Habitat Suitability of the Squid Sthenoteuthis oualaniensis in Northern Indian Ocean Based on Different Weights
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Source
2.2. Analysis Method
2.2.1. Calculation
2.2.2. Single-Factor Suitability Index (SI) Model
2.2.3. Habitat Suitability Index (HSI) Model
2.2.4. HSI Model Screening and Verification
3. Results and Analysis
3.1. SI Model
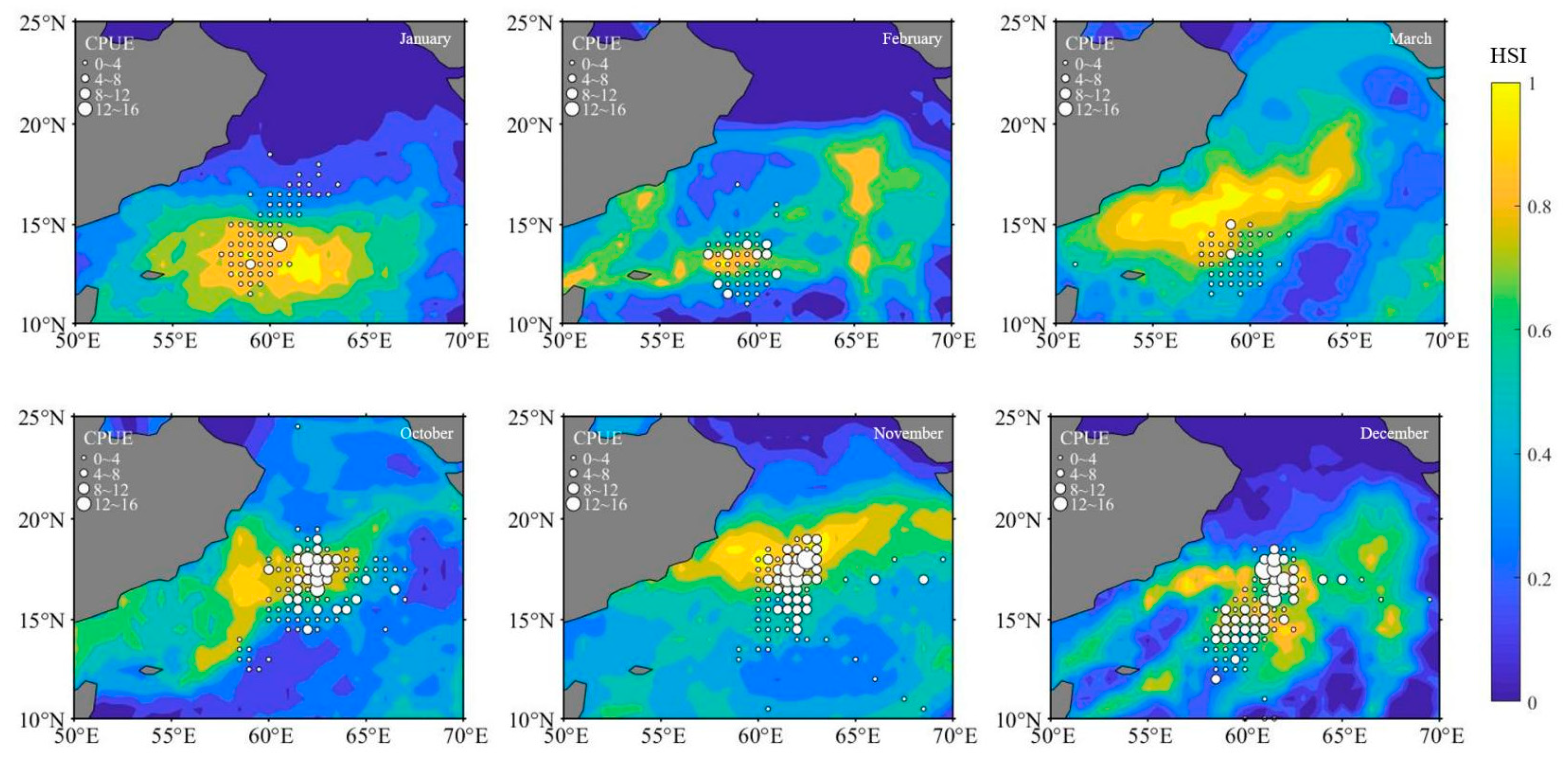
3.2. HSI Model Analysis
3.3. Model Verification and Screening Results
4. Discussion
4.1. Effects of Environmental Factors on the Spatial Distribution of the Habitat of S. oualaniensis
4.2. Analysis of Habitat Suitability Index Model of S. oualaniensis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, Z.Z. Biology of Oceanic Economic Cephalopod in the World; Shandong Science and Technology Press: Jinan, China, 1991; pp. 91–94. [Google Scholar]
- Chen, X.J.; Ye, X.C. Preliminary study on the relationship between fishing ground of Sthenoteuthis oualaniensis and environmental factors in northwestern Indian Ocean. J. Shanghai Fish. Univ. 2005, 14, 55–60. [Google Scholar] [CrossRef]
- Voss, G.L. Cephalopod resources of the world. FAO Fish Circ. 1973, 10, 75. [Google Scholar]
- Chen, X.J.; Liu, B.L.; Wang, Y.G. Cephalopods of the World; China Ocean Press: Beijing, China, 2009; pp. 312–442. [Google Scholar]
- Vecchione, M. Extraordinary abundance of squid paralarvae in the tropical eastern Pacific Ocean during El Niño of 1987. Fish. Bull. 1999, 97, 1025–1030. [Google Scholar]
- Sajikumar, K.K.; Ragesh, N.; Venkatesan, V.; Koya, K.P.; Sasikumar, G.; Kripa, V.; Mohamed, K.S. Morphological development and distribution of paralarvae and juveniles of purpleback flying squid Sthenoteuthis oualaniensis (Ommastrephidae), in South-eastern Arabian Sea. Vie Milieu-Life Environ. 2018, 68, 75–86. [Google Scholar]
- Chen, X.J.; Shao, F. Study on the Resource Characteristics of Sthenoteuthis oualaniensis and Their Relationships with the Sea Conditions in the High Sea of the Northwestern Indian Ocean. Period. Ocean Univ. China 2006, 36, 611–616. [Google Scholar]
- Schott, F.A.; McCreary, J.P., Jr. The monsoon circulation of the Indian Ocean. Prog. Oceanogr. 2001, 51, 1–123. [Google Scholar] [CrossRef]
- Schott, F.A.; Xie, S.P.; Mccreary, J.P. Indian Ocean circulation and climate variability. Rev. Geophys. 2009, 47, 549. [Google Scholar] [CrossRef]
- Roxy, M.K.; Ritika, K.; Terray, P.; Murtugudde, R.; Ashok, K.; Goswami, B.N. Drying of Indian subcontinent by rapid Indian Ocean warming and a weakening land sea thermal gradient. Nat. Commun. 2015, 6, 7423. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhang, H.; Fang, Z.; Chen, X. Preliminary standardization of Sthenoteuthis oualaniensis in Northern Indian Ocean. Trans. Oceanol. Limnol. 2022, 44, 89–97. [Google Scholar]
- US Fish and Wildlife Service. 101ESM Habitat as a Base for Environmental Assessment; Division of Ecological: Washington, DC, USA, 1980.
- Yu, W.; Guo, A.; Zhang, Y.; Chen, X.; Qian, W.; Li, Y. Climate-induced habitat suitability variations of chub mackerel Scomber japonicus in the East China Sea. Fish. Res. 2018, 207, 63–73. [Google Scholar] [CrossRef]
- Feng, Z.P.; Yu, W.; Chen, X.J.; Liu, B.L.; Zhang, Z. Analysis of fishing ground of jumbo flying squid Dosidicus gigas in the southeast Pacific Ocean off Peru based on weighting-based habitat suitability index model. J. Shanghai Ocean Univ. 2021, 29, 878–888. [Google Scholar]
- Gong, C.X.; Chen, X.J.; Gao, F.; Guan, W.J.; Lei, L. Review on habitat suitability index in fishery science. J. Shanghai Ocean Univ. 2011, 20, 260–269. [Google Scholar]
- Liu, H.W.; Yu, W.; Chen, X.J.; Zhu, W.B. Construction of habitat suitability index model for Illex argentinus based on vertical water temperature at different depths. J. Dalian Ocean Univ. 2021, 36, 1035–1043. [Google Scholar]
- Wang, Y.F.; Chen, X.J. Comparisons of habitat suitability index models of skipjack tuna in the Western and Central Pacific Ocean. J. Shanghai Ocean Univ. 2017, 26, 743–750. [Google Scholar]
- Mohri, M. Seasonal changes in Bigeye tuna fishing areas in relation to the oceanographic parameters in the Indian Ocean. J. Natl. Fish. Univ. 1999, 47, 43–54. [Google Scholar]
- Fang, X.Y.; Chen, X.J.; Ding, Q. Optimization Fishing Ground Prediction Models of Dosidicus gigas in the High Sea off Chile Based on Habitat Suitability Index. J. Guangdong Ocean Univ. 2014, 34, 67–73. [Google Scholar]
- Yu, W.; Yi, Q.; Chen, X.; Chen, Y. Modelling the effects of climate variability on habitat suitability of jumbo flying squid, Dosidicus gigas, in the Southeast Pacific Ocean off Peru. ICES J. Mar. Sci. 2016, 73, 239–249. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X.J.; Zhang, Y. Seasonal habitat patterns of jumbo flying squid Dosidicus gigas off Peruvian waters. J. Mar. Syst. 2019, 194, 41–51. [Google Scholar] [CrossRef]
- Ichii, T.; Mahapatra, K.; Sakai, M.; Wakabayashi, T.; Okamura, H.; Igarashi, H.; Inagake, D.; Okada, Y. Changes in abundance of the neon flying squid Ommastrephes bartramii in relation to climate change in the central North Pacific Ocean. Mar. Ecol. Prog. Ser. 2011, 441, 151–164. [Google Scholar] [CrossRef]
- Alabia, I.D.; Saitoh, S.I.; Igarashi, H.; Ishikawa, Y.; Usui, N.; Kamachi, M.; Awaji, T.; Seito, M. Future projected impacts of ocean warming to potential squid habitat in western and central North Pacific. ICES J. Mar. Sci. 2016, 73, 1343–1356. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X.J. Analysis on Habitat Suitability Index of Sthenoteuthis oualaniensis in Northwestern Indian Ocean from September to October. J. Guangdong Ocean Univ. 2012, 32, 74–80. [Google Scholar]
- Hood, R.R.; Beckley, L.E.; Wiggert, J.D. Biogeochemical and Ecological Impacts of Boundary Currents in the Indian Ocean. Prog. Oceanogr. 2017, 156, 290–325. [Google Scholar] [CrossRef]
- Wiggert, J.D.; Jones, B.H.; Dickey, T.D.; Brink, K.H.; Weller, R.A.; Marra, J.; Codispoti, L.A. The northeast monsoon’s impact on mixing, phytoplankton biomass and nutrient cycling in the Arabian Sea. Deep Sea Res. Part II Top. Stud. Oceanogr. 2000, 47, 1353–1385. [Google Scholar] [CrossRef]
- Hastenrath, S. Regional Circulation Systems. Clim. Dyn. Trop. 1991, 8, 114–218. [Google Scholar]
- Sims, D.W.; Genner, M.J.; Southward, A.J.; Hawkins, S.J. Timing of squid migration reflects North Atlantic climate variability. Proc. R. Soc. B Biol. Sci. 2001, 268, 2607–2611. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Alfonso, A.M.; Ganzedo, U.; Trujillo-Santana, A.; Polanco, J.; del Pino, A.S.; Ibarra-Berastegi, G.; Castro-Hernández, J.J. The role of climatic variability on the short term fluctuations of octopus captures at the Canary Islands. Fish. Res. 2010, 102, 258–265. [Google Scholar] [CrossRef]
- Zhou, W.F.; Xu, H.Y.; Li, A.Z.; Cui, X.S.; Chen, G.B. Comparison of habitat suitability index models for purpleback flying squid (Sthenoteuthis oualaniensis) in the open South China sea. Appl. Ecol. Environ. Res. 2019, 17, 4903–4913. [Google Scholar] [CrossRef]
- Trotsenko, B.G.; Pinchukov, M.A. Mesoscale distribution features of the purpleblack squid Sthenoteuthis Ouslsniensis with reference to the structure of the upper quasi homogeneous layer in the western India Ocean. Oceanology 1994, 34, 380–385. [Google Scholar]
- Yang, X.M.; Chen, X.J.; Zhou, Y.Q.; Tian, S.Q. A marine remote sensing-based preliminary analysis on the fishing ground of purple flying squid Sthenoteuthis oualaniensis in the northwest Indian Ocean. J. Shanghai Fish. Univ. 2006, 30, 669–675. [Google Scholar]
- Qian, M.; Gong, J.; Fan, J.; Yu, W.; Chen, X.; Qian, W. Variations in the abundance and spatial distribution of Ommastrephes bartramii in the Northwest Pacific Ocean based on photosynthetic active radiation. Acta Oceanol. Sin. 2020, 42, 44–53. [Google Scholar]
- Lu, H.J.; Ou, Y.Z.; He, J.R.; Zhao, M.L.; Chen, Z.Y.; Chen, X.J. Age, growth and population structure analyses of the purpleback flying squid Sthenoteuthis oualaniensis in the Northwest Indian Ocean by beak microstructure. J. Mar. Sci. Eng. 2022, 10, 1094. [Google Scholar] [CrossRef]
- Jeena, N.S.; Sajikumar, K.K.; Rahuman, S.; Ragesh, N.; Koya, K.S.; Chinnadurai, S.; Sasikumar, G.; Mohamed, K.S. Insights into the divergent evolution of the oceanic squid Sthenoteuthis oualaniensis (Cephalopoda: Ommastrephidae) from the Indian Ocean. Integr. Zool. 2023, 18, 924–948. [Google Scholar] [CrossRef] [PubMed]

| Scenarios | kSST | kWS | kPAR |
|---|---|---|---|
| Case1 | 0.5 | 0.25 | 0.25 |
| Case2 | 0.333 | 0.333 | 0.333 |
| Case3 | 0.25 | 0.5 | 0.25 |
| Case4 | 0.25 | 0.25 | 0.5 |
| Case5 | 0.1 | 0.1 | 0.8 |
| Case6 | 0.1 | 0.8 | 0.1 |
| Case7 | 0 | 0 | 1 |
| Case8 | 0 | 1 | 0 |
| Environmental Factors | Month | SI Model | R2 | p |
|---|---|---|---|---|
| SST | January | SISST = exp [−4.012 × (XSST − 25.877)2] | 0.981 | 0.006 |
| February | SISST = exp [−7.845 × (XSST − 25.498)2] | 0.982 | 0.001 | |
| March | SISST = exp [−11.417 × (XSST − 26.756)2] | 0.999 | 0.008 | |
| October | SISST = exp [−4.696 × (XSST − 28.089)2] | 0.997 | 0.001 | |
| November | SISST = exp [−7.814 × (XSST − 27.889)2] | 0.760 | 0.018 | |
| December | SISST = exp [−6.75 × (XSST − 26.459)2] | 0.925 | 0.001 | |
| WS | January | SIWS = exp [−0.919 × (XWS − 6.926)2] | 0.747 | 0.001 |
| February | SIWS = exp [−14.23 × (XWS − 5.229)2] | 0.549 | 0.005 | |
| March | SIWS = exp [−1.896 × (XWS − 4.035)2] | 0.689 | 0.031 | |
| October | SIWS = exp [−9.212 × (XWS − 3.264)2] | 0.955 | 0.001 | |
| November | SIWS = exp [−1.029 × (XWS − 4.987)2] | 0.623 | 0.017 | |
| December | SIWS = exp [−4.895 × (XWS − 7.45)2] | 0.826 | 0.016 | |
| PAR | January | SIPAR = exp [−0.564 × (XPAR − 43.059)2] | 0.742 | 0.003 |
| February | SIPAR = exp [−0.501 × (XPAR − 48.204)2] | 0.536 | 0.009 | |
| March | SIPAR = exp [−1.061 × (XPAR − 54.238)2] | 0.814 | 0.001 | |
| October | SIPAR = exp [−6.686 × (XPAR − 47.041)2] | 0.756 | 0.001 | |
| November | SIPAR = exp [−0.547 × (XPAR − 41.682)2] | 0.733 | 0.001 | |
| December | SIPAR = exp [−1.552 × (XPAR − 39.563)2] | 0.613 | 0.001 |
| Month | HSI | Case1 | Case2 | Case3 | Case4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Catch/% | Effort/% | Catch/% | Effort/% | Catch/% | Effort/% | Catch/% | Effort/% | ||
| January | [0–0.2] | 0.21 | 1.73 | 0.16 | 1.64 | 0.15 | 1.51 | 0.18 | 1.68 |
| (0.2–0.6) | 36.98 | 39.76 | 24.17 | 28.86 | 17.97 | 22.21 | 37.46 | 36.97 | |
| [0.6–1] | 62.82 | 58.51 | 75.67 | 69.50 | 81.88 | 76.29 | 62.36 | 61.35 | |
| February | [0–0.2] | 2.65 | 1.74 | 2.76 | 1.39 | 3.24 | 2.44 | 0.60 | 0.60 |
| (0.2–0.6) | 29.97 | 23.28 | 42.50 | 44.78 | 38.81 | 46.24 | 47.46 | 38.36 | |
| [0.6–1] | 67.38 | 74.98 | 54.74 | 53.83 | 57.95 | 51.32 | 51.94 | 61.04 | |
| March | [0–0.2] | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| (0.2–0.6) | 29.85 | 25.16 | 11.27 | 13.57 | 8.53 | 14.50 | 36.51 | 32.96 | |
| [0.6–1] | 70.15 | 74.84 | 88.73 | 86.43 | 91.47 | 85.50 | 63.49 | 67.04 | |
| October | [0–0.2] | 3.56 | 3.38 | 1.84 | 2.06 | 6.77 | 7.60 | 7.85 | 8.74 |
| (0.2–0.6) | 38.91 | 43.70 | 40.62 | 45.02 | 43.33 | 41.68 | 46.20 | 51.95 | |
| [0.6–1] | 57.53 | 52.92 | 57.53 | 52.92 | 49.90 | 50.72 | 45.95 | 39.31 | |
| November | [0–0.2] | 5.69 | 10.68 | 0.76 | 2.73 | 0.85 | 3.13 | 0.85 | 3.09 |
| (0.2–0.6) | 19.66 | 20.17 | 35.93 | 40.22 | 36.05 | 40.22 | 23.84 | 30.55 | |
| [0.6–1] | 74.64 | 69.16 | 63.31 | 57.05 | 63.10 | 56.65 | 75.26 | 66.32 | |
| December | [0–0.2] | 5.03 | 5.75 | 2.85 | 3.77 | 4.28 | 5.40 | 3.75 | 4.39 |
| (0.2–0.6) | 55.44 | 57.08 | 55.69 | 58.01 | 44.04 | 46.85 | 59.82 | 59.68 | |
| [0.6–1] | 39.53 | 37.17 | 41.46 | 38.22 | 51.68 | 47.74 | 36.43 | 35.93 | |
| Month | HSI | Case5 | Case6 | Case7 | Case8 | ||||
| Catch/% | Effort/% | Catch/% | Effort/% | Catch/% | Effort/% | Catch/% | Effort/% | ||
| January | [0–0.2] | 6.20 | 9.04 | 1.14 | 5.59 | 10.28 | 12.06 | 1.95 | 6.91 |
| (0.2–0.6) | 32.69 | 32.89 | 18.96 | 22.70 | 33.57 | 30.85 | 8.71 | 13.74 | |
| [0.6–1] | 61.10 | 58.07 | 79.90 | 71.72 | 56.16 | 57.09 | 89.35 | 79.34 | |
| February | [0–0.2] | 1.83 | 0.90 | 37.82 | 46.87 | 17.47 | 11.89 | 42.05 | 53.68 |
| (0.2–0.6) | 42.01 | 28.51 | 4.23 | 6.82 | 22.97 | 15.22 | 0.00 | 0.00 | |
| [0.6–1] | 56.16 | 70.60 | 57.95 | 46.32 | 59.56 | 72.89 | 57.95 | 46.32 | |
| March | [0–0.2] | 7.84 | 6.69 | 0.00 | 0.00 | 24.38 | 15.56 | 0.00 | 0.00 |
| (0.2–0.6) | 38.69 | 35.55 | 9.24 | 19.44 | 29.18 | 36.93 | 9.20 | 19.25 | |
| [0.6–1] | 53.46 | 57.76 | 90.76 | 80.56 | 46.44 | 47.51 | 90.80 | 80.75 | |
| October | [0–0.2] | 32.71 | 41.85 | 14.69 | 19.72 | 36.65 | 45.76 | 19.05 | 25.73 |
| (0.2–0.6) | 17.23 | 14.54 | 31.57 | 25.65 | 13.30 | 10.63 | 27.20 | 19.63 | |
| [0.6–1] | 50.05 | 43.61 | 53.74 | 54.63 | 50.05 | 43.61 | 53.75 | 54.64 | |
| November | [0–0.2] | 1.21 | 3.70 | 1.43 | 4.10 | 13.63 | 17.76 | 1.50 | 4.92 |
| (0.2–0.6) | 34.03 | 34.04 | 10.58 | 15.92 | 21.12 | 19.23 | 10.44 | 14.81 | |
| [0.6–1] | 64.76 | 62.26 | 87.99 | 79.98 | 65.25 | 63.01 | 88.06 | 80.27 | |
| December | [0–0.2] | 34.76 | 31.45 | 27.22 | 29.28 | 41.91 | 40.71 | 26.84 | 28.77 |
| (0.2–0.6) | 23.57 | 24.38 | 8.28 | 11.59 | 7.16 | 7.43 | 7.82 | 11.63 | |
| [0.6–1] | 41.67 | 44.17 | 64.50 | 59.14 | 50.93 | 51.87 | 65.34 | 59.6 | |
| Month | HSI | CPUE (t/time) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Case1 | Case2 | Case3 | Case4 | Case5 | Case6 | Case7 | Case8 | ||
| January | [0–0.2] | 0.52 | 0.26 | 0.25 | 0.40 | 1.58 | 0.94 | 1.75 | 0.99 |
| (0.2–0.6) | 2.11 | 1.88 | 1.82 | 2.00 | 1.98 | 2.05 | 2.09 | 1.95 | |
| [0.6–1] | 2.82 | 3.06 | 2.93 | 2.99 | 3.08 | 2.87 | 2.82 | 2.94 | |
| February | [0–0.2] | 3.18 | 4.42 | 2.23 | 2.57 | 6.59 | 1.65 | 5.12 | 1.90 |
| (0.2–0.6) | 2.66 | 2.16 | 1.87 | 2.46 | 4.07 | 3.69 | 4.17 | 0.00 | |
| [0.6–1] | 2.29 | 2.86 | 3.79 | 2.39 | 1.68 | 3.79 | 1.78 | 3.79 | |
| March | [0–0.2] | 0.00 | 0.00 | 0.00 | 0.00 | 2.01 | 0.00 | 2.61 | 0.00 |
| (0.2–0.6) | 2.52 | 2.07 | 1.93 | 2.23 | 2.37 | 1.61 | 1.98 | 1.70 | |
| [0.6–1] | 2.04 | 2.23 | 2.28 | 2.17 | 2.07 | 2.32 | 2.18 | 2.29 | |
| October | [0–0.2] | 5.46 | 5.20 | 5.36 | 5.43 | 5.23 | 5.29 | 5.28 | 5.33 |
| (0.2–0.6) | 5.38 | 5.42 | 5.50 | 5.46 | 6.52 | 5.97 | 6.72 | 6.00 | |
| [0.6–1] | 7.40 | 7.40 | 6.74 | 7.31 | 6.91 | 6.28 | 6.91 | 6.28 | |
| November | [0–0.2] | 4.10 | 2.89 | 2.68 | 2.33 | 3.92 | 3.71 | 4.97 | 3.25 |
| (0.2–0.6) | 5.78 | 5.86 | 5.87 | 5.52 | 6.21 | 5.45 | 6.70 | 5.91 | |
| [0.6–1] | 7.92 | 7.67 | 7.97 | 7.81 | 7.23 | 7.89 | 6.97 | 7.81 | |
| December | [0–0.2] | 5.17 | 4.81 | 4.88 | 5.10 | 6.51 | 5.39 | 6.58 | 5.36 |
| (0.2–0.6) | 5.94 | 5.75 | 5.65 | 5.93 | 5.81 | 5.51 | 4.80 | 4.87 | |
| [0.6–1] | 6.16 | 7.13 | 7.14 | 6.16 | 5.10 | 6.65 | 5.25 | 6.78 | |
| Month | HSI | Catch/% | Effort/% | CPUE | Prediction Accuracy |
|---|---|---|---|---|---|
| [0–0.2] | 0.02 | 0.02 | 4.03 | 100.00% | |
| January | (0.2–0.6) | 27.41 | 18.01 | 5.12 | |
| [0.6–1] | 72.57 | 81.97 | 5.83 | ||
| [0–0.2] | 6.15 | 6.97 | 3.83 | 56.82% | |
| February | (0.2–0.6) | 28.15 | 28.54 | 4.01 | |
| [0.6–1] | 65.70 | 64.49 | 5.40 | ||
| [0–0.2] | 2.12 | 3.71 | 4.34 | 93.51% | |
| March | (0.2–0.6) | 8.83 | 8.47 | 4.84 | |
| [0.6–1] | 89.05 | 87.82 | 4.86 | ||
| [0–0.2] | 0.31 | 0.32 | 3.86 | 80.80% | |
| October | (0.2–0.6) | 13.45 | 18.52 | 4.12 | |
| [0.6–1] | 86.24 | 81.16 | 4.54 | ||
| [0–0.2] | 0.44 | 0.57 | 3.13 | 83.65% | |
| November | (0.2–0.6) | 15.39 | 20.86 | 3.60 | |
| [0.6–1] | 84.17 | 78.57 | 4.42 | ||
| [0–0.2] | 1.76 | 3.36 | 3.53 | 100.00% | |
| December | (0.2–0.6) | 34.24 | 34.72 | 4.45 | |
| [0.6–1] | 64.00 | 61.92 | 5.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Wen, L.; Liu, S.; Zhang, H.; Fang, Z. Habitat Suitability of the Squid Sthenoteuthis oualaniensis in Northern Indian Ocean Based on Different Weights. Fishes 2024, 9, 107. https://doi.org/10.3390/fishes9030107
Yu J, Wen L, Liu S, Zhang H, Fang Z. Habitat Suitability of the Squid Sthenoteuthis oualaniensis in Northern Indian Ocean Based on Different Weights. Fishes. 2024; 9(3):107. https://doi.org/10.3390/fishes9030107
Chicago/Turabian StyleYu, Jun, Lihong Wen, Siyuan Liu, Heng Zhang, and Zhou Fang. 2024. "Habitat Suitability of the Squid Sthenoteuthis oualaniensis in Northern Indian Ocean Based on Different Weights" Fishes 9, no. 3: 107. https://doi.org/10.3390/fishes9030107
APA StyleYu, J., Wen, L., Liu, S., Zhang, H., & Fang, Z. (2024). Habitat Suitability of the Squid Sthenoteuthis oualaniensis in Northern Indian Ocean Based on Different Weights. Fishes, 9(3), 107. https://doi.org/10.3390/fishes9030107







