Simple Summary
Enteromorpha prolifera is a type of green marine macroalgae that can cause green tides due to eutrophication. However, aquafeeds demand for E. prolifera meal has gradually exceeded annual E. prolifera production. Therefore, novel extraction methods should be applied to increase the bioactive compounds of E. prolifera at the level of large-scale industrialization to support the efficient utilization of E. prolifera in aquafeeds. According to biological activities of E. prolifera, the growth, feed intake, lipid metabolism, antioxidant, immune parameters and stress resistance were investigated in Pacific white shrimp. It confirmed that bio-products of E. prolifera based on different processing procedures could be used to reduce the amount of E. prolifera in feed without compromising its biological function. It will helpful to improve the application of E. prolifera in aquaculture.
Abstract
A feeding trial was conducted to evaluate the biological activities of Enteromorpha prolifera bio-products in the diets of Pacific white shrimp (Litopenaeus vannamei). Bio-products of E. prolifera included E. prolifera meal, E. prolifera hydrolysate and E. prolifera polysaccharide, which was supplied using different processing procedures. The control diet was supplemented without any E. prolifera bio-products or dietary attractants. Experimental diets were formulated to contain 0.2% and 0.4% of E. prolifera hydrolysate (EPH0.2% and EPH0.4%), 0.03% of E. prolifera polysaccharide (EPP0.03%), 3% of E. prolifera meal (EPM3%), 0.1% of dimethyl-β-propiothetn (DMPT0.1%) and 1% of squid paste (SP1%). Shrimp (~8 g) were randomly distributed in 21 tanks and fed for 44 days. Feed intake showed that 3% of E. prolifera meal and 0.4% of E. prolifera hydrolysate in diets exhibited similar attractant effects as 0.1% of DMPT. Gross qualitative observation showed that the abundance of lipid droplets decreased in hepatopancreas of the EPH0.4% and EPM3% groups, which were supported by hepatopancreas triacylglycerol (TG), where significantly lower concentrations were observed in the EPH0.4% and EPM3% groups compared with the control group. Similarly, TG and low-density lipoprotein cholesterol in serum significantly decreased in the EPH0.4%, EPP0.03% and EPM3% groups compared to the control group. Supplemental E. prolifera bio-products resulted in significantly higher serum glutathione level of EPP0.03% or superoxide dismutase activities of EPH0.4% and EPM3%, but significantly lower serum malondialdehyde level of EPM3%. In addition, tnf-α expression in hepatopancreas was significantly down-regulated in shrimp fed the EPH0.2%, EPH0.4% and EPM3% diets. Based on survival analysis, E. prolifera bio-products improved the resistance of shrimp to hypoxic stresses. Thus, this study confirmed that bio-products of E. prolifera supplied using different processing procedures could be used to reduce the amount of E. prolifera in feed of shrimp without compromising their biological functions.
Keywords:
Pacific white shrimp; Enteromorpha prolifera; feed intake; lipid metabolism; antioxidant; stress resistance Key Contribution:
This study provided three types of Enteromorpha prolifera bio-products. E. prolifera hydrolysate and E. prolifera polysaccharide can be used to reduce the amount of E. prolifera meal in feed of Pacific white shrimp (Litopenaeus vannamei) without compromising their biological functions.
1. Introduction
Enteromorpha prolifera is a type of green marine macroalgae that can cause green tides due to eutrophication [1]. Considering the potential of a detrimental effect on the environment, this algal blooms in coastal areas of Qingdao of China in 2008 caused a great concern for the local government and environmentalists [2]. After that, specialized commercial companies from Qingdao were established to clean up E. prolifera. An important commercial use of E. prolifera collected from the sea was to become feed ingredients [3]. Traditionally, macroalgae including E. prolifera were used as a seaweed meal in compound animal feed by drying and grinding to a fine powder [4]. However, aquafeeds demand for E. prolifera meal has gradually exceeded annual E. prolifera production. Thus, except for the drying technique, other effective processing technologies should be used to obtain extracted bioactive compounds of E. prolifera for high-value applications in aquaculture [4].
E. prolifera contains sulfated polysaccharides mainly composed of rhamnose, glucuronic acid and xylose, which are regarded as the biologically active molecules [5,6]. Because of these, E. prolifera polysaccharide has been used as the feed additive to regulate various biological activities, such as immunomodulatory, antioxidant, antimicrobial, and lipid metabolism [7,8]. These biological activities of E. prolifera polysaccharide were not only confirmed in terrestrial animals [5,9], but also in fish and shrimp [2,10]. In addition, some studies found that seaweed polysaccharide could mitigate various stress responses, such as air exposure, heat stress and ammonia stress [11,12,13,14]. Except for the polysaccharide, E. prolifera had been shown to enhance feed intake of fish due to its potential feeding attractants, such as betaine [2,15]. However, aforementioned studies with E. prolifera meal or E. prolifera polysaccharide were mainly based on the traditional drying process or extraction procedures on a laboratory scale. Novel extraction methods should be applied to increase the bioactive compounds of E. prolifera at the level of large-scale industrialization to support the efficient utilization of E. prolifera in aquafeeds.
Pacific white shrimp (Litopenaeus vannamei) is an important cultured shrimp species, accounting for 70% of the total shrimp production in the world [16]. As a feed ingredient, E. prolifera meal was mainly used in shrimp feed. E. prolifera inclusion level in aquaculture feed has to be decreased because its production is finite, but the aquaculture industry has still been expanding so far. Thus, the aim of this study was to evaluate three types of bio-products of E. prolifera (E. prolifera meal, E. prolifera hydrolysate and E. prolifera polysaccharide), which is supplied on a large scale by a commercial company. According to biological activities of E. prolifera, the growth, feed intake, lipid metabolism, antioxidant, immune parameters and stress resistance were investigated in Pacific white shrimp.
2. Materials and Methods
2.1. Preparation of Bio-Products of Enteromorpha prolifera
In this study, fresh Enteromorpha prolifera was collected from the coast of Qingdao in China (July in 2022) and used to be prepared for the production of bio-products of E. prolifera. Bio-products of E. prolifera included E. prolifera meal, E. prolifera hydrolysate and E. prolifera polysaccharide, which were provided by Qingdao Seawin Biotech Group Co., Ltd. (Qingdao, China). E. prolifera meal (crude protein 9.26% of dry matter, crude lipid 1.70% of dry matter and carbohydrate 39.37% of dry matter) is obtained by washing, drying and grinding fresh E. prolifera. E. prolifera hydrolysate (crude protein 7.93% of dry matter, crude lipid 0.28% of dry matter and carbohydrate 43.72% of dry matter) is obtained by hydrolyzing E. prolifera meal with polysaccharide-degrading enzymes and polysaccharide lyases. E. prolifera polysaccharide (crude protein 5.74% of dry matter, crude lipid 0% of dry matter and carbohydrate 50.40% of dry matter) is obtained using enzymatic hydrolysis extraction, purification, concentration and spray-drying of E. prolifera meal, which was sulfated polysaccharides composed mainly of rhamnose sulfate, glucuronic acid and xyloseo.
2.2. Experimental Diets
The control diet contained fish meal, wheat gluten, peanut meal and soybean meal as the main protein source and was supplemented without any bio-products of E. prolifera or dietary attractants. For experimental diets, 0.2% and 0.4% of E. prolifera hydrolysate (EPH0.2% and EPH0.4%), 0.03% of E. prolifera polysaccharide (EPP0.03%), as well as 3% of E. prolifera meal (EPM3%) were added to the control diet, respectively. All bio-products of E. prolifera were added in the experimental diets based on the recommended amounts of their production enterprise (Qingdao Seawin Biotech Group Co., Ltd.), and ensured that the cost of all added E. prolifera bio-products were similar among all the experimental diets. In addition, considering E. prolifera potentially functioned as a feeding attractant, two attractants for pacific white shrimp (Litopenaeus vannamei), 0.1% of dimethyl-β-propiothetn (DMPT0.1%) and 1% of squid paste were added to the control diet to evaluate the potential feeding attractant function of E. prolifera bio-products [17,18,19]. Formulation and proximate composition of the experimental diets are presented in Table 1.

Table 1.
Formulation and proximate composition of the experimental diets (% dry matter).
2.3. Experimental Shrimp and Feeding Trial
Pacific white shrimp were provided by Center for Marine Fisheries Science Research of Yellow Sea Fisheries Research Institute (Qingdao, China), and then were transferred to the experimental culture system and fed a commercial diet for one week to adapt to the experimental conditions. The feeding trial was also conducted at Center for Marine Fisheries Science Research of Yellow Sea Fisheries Research Institute. A total of 420 shrimp with an average body weight of 8 g were randomly assigned to 21 tanks (diameter, 90 cm; height, 80 cm) with 20 shrimp in each tank. Each diet was assigned to triplicate tanks. Shrimp were fed by hand three 3 times a day (07:30, 12:30 and 18:30). The feeding trial lasted for 44 days. During the period of the feeding trial, water-quality parameters were monitored twice a week, and water temperature was 26.0–29.2 °C, salinity was 22–29, pH was 7.5–8.0, and dissolved oxygen was 6–8 mg/L.
2.4. Sample Collection
At the end of the feeding trial, all shrimp were deprived of food for 24 h, and then were bulk-weighted to calculate the growth parameters. Before the sampling, shrimp were anesthetized with tricaine methanesulfonate (MS-222) (50 mg/L). Then, six shrimp per tank were collected to take hemolymph from ventral sinus using 1 mL syringes with a 26-gauge needle. Hemolymph samples were collected and mixed with SIC-EDTA anticoagulant (10 mmol/L of ethylenediaminetetraacetic acid (EDTA)-Na2, 450 mmol/L NaCl, 10 mmol/L KCl, 10 mmol/L 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES), and pH 7.0) at a 1:2 ratio, and kept at −4 °C for 3 h [20]. And then, they were centrifuged at 3000× g for 10 min, and the supernatant was used as the plasma samples and stored at −80 °C for further analysis. After hemolymph sampling, six shrimp were euthanized with lethal dose of MS-222 (500 mg/L) and dissected to collect hepatopancreas. A hepatopancreas sample from three of them was collected, frozen in liquid nitrogen and stored at −80 °C for the analysis of gene expression and triglycerides contents, and the other three were fixed in Bouin’s solution for histological processing. In addition, two shrimp per tank were sampled for the analysis of body composition. In the current experiment, all procedures were performed with the permission of Institutional Animal Care and Use Committee of the Yellow Sea Fisheries Research Institute.
2.5. Analytical Methods
2.5.1. Chemical Analysis
Moisture, crude protein, crude lipid and ash of experimental diets and whole body weight were analyzed based on the standard methods [21]. Briefly, moisture content was calculated after keeping the sample at 105 °C for 24 h at a constant weight. Crude protein content (N × 6.25) was determined using a Foss Kjeltec 2300 analyzer (Foss, Högänas, Sweden) using the Kjeldahl method. Crude lipid content was determined using petroleum ether at a boiling point of 30–60 °C using a Foss Soxtec 2050 auto-extractor (Foss, Högänas, Sweden). Ash content was determined through incineration at 550 °C in a muffle furnace for 6 h.
2.5.2. Plasma or Hepatopancreas Biochemical, Antioxidant and Immunological Parameters
Triglycerides (TG) of hepatopancreas and plasma were determined using a Triglycerides assay kit (A110-1-1) based on the manufacturer’s protocols. Other biochemical parameters of plasma including glucose, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) were measured using their respective commercial kits (glucose assay kit, F006-1-1; high-density lipoprotein cholesterol assay kit, A112-1-1; low-density lipoprotein cholesterol assay kit, A113-1-1). Plasma enzymes related to antioxidant capacity including superoxide dismutase (SOD), glutathione peroxidase (GPx), malondialdehyde (MDA), reduced glutathione (GSH) were measured using their respective commercial kits (superoxide dismutase typed assay kit, A001-2-1; glutathione peroxidase assay kit, A005-1-1; malondialdehyde assay kit, A003-1-1; reduced glutathione assay kit, A006-2-1). Plasma-immunity-related enzymes including glutamic oxalic aminotransferase (GOP), glutamic pyruvic aminotransferase (GPT), alkaline phosphatase (AKP), acid phosphatase (ACP), lysozyme (LZM) and phenoloxidase (PO) were measured using their respective commercial kits (aspartate aminotransferase assay kit, C010-2-1; alanine aminotransferase assay kit, C009-2-1; alkaline phosphatase assay kit, A059-2-2; acid phosphatase assay kit, A060-2-1; lysozyme assay kit, A050-1-1; phenoloxidase, H247). All commercial kits mentioned above were purchased from NanJing JianCheng Bioengineering Institute (NanJing JianCheng Bioengineering Institute, Nanjing, China).
2.5.3. Oil Red O Staining
After starvation for 24 h, hepatopancreas tissues of three shrimp per tank were fixed in Bouin’s solution. And then, samples were stained with oil red O and then separated with ethanol. After washing using distilled water, the nuclei of the hepatopancreas was dyed with hematoxylin. Finally, hepatopancreas sections were washed with distilled water until blue and sealed with glycerin gelatin. The sections were photographed using a light microscope (an Eclipse 80i/90i microscope, Nikon, Tokyo, Japan).
2.6. RNA Extraction and Gene Expression Assay
Total RNA from the hepatopancreas was isolated using an RNAiso Plus kit (Takara, Kusatsu, Japan). The purity and concentration of RNA were detected using a Colibri Microvolume Spectrometer (Titertek Berthold, Berthold Detection Systems GmbH, Pforzheim, Germany). The A260/A280 ratio for RNA samples was in the range of 1.8–2.0, indicating that the extracted RNA samples were high purity and could be used for reverse transcription. cDNA was synthesized with an Evo M-MLV RT Mix kit with gDNA Clean (Accurate Biotechnology, Changsha, China). A Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR) was performed on a Roche LightCycler 96 system (Roche, Basel, Switzerland) using SYBR Green Premix Pro Taq HS qPCR Kit (Accurate Biotechnology, China) based on the manufacturer’s protocol. β-actin and glyceraldehyde-3-phosphate dehydrogenase (gapdh) were used as reference genes in this study. Relative mRNA expression was calculated using 2−ΔΔCt method [22]. Target expression was examined with normalization to the geometric mean of the Ct values from two reference genes. All target genes and internal reference genes are shown in Table 2.

Table 2.
Primer sequences used for qRT-PCR.
2.7. Hypoxia Stress
At the end of feeding trial, six shrimp from each tank were transported to a new bucket with 2.5 L of water used as hypoxic stress. The water of the bucket was not aerated and dissolved oxygen was gradually reduced by shrimp breathing. In the process of hypoxia stress, initial oxygen concentration in the water is 4.7 mg/L, and then dissolved oxygen and the number of dead shrimp were recorded twice an hour for survival analysis. And, all shrimp were fed their allocated diets and the hypoxic exposure would be ended until all the shrimp died.
2.8. Calculations
The growth parameters were calculated from the following formulas:
Survival rate (SR, %) = 100 × (final shrimp number/initial shrimp number),
Weight gain (WG, %) = 100 × (final body weight − initial body weight)/initial body weight,
Specific growth ratio (SGR, %/d) = 100 × [ln (final body weight) − ln (initial body weight)]/feeding days,
Feed intake (FI, %/d) = 100 × total feed intake/[feeding days × (final body weight + initial body weight)/2],
Feed conversion ratio (FER) = dry feed intake/body weight gain,
Protein efficiency ratio (PER) = (final body weight − initial body weight)/protein intake,
Protein productive value (PPV, %) = 100 × (final body protein content − initial body protein content)/protein intake.
2.9. Statistical Analysis
SPSS 26.0 software (SPSS Company, Chicago, IL, USA) was used for all statistical analyses. All data were presented as mean values ± standard error. A one-way analysis of variance (ANOVA) test was performed on all experimental data except for the survival after hypoxia test. The survival analysis after hypoxia test was assessed using Kaplan–Meier analysis with a log-rank. When there were significant differences between different treatments (p < 0.05), Tukey’s method was used for multiple comparison.
3. Results
3.1. The Cumulative Feed Intake Per Shrimp during the 14 Days
The cumulative feed intake per shrimp from the first feeding meal (FF), first day after feeding (1DAF), and 3DAF was not significantly different between treatments (p > 0.05) (Table 3). However, from 7 days to 14 days after feeding, the cumulative feed intake per shrimp from all the experimental diets was higher than that of the control diet, especially for 0.4% of E. prolifera hydrolysate (p < 0.05).

Table 3.
The cumulative feed intake per shrimp during 14 d following the initiation of feeding trial 1.
3.2. Growth Performance
Survival rate ranged from 93.33 to 98.33%, and was not significantly different among all the groups (p > 0.05) (Table 4). The highest level of final body weight (FBW), weight gain (WG) or specific growth rate (SGR) was observed in shrimp fed the EPH0.4% diet, which was significantly higher than that of shrimp fed the DMPT0.1% diet (p < 0.05). As for bio-products of E. prolifera, shrimp fed 0.4% of E. prolifera hydrolysate exhibited the highest level of FBW, WG or SGR, following by 3% of E. prolifera meal, 0.2% of E. prolifera hydrolysate, and 0.03% of E. prolifera polysaccharide. Feed intake of shrimp fed the EPH0.4%, EPM3%, and DMPT0.1% diets was significantly higher than that of shrimp fed the control diet (p < 0.05). Feed conversion ratio was significantly affected by dietary treatments, where shrimp fed the EPH0.2% diet had a lower ratio than that of shrimp fed the DMPT0.1% diet (p < 0.05). There were no significant differences in protein-efficiency ratio and protein productive value among all the groups (p > 0.05).

Table 4.
Growth and feed utilization in fish fed experimental diets 1.
3.3. Shrimp Whole-Body Proximate Composition
Proximate composition of the shrimp’s whole-body is shown in Table 5. The contents of crude protein, crude lipid, moisture and ash were not affected by dietary treatments (p > 0.05).

Table 5.
The composition of shrimp’s whole body (% wet weight).
3.4. Lipid-Metabolism-Related Biochemical Parameters in Hepatopancreas and Plasma
Oil red O staining of the hepatopancreas is presented in Figure 1. Gross qualitative observation showed that histological analysis in the hepatopancreas of shrimp fed bio-products of E. prolifera had lower abundance of lipid droplets compared to the control group. This result was supported by the triacylglycerols (TG) of hepatopancreas (Table 6), where its concentration was in the range from 4.41 to 5.93 mmol/L among all the E. prolifera bio-product groups, which was lower or significantly lower (p < 0.05) than that in the control group (9.34 mmol/L).
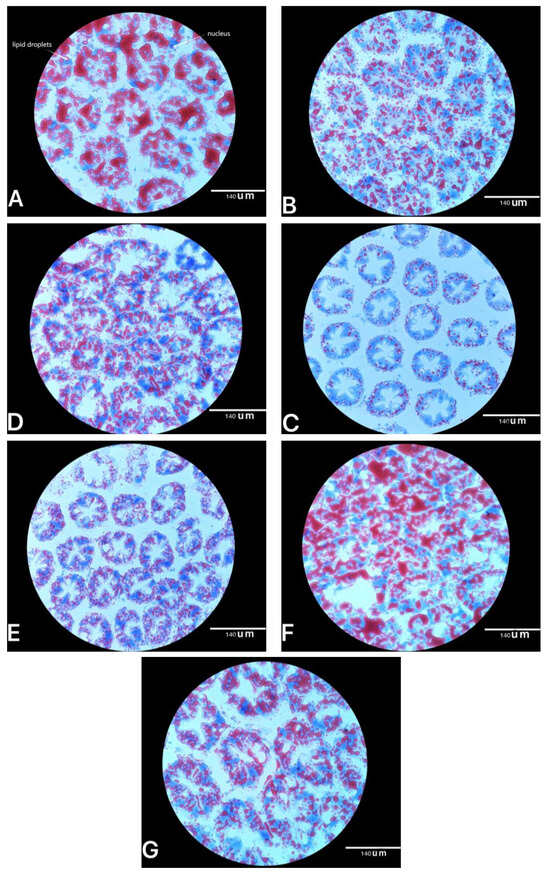
Figure 1.
Example images of hepatopancreatic histology from shrimp fed experimental diets. (A) (Control), (B) (EPH0.2%), (C) (EPH0.4%), (D) (EPP0.03%), (E) (EPM3%), (F) (DMPT0.01%) and (G) (SP1%) were measured at a 40× objective magnification. The lipid droplet is orange–red or bright red, and the nucleus is blue.

Table 6.
Lipid-metabolism-related biochemical parameters in plasma and hepatopancreas (mmol/L) 1.
Plasma lipid-metabolism-related biochemical parameters are presented in Table 6. Plasma TG concentration in fish fed E. prolifera bio-products, especially the EPH0.4%, EPP0.03% and EPM3% diets, significantly decreased compared to fish fed the control and DMPT0.1% diets (p < 0.05). In addition, the concentration of plasma LDL-C from the E. prolifera bio-product groups showed a decreased trend compared to the control diet (p > 0.05), and was significantly lower than that of the DMPT0.1% group (p < 0.05). However, HDL-C in plasma was not affected by dietary treatments (p > 0.05).
3.5. Antioxidant and Immune Parameters in Plasma
Plasma antioxidant parameters are presented in Table 7. Compared with the control group, bio-products of E. prolifera increased the content of plasma GSH to some extent, especially the 0.03% of E. prolifera polysaccharide (p < 0.05). However, the activity of GPx in plasma was not affected by dietary treatments (p > 0.05). The activity of plasma SOD in shrimp fed bio-products of E. prolifera increased or significantly (p < 0.05) increased compared to the control diet, and on the contrary, decreased the concentration of plasma MDA.

Table 7.
Plasma antioxidant and immune parameters 1.
Plasma immune parameters are presented in Table 7. Compared with the control group, 0.4% of E. prolifera hydrolysate and 0.03% of E. prolifera polysaccharide significantly reduced the activity of AKP (p < 0.05), but bio-products of E. prolifera did not affect the activities of ACP, GPT, GOT and LZM (p > 0.05). In addition, the activity of PO in shrimp fed E. prolifera hydrolysate, especially the EPH0.2% diet, was higher than that of shrimp fed the control diet.
3.6. Relative mRNA Expression of Tumor Necrosis Factor-α in Hepatopancreas
Gene expression of the pro-inflammation cytokine (tumor necrosis factor-α, tnf-α) was determined in the hepatopancreas (Figure 2). Bio-products of E. prolifera (EPH0.2%, EPH0.4% and EPM3%) significantly down-regulated the expression of tnf-α compared to fish fed the control diet (p < 0.05). In addition, hepatopancreatic tnf-α expression showed a slight decrease in the EPP0.03% group, although there was no statistically difference compared with the control group (p > 0.05).
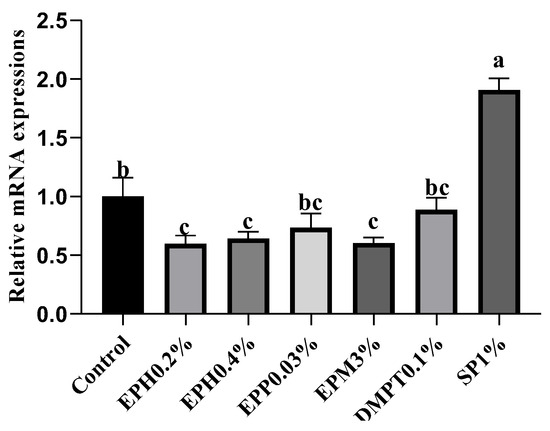
Figure 2.
Relative mRNA expression of tumor necrosis factor-α (tnf-α) in hepatopancreas. Data are presented as mean values ± standard error (n = 3). Bars with same letters are not significantly different using Tukey’s test (p > 0.05).
3.7. Hypoxia Stress
Survival analyses after hypoxia stress have been assessed using a Kaplan–Meier survival curve in Figure 3. Although there was no statistical difference, the log-rank test (p = 0.073) indicated that survival curves were to some extent affected by dietary treatments. For example, when the dissolved oxygen in the water was 0.6 mg/L at 6 h after hypoxic stress, shrimp in the control and DMPT0.01% groups began to die. Dead shrimp were observed in all the groups when hypoxic stress lasted for 7 h and the dissolved oxygen in the water was still 0.6 mg/L. At this time point (7 h), survival probability in shrimp fed bio-products of E. prolifera and squid paste groups was higher than 80%, but the control and DMPT0.01% groups reached 78% and 72%, respectively. Until 10 h of hypoxic stress, dissolved oxygen in water decreased to 0.3 mg/L, and survival probability of shrimp fed bio-products of E. prolifera was higher than that of the control, DMPT 0.01%, and SP1% groups. Finally, when dissolved oxygen in the water was 0.2 mg/L at 11.5 h of hypoxia stress, survival probability of the EPP0.03% and EPM3% were still 17% and 6%, respectively, while those of the other groups were 0. It indicated that after hypoxia stress, the average survival time of shrimp fed diets containing bio-products of E. prolifera was longer when compared to the control diet.
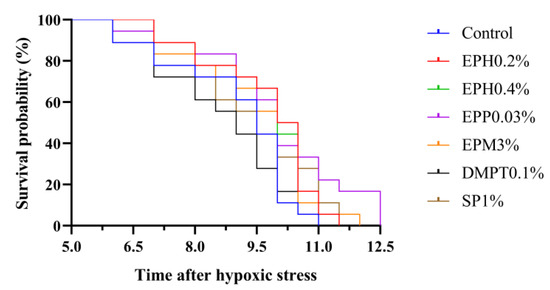
Figure 3.
Survival analysis of Pacific white shrimp after hypoxia test. Kaplan–Meier survival curve comparing overall survival estimation between treatments.
4. Discussion
E. prolifera is a green algal species, which contains various feeding attractants such as dimethyl-β-propiothetin (DMPT), dimethylsulfoniopropionate (DMSP), and betaine [15,23,24,25]. Asino [2] reported that E. prolifera showed a positive effect on the growth performance of the larger yellow croaker (Pseudosciaena crocea), which was attributed, to some extent, to increasing feed intake. Thus, in the present study, the cumulative feed intake per shrimp for 14 days after the beginning of the feeding trial and after feed intake of the entire feeding period were determined to evaluate the attractant effects of E. prolifera bio-products. Results of the cumulative feed intake per shrimp showed that statistical difference was observed 7 days after feeding. For aquatic animals, there were two main sensorial stimuli (olfaction and gustation) to distinguish the same type of feed [23]. Thus, it could be speculated that bio-products of E. prolifera mainly affected the gustation rather than the olfaction, thereby improving the feeding response of Pacific white shrimp [26]. In addition, compared with the control diet, only the EPH0.4%, EPM3%, and DMPT0.1% groups showed significantly higher feed intake than the control group. It indicated that 3% of E. prolifera meal and 0.4% of E. prolifera hydrolysate in diets could achieve similar attractant effects as 0.1% of DMPT on Pacific white shrimp. Meanwhile, there was no significant difference in feed intake between the EPP0.03% and control groups. Considering that shrimp fed the EPP0.03% diet presented a numerically higher feed intake than the control group, it did not mean that 0.03% of E. prolifera polysaccharide was not an attractant effect, but might be related to its volume in feed. The results of E. prolifera hydrolysate supported this hypothesis, where feeding intake of the control group was not significantly different from the 0.2% of E. prolifera hydrolysate group, but was numerically lower, and showed a significant decrease compared to the 0.4% of E. prolifera hydrolysate group. Taken together, consistent with the larger yellow croaker, the results of growth including final body weight, weight gain, and specific growth rate were the highest in the 0.4% of E. prolifera hydrolysate group, which further verified that bio-products of E. prolifera promoted growth of shrimp partly due to the increased feeding intake [2].
In rats, sulfated polysaccharides from E. prolifera exhibited the hypolipidaemic activity by regulating the lipid metabolism [27]. Oral administration of E. prolifera polysaccharide at a dosage of 100, 200 and 300 mg/kg body weight successfully reduced plasma total cholesterol, TG and LDL-C in a dose-dependent manner in mice fed high-fat diet [5]. Subsequent studies revealed the underlying mechanism that E. prolifera reduced cholesterol content by inhibiting sterol regulatory element binding protein-2 (SREBP-2) and 3-hydroxy-3-methylglutaryl-CoA reductase [28], while reducing TG content by regulating SREBP-1c and acetyl-CoA carboxylase [29]. Similar to results in mice, the present study showed that either E. prolifera hydrolysate, E. prolifera polysaccharide or E. prolifera meal decreased TG and LDL-C in plasma. It indicated that bio-products of E. prolifera obtained from the three types of the drying process or extraction procedure had similar hypolipidaemic effects on Pacific white shrimp as those in mice. In addition, oil red O staining of the hepatopancreas showed that the abundance of lipid droplets in shrimp fed the bio-products of E. prolifera, especially 0.4% of E. prolifera hydrolysate and 3% of E. prolifera meal, significantly decreased compared with the control group. Those results were supported by the hepatopancreas TG concentrations, which indicated that three types of E. prolifera bio-products could effectively reduce the accumulation of lipids in hepatopancreas [27].
It had been reported that E. prolifera had antioxidant, immunomodulatory and anti-inflammatory properties in many aquatic animals, such as banana shrimp (Fenneropenaeus merguiensis), red tilapia (Oreochromis mossambicus × Oreochromis niloticus) and crucian carp (Carassius auratus) [6,10,30]. Reactive oxygen species (ROS) are by-products of aerobic metabolism, which can cause oxidative damage by increasing their intracellular levels [31]. To avoid the damaging effects of naturally producing ROS, the living organisms including aquatic animals possess two distinct systems of antioxidant defense, the enzymatic antioxidant system such as SOD and GPx as well as the non-enzyme antioxidants such as GSH [32,33]. Many studies had demonstrated the involvement of antioxidant systems in repairing or preventing oxidative stress [33]. In our study, three types of E. prolifera bio-products increased the content of plasma GSH or the activity of plasma SOD, especially 0.03% of E. prolifera polysaccharide for GSH or 0.4% of E. prolifera hydrolysate and 3% of E. prolifera meal for SOD. It indicated that bio-products of E. prolifera based on different processing procedures could regulate some antioxidant enzymes and non-enzymatic antioxidant substances in Pacific white shrimp to some extent, which was able to eliminate the excess of superoxide or free radicals, thus resulting in improving the antioxidant capacity. The significantly lower level of plasma MDA (a product of lipid peroxidation) was observed in fish fed the EPM3% diet compared with the control diet, which supported the conclusion that E. prolifera bio-products could improve antioxidant defense. As regards to the non-specific immunity of shrimp, most studies have evaluated the immunity by measuring the activities of immune-related enzymes in plasma, such as ACP, AKP, GPT GOP, LZM and PO [34,35,36]. In the present study, however, the changes of most immune-related enzyme activities in the plasma of shrimp fed E. prolifera bio-products were not found to improve the immunity of shrimp compared with the control group. Only the activity of PO in shrimp fed 0.2% of E. prolifera hydrolysate was significantly higher than that of the control group. But, given that it had been well established that polysaccharides from E. prolifera could enhance immunity [7,10], the results of our study could not negate this conclusion solely on the basis of the results of immune-related enzyme activities in plasma. Therefore, in future studies, a pathogen challenge test needs to be conducted to confirm whether polysaccharides from E. prolifera could improve the immunity of Pacific white shrimp. In addition, in this study, the inflammatory status of shrimp was evaluated by analyzing the expression of a tumor necrosis factor-α (tnf-α) in the hepatopancreas. tnf-α is a typical pro-inflammatory cytokine produced by immune cells [37], and previous studies have found that polysaccharides from Atractylodis macrocephalae (Chinese herbal medicine) could decrease its expression in the hepatopancreas of Pacific white shrimp [38]. In broilers, heat stress caused a significant up-regulation of tnf-α expression, indicating that it induced inflammatory responses. However, polysaccharides from E. prolifera effectively decreased the level of tnf-α expression, which demonstrated that it might play an important role in suppressing inflammatory responses [11]. In our study, although it could not be confirmed whether inflammation occurred in the control group during the feeding trial, combined with the above studies, it partially indicated that the down-regulation of tnf-α expression by E. prolifera bio-products (EPH0.2%, EPH0.4% and EPM3%) could reduce the risk of inflammation in shrimp.
Apart from the lipid metabolism, antioxidant, and immune effects, the stress resistance of E. prolifera bio-products was evaluated in this study. Polysaccharides from seaweed could improve survival rate of aquatic animals under various stress, such as air exposure, ammonia stress and heat stress [12,13,14]. Survival rate was used as an important index for comprehensive evaluation of resistance to stress because its challenge tests reflected non-specific immune and anti-oxidation functions of the organisms [39]. In the present study, based on Kaplan–Meier survival curve, survival probability in shrimp fed E. prolifera bio-products (EPH0.2%: 89%; EPH0.4%: 89%; EPP0.03%: 89%; EPM3%: 83%) in 0.6 mg/L at 7 h after hypoxic stress was higher than that of the control diet (78%), and this trend persisted until the dissolved oxygen reached 0.2 mg/L at 10.5 h after hypoxic stress. It indicated that E. prolifera bio-products could improve the resistance of shrimp to hypoxic stress.
5. Conclusions
In conclusion, the amounts of E. prolifera bio-products added to the feed, from E. prolifera meal (3%), E. prolifera hydrolysate (0.2–0.4%) to E. prolifera polysaccharide (0.03%), was reduced by almost orders of magnitude. However, there were no distinct differences in biological activities of E. prolifera between treatments, including the attractant effect, hypolipidaemic activity, antioxidant, immunomodulatory, and stress resistance. It confirmed that bio-products of E. prolifera based on different processing procedures could be used to reduce the amount of E. prolifera in feed without compromising their biological functions.
Author Contributions
Conceptualization, Y.W., M.L., H.X. and Q.M.; methodology, Z.Z., L.Z., Q.G., Y.L., R.X. and M.P.; formal analysis, Z.Z.; resources, L.W., M.D. and P.W.; writing—original draft preparation, Z.Z.; writing—review and editing, Y.W.; funding acquisition, Y.W. and M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program of China (2022YFD2400202), Central Public-interest Scientific Institution Basal Research Fund, CAFS (2023TD52), and Taishan industry leading talent blue talent project (2020).
Institutional Review Board Statement
This study protocol was approved by Institutional Animal Care and Use Committee of the Yellow Sea Fisheries Research Institute (approval code 352022031, approved on 20 August 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lin, G.; Sun, F.; Wang, C.; Zhang, L.; Zhang, X. Assessment of the effect of Enteromorpha prolifera on bacterial community structures in aquaculture environment. PLoS ONE 2017, 12, e0179792. [Google Scholar] [CrossRef]
- Asino, H.; Ai, Q.; Mai, K. Evaluation of Enteromorpha prolifera as a feed component in large yellow croaker (Pseudosciaena crocea, Richardson, 1846) diets. Aquac. Res. 2011, 42, 525–533. [Google Scholar] [CrossRef]
- Aquino, J.I.; Serrano, A.E., Jr.; Corre, V.L., Jr. Dried Enteromorpha intestinalis could partially replace soybean meal in the diet of juvenile Oreochromis niloticus. ABAH Bioflux. 2014, 6, 95–101. [Google Scholar]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, H.; Wang, S.; Wen, S.; Qin, S. Hypolipidemic and antioxidant properties of a polysaccharide fraction from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 58, 186–189. [Google Scholar] [CrossRef]
- Zhou, Z.; Pan, S.; Wu, S. Modulation of the growth performance, body composition and nonspecific immunity of crucian carp Carassius auratus upon Enteromorpha prolifera polysaccharide. Int. J. Biol. Macromol. 2020, 147, 29–33. [Google Scholar] [CrossRef]
- Wassie, T.; Lu, Z.; Duan, X.; Xie, C.; Gebeyew, K.; Yumei, Z.; Yin, Y.; Wu, X. Dietary Enteromorpha polysaccharide enhances intestinal immune response, integrity, and caecal microbial activity of broiler chickens. Front. Nutr. 2021, 8, 783819. [Google Scholar] [CrossRef]
- Wassie, T.; Niu, K.; Xie, C.; Wang, H.; Xin, W. Extraction techniques, biological activities and health benefits of marine algae Enteromorpha prolifera polysaccharide. Front. Nutr. 2021, 8, 747928. [Google Scholar] [CrossRef]
- Wassie, T.; Cheng, B.; Zhou, T.; Gao, L.; Lu, Z.; Wang, J.; Mulu, B.; Taye, M.; Wu, X. Enteromorpha polysaccharide and yeast glycoprotein mixture improves growth, antioxidant activity, serum lipid profile and regulates lipid metabolism in broiler chickens. Poult. Sci. 2022, 101, 102064. [Google Scholar] [CrossRef]
- Liu, W.C.; Zhou, S.H.; Balasubramanian, B.; Zeng, F.Y.; Sun, C.B.; Pang, H.Y. Dietary seaweed (Enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish Shellfish Immunol. 2020, 104, 202–212. [Google Scholar] [CrossRef]
- Liu, W.C.; Ou, B.H.; Liang, Z.L.; Zhang, R.; Zhao, Z.H. Algae-derived polysaccharides supplementation ameliorates heat stress-induced impairment of bursa of Fabricius via modulating NF-κB signaling pathway in broilers. Poult. Sci. 2021, 100, 101139. [Google Scholar] [CrossRef]
- Sun, L.; Guo, Y.; Ma, S.; Fan, W.; Liu, Y.; Liu, D.; Zhang, Y.; Zhang, W.; Mai, K. Replacement of dietary kelp meal with three macroalgae sources on the growth performance, immune responses and anti-stress capacity of abalone Haliotis discus hannai. J. Appl. Phycol. 2021, 33, 4051–4065. [Google Scholar] [CrossRef]
- Wassef, E.A.; El-Sayed, A.F.M.; Sakr, E.M. Pterocladia (Rhodophyta) and Ulva (Chlorophyta) as feed supplements for European seabass, Dicentrarchus labrax L., fry. J. Appl. Phycol. 2013, 25, 1369–1376. [Google Scholar] [CrossRef]
- Zuo, Z.; Wang, S.; Wang, Q.; Wang, D.; Wu, Q.; Xie, S.; Zou, J. Effects of partial replacement of dietary flour meal with seaweed polysaccharides on the resistance to ammonia stress in the intestine of hybrid snakehead (Channa maculatus♀ × Channa argus♂). Fish Shellfish Immunol. 2022, 127, 271–279. [Google Scholar] [CrossRef]
- Han, L.; Fan, X.; Yan, X. The betaines from Chinese seaweeds. Chin. J. Oceanol. Limnol. 2002, 20, 97–100. [Google Scholar]
- Li, E.; Xu, C.; Wang, X.; Wang, S.; Zhao, Q.; Zhang, M.; Qin, J.; Chen, L. Gut microbiota and its modulation for healthy farming of Pacific white shrimp Litopenaeus vannamei. Rev. Fish. Sci. Aquac. 2018, 26, 381–399. [Google Scholar] [CrossRef]
- Cao, M.; Wang, X.Q.; Yan, B.L. The Nutritional Control of Litopenaeus vannamei Culture Pollution. Adv. Mat. Res. 2012, 554, 1439–1442. [Google Scholar] [CrossRef]
- Rajauria, G. Seaweeds: A sustainable feed source for livestock and aquaculture. In Seaweed Sustainability, Food and Non-Food Applications; Elsevier Inc., University College Dublin, Lyons Research Farm, Newcastle, Co.: Dublin, Ireland, 2015; pp. 89–420. [Google Scholar]
- Zhu, T.; Morais, S.; Luo, J.; Jin, M.; Lu, Y.; Le, Y.; Zhou, Q. Functional palatability enhancer improved growth, intestinal morphology, and hepatopancreas protease activity, replacing squid paste in white shrimp, Litopenaeus vannamei, diets. J. World Aquac. Soc. 2019, 50, 1064–1077. [Google Scholar] [CrossRef]
- Li, J.; Tan, B.; Mai, K. Dietary probiotic Bacillus OJ and isomaltooligosaccharides influence the intestine microbial populations, immune responses and resistance to white spot syndrome virus in shrimp (Litopenaeus vannamei). Aquaculture 2009, 291, 35–40. [Google Scholar] [CrossRef]
- AOAC International. Official Method of Analysis, 18th ed.; AOAC: Gaithersburg, ML, USA, 2005. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hancz, C. Feed efficiency, nutrient sensing and feeding stimulation in aquaculture: A review. Acta Agrar. Kaposváriensis 2020, 24, 35–54. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z.; Roje, M.; Kuś, P.M.; Jokić, S.; Čož-Rakovac, R. Phytochemical study of the headspace volatile organic compounds of fresh algae and seagrass from the Adriatic Sea (single point collection). PLoS ONE 2018, 13, e0196462. [Google Scholar] [CrossRef]
- Kawabe, T.; Nagaoka, T.; Nagahama, G.; Morita, H.; Ohbayashi, A. Generation of Dimethyl Sulfide from dimethyl-β-propiothetin in an extract of green alga Monostroma nitidum and its retention by cyclodextrin. Agric. Bioi. Chem. 1989, 53, 2587–2591. [Google Scholar]
- Morais, S. The physiology of taste in fish: Potential implications for feeding stimulation and gut chemical sensing. Rev. Fish. Sci. Aquac. 2017, 25, 133–149. [Google Scholar] [CrossRef]
- Teng, Z.; Qian, L.; Zhou, Y. Hypolipidemic activity of the polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 62, 254–256. [Google Scholar] [CrossRef]
- Ren, R.; Gong, J.; Zhao, Y.; Zhuang, X.; Ye, Y.; Lin, W. Sulfated polysaccharides from Enteromorpha prolifera suppress SREBP-2 and HMG-CoA reductase expression and attenuate non-alcoholic fatty liver disease induced by a high-fat diet. Food Funct. 2017, 8, 1899–1904. [Google Scholar] [CrossRef]
- Ren, R.; Gong, J.; Zhao, Y.; Zhuang, X.; Ye, Y.; Huang, F.; Lin, W. Sulfated polysaccharide from Enteromorpha prolifera suppresses SREBP-1c and ACC expression to lower serum triglycerides in high-fat diet-induced hyperlipidaemic rats. J. Funct. Foods 2018, 40, 722–728. [Google Scholar] [CrossRef]
- Yang, H.; Li, Z.B.; Chen, Q.; Li, W.J.; Sun, Y.Z.; Lu, J. Effect of fermented Enteromopha prolifera on the growth performance, digestive enzyme activities and serum non-specific immunity of red tilapia (Oreochromis mossambicus × Oreochromis niloticus). Aquac. Res. 2016, 47, 4024–4031. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Gu, Z.; Jia, R.; He, Q.; Cao, L.; Du, J.; Feng, W.; Jeney, G.; Xu, P.; Yin, G. Alteration of lipid metabolism, autophagy, apoptosis and immune response in the liver of common carp (Cyprinus carpio) after long-term exposure to bisphenol A. Ecotoxicol. Environ. Saf. 2021, 211, 111923. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative stress and antioxidant defense in fish: The implications of probiotic, prebiotic, and synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Ayiku, S.; Shen, J.F.; Tan, B.P.; Dong, X.H.; Liu, H.Y. Effects of dietary yeast culture on shrimp growth, immune response, intestinal health and disease resistance against Vibrio harveyi. Fish Shellfish Immunol. 2020, 102, 286–295. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Yuan, X.; Wang, L.; Lu, K.; Song, K.; Zhang, C. Chitooligosaccharide supplementation in low-fish meal diets for Pacific white shrimp (Litopenaeus vannamei): Effects on growth, innate immunity, gut histology, and immune-related genes expression. Fish Shellfish Immunol. 2018, 80, 405–415. [Google Scholar] [CrossRef]
- Wu, J.; Tian, S.; Luo, K.; Zhang, Y.; Pan, H.; Zhang, W.; Mai, K. Dietary recombinant human lysozyme improves the growth, intestinal health, immunity and disease resistance of Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2022, 121, 39–52. [Google Scholar] [CrossRef]
- Mehaffey, E.; Majid, D.S. Tumor necrosis factor-α, kidney function, and hypertension. Am. J. Physiol. Renal Physiol. 2017, 313, F1005–F1008. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, X.; Jiao, L.; Tao, X.; Li, M.; Lu, J.; Zhou, Q. The regulation effect of Atractylodis macrocephalae polysaccharides on the growth performance, antioxidant capacity and immune function in Litopenaeus vannamei. Aquac. Rep. 2023, 31, 101641. [Google Scholar] [CrossRef]
- Ma, S.; Guo, Y.; Sun, L.; Fan, W.; Liu, Y.; Liu, D.; Mai, K. Over high or low dietary protein levels depressed the growth, TOR signaling, apoptosis, immune and anti-stress of abalone Haliotis discus hannai. Fish Shellfish Immunol. 2020, 106, 241–251. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).