Age, Growth, and Mortality of Pontic Shad, Alosa immaculata Bennett, 1835, in the Danube River, Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Determination of Sex and Age
2.3. Length–Weight Relationship
2.4. Estimation of Growth Parameters
2.5. Total Mortality, Fishing Mortality, and Exploitation Rate
2.6. Probability of Capture, Relative Yield per Recruit (Y/R), Relative Biomass per Recruit (B`/R), and Virtual Population Analysis (VPA)
2.7. Statistical Analyses
3. Results
3.1. Sex Structure of Pontic Shad Population
3.2. Length–Weight Relationship (L-W)
3.3. Growth and Mortality Parameters
3.4. Total Mortality, Fishing Mortality, and Exploitation Rate
3.5. Probability of Capture, Relative Yield per Recruit (Y`/R), and Relative Biomass per Recruit (B`/R)
4. Discussion
| Growth Parameters | Mortality and Exploitation Rates | Area | Reference | |||||
|---|---|---|---|---|---|---|---|---|
| L∞ | k | t0 | Z | M | F | E | ||
| 36.75 | 0.68 | −0.67 | 2.76 | 0.89 | 1.87 | 0.68 | Danube River, Romania | Our study |
| 48.10 | 0.2 | −1.58 | - | - | - | - | Danube River, Romania | [9] |
| 43.05 | 0.51 | −0.53 | 2.32 | 0.77 | 1.55 | 0.67 | Danube River, Romania | [37] |
| 36.75 | 0.66 | - | 1.83 | 0.87 | 0.96 | 0.53 | Danube River, Romania | [6] |
| 40.43 | 0.38 | −0.08 | 1.54 | 0.58 | 0.95 | 0.61 | Danube River, Romania | [44] |
| 35.74 | 0.49 | 0.341 | - | - | - | - | Danube River, Bulgaria | [1] |
| 40.43 | 0.27 | −0.218 | - | - | - | - | Danube River | [51] |
| 57.38 | 0.10 | 1727 | - | - | - | - | Danube River | [50] |
| 41.5 | 0.38 | −0.35 | 1.71 | 0.58 | 1.13 | 0.66 | Black Sea, Romania | [45] |
| 41.5 | 0.38 | −0.34 | 1.71 | 0.63 | 1.07 | 0.625 | Black Sea, Romania | [45] |
| 37.8 | 0.87 | −0.69 | 3.03 | 1.12 | 1.01 | - | Black Sea | [2] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rozdina, D.; Raikova-Petrova, G.; Mirtcheva, P. Age composition and growth rate of the spawning part of the population of pontic shad Alosa immaculata (Bennett, 1835) in the Bulgarian sector of Danube River. Bulg. J. Agric. Sci. 2013, 19 (Suppl. S1), 118–125. [Google Scholar]
- Țiganov, G.; Grigoraș, D.; Năstase, A.; Păun, C.; Galațchi, M. Assesing of Pontic shad (Alosa immaculata, Bennett 1835) stock status from Romanian Black Sea Coast. Turkish J. Fish. Aquat. Sci. 2023, 23. Available online: https://www.trjfas.org/uploads/pdf_14965.pdf (accessed on 22 January 2023).
- Bănărescu, P. Fauna of Romanian Popular Republic. Pisces-Osteichthyes; Romanian Academy Publishing House: Bucharest, Romania, 1964; p. 962. [Google Scholar]
- Lenhardt, M.; Cakić, P.; Kolarević, J. Influence of the HEPS Djerdap I and Djerdap II dam construction on catch of economically important fish species in the Danube River. Ecohydrol. Hydrobiol. 2004, 4, 499–502. [Google Scholar]
- Năvodaru, I. Exploitation of Alosa pontica in the Danube Delta, Romania. In Stock Assessment in Inland Fisheries; Cowx, I.G., Ed.; Fishing New Books: Oxford, UK, 1996; pp. 448–453. [Google Scholar]
- Leonov, C.M.; Stroe, M.D.; Dima, F.M.; Vidu, L.; Nicolae, C.G. Assessment of growth and mortality parameters of Alosa immaculata (Bennet, 1835) from the Danube Delta. Sci. Pap. Ser. D Anim. Sci. 2023, 66, 596–601. Available online: https://animalsciencejournal.usamv.ro/pdf/2023/issue_1/Art78.pdf (accessed on 5 February 2023).
- NAFA, National Agency of Fishery and Aquaculture. Available online: https://www.anpa.ro/wp-content/uploads/2022/02/pescuit-comercial-ape-interioare.pdf (accessed on 1 February 2023).
- Năvodaru, I.; Staraș, M.; Cernișencu, I. Influence of the hydrological regime of the Danube on the annual variation of the Pontic shad (Alosa pontica Eichvald). In Analalele Științífice ale Institutului Delta Dunării; Institutul Delta Dunării: Tulcea, Romania, 1995; Volume III/1, pp. 215–221. [Google Scholar]
- Năvodaru, I. The Evolution of the Pontic Shad Populations in the New Ecological Conditions of the River and Measures to Maintain Them. Ph.D. Thesis, Dunărea de Jos, University of Galați, Galați, Romania, 1997. [Google Scholar]
- Smederevac-Lalić, M.; Kalauzi, A.; Regner, S.; Navodaru, I.; Višnjić-Jeftić, Ž.; Gačić, Z.; Lenhardt, M. Analysis and forecast of Pontic shad (Alosa immaculata) catch in the Danube River. Iran. J. Fish. Sci. 2018, 17, 443–457. [Google Scholar] [CrossRef]
- Freyhof, J.; Kottelat, M. Alosa Immaculata. The IUCN Red List of Threatened Species 2008: E.T907A13093654. Available online: https://doi.org/10.2305/IUCN.UK.2008.RLTS.T907A13093654.en (accessed on 19 December 2023).
- Habitats Directive 92/43 EEC. Available online: https://eur-lex.europa.eu/legal-content/RO/TXT/?uri=celex%3A31992L0043 (accessed on 22 January 2023).
- Emergency Government Ordinance 57/2007. Available online: https://legislatie.just.ro/Public/DetaliiDocumentAfis/83289 (accessed on 22 January 2023).
- Allen, M.S.; Hightower, J.E. Chapter 2. Fish population dynamics: Mortality, growth, and recruitment. In Inland Fisheries Management in North America, 3rd ed.; American Fisheries Society: Bethesda, MD, USA, 2010; pp. 43–79. [Google Scholar]
- Yilmaz, S.; Polat, N. Age determination of Shad (Alosa pontica, Eichwald, 1838) inhabiting the Black Sea. Turk. J. Zool. 2002, 26, 393–398. Available online: https://journals.tubitak.gov.tr/cgi/viewcontent.cgi?article=2563&context=zoology (accessed on 22 January 2023).
- Bolat, Y.; Yağci, A. A comparative study on age determination of carp (Cyprinus carpio Linnaeus, 1758) in Lake Eğirdir using otolith, vertebrae and scale counts. J. Agric. Sci. 2018, 24, 199–204. [Google Scholar] [CrossRef]
- de Moraes Vazzoler, A.E.A. Biologia da Reprodução de Peixes Teleósteos: Teoria e Prática; Eduem: Maringá, Brazil, 1996; 169p. [Google Scholar]
- Ricker, W.E. Computation and Interpretation of Biological Statistics of Fish Populations. J. Fish. Res. Board Can. 1975, 191, 1–382. [Google Scholar]
- Froese, R.; Tsikliras, A.C.; Stergiou, K.I. Editorial note on weight-length relations of fishes. Acta Ichthyol. Piscat. 2011, 41, 261–263. [Google Scholar] [CrossRef]
- Pauly, D. Some simple methods for the assessment of tropical fish stocks. FAO Fish. Tech. Pap. 1983, 234, 52. [Google Scholar]
- Pauly, D.; Munro, J.L. Once More on the Comparison of Growth in Fish and Invertebrates. ICLARM Fishbyte 1984, 2, 21. [Google Scholar]
- Ragonese, S.; Vitale, S.; Mazzola, S.; Pagliarino, E.; Bianchini, M.L. Behavior of some growth performance indexes for exploited Mediterranean hake. Acta Adriat. 2012, 53, 105–122. Available online: https://hrcak.srce.hr/file/132894 (accessed on 5 February 2023).
- Gayanilo, F.C., Jr.; Sparre, P.; Pauly, D. FAO-ICLARM Stock Assessment Tools II (FiSAT II). Revised Version, User’s Guide; FAO Computerized Information Series (Fisheries); FAO: Rome, Italy, 2005; 168p. [Google Scholar]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Then, A.Y.; Honeig, J.M.; Hall, N.G.; Hewitt, D.A. Evaluating the predictive performance of empirical estimators of natural mortality rate using information on over 200 fish species. ICES J. Mar. Sci. 2015, 72, 82–92. [Google Scholar] [CrossRef]
- Gulland, J.A. The Fish Resources of the Ocean; Fishing News (Books) Ltd.: Chichester, UK, 1971; Available online: https://www.fao.org/3/al937e/al937e.pdf (accessed on 22 January 2023).
- Balik, İ. Population parameters of the Pontic shad, Alosa immaculata Bennett, 1835 in the Fatsa coast of the south-eastern Black Sea. EgeJFAS 2019, 36, 319–324. Available online: http://www.egejfas.org/tr/download/article-file/751645 (accessed on 5 February 2023). [CrossRef]
- Beverton, R.J.H.; Holt, S.J. Manual of Methods for Fish Stock Assessment, Part II, Tables of Yield Function; Fisheries Technical Paper No. 38, Review 1; FAO: Rome, Italy, 1966. [Google Scholar]
- Basilone, G.; Ferreri, R.; Bonanno, A.; Genovese, S.; Barra, M.; Aronica, S. Age and Growth of European Sardine (Sardina pilchardus) in the Central Mediterranean Sea: Implication for Stock Assessment. Fishes 2023, 8, 202. [Google Scholar] [CrossRef]
- Tah, L.; Joanny, T.G.; N’Douba, V.; Kouassi, N.J.; Moreau, J. Preliminary Estimates of the Population Parameters of Major Fish Species in Lake Ayamé I (Bia basin; Cơte d’Ivoire). Appl. Ichthyol. 2010, 26, 57–63. [Google Scholar] [CrossRef]
- Li, P.; Liu, J.; Wang, T.; Wang, J. Estimates of the Age, Growth, and Mortality of Triplophysa scleroptera (Herzenstein, 1888) in the Upper Reaches of the Yellow River, China. Fishes 2023, 8, 457. [Google Scholar] [CrossRef]
- Kolarov, P.P. Biological Characteristics and Population Dynamic of Anadromous Fish Specie. Ph.D. Thesis, Institute for Fish Resources, Varna, Bulgaria, 1985. [Google Scholar]
- Schmutz, S. Assessment of the Potential Transboundary Effects of the Construction of the Bystre Deep-Water Navigation Channel on Fish and Fisheries. Report to the ESPOO Inquiry Commission Vienna. 2006, p. 56. Available online: https://unece.org/DAM/env/eia/documents/inquiry/Final%20Report%20Schmutz.pdf (accessed on 22 January 2023).
- Available online: https://www.afdj.ro/ro/cotele-dunarii (accessed on 5 February 2023).
- Niculescu-Duvăz, M.; Nalbant, T. Consideratii Asupra Sistematicii Scrumbiei de Dunare (Alosa Pontica Pontica Eichw.) si Asupra unor Fenomene Specifice Legate de Migratia si Prognoza Acestei Specii în Apele Dunarii. Bul. Inst. Cercet. Pentru Pescuit Si Piscic. XXIV 1 1965, 24, 15–25. (In Romanian) [Google Scholar]
- Năvodaru, I.; Năstase, A. New data on pontic shad (Alosa immaculata Bennet 1835) migration and drifting larvae in Danube River, Deltaica. In Noi Date Asupra Prezenţei Marilor Peşti Migratory Anadromi în Marea Neagră—Zona Marină a Rezervaţiei Biosferei Delta Dunării (New Data on Presence of the Great Anadroumous Migratory Fishes in Black Sea—Marine Zone of Danube Delta Biosphere Reserve); Torok, L., Ed.; Institutul Naţional de Cercetare-Dezvoltare “Delta Dunării”—Tulcea: Tulcea, Romania, 2014; Volume 3. [Google Scholar] [CrossRef]
- Mocanu, M.; Oprea, L.; Cordeli, A.N.; Crețu, M. Estimation of growth parameters and mortality rate of Pontic shad (Alosa immaculata, Bennett, 1835) in the Romanian sector of the Danube River, km 169–km 197. Sci. Pap. Ser. D Anim. Sci. 2021, 64, 448–453. [Google Scholar]
- Ciolac, A.; Patriche, N. Structure of Danube shad (Alosa pontifica Eichwald, 1838) spawner flocks migrating for reproduction in Danube River. Appl. Ecol. Environ. Res. 2003, 2, 53–58. [Google Scholar] [CrossRef]
- Tiganov, G.; Năvodaru, I.; Cernișencu, I.; Năstase, A.; Maximov, V.; Oprea, L. Preliminary Data on the Studies of Alosa immaculata in Romanian marine waters. Sci. Ann. Danub. Delta Inst. 2016, 22, 141–148. Available online: https://ddniscientificannals.ddni.ro/images//22_17.pdf (accessed on 5 February 2023).
- Trindade-Santos, I.; Freire, K.M.F. Analysis of reproductive patterns of fishes from three large marine ecosystems. Front Mar. Sci. 2015, 2, 38. [Google Scholar] [CrossRef]
- Stroe, M.D.; Crețu, M.; Ion, G.; Mirea, D.; Savin, V.; Tenciu, M.; Patriche, N. Population structure and growth parameters of Alosa immaculata species, Bennett, 1835 (Pontic shad) on the Danube sector km 169–km 197 in 2020. Sci. Pap.-Anim. Sci. Ser. Lucr. Ştiinţifice Ser. Zooteh. 2021, 75, 265–270. Available online: https://www.uaiasi.ro/firaa/Pdf/Pdf_Vol_75/Desimira_Stroe.pdf (accessed on 5 February 2023).
- Năstase, A.; Năvodaru, I.; Cernișencu, I.; Țiganov, G.; Popa, L. Pontic shad (Alosa immaculata) migrating upstream the Danube River and larval drift downstream to the Black Sea in 2016. Sci. Ann. Danub. Delta Inst. 2018, 23, 57–68. Available online: https://www.ddniscientificannals.ro/images/23_08.pdf (accessed on 22 January 2023).
- Battes, K.W.; Pricope, F.; Ureche, D.; Ureche, C.; Stoica, I.; Răducanu, D.; Dogaru, N. Evaluarea stării resurselor pescăreşti şi capturilor durabile din apele interioare. In Estimarea Stocurilor de Peşti şi Pescăriilor; Năvodaru, I., Ed.; Ed. Dobrogea: Constanţa, Romania, 2008; pp. 275–288. [Google Scholar]
- Ibănescu, D.C.; Popescu, A.; Nica, A. Estimation of growth and mortality parameters of the Pontic shad (Alosa immaculata Bennett, 1835) in Romanian section of the Danube River. Sci. Pap.-Zootech. Univ. Agric. Sci. Vet. Med. Iași 2017, 67, 285–289. Available online: https://www.uaiasi.ro/firaa/Pdf/Pdf_Vol_67/Daniela_Ibanescu.pdf (accessed on 5 February 2023).
- Tiganov, G.; Nenciu, M.I.; Danilov, C.S.; Nita, V.N. Estimates of the population parameters and exploitation rate of pontic shad (Alosa immaculata Bennett, 1835) in the Romanian Black Sea coast. Sciendo Agric. Life Life Agric. Conf. Proc. 2018, 1, 162–167. [Google Scholar] [CrossRef][Green Version]
- Vieira, R.P.; Monteiro, P.; Ribeiro, J.; Bentes, L.; Oliveira, F.; Erzini, K.; Gonçalves, J.M.D.S. Length-weight relationships of six syngnathid species from Ria Formosa, SW Iberian coast. Cah. Biol. Mar. 2014, 55, 9–12. Available online: https://sapientia.ualg.pt/bitstream/10400.1/8805/1/2014%20Vieira%20et%20al_WL%20relationships%20Syngnathidae.pdf (accessed on 22 January 2023).
- Li, Y.; Feng, M.; Huang, L.; Zhang, P.; Wang, H.; Zhang, J.; Xu, J. Weight–Length Relationship Analysis Revealing the Impacts of Multiple Factors on Body Shape of Fish in China. Fishes 2023, 8, 269. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Z.; Liu, M.; Liu, W.; Zhao, W.; Liu, H.; Zhang, P.; You, F. Length-weight, length-length relationships, and condition factors of black rockfish Sebastes schlegelii Hilgendorf, 1880 in Lidao Bay, China. Thalass. Int. J. Mar. Sci. 2017, 33, 57–63. [Google Scholar] [CrossRef]
- Morado, C.N.; Araújo, F.G.; Gomes, I.D. The use of biomarkers for assessing effects of pollutant stress on fish species from a tropical river in Southeastern Brazil. Acta Sci. Biol. Sci. 2017, 39, 431–439. [Google Scholar] [CrossRef]
- Kolarov, P. Particularities of Pontic shad (Alosa kessleri pontica Eichw.) in 1979 in Bulgarian aquatory. Fisheries 1980, 27, 7–19. [Google Scholar]
- Kolarov, P. Some basic parameters of the Pontic shad (Alosa kesleri pontica Eichw.) population. Hydrobiologiya 1983, 19, 60–69. [Google Scholar]
- Tsikliras, A.C.; Froese, R. Maximum sustainable yield. Encycl. Ecol. 2019, 1, 108–115. [Google Scholar] [CrossRef]
- Santos, R.; Peixoto, U.I.; Medeiros-Leal, W.; Novoa-Pabon, A.; Pinho, M. Growth Parameters and Mortality Rates Estimated for Seven Data-Deficient Fishes from the Azores Based on Length-Frequency Data. Life 2022, 12, 778. [Google Scholar] [CrossRef] [PubMed]
- Gulland, J.A. Fish Stock Assessment. A Manual of Basic Method; FAO/Wiley Series on Food and Agriculture; FAO: Rome, Italy, 1983; 64p. [Google Scholar]
- Gulland, J.A.; Holt, S.J. Estimation of Growth Parameters for Data at Unequal Time Intervals. ICES J. Mar. Sci. 1959, 25, 47–49. [Google Scholar] [CrossRef]
- Etim, L.; Lebo, P.E.; King, R.P. The dynamics of an exploited population of a Silurid catfish (Schilbe intermidius, Reupell 1832) in the Cross River, Nigeria. Fish. Res. 1999, 40, 295–307. [Google Scholar] [CrossRef]
- Tsikliras, A.C.; Antonopoulou, E. Reproductive biology of round sardinella (Sardinella aurita) in north-eastern Mediterranean. Sci. Mar. 2006, 70, 281–290. Available online: https://scientiamarina.revistas.csic.es/index.php/scientiamarina/article/view/155/152 (accessed on 5 February 2023). [CrossRef]
- Jennings, S.; Kaiser, M.J.; Reynolds, J.D. Marine Fisheries Ecology; Blackwell Science: London, UK, 2009; 393p. [Google Scholar]

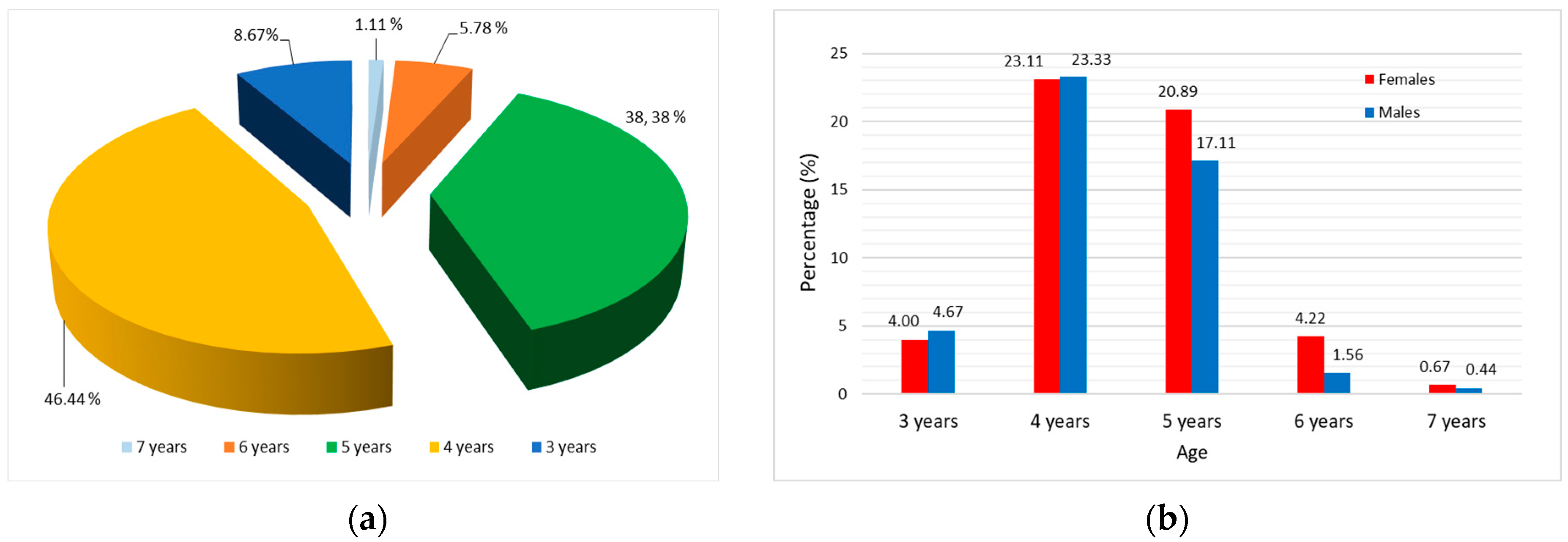

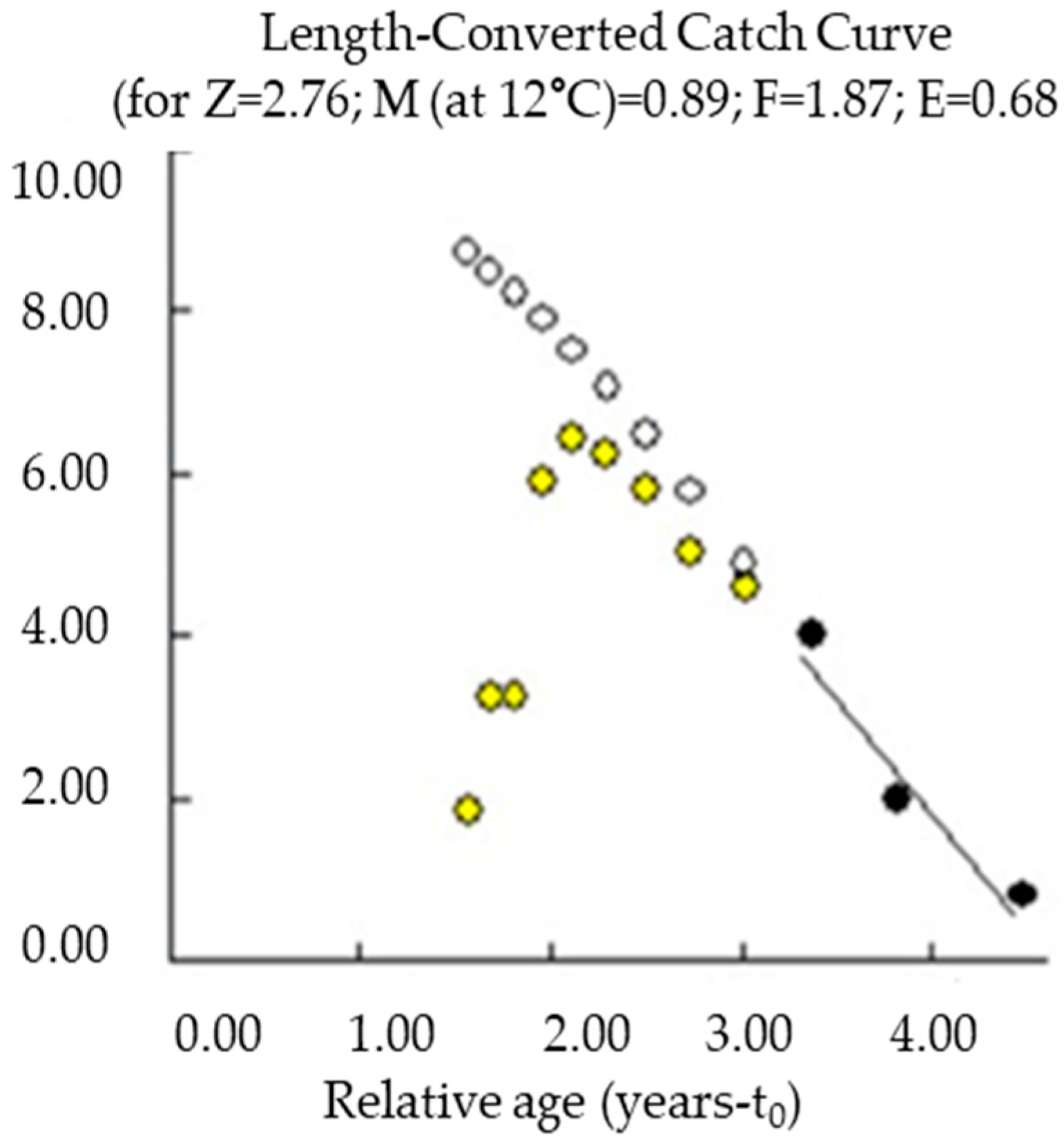
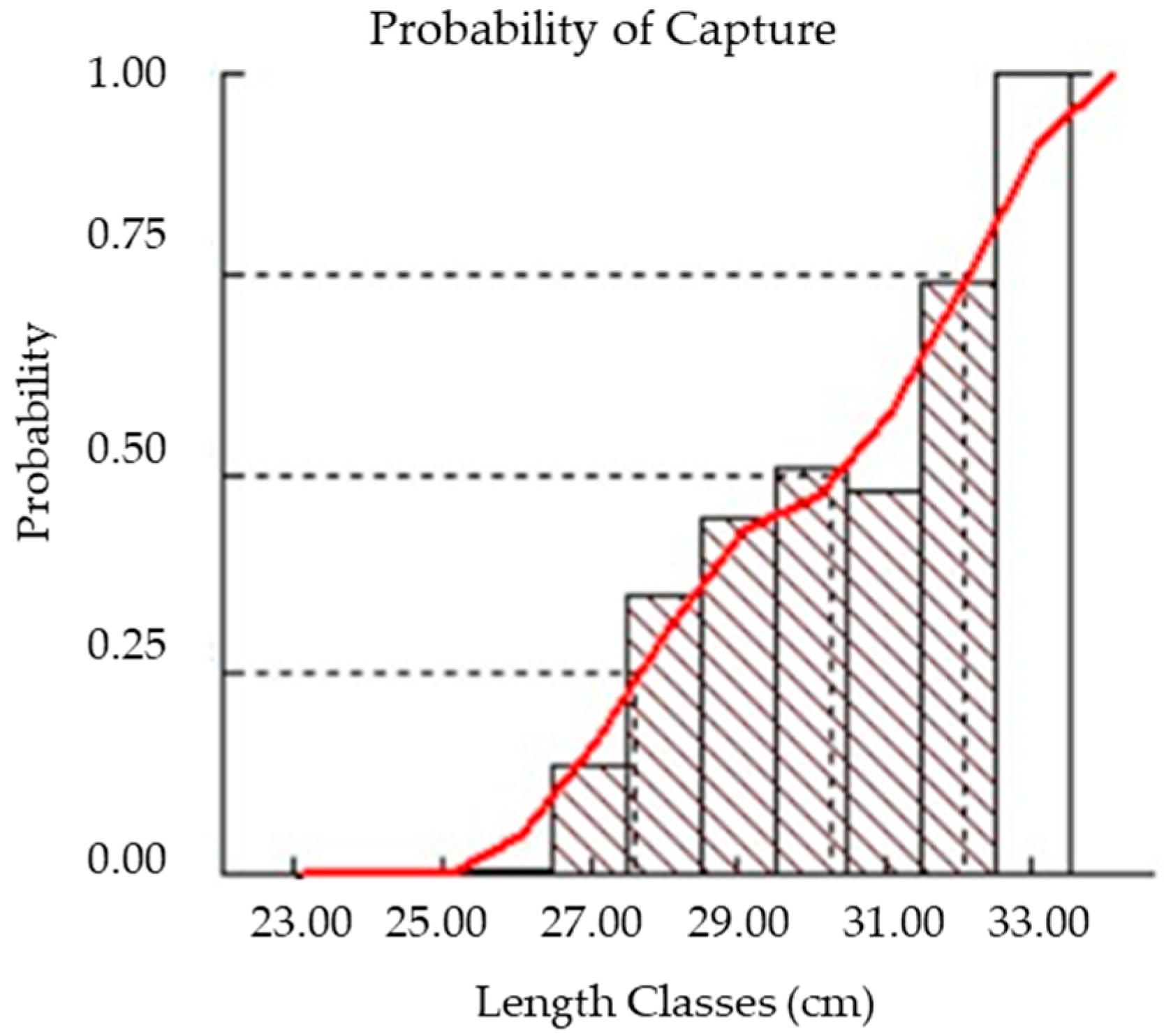
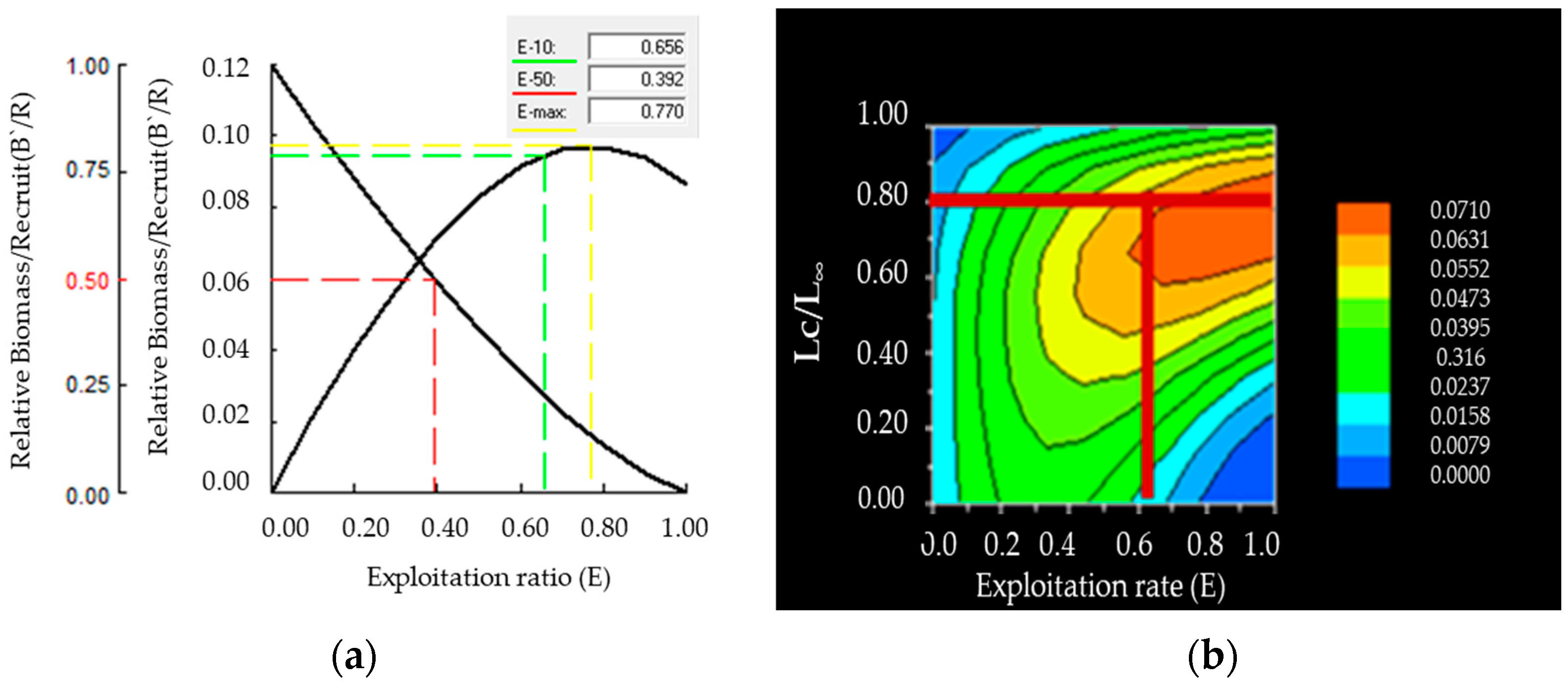

| Sex | N | Mean W (g) | Mean Lt (cm) | Mean Ls (cm) | Mean Lf (cm) | Mean H (cm) |
|---|---|---|---|---|---|---|
| Females | 238 | 247.88 ± 45.30 | 30.47 ± 1.76 | 26.97 ± 1.63 | 27.25 ± 1.68 | 6.15 ± 0.53 |
| Males | 212 | 196.13 ± 23.90 | 28.46 ± 1.09 | 25.21 ± 1.07 | 25.34 ± 1.06 | 5.55 ± 0.49 |
| Both sexes | 450 | 223.5 ± 44.95 | 29.53 ± 1.79 | 26.14 ± 1.65 | 26.35 ± 1.71 | 5.87 ± 0.59 |
| Sex | Number of Fish | Equation | R2 |
|---|---|---|---|
| Males | 212 | W = 0.32 × Lt2.92 | 0.63 |
| Females | 238 | W = 0.0258 × Lt 2.68 | 0.74 |
| Males + Females | 450 | W = 0.021 × Lt2.72 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stroe, D.M.; Cretu, M.; Tenciu, M.; Dima, F.M.; Patriche, N.; Tiganov, G.; Dediu, L. Age, Growth, and Mortality of Pontic Shad, Alosa immaculata Bennett, 1835, in the Danube River, Romania. Fishes 2024, 9, 128. https://doi.org/10.3390/fishes9040128
Stroe DM, Cretu M, Tenciu M, Dima FM, Patriche N, Tiganov G, Dediu L. Age, Growth, and Mortality of Pontic Shad, Alosa immaculata Bennett, 1835, in the Danube River, Romania. Fishes. 2024; 9(4):128. https://doi.org/10.3390/fishes9040128
Chicago/Turabian StyleStroe, Desimira Maria, Mirela Cretu, Magdalena Tenciu, Floricel Maricel Dima, Neculai Patriche, George Tiganov, and Lorena Dediu. 2024. "Age, Growth, and Mortality of Pontic Shad, Alosa immaculata Bennett, 1835, in the Danube River, Romania" Fishes 9, no. 4: 128. https://doi.org/10.3390/fishes9040128
APA StyleStroe, D. M., Cretu, M., Tenciu, M., Dima, F. M., Patriche, N., Tiganov, G., & Dediu, L. (2024). Age, Growth, and Mortality of Pontic Shad, Alosa immaculata Bennett, 1835, in the Danube River, Romania. Fishes, 9(4), 128. https://doi.org/10.3390/fishes9040128







