Aurantiochytrium sp. Meal as Feed Additive for Pacific White Shrimp Reared under Low Temperature and Challenged by WSSV in Association with Thermal Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Shrimps, Feeding and Maintance
2.2. Proximal Analysis of Aurantiochytrium sp.
2.3. Experimental System
2.4. Growth Performance
2.5. Microbiological Parameters
2.6. White Spot Syndrome Virus (WSSV) Challenge with Thermal Stress
2.7. Hemato-Immunological Assessment
2.8. Statistical Analysis
3. Results
3.1. Experimental System and Growth Performance
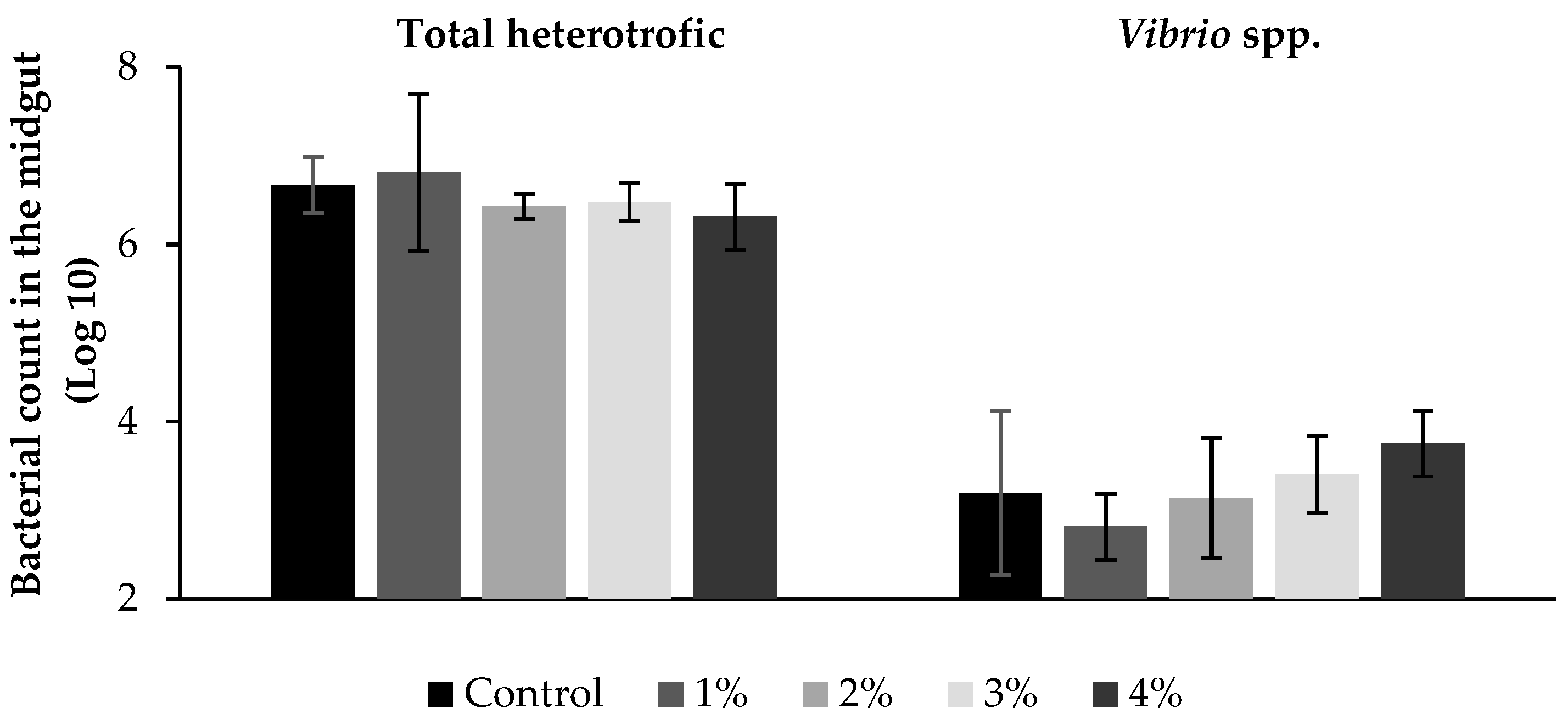
3.2. Microbiological Analysis
3.3. WSSV Challenge Associated with Thermal Stress
3.4. Hemato-Immunological Parameters
4. Discussion
4.1. Growth Performance and Diet Composition
4.2. Microbiological and Hemato-Immunological and Analyses and Challenge with WSSV in Association with Thermal Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walker, S.J.; Neill, W.H.; Lawrence, A.L.; Gatlin, D.M. Effects of Temperature and Starvation on Ecophysiological Performance of the Pacific White Shrimp (Litopenaeus Vannamei). Aquaculture 2011, 319, 439–445. [Google Scholar] [CrossRef]
- Wyban, J.; Walsh, W.A.; Godin, D.M. Temperature Effects on Growth, Feeding Rate and Feed Conversion of the Pacific White Shrimp (Penaeus Vannamei). Aquaculture 1995, 138, 267–279. [Google Scholar] [CrossRef]
- Ponce-Palafox, J.; Martinez-Palacios, C.A.; Ross, L.G. The Effects of Salinity and Temperature on the Growth and Survival Rates of Juvenile White Shrimp, Penaeus Vannamei, Boone, 1931. Aquaculture 1997, 157, 107–115. [Google Scholar] [CrossRef]
- Flegel, T.W. Review of Disease Transmission Risks from Prawn Products Exported for Human Consumption. Aquaculture 2009, 290, 179–189. [Google Scholar] [CrossRef]
- Peruzza, L.; Shekhar, M.S.; Kumar, K.V.; Swathi, A.; Karthic, K.; Hauton, C.; Vijayan, K.K. Temporal Changes in Transcriptome Profile Provide Insights of White Spot Syndrome Virus Infection in Litopenaeus Vannamei. Sci. Rep. 2019, 9, 13509. [Google Scholar] [CrossRef] [PubMed]
- Lightner, D. V Virus Diseases of Farmed Shrimp in the Western Hemisphere (the Americas): A Review. J. Invertebr. Pathol. 2011, 106, 110–130. [Google Scholar] [CrossRef] [PubMed]
- Millard, R.S.; Ellis, R.P.; Bateman, K.S.; Bickley, L.K.; Tyler, C.R.; van Aerle, R.; Santos, E.M. How Do Abiotic Environmental Conditions Influence Shrimp Susceptibility to Disease? A Critical Analysis Focussed on White Spot Disease. J. Invertebr. Pathol. 2021, 186, 107369. [Google Scholar] [CrossRef]
- Zhang, J.S.; Li, Z.J.; Wen, G.L.; Wang, Y.L.; Luo, L.; Zhang, H.J.; Dong, H.B. Relationship between White Spot Syndrome Virus (WSSV) Loads and Characterizations of Water Quality in Litopenaeus Vannamei Culture Ponds during the Tropical Storm. Iran. J. Vet. Res. 2016, 17, 210. [Google Scholar]
- Vidal, O.M.; Granja, C.B.; Aranguren, F.; Brock, J.A.; Salazar, M. A Profound Effect of Hyperthermia on Survival of Litopenaeus Vannamei Juveniles Infected with White Spot Syndrome Virus. J. World Aquac. Soc. 2001, 32, 364–372. [Google Scholar] [CrossRef]
- Abdelrahman, H.A.; Abebe, A.; Boyd, C.E. Influence of Variation in Water Temperature on Survival, Growth and Yield of Pacific White Shrimp Litopenaeus Vannamei in Inland Ponds for Low-salinity Culture. Aquac. Res. 2019, 50, 658–672. [Google Scholar] [CrossRef]
- Prates, E.; Holanda, M.; Pedrosa, V.F.; Monserrat, J.M.; Wasielesky, W. Compensatory Growth and Energy Reserves Changes in the Pacific White Shrimp (Litopenaeus Vannamei) Reared in Different Temperatures and under Feed Restriction in Biofloc Technology System (BFT). Aquaculture 2023, 562, 738821. [Google Scholar] [CrossRef]
- Hayward, S.A.L.; Manso, B.; Cossins, A.R. Molecular Basis of Chill Resistance Adaptations in Poikilothermic Animals. J. Exp. Biol. 2014, 217, 6–15. [Google Scholar] [CrossRef]
- Chowanski, S.; Lubawy, J.; Spochacz, M.; Ewelina, P.; Grzegorz, S.; Rosinski, G.; Slocinska, M. Cold Induced Changes in Lipid, Protein and Carbohydrate Levels in the Tropical Insect Gromphadorhina Coquereliana. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 183, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Glencross, B.D. Exploring the Nutritional Demand for Essential Fatty Acids by Aquaculture Species. Rev. Aquac. 2009, 1, 71–124. [Google Scholar] [CrossRef]
- COUNCIL, N.R. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Das, U. Essential Fatty Acids—A Review. Curr. Pharm. Biotechnol. 2006, 7, 467–482. [Google Scholar] [CrossRef]
- Harwood, J.L.; Guschina, I.A. The Versatility of Algae and Their Lipid Metabolism. Biochimie 2009, 91, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Perini, J.Â.D.L.; Stevanato, F.B.; Sargi, S.C.; Visentainer, J.E.L.; Dalalio, M.M.D.O.; Matshushita, M.; De Souza, N.E.; Visentainer, J.V. Ácidos Graxos Poli-Insaturados n-3 e n-6: Metabolismo Em Mamíferos e Resposta Imune. Rev. Nutr. 2010, 23, 1075–1086. [Google Scholar] [CrossRef]
- Qiu, X. Biosynthesis of Docosahexaenoic Acid (DHA, 22:6-4, 7,10,13,16,19): Two Distinct Pathways. Prostaglandins Leukot. Essent. Fatty Acids 2003, 68, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Barreira, L.; Figueiredo, F.; Custódio, L.; Vizetto-Duarte, C.; Polo, C.; Rešek, E.; Engelen, A.; Varela, J. Polyunsaturated Fatty Acids of Marine Macroalgae: Potential for Nutritional and Pharmaceutical Applications. Mar. Drugs 2012, 10, 1920–1935. [Google Scholar] [CrossRef]
- Wang, K.; Sun, T.; Cui, J.; Liu, L.; Bi, Y.; Pei, G.; Chen, L.; Zhang, W. Screening of Chemical Modulators for Lipid Accumulation in Schizochytrium Sp. S31. Bioresour. Technol. 2018, 260, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Marchan, L.F.; Chang, K.J.L.; Nichols, P.D.; Mitchell, W.J.; Polglase, J.L.; Gutierrez, T. Taxonomy, Ecology and Biotechnological Applications of Thraustochytrids: A Review. Biotechnol. Adv. 2018, 36, 26–46. [Google Scholar] [CrossRef]
- Jaritkhuan, S.; Suanjit, S. Species Diversity and Polyunsaturated Fatty Acid Content of Thraustochytrids from Fallen Mangrove Leaves in Chon Buri Province, Thailand. Agric. Nat. Resour. 2018, 52, 24–32. [Google Scholar] [CrossRef]
- Shene, C.; Paredes, P.; Flores, L.; Leyton, A.; Asenjo, J.A.; Chisti, Y. Dynamic Flux Balance Analysis of Biomass and Lipid Production by Antarctic Thraustochytrid Oblongichytrium Sp. RT2316-13. Biotechnol. Bioeng. 2020, 117, 3006–3017. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.A.O.; Koshio, S.; Esteban, M.Á. Beneficial Roles of Feed Additives as Immunostimulants in Aquaculture: A Review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Samocha, T.M.; Patnaik, S.; Davis, D.A.; Bullis, R.A.; Browdy, C.L. Use of Commercial Fermentation Products as a Highly Unsaturated Fatty Acid Source in Practical Diets for the Pacific White Shrimp Litopenaeus Vannamei. Aquac. Res. 2009, 41, 961–967. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Filer, K.; Xue, Y.; Ai, Q.; Mai, K. Replacement of Fish Oil with a DHA-Rich Schizochytrium Meal on Growth Performance, Activities of Digestive Enzyme and Fatty Acid Profile of Pacific White Shrimp (Litopenaeus Vannamei) Larvae. Aquac. Nutr. 2017, 23, 1113–1120. [Google Scholar] [CrossRef]
- Kumar, V.; Habte-Tsion, H.; Allen, K.M.; Bowman, B.A.; Thompson, K.R.; El-Haroun, E.; Filer, K.; Tidwell, J.H. Replacement of Fish Oil with Schizochytrium Meal and Its Impacts on the Growth and Lipid Metabolism of Pacific White Shrimp (Litopenaeus Vannamei). Aquac. Nutr. 2018, 24, 1769–1781. [Google Scholar] [CrossRef]
- Nobrega, R.O.; Batista, R.O.; Corrêa, C.F.; Mattioni, B.; Filer, K.; Pettigrew, J.E.; Fracalossi, D.M. Dietary Supplementation of Aurantiochytrium Sp. Meal, a Docosahexaenoic-Acid Source, Promotes Growth of Nile Tilapia at a Suboptimal Low Temperature. Aquaculture 2019, 507, 500–509. [Google Scholar] [CrossRef]
- Chang, K.J.L.; Parrish, C.C.; Simon, C.J.; Revill, A.T.; Nichols, P.D. Feeding Whole Thraustochytrid Biomass to Cultured Atlantic Salmon (Salmo Salar) Fingerlings: Culture Performance and Fatty Acid Incorporation. J. Mar. Sci. Eng. 2020, 8, 207. [Google Scholar] [CrossRef]
- Moi, I.M.; Leow, A.T.C.; Ali, M.S.M.; Rahman, R.N.Z.R.A.; Salleh, A.B.; Sabri, S. Polyunsaturated Fatty Acids in Marine Bacteria and Strategies to Enhance Their Production. Appl. Microbiol. Biotechnol. 2018, 102, 5811–5826. [Google Scholar] [CrossRef]
- Nagano, N.; Sakaguchi, K.; Taoka, Y.; Okita, Y.; Honda, D.; Ito, M.; Hayashi, M. Detection of Genes Involved in Fatty Acid Elongation and Desaturation in Thraustochytrid Marine Eukaryotes. J. Oleo Sci. 2011, 60, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, P.; Scarpa, J. Water Quality Requirements and Management. In Farming Marine Shrimp in Recirculating Freshwater Systems; Van Wyk, P., Scarpa, J., Eds.; Harbor Branch Oceanographic Institution: Fort Pierce, FL, USA, 1999; Volume 13, pp. 141–162. [Google Scholar]
- Simon, C.J.; Truong, H.H.; Noble, T.H.; Osborne, S.A.; Wynne, J.W.; Wade, N.M. Microbial Biomass, Marine Invertebrate Meals and Feed Restriction Influence the Biological and Gut Microbiota Response of Shrimp Penaeus Monodon. Aquaculture 2020, 520, 734679. [Google Scholar] [CrossRef]
- Coutteau, P.; Sorgeloos, P. Intercalibration Exercise on the Qualitative and Quantitative Analysis of Fatty Acids in Artemia and Marine Samples Used in Mariculture. ICES Coop. Res. Rep. 1995, 211, 34. [Google Scholar]
- APHA American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, Eaton, A.D., Clesceri, L.S., Rice, E.W., Greenberg, A.E., Eds.; 21st ed.; American Public Health Association: Washington DC, USA, 2005. [Google Scholar]
- Bagenal, T.B.; Tesch, F.W. Methods for Assessment of Fish Production in Fresh Waters, 3rd ed.; Bagenal, T., Ed.; Blackwell Scientific: Hoboken, NJ, USA, 1978. [Google Scholar]
- Rezende, P.C.; Miranda, C.; Fracalossi, D.M.; Hayashi, L.; Seiffert, W.Q.; do Nascimento Vieira, F.; Schleder, D.D. Brown Seaweeds as a Feed Additive for Litopenaeus Vannamei Reared in a Biofloc System Improved Resistance to Thermal Stress and White Spot Disease. J. Appl. Phycol. 2022, 34, 2603–2614. [Google Scholar] [CrossRef]
- Schleder, D.D.; Blank, M.; Peruch, L.G.B.; Poli, M.A.; Gonçalves, P.; Rosa, K.V.; Fracalossi, D.M.; do Nascimento Vieira, F.; Andreatta, E.R.; Hayashi, L. Impact of Combinations of Brown Seaweeds on Shrimp Gut Microbiota and Response to Thermal Shock and White Spot Disease. Aquaculture 2020, 519, 734779. [Google Scholar] [CrossRef]
- de Souza Valente, C.; Oliveira Ortiz, K.; Depperschmidt, R.; de Medeiros Fraga, A.P.; do Nascimento Vieira, F.; Freire Marques, M.R. Transcription of Defense Related Genes in Pacific White Shrimp, Litopenaeus Vannamei, Kept in Biofloc and in Clear Seawater and Challenged with the White Spot Syndrome Virus. Aquac. Int. 2020, 28, 293–307. [Google Scholar] [CrossRef]
- Bolívar-Ramírez, N.C.; Mallmann, A.S.; Schleder, D.D.; Machado, C.; Seiffert, W.Q.; do Nascimento Vieira, F. Tannins as a Food Additive in Pacific White Shrimp Diet. Aquaculture 2022, 556, 738232. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice-Hall/Pearson: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Boyd, C.E.; Gautier, D. GAA’S, Implementing. Effluent Composition &Water Quality Standards. Glob. Aquac. Advocate 2000, 3, 61–66. [Google Scholar]
- Glencross, B.D.; Smith, D.M.; Thomas, M.R.; Williams, K.C. Optimising the Essential Fatty Acids in the Diet for Weight Gain of the Prawn, Penaeus Monodon. Aquaculture 2002, 204, 85–99. [Google Scholar] [CrossRef]
- Batista, R.O.; Richter, B.L.; Banze, J.F.; Schleder, D.D.; Salhi, M.; Nobrega, R.O.; da Silva, M.F.O.; Mattioni, B.; Pettigrew, J.E.; Fracalossi, D.M. Soy Lecithin Supplementation Promotes Growth and Increases Lipid Digestibility in GIFT Nile Tilapia Raised at Suboptimal Temperature. Fishes 2023, 8, 404. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Wu, X.; Zhong, W.; Xian, J.; Liao, S.; Miao, Y.; Wang, A. Effects of Different Dietary Lipid Level on the Growth, Survival and Immune-Relating Genes Expression in Pacific White Shrimp, Litopenaeus Vannamei. Fish. Shellfish. Immunol. 2013, 34, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.-H.; To, V.-A.; Zhang, Z.-F.; Lin, Y.-H. The Effect of Dietary Lecithin and Lipid Levels on the Growth Performance, Body Composition, Hemolymph Parameters, Immune Responses, Body Texture, and Gene Expression of Juvenile White Shrimp (Litopenaeus Vannamei). Aquaculture 2023, 567, 739260. [Google Scholar] [CrossRef]
- González-Félix, M.L.; Lawrence, A.L.; Gatlin, D.M.; Perez-Velazquez, M. Growth, Survival and Fatty Acid Composition of Juvenile Litopenaeus Vannamei Fed Different Oils in the Presence and Absence of Phospholipids. Aquaculture 2002, 205, 325–343. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, J.; Li, J.; Lv, W.; Zou, J.; Fan, L. A New Insight into the Intestine of Pacific White Shrimp: Regulation of Intestinal Homeostasis and Regeneration in Litopenaeus Vannamei during Temperature Fluctuation. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2020, 35, 100687. [Google Scholar] [CrossRef]
- Glencross, B.D.; Smith, D.M. Optimizing the Essential Fatty Acids, Eicosapentaenoic and Docosahexaenoic Acid, in the Diet of the Prawn, Penaeus Monodon. Aquac. Nutr. 2001, 7, 101–112. [Google Scholar] [CrossRef]
- Corrêa, C.F.; Nobrega, R.O.; Mattioni, B.; Block, J.M.; Fracalossi, D.M. Dietary Lipid Sources Affect the Performance of Nile Tilapia at Optimal and Cold, Suboptimal Temperatures. Aquac. Nutr. 2017, 23, 1016–1026. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, A.-L.; Xian, J.-A. Variation of Free Amino Acid and Carbohydrate Concentrations in White Shrimp, Litopenaeus Vannamei: Effects of Continuous Cold Stress. Aquaculture 2011, 317, 182–186. [Google Scholar] [CrossRef]
- González-Félix, M.L.; Gatlin, D.M.; Lawrence, A.L.; Perez-Velazquez, M. Effect of Various Dietary Lipid Levels on Quantitative Essential Fatty Acid Requirements of Juvenile Pacific White Shrimp Litopenaeus Vannamei. J. World Aquac. Soc. 2002, 33, 330–340. [Google Scholar] [CrossRef]
- Sánchez-Paz, A. White Spot Syndrome Virus: An Overview on an Emergent Concern. Vet. Res. 2010, 41, 43. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.M.; Schleder, D.D.; Nagata, M.; Nóbrega, R.O.; Fracalossi, D.M.; Seiffert, W.Q.; Vieira, F.N. Aurantiochytrium sp. Meal Can Replace Fish Oil in Practical Diets for the Juvenile Pacific White Shrimp. Aquac. Nutr. 2019, 25, 798–807. [Google Scholar] [CrossRef]
- Sheikh, H.I.; Najiah, M.; Fadhlina, A.; Laith, A.A.; Nor, M.M.; Jalal, K.C.A.; Kasan, N.A. Temperature Upshift Mostly but Not Always Enhances the Growth of Vibrio Species: A Systematic Review. Front. Mar. Sci. 2022, 9, 959830. [Google Scholar] [CrossRef]
- Bolívar-Ramírez, N.C.; Rodrigues, M.S.; Guimarães, A.M.; Guertler, C.; Rosa, J.R.; Seiffert, W.Q.; Andreatta, E.R.; do Nascimento Vieira, F. Effect of Dietary Supplementation with Butyrate and Probiotic on the Survival of Pacific White Shrimp after Challenge with Vibrio Alginolyticus. Rev. Bras. Zootec. 2017, 46, 471–477. [Google Scholar] [CrossRef]
- da Rosa Coelho, J.; Rosa, K.V.; Rocha, J.S.; Ramírez, N.C.B.; Maraschin, M.; do Nascimento Vieira, F. In Vitro Antimicrobial Activity Of Carvacrol against Shrimp Pathogens and Its Use As Feed Additive for the Pacific White Shrimp. Bol. Inst. Pesca 2021, 47, e645. [Google Scholar] [CrossRef]
- Rojo, A.; Ibarra, L.; Brown, J.M.M.; Velasco, G.; Martínez-Téllez, M.Á.; Medina-Jasso, M.A.; Nieves-Soto, M.; Quintana-Zavala, D. Potential of Nannochloropsis in Beta Glucan Production. In Nannochloropsis: Biology, Biotechnological, Potential and Challenges; Jan, M., Kazik, P., Eds.; Nova Sciences: Hauppauge, NY, USA, 2017; pp. 181–225. [Google Scholar]
- Li, C.; Weng, S.; He, J. WSSV–Host Interaction: Host Response and Immune Evasion. Fish. Shellfish. Immunol. 2019, 84, 558–571. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-S.; Liau, S.-Y.; Huang, C.-T.; Nan, F.-H. Beta 1,3/1,6-Glucan and Vitamin C Immunostimulate the Non-Specific Immune Response of White Shrimp (Litopenaeus Vannamei). Fish. Shellfish. Immunol. 2016, 57, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Diaz, D.A.; Schmidt, J.G.; Vera-Jiménez, N.I.; Steinhagen, D.; Nielsen, M.E. β-Glucan Enriched Bath Directly Stimulates the Wound Healing Process in Common Carp (Cyprinus Carpio L.). Fish. Shellfish. Immunol. 2013, 35, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Gu, M.; Zhang, W.; Xu, W.; Mai, K. Effects of β-Glucan Derivatives on the Immunity of White Shrimp Litopenaeus Vannamei and Its Resistance against White Spot Syndrome Virus Infection. Aquaculture 2014, 426–427, 66–73. [Google Scholar] [CrossRef]
- Ochoa-Álvarez, N.A.; Casillas-Hernández, R.; Magallón-Barajas, F.J.; Ramirez-Orozco, J.M.; Carbajal-Millán, E. Protector Effect of Beta-Glucans from Shrimp Pond-Related Yeasts in Penaeus Vannamei Rearing under White Spot Syndrome Virus Presence. Lat. Am. J. Aquat. Res. 2021, 49, 18–28. [Google Scholar] [CrossRef]
- Yang, H.; Huang, Y.; Li, Z.; Guo, Y.; Li, S.; Huang, H.; Yang, X.; Li, G.; Chen, H. Effects of Dietary Supplementation with Aurantiochytrium Sp. on Zebrafish Growth as Determined by Transcriptomics. Animals 2022, 12, 2794. [Google Scholar] [CrossRef]
- Watanabe, K.; Arafiles, K.H.V.; Higashi, R.; Okamura, Y.; Tajima, T.; Matsumura, Y.; Nakashimada, Y.; Matsuyama, K.; Aki, T. Isolation of High Carotenoid-producing Aurantiochytrium sp. Mutants and Improvement of Astaxanthin Productivity Using Metabolic Information. J. Oleo Sci. 2018, 67, 571–578. [Google Scholar] [CrossRef]
- TEETS, N.M.; DENLINGER, D.L. Physiological Mechanisms of Seasonal and Rapid Cold-hardening in Insects. Physiol. Entomol. 2013, 38, 105–116. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and Functions of Lipids and Fatty Acids in Teleosts Fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Wang, Y.; Chen, W.; Jin, Z.; Yao, Z.; Zhang, D. Strain-Specific Changes in the Gut Microbiota Profiles of the White Shrimp Litopenaeus Vannamei in Response to Cold Stress. Aquaculture 2019, 503, 357–366. [Google Scholar] [CrossRef]
- Abdel-Ghany, H.M.; El-Sayed, A.-F.M.; Ezzat, A.A.; Essa, M.A.; Helal, A.M. Dietary Lipid Sources Affect Cold Tolerance of Nile Tilapia (Oreochromis Niloticus). J. Therm. Biol. 2019, 79, 50–55. [Google Scholar] [CrossRef]
- Kostetsky, E.Y.; Velansky, P.V.; Sanina, N.M. Phase Transitions of Phospholipids as a Criterion for Assessing the Capacity for Thermal Adaptation in Fish. Russ. J. Mar. Biol. 2013, 39, 214–222. [Google Scholar] [CrossRef]
- Mai, W.; Wang, W. Protection of Blue Shrimp (Litopenaeus Stylirostris) against the White Spot Syndrome Virus (WSSV) When Injected with Shrimp Lysozyme. Fish. Shellfish. Immunol. 2010, 28, 727–733. [Google Scholar] [CrossRef]
- Ji, P.F.; Yao, C.L.; Wang, Z.Y. Reactive Oxygen System Plays an Important Role in Shrimp Litopenaeus Vannamei Defense against Vibrio Parahaemolyticus and WSSV Infection. Dis. Aquat. Organ. 2011, 96, 9–20. [Google Scholar] [CrossRef]
- Immanuel, G.; Sivagnanavelmurugan, M.; Marudhupandi, T.; Radhakrishnan, S.; Palavesam, A. The Effect of Fucoidan from Brown Seaweed Sargassum Wightii on WSSV Resistance and Immune Activity in Shrimp Penaeus Monodon (Fab). Fish. Shellfish. Immunol. 2012, 32, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Schleder, D.D.; Guertler, C.; Perazzolo, L.M.; Vinatea, L. Hypoxia Increases Susceptibility of Pacific White Shrimp to Whitespot Syndrome Virus (WSSV). Arq. Bras. Med. Vet. Zootec. 2016, 68, 397–403. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Yavari, V.; Hoseini, S.J.; Nafisi, M.; Mozanzadeh, M.T. Effects of Different Carbon Sources and Dietary Protein Levels in a Biofloc System on Growth Performance, Immune Response against White Spot Syndrome Virus Infection and Cathepsin L Gene Expression of Litopenaeus Vannamei. Aquac. Res. 2019, 50, 1162–1176. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, W.-N.; Wang, L.; Liu, Y.-F.; Wang, A.-L. Oxidative Stress, DNA Damage and Osmolality in the Pacific White Shrimp, Litopenaeus Vannamei Exposed to Acute Low Temperature Stress. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2011, 154, 36–41. [Google Scholar] [CrossRef]
- Zhou, M.; Wu, Z.; Liang, R.; Gu, N. Effects of Dietary Taurine, Carnitine and Cholesterol Supplementation on Growth Performance and Immunological Status of Litopenaeus Vannamei under Cold Exposure. Aquac. Res. 2017, 48, 1279–1290. [Google Scholar] [CrossRef]


| Ingredient (g/kg as Fed) | % Inclusion of Aurantiochytrium sp. Meal | ||||
|---|---|---|---|---|---|
| Control | 1 | 2 | 3 | 4 | |
| Soybean meal | 300 | 290 | 290 | 290 | 290 |
| Fishmeal | 160 | 160 | 160 | 160 | 160 |
| Wheat meal | 159 | 166 | 165 | 165 | 165 |
| Poultry meal | 140 | 140 | 140 | 140 | 140 |
| Kaolin | 100 | 100 | 91.5 | 83 | 75.5 |
| Vitamin and mineral premix a | 25 | 25 | 25 | 25 | 25 |
| Soy lecithin | 22 | 15 | 15 | 13.5 | 11 |
| Fish oil | 20 | 25 | 27.5 | 30 | 30 |
| Soybean oil | 10 | 5 | 2.5 | 0 | 0 |
| Magnesium sulphate | 15 | 15 | 15 | 15 | 15 |
| Monocalcium phosphate | 10 | 10 | 10 | 10 | 10 |
| Sodium chloride | 15 | 15 | 15 | 15 | 15 |
| Potassium chloride | 15 | 15 | 15 | 15 | 15 |
| Carboxymethylcellulose | 5 | 5 | 5 | 5 | 5 |
| DL-Methionine | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Vitamin C | 1 | 1 | 1 | 1 | 1 |
| Choline hydrochloride | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Aurantiochytrium sp meal. b | 0 | 10 | 20 | 30 | 40 |
| Chemical Composition (%) | % Inclusion of Aurantiochytrium sp. Meal | ||||
|---|---|---|---|---|---|
| Control | 1 | 2 | 3 | 4 | |
| Moisture content | 9.5 | 9.5 | 9.5 | 8.5 | 9.6 |
| Crude protein | 40.8 | 40.0 | 40.4 | 40.1 | 40.3 |
| Crude lipid | 8.1 | 8.9 | 9.7 | 10.5 | 10.8 |
| Carbohydrate | 25.3 | 25.5 | 25.7 | 24.7 | 25.8 |
| Ash | 16.3 | 16.1 | 14.7 | 16.2 | 13.5 |
| Dry matter (%) | 90.5 | 90.5 | 90.5 | 91.5 | 90.4 |
| Gross energy (MJ/kg) a | 16.6 | 16.3 | 16.6 | 16.6 | 16.7 |
| Fatty acids (% DM) | |||||
| 14:0 | 0.12 | 0.17 | 0.22 | 0.27 | 0.30 |
| 16:0 | 1.89 | 2.42 | 2.99 | 3.5 | 3.85 |
| 18:0 | 0.60 | 0.62 | 0.64 | 0.69 | 0.66 |
| 18:1 n-9 | 2.14 | 2.23 | 2.24 | 2.31 | 2.19 |
| 18:2 n-6 | 2.22 | 1.95 | 1.79 | 1.66 | 1.63 |
| 18:3 n-3 | 0.20 | 0.17 | 0.15 | 0.15 | 0.14 |
| ARA b | 0.06 | 0.06 | 0.07 | 0.07 | 0.07 |
| EPA c | 0.08 | 0.10 | 0.11 | 0.12 | 0.12 |
| DHA d | 0.25 | 0.48 | 0.73 | 0.91 | 1.11 |
| SFA | 2.77 | 3.4 | 4.05 | 4.68 | 5.04 |
| MUFA | 2.47 | 2.62 | 2.65 | 2.78 | 2.63 |
| PUFA | 2.88 | 2.86 | 2.95 | 3.03 | 3.17 |
| LC-PUFA | 0.33 | 0.58 | 0.84 | 1.03 | 1.23 |
| n-3 | 0.53 | 0.76 | 1.0 | 1.19 | 1.37 |
| n-6 | 2.31 | 2.05 | 1.9 | 1.78 | 1.74 |
| n-3:n-6 | 0.23 | 0.37 | 0.53 | 0.67 | 0.79 |
| DHA:EPA | 3.13 | 4.80 | 6.64 | 7.58 | 9.25 |
| PUFA:SFA | 1.04 | 0.84 | 0.73 | 0.65 | 0.63 |
| Aurantiochytrium sp. ALL-G-RICH | |||||
|---|---|---|---|---|---|
| Chemical Composition g kg−1 | Minerals mg kg−1 | ||||
| Moisture content | 24.65 | P | 1798.83 | Na | 1015.46 |
| Crude protein | 108.68 | Ca | 2898.55 | Fe | 13.89 |
| Lipid | 688.73 | Mg | 2496.27 | Al | 7.87 |
| Ash | 30.64 | K | 1519.93 | Zn | 12.43 |
| Carbohydrate | 147.30 | S | 4964.37 | Si | 208.24 |
| Dry matter | 975.35 | - | - | ||
| Gross energy a | 31.90 | - | - | ||
| Fatty acids composition % | |||||
| 14:00 | 5.34 | 18:4 n-3 | 0.26 | MUFA | 0.85 |
| 16:00 | 62.98 | ARA b | 0.82 | PUFA | 28.39 |
| 16:1 n-7 | 0.11 | EPA c | 0.12 | LC-PUFA | 27.13 |
| 18:1 n-9 | 0.33 | DHA d | 26.62 | n-3 | 27.39 |
| 18:2 n-6 | 0.29 | SFA | 68.61 | n-6 | 1.26 |
| Treatment | Initial Weight (g−1) | Final Weight (g−1) | Weekly Weight Gain (g−1) | Yield (kg−1/m3) | Survival (%) | Feed Conversion Ratio (kg−1) |
|---|---|---|---|---|---|---|
| Control | 3.8 ± 0.01 | 13.1 ± 0.67 | 1.04 ± 0.07 | 1.35 ± 0.10 | 100.0 ± 0.0 | 2.16 ± 0.15 |
| 1% | 3.8 ± 0.02 | 12.6 ± 0.78 | 0.98 ± 0.08 | 1.24 ± 0.04 | 97.5 ± 2.5 | 2.32 ± 0.07 |
| 2% | 3.8 ± 0.03 | 12.7 ± 0.78 | 0.99 ± 0.09 | 1.25 ± 0.08 | 98.3 ± 1.4 | 2.26 ± 0.13 |
| 3% | 3.8 ± 0.04 | 13.9 ± 0.07 | 1.10 ± 0.03 | 1.34 ± 0.02 | 96.7 ± 1.4 | 2.16 ± 0.07 |
| 4% | 3.8 ± 0.02 | 12.9 ± 0.41 | 1.01 ± 0.04 | 1.25 ± 0.02 | 97.5 ± 2.5 | 2.26 ± 0.03 |
| p value | 0.9931 | 0.2411 | 0.2364 | 0.1364 | 0.5251 | 0.3025 |
| Treatments | Suboptimal Temperature Phase (22 °C) | Thermal Increment Phase | Optimal Temperature Phase (28 °C) | Cumulative Total Mortality |
|---|---|---|---|---|
| Negative control * | 0 | 0 | 0 | 0 |
| Control | 40.8 ± 21.3 a | 0 | 20.8 ± 16.6 a | 61.7 ± 12.6 a |
| 1% | 26.7 ± 23 a | 0 | 20.0 ± 17.3 a | 46.7 ± 40.4 a |
| 2% | 21.9 ± 11.7 a | 3.7 ± 6.4 | 28.5 ± 5.7 a | 54.1 ± 13.4 a |
| 3% | 10.7 ± 1.0 b | 0 | 46.3 ± 3.2 b | 57.0 ± 2.6 a |
| 4% | 16.7 ± 15.3 b | 3.3 ± 5.7 | 16.7 ± 20.8 a | 36.7 ± 35.1 b |
| Time (h) | 108 | 12 | 48 | 168 |
| p value | 0.0184 | - | 0.0170 | 0.0006 |
| Treatments | THC (106) | Protein Concentration (mg mL−1) | PO activity (U min−1 mg−1 Protein) | Agglutination Titer (Log2) | |
|---|---|---|---|---|---|
| 1 st sample: after 9 weeks of trial, before infection WSSV (22 °C) | Control | 53.90 ± 5.77 B | 167.9 ± 43.8 | 60.9 ± 43.6 | 9.33 ± 0.58 ABC |
| 1% | 100.78 ± 19.44 A | 163.4 ± 48.9 | 87.7 ± 37.8 | 10.33 ± 0.58 A | |
| 2% | 53.53 ± 20.43 B | 161.6 ± 10.0 | 59.4 ± 21.6 | 9.00 ± 1.00 ABC | |
| 3% | 55.76 ± 12.47 B | 164.7 ± 37.7 | 49.6 ± 26.5 | 9.91 ± 0.87 AB | |
| 4% | 57.13 ± 30.52 B | 199.4 ± 63.2 | 47.0 ± 19.9 | 9.77 ± 0.88 AB | |
| 2nd sample: 4.5 days after infection WSSV (22 °C) | Negative control | 27.58 ± 13.14 | 143.6 ± 9.3 | 30.0 ± 11.2 | 8.74 ± 1.08 |
| Control | 23.21 ± 7.12 B | 135.4 ± 17.7 | 38.9 ± 21.0 | 10.02 ± 0.58 AB | |
| 1% | 25.89 ± 12.90 B | 139.1 ± 13.9 | 25.4 ± 13.1 | 8.02 ± 0.73 BC | |
| 2% | 21.00 ± 8.77 B | 131.1 ± 12.5 | 19.8 ± 1.8 | 8.45 ± 0.56 ABC | |
| 3% | 27.73 ± 15.22 B | 109.5 ± 24.9 | 43.3 ± 16.5 | 8.42 ± 0.38 ABC | |
| 4% | 16.83 ± 4.90 BC | 99.4 ± 11.5 | 32.3 ± 2.9 | 8.06 ± 0.93 BC | |
| 3 rd sample: 7 days after infection WSSV (28 °C) | Negative control | 14.71 ± 5.68 | 115.2 ± 6.0 | 15.4 ± 4.5 | 8.63 ± 0.40 |
| Control | 3.76 ± 3.54 C | 117.1 ± 14.5 | 14.8 ± 8.0 | 8.4 ± 0.65 ABC | |
| 1% | 6.03 ± 6.88 C | 114.2 ± 44.0 | 18.4 ± 11.6 | 7.37 ± 0.48 C | |
| 2% | 8.28 ± 4.40 C | 115.9 ± 5.8 | 21.3 ± 9.3 | 8.09 ± 0.36 BC | |
| 3% | 4.70 ± 7.08 C | 134.1 ± 24.3 | 22.4 ± 11.3 | 8.0 ± 1.00 BC | |
| 4% | 9.07 ± 7.11 C | 118.4 ± 23.9 | 13.3 ± 3.5 | 9.24 ± 0.54 ABC | |
| Factor 1: Feed | 0.0207 | 0.9538 | 0.7044 | 0.1715 | |
| Factor 2: Time infection/thermal stress | <0.01 | <0.01 | <0.01 | <0.01 | |
| Interaction between 1 and 2 | 0.0309 | 0.8450 | 0.4169 | <0.01 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffling, F.B.; Marquezi, A.S.; Pinheiro, I.; Simon, C.; Rombenso, A.N.; Seiffert, W.Q.; Vieira, F.d.N.; Schleder, D.D. Aurantiochytrium sp. Meal as Feed Additive for Pacific White Shrimp Reared under Low Temperature and Challenged by WSSV in Association with Thermal Stress. Fishes 2024, 9, 108. https://doi.org/10.3390/fishes9030108
Hoffling FB, Marquezi AS, Pinheiro I, Simon C, Rombenso AN, Seiffert WQ, Vieira FdN, Schleder DD. Aurantiochytrium sp. Meal as Feed Additive for Pacific White Shrimp Reared under Low Temperature and Challenged by WSSV in Association with Thermal Stress. Fishes. 2024; 9(3):108. https://doi.org/10.3390/fishes9030108
Chicago/Turabian StyleHoffling, Flávia Banderó, Alex Silva Marquezi, Isabela Pinheiro, Cedric Simon, Artur Nishioka Rombenso, Walter Quadros Seiffert, Felipe do Nascimento Vieira, and Delano Dias Schleder. 2024. "Aurantiochytrium sp. Meal as Feed Additive for Pacific White Shrimp Reared under Low Temperature and Challenged by WSSV in Association with Thermal Stress" Fishes 9, no. 3: 108. https://doi.org/10.3390/fishes9030108
APA StyleHoffling, F. B., Marquezi, A. S., Pinheiro, I., Simon, C., Rombenso, A. N., Seiffert, W. Q., Vieira, F. d. N., & Schleder, D. D. (2024). Aurantiochytrium sp. Meal as Feed Additive for Pacific White Shrimp Reared under Low Temperature and Challenged by WSSV in Association with Thermal Stress. Fishes, 9(3), 108. https://doi.org/10.3390/fishes9030108








