural Control Agents and Mobile Vectors for Invasive Algae in a Hawaiian Marine Reserve. Mar. Biol. 2017, 164, 25. [Google Scholar] [CrossRef]
- Mumby, P.J.; Edwards, A.J.; Arias-Gonazalez, E.J.; Lindeman, K.C.; Blackwell, P.G.; Gall, A.; Gorczynska, M.I.; Harborne, A.R.; Pescod, C.L.; Renken, H.; et al. Mangroves Enhance the Biomass of Coral Reef Fish Communities in the Caribbean. Nature 2004, 427, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Serafy, J.E.; Shideler, G.S.; Araújo, R.J.; Nagelkerken, I. Mangroves Enhance Reef Fish Abundance at the Caribbean Regional Scale. PLoS ONE 2015, 10, e0142022. [Google Scholar] [CrossRef] [PubMed]
- Lugendo, B.R.; Nagelkerken, I.; van der Velde, G.; Mgaya, Y.D. The Importance of Mangroves, Mud and Sand Flats, and Seagrass Beds as Feeding Areas for Juvenile Fishes in Chwaka Bay, Zanzibar: Gut Content and Stable Isotope Analyses. J. Fish Biol. 2006, 69, 1639–1661. [Google Scholar] [CrossRef]
- Clements, K.D.; German, D.P.; Piché, J.; Tribollet, A.; Choat, J.H. Integrating Ecological Roles and Trophic Diversification on Coral Reefs: Multiple Lines of Evidence Identify Parrotfishes as Microphages. Biol. J. Linn. Soc. 2017, 120, 729–751. [Google Scholar] [CrossRef]
- Nicholson, G.M.; Clements, K.D. Resolving Resource Partitioning in Parrotfishes (Scarini) Using Microhistology of Feeding Substrata. Coral Reefs 2020, 39, 1313–1327. [Google Scholar] [CrossRef]
- Russ, G.R.; Questel, S.L.A.; Rizzari, J.R.; Alcala, A.C. The Parrotfish–Coral Relationship: Refuting the Ubiquity of a Prevailing Paradigm. Mar. Biol. 2015, 162, 2029–2045. [Google Scholar] [CrossRef]
- Carlson, P.M.; Davis, K.; Warner, R.R.; Caselle, J.E. Fine-Scale Spatial Patterns of Parrotfish Herbivory Are Shaped by Resource Availability. Mar. Ecol. Prog. Ser. 2017, 577, 165–176. [Google Scholar] [CrossRef]
- Eggertsen, L.; Goodell, W.; Cordeiro, C.A.M.M.; Mendes, T.C.; Longo, G.O.; Ferreira, C.E.L.; Berkström, C. Seascape Configuration Leads to Spatially Uneven Delivery of Parrotfish Herbivory across a Western Indian Ocean Seascape. Diversity 2020, 12, 434. [Google Scholar] [CrossRef]
- Fulton, C.J.; Bellwood, D.R. Patterns of Foraging in Labrid Fishes. Mar. Ecol. Prog. Ser. 2002, 226, 135–142. [Google Scholar] [CrossRef]
- Wainwright, P.C.; Bellwood, D.R.; Westneat, M.W. Ecomorphology of Locomotion in Labrid Fishes. Environ. Biol. Fishes 2002, 65, 47–62. [Google Scholar] [CrossRef]
- Russ, G.R.; Lowe, J.R.; Rizzari, J.R.; Bergseth, B.J.; Alcala, A.C. Partitioning No-Take Marine Reserve (NTMR) and Benthic Habitat Effects on Density of Small and Large-Bodied Tropical Wrasses. PLoS ONE 2017, 12, e0188515. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.R.; Williamson, D.H.; Ceccarelli, D.M.; Evans, R.D.; Russ, G.R. Responses of Coral Reef Wrasse Assemblages to Disturbance and Marine Reserve Protection on the Great Barrier Reef. Mar. Biol. 2019, 166, 119. [Google Scholar] [CrossRef]
- Green, A.L. Spatial, Temporal and Ontogenetic Patterns of Habitat Use by Coral Reef Fishes (Family Labridae). Mar. Ecol. Prog. Ser. 1996, 133, 1–11. [Google Scholar] [CrossRef]
- Berkström, C.; Jones, G.P.; McCormick, M.I. Trade-Offs in the Ecological Versatility of Juvenile Wrasses: An Experimental Evaluation. J. Exp. Mar. Biol. Ecol. 2014, 453, 91–97. [Google Scholar] [CrossRef]
- Berkström, C.; Jones, G.P.; McCormick, M.I.; Srinivasan, M. Ecological Versatility and Its Importance for the Distribution and Abundance of Coral Reef Wrasses. Mar. Ecol. Prog. Ser. 2012, 461, 151–163. [Google Scholar] [CrossRef]
- Kramer, M.J.; Bellwood, O.; Bellwood, D.R. Foraging and Microhabitat Use by Crustacean-Feeding Wrasses on Coral Reefs. Mar. Ecol. Prog. Ser. 2016, 548, 277–282. [Google Scholar] [CrossRef]
- Shibuno, T.; Nakamura, Y.; Horinouchi, M.; Sano, M. Habitat Use Patterns of Fishes across the Mangrove-Seagrass-Coral Reef Seascape at Ishigaki Island, Southern Japan. Ichthyol. Res. 2008, 55, 218–237. [Google Scholar] [CrossRef]
- Gullström, M.; Berkström, C.; Öhman, M.C.; Bodin, M.; Dahlberg, M. Scale-Dependent Patterns of Variability of a Grazing Parrotfish (Leptoscarus vaigiensis) in a Tropical Seagrass-Dominated Seascape. Mar. Biol. 2011, 158, 1483–1495. [Google Scholar] [CrossRef]
- Tano, S.A.; Eggertsen, M.; Wikstrom, S.; Berkstrom, C.; Buriyo, A.; Halling, C. Tropical Seaweed Beds as Important Habitats for Juvenile Fish. Mar. Freshw. Res. 2017, 68, 1921–1934. [Google Scholar] [CrossRef]
- Wilson, S.K.; Depczynski, M.; Holmes, T.H.; Noble, M.M.; Radford, B.T.; Tinkler, P.; Fulton, C.J. Climatic Conditions and Nursery Habitat Quality Provide Indicators of Reef Fish Recruitment Strength. Limnol. Oceanogr. 2017, 62, 1868–1880. [Google Scholar] [CrossRef]
- Wilson, S.K.; Depczynski, M.; Fisher, R.; Holmes, T.H.; O’Leary, R.A.; Tinkler, P. Habitat Associations of Juvenile Fish at Ningaloo Reef, Western Australia: The Importance of Coral and Algae. PLoS ONE 2010, 5, e15185. [Google Scholar] [CrossRef] [PubMed]
- Olds, A.D.; Connolly, R.M.; Pitt, K.A.; Maxwell, P.S.; Aswani, S.; Albert, S. Incorporating Surrogate Species and Seascape Connectivity to Improve Marine Conservation Outcomes. Conserv. Biol. 2014, 28, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Grober-Dunsmore, R.; Frazer, T.K.; Beets, J.P.; Lindberg, W.J.; Zwick, P.; Funicelli, N.A. Influence of Landscape Structure on Reef Fish Assemblages. Landsc. Ecol. 2008, 23, 37–53. [Google Scholar] [CrossRef]
- Moustaka, M.; Evans, R.D.; Kendrick, G.A.; Hyndes, G.A.; Cuttler, M.V.W.; Bassett, T.J.; O’Leary, M.J.; Wilson, S.K. Local Habitat Composition and Complexity Outweigh Seascape Effects on Fish Distributions across a Tropical Seascape. Landsc. Ecol. 2024, 39, 28. [Google Scholar] [CrossRef]
- Alcala, A.C.; Russ, G.R. A Direct Test of the Effects of Protective Management on a Tropical Marine Reserve. ICES J. Mar. Sci. 1990, 46, 40–47. [Google Scholar] [CrossRef]
- Russ, G.R.; Alcala, A.C. Marine Reserves: Long-Term Protection Is Required for Full Recovery of Predatory Fish Populations. Oecologia 2004, 138, 622–627. [Google Scholar] [CrossRef]
- Russ, G.R.; Rizzari, J.R.; Abesamis, R.A.; Alcala, A.C. Coral Cover a Stronger Driver of Reef Fish Trophic Biomass than Fishing. Ecol. Appl. 2021, 31, e02224. [Google Scholar] [CrossRef] [PubMed]
- Verweij, M.C.; Nagelkerken, I. Short and Long-Term Movement and Site Fidelity of Juvenile Haemulidae in Back-Reef Habitats of a Caribbean Embayment. Hydrobiologia 2007, 592, 257–270. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Bothwell, J.; Nemeth, R.S.; Pitt, J.M.; van der Velde, G. Interlinkage between Caribbean Coral Reefs and Seagrass Beds through Feeding Migrations by Grunts (Haemulidae) Depends on Habitat Accessibility. Mar. Ecol. Prog. Ser. 2008, 368, 155–164. [Google Scholar] [CrossRef]
- Sekund, L.; Pittman, S. Explaining Island-wide Geographical Patterns of Caribbean Fish Diversity: A Multi-scale Seascape Ecology Approach. Mar. Ecol. 2017, 38, e12434. [Google Scholar] [CrossRef]
- Jackson, H.B.; Fahrig, L. Are Ecologists Conducting Research at the Optimal Scale? Glob. Ecol. Biogeogr. 2015, 24, 52–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).