Abstract
The research aimed to analyze the influences of adding marine green algae Chaetomorpha aerea to the diet of guppy fish (Poecillia reticulate) on growth, immunological responses in skin mucus, total carotenoid content, and sexual characteristics. A total of 450 fish, with a mean body weight of 0.19 ± 0.1 g and 30 fish per tank (triplicate), were randomly fed into 15 experimental tanks, each containing 50 L. Five different diets with 0, 1, 2, 4, 8, and 10% of C. aerea g/kg diets were fed to P. reticulate for 30 days. After 30 days, growth, immunological responses in skin mucus, total carotenoid content, and sexual characteristics were investigated. The results observed that the feed conversion rate and fry output were significantly (p > 0.05) decreased in experimental groups compared to the control group. The results revealed that the dietary inclusion of C. aerea algal significantly increased (p < 0.05) in mucosal immunological parameters containing lysozyme activity, myeloperoxidase activity, total immunoglobulins, and alternative complement activity, which were the highest in the group with 4% of C. aerea g/kg. Additionally, lateral skin and the caudal fin of fish had higher total carotenoid levels from the dietary C. aerea algae diet than the control group, which were the highest in the groups with 4%. Among them, 4 and 8% of C. aerea g/kg diet resulted in better growth performance and feed conversion ratio. Thus, the study suggested that 4% of C. aerea g/kg diet has enrichment of immunity, total carotenoid concentrations, and skin mucus immunity of P. reticulate.
Key Contribution:
Marine macro algae, C. aerea, can be incorporated into fish diets as safe functional feeds. C. aerea enhanced the growth and skin mucus immune response of guppy fish. C. aerea regulated the sex steroid hormones and total carotenoid content levels. C. aerea macroalgae-enriched diet exerts an apparent immunomodulatory effect in guppy fish and thus can be suggested as a potential health-promoting fish feed supplement.
1. Introduction
Ornamental fish species are produced by an aquaculture industry segment that is expanding substantially for aquarium hobbyists, which lessens the strain on declining wild stock capture rates and significantly boosts the industry’s economic growth [1]. The most important tropical species traded globally is the ornamental guppy fish, Poecillia reticulata, a freshwater fish native to Central America, the Caribbean, and South America. It is widespread among aquarists because of its low maintenance requirements and its appealing and variegated colors [2]. Due to water scarcity in certain regions of the world, the requirement for supervised culture conditions, including stable temperatures for tropical ornamental fish and the wish to lower the chance of disease introduction, the production of ornamental fish within closed intensive culture conditions is becoming widespread. The quality of the feed has a considerable effect on growth rate, reproductive success, and overall health since fish are raised in vast numbers on artificial feed. According to Lim et al. [3], modern techniques for ornamental fish packaging are distinguished by abnormally high fish-carrying ratios and a buildup of metabolic by-products in the transport environment after transportation. The latter is essential since ornamental fish are exported and need to be able to tolerate protracted air travel. It is well-recognized that nutritional manipulation, such as dietary supplementation with macro- and micro-elements, increases the immune response in fish, which has an impact on a fish’s appropriateness for shipping. A well-known method for reducing post-shipment mortality is nutritional prophylaxis, which involves stimulating the immune systems of fish by supplementing essential nutrients to their feed [3].
The ornamental sector is impacted by the global health crisis in several ways. However, the frequency of illnesses in the agricultural industry, especially, can result in large financial losses, impeding the development of the ornamental sector [4]. A promising safe and sustainable alternative to antibiotics and vaccines is provided by medicinal plants and algae, which also boost fishes’ non-specific defense immunity [5]. Ornamental fish aquaculture practices utilized a variety of herbal plants and algae for disease management and prevention of various ailments, as well as the promotion of health. In accordance with Dawood et al. [6], macro- and microalgae are safe for the environment, efficacious, quickly biodegradable, non-narcotic, non-habit-forming, and free of negative side effects. Recently, their value as a source of novel bioactive substances has grown rapidly, and researchers have revealed that marine algal-originated compounds exhibit various biological activities [7,8]. The host organism biosynthesizes these compounds as non-primary or secondary metabolites to protect themselves and maintain homeostasis in their environment; some of these secondary metabolites offer avenues for developing cost-effective, safe, and potent drugs. Those compounds already isolated from seaweeds are providing valuable ideas for the development of new drugs against cancer, microbial infections, and inflammation [9], apart from their potential ecological and industrial significance such as controlling reproduction, settlement, and biofouling, and serving as feeding deterrents [10]. Aquaculture organisms’ immunity has long been studied through the use of algae meal or their extracts as a feed additive [11]. Feeds from algae have also been investigated as a likely fish feed replacement to lower the cost of making fish feed [12,13]. Sulfur-containing polysaccharides, which are only found in algal meal, are absent from terrestrial plants.
Chaetomorpha is a common and widespread green seaweed genus characterized by unbranched filaments [14]. Chaetomorpha, also known as Spaghetti algae, contains vitamins C and A [15]. Some species are edible, such as C. crassa, C. linum, and C. brachygona. C. crassa is consumed as salad or dessert in Far Eastern countries due to its characteristic of gelatinization [16]. Apparently, there has been no study done so far on skin mucus immunity and sex steroid hormones in guppy fish of filamentous green seaweeds from India. Tsutsui et al. [17] and Sattanathan et al. [18] reported that Chaetomorpha sp. is an effective supplement that improves the growth performance and feed conversion rates of Penaeus monodon and Labeo rohita, respectively. While C. antennina had access to the methanolic extract, L. rohita showed increased specific and non-specific immunity [19]. Similar conclusions were drawn about how a meal including C. linum and Zostera marina affected the sea cucumber Apostichopus japonicas’ growth and development, food consumption, and energy levels [20]. Therefore, the focal aim of this pilot study examined the effects of C. aerea on P. reticulate growth, sexual hormone levels, total carotenoids, and skin mucus immunological response.
2. Materials and Methods
2.1. Experimental Fish and Their Maintanance
Guppies in good health were obtained from Dimapur, Nagaland, after being bought from a local aquarium fish farm, and they were then kept for two weeks in a lab environment for acclimatization in tanks with 50 L capacity. Weekly, twice-syphoning with a 50% water exchange was employed throughout the trial to clean the tanks and eliminate residual feed and waste. After acclimatization, the fish, with an average body weight of 0.19 ± 0.1 g, were divided into five major treatment groups for the administration of different dosages of C. aerea through feed. Experiments were performed in rectangular plastic tanks (95 × 70 × 60 cm, 180 L), and dechlorinated water was used for rearing. Guppy fish (n = 450) were distributed into 15 tanks, with each tank containing 30 (1:1 ratio of male and female fish) fish and maintained in triplicate. Water quality was monitored throughout the experiment. The temperature was 28 ± 2 °C, dissolved oxygen concentration was 5.8 ± 0.3 mg/L, and ammonia–nitrogen concentration was 0.032 ± 0.001 mg/L during the experiment period.
2.2. Collection and Preparation of Seaweed
Algae of the species C. aerea were gathered in Parangipettai, Chidambaram, Tamil Nadu, India. After being thoroughly rinsed with tap water two or three times, the collected marine green algae were immersed in distilled water for 30 min to eliminate soil particles. After drying for seven days in the shade, they were ground. After being ground, the algal dry powder was kept chilled at 4 °C.
2.3. Diet Preparation and Experimental Design
The experimental composition of the basal diet was dry matter 61.43 ± 2.45; ash content 2.50 ± 0.45; total protein 40.12 ± 2.14; total lipid 3.91 ± 1.01; total fiber content 3.50 ± 0.87. Five experimental diets were prepared by incorporating C. aerea at concentrations of 0 (control), 1, 2, 4, and 8 g/kg feed in basal ingredients such as rice bran, soyabean meal, fish meal, corn flour, wheat flour, iodine salt, vegetable oil, and vitamin and mineral mixture (Table 1). First, dry ingredients were mixed thoroughly, and the required water was added and mixed thoroughly in a mixer for 30 min. The resulting dough was pelleted, dried at room temperature for two days, and then stored in airtight sterile containers at room temperature until feeding [18]. Feeding rate was adjusted by monitoring daily feed intake, and accordingly, each of the diets was fed to the fish in all triplicate tanks of every treatment group at a feeding rate 2% of body weight per day for 30 days. The daily ration was subdivided into two feeds at 09.00 h and 17:00 h. The institutional ethical clearance committee from St. Joseph University endorsed all the testing procedures.

Table 1.
Composition of ingredients in experimental diets with desired crude protein and lipid levels (for 1 kg).
2.4. Growth Performance and Fry Production
Both at the beginning and at the end of the experiment, the fish were weighted on a digital balance accurate to 0.0001 g. Weight gain, specific growth rate (SGR), feed conversion ratio (FCR), and fry production were calculated as follows [21].
Weight gain (g) = Final weight − Initial weight
FCR (g) = [Feed given (Dry weight)]/[Body weight gain (Wet weight)]
SGR (%) = {[Ln (Final weight) − Ln (Initial weight)]/Total days of experiment} × 100
Fry production = Number of produced fry/Number of female fishes
Survival rate (%) = Total fish harvested/Total fish stocked × 100
2.5. Fish Skin Mucus Collection
The collection of mucus from fish skin was performed as per the methods described by Bishat et al. [21]. In this modified method, guppies (n = 10) were anesthetized with clove oil (5 mL−1) and placed in vicinity of 10 mL of 50 mM NaCl in individual plastic bags. Then, these were gently rubbed inside the plastic for 2 min. Mucus samples were centrifuged at 2000× g for 8 min at 4 °C. The supernatant was collected and stored at −80 °C until further analysis.
2.6. Skin Mucus Immunological Parameters
2.6.1. Lysozyme Activity
Lysozyme activity was measured based on the lysis of the lysozyme-sensitive gram-positive bacterium Microbacterium lysodeikticus (Sigma, Saint Louis, MO, USA), according to the method [22]. In summary, 25 µL of plasma was added to 96-well ELISA plates. Then, 175 µL of an M. lysodeikticus bacterial suspension was added, and optical density was measured on a spectrophotometer at 450 nm.
2.6.2. Alternative Complement Activity
Alternative complement activity was determined according to the method by Yano [23] in an altered manner. Briefly, human red blood cells (HuRBCs) were collected from a volunteer. Fish mucus samples were added to Hanks balanced salt solution (HBSS), containing magnesium ion (1 mM Mg2+), 10 mM EGTA (Ethylene glycol tetraacetic acid), and 6.7 mM HEPES (N-(2-Hydroxyethyl) piper azine-N(2-ethanesulfonic acid). By adding 100 µL of HuRBCs to the reaction mixture, it was maintained at 22 °C for 90 min. The reaction was interrupted by adding HBSS containing EDTA (Etyelene glycol tetraacetic acid) and centrifuging at 600× g rpm for 5 min. At 414 nm, the absorbance of the supernatant was measured.
2.6.3. Myeloperoxidase Activity
Myeloperoxidase (MPO) content was determined by the method described by Quade and Roth [24] with slight modifications. In a sterile 96-well plate, a mixture of fish mucus (10 µL) mixed with 90 µL of phenol-free HBSS, 25 µL of 20 mM TMB (3,3′, 5,5′-tetramethyl benzidine hydrochloride), and 25 µL of 5 mM H2O2 was taken and incubated for 15 min at 30 °C. Later, 50 µL of 2 mM H2SO4 was used to stop the reaction, and the absorbance was measured at 450 nm using a plate reader.
2.6.4. Total Immunoglobulin
The total immunoglobulin (Ig) concentration in plasma was determined using the Siwicki and Anderson [25] method. The technique was based on measuring total protein levels in plasma using Lowry’s micro protein determination method before and after precipitating immunoglobulin molecules with a 12% polyethylene glycol solution (Sigma). The difference in protein content was used to calculate the Ig content of guppy fish.
2.6.5. Total Carotenoid Concentrations
The total carotenoids in fish tissue samples were determined using the procedures reported by Lee [26], with some modifications. Samples of fish skin from both lateral sides and the whole caudal fin were obtained separately. Then, 20 mL of acetone and methanol (1:1 v/v) was used to extract samples for 30 min. After that, the extracts were mixed with 20 mL of petroleum ether in a separation funnel. Following this, the upper phase was separated, and then one-third of distilled water was added and vacuum dried at 40 °C. Finally, the absorbance of the residue in petroleum ether was read at 450 nm using a spectrophotometer (Systronic, Digital 108, Gujarat, India).
2.6.6. Evaluation of Sex Steroid Hormones
Whole-body homogenate was examined for the presence of steroids at the end of the trial. All female (n = 15) guppies were homogenized with 25 mM Tris-HCl buffer, 0.01% aprotinin, and 0.5 mM PMSF (pH 8.0), after which the supernatant was separated and kept at −80 °C after being centrifuged at 100,000 × g for 1 h. The protocol described by Feist et al. [27] was used to extract sex steroids from the homogenate. Radioimmunoassay was used to measure hormone levels [28]. Samples and standard solutions totaling 40–50 µL were added to tubes coated with mouse polyclonal antibodies. All experimental tubes were then filled with 500 l of iodine-labeled hormones (estradiol (E2), 170 kBq, 200 kBq, and progesterone (P), 185 kBq, Mimotec, Sion, Switzerland) or 1 mL of 17-hydroxyprogestrone (17-OHP), 185 kBq, (Sigma Chemicals, Saint Louis, Missouri, USA) and incubated in a water bath for 30 min at 40 °C. The level of radioactive activity was measured using a gamma counter (RX-105, Taipei, Taiwan) after washing with phosphate buffer. Estradiol had a standard concentration range of 0 to 430 nm/mL, testosterone 0 to 18.7, progesterone 0 to 71 ng/mL, and 17-hydroxyprogesterone 0 to 96 ng/mL.
2.7. Statistical Analysis
Each assay was repeated three times, and a statistically significant difference between the control and treatment groups was detected. The studies’ triplicate data were evaluated, and the results are expressed as mean ± standard error (SE) values with significant levels (p < 0.05) using one-way ANOVA and the DMRT HSD test.
3. Results
3.1. Growth Parameters
Gradual increases in the growth performance of guppy fish treated with C. aerea algae were observed and tabulated in Table 2. Although there was significantly decrease in fry production, there was substantial variation (p > 0.05) in growth rate, SGR, FCR, and survival rate when utilizing the C. aerea algae supplement diet, as well as significant variations in fry production between the experimental groups. No mortality was observed during the experimental study.

Table 2.
Weight gain, specific growth rate, feed conversion rate, fry production of guppy fish fed with diets supplemented with varying levels of C. aerea. The values represented were the means ± SE. Values in the same row with different superscripts are significantly different (p < 0.05).
3.2. Skin Mucus Immunity
The findings of this investigation revealed that the various amounts of C. aerea algae greatly raised the activity of lysozyme and myeloperoxidase, alternative complement activity (ACH50), and Ig (Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5). The T3 treatment group, which received 4% more C. aerea algae, had the greatest level of lysozyme. With an increase in C. aerea algae content, lysozyme activity rose considerably (p < 0.05). Similarly, guppy mucus from C. aerea algae-treated fish showed a considerable increase in alternative complement activity; the greatest value was noted in response to a 4% diet treatment. The MPO activity values in fish considerably increased (p < 0.05), with diet concentrations of C. aerea algae increasing from 2 to 4%. Both the 4 and 8% treatments considerably raised the Ig content.
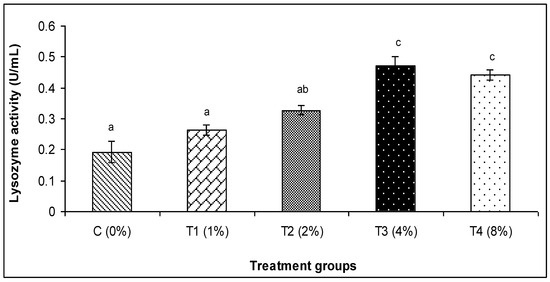
Figure 1.
Effect of marine algae C. aerea on lysozyme activity of Poecilia reticulate. The values represented were the means ± SE. Values in the same row with different superscripts are significantly different (p < 0.05).
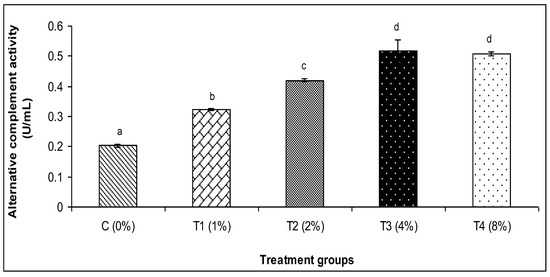
Figure 2.
Effect of marine algae C. aerea on alternative complement (ACH50) activity of Poecilia reticulate. The values represented were the means ± SE. Values in the same row with different superscripts are significantly different (p < 0.05).
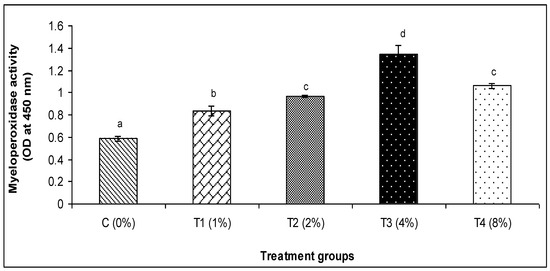
Figure 3.
Effect of marine algae C. aerea on myeloperoxidase activity of Poecilia reticulate. The values represented were the means ± SE. Values in the same row with different superscripts are significantly different (p < 0.05).
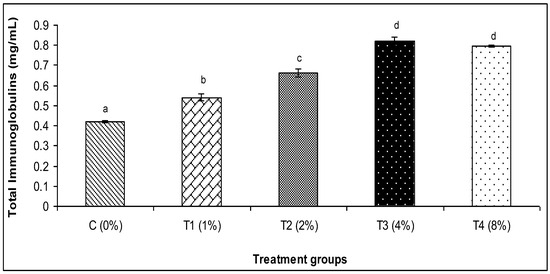
Figure 4.
Effect of marine algae C. aerea on Ig levels of Poecilia reticulate. The values represented were the means ± SE. Values in the same row with different superscripts are significantly different (p < 0.05).

Figure 5.
Effect of marine algae C. aerea on total mucus protein (mg/mL) levels of Poecilia reticulate. The values represented were the means ± SE. Values in the same row with different superscripts are significantly different (p < 0.05).
3.3. Sex Steroid Hormones
The levels of the steroid hormones (E2, T, 17-OHP, and P) in P. reticulate fish provided with various levels of C. aerea algae are shown in Figure 6. All sex steroid hormone concentrations were considerably higher (p < 0.05) in fish provided with C. aerea algae. The group that received the T3 treatment had the greatest levels of sex steroid hormones.
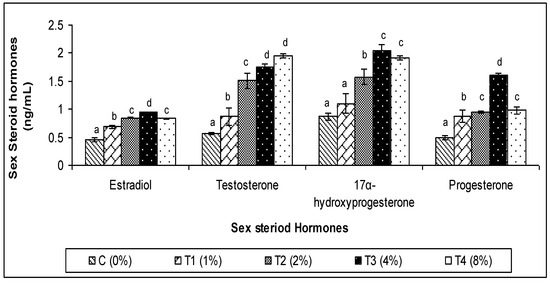
Figure 6.
Effect of marine algae C. aerea on sex steroid hormone concentrations of Poecilia reticulate. The values represented were the means ± SE. Values in the same row with different superscripts are significantly different (p < 0.05).
3.4. Total Carotenoid Content
Additionally, total carotenoids were found in considerably higher amounts in both the skin and caudal fin of guppy fish diets including algae C. aerea (p < 0.05, Figure 7). Therefore, among the several treated groups, the fish that were fed 4% of C. aerea algae had the greatest total carotenoid contents in both tissues.
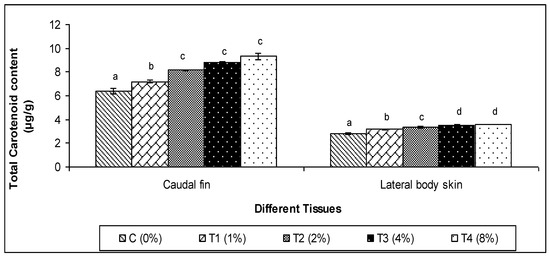
Figure 7.
Effect of marine algae C. aerea on total carotenoid content in lateral skin and caudal fin of Poecilia reticulate. The values represented were the means ± SE. Values in the same row with different superscripts are significantly different (p < 0.05).
4. Discussion
The increasing interest in the use of herbs for boosting growth and immunity in fish has originated recently. Many plant- and algae-derived compounds have been found to trigger both specific and non-specific immune responses in animals, which are more evaluated in finfish [29,30,31]. It is uncertain whether clay and marine algae are used together as an additional feed source for aquatic life. Current research has revealed that dietary supplementation with C. aerea of green marine algae has been researched and demonstrated to improve fish and prawn growth performance and food utilization [17,18]. However, this investigation study reveals that fish provided with C. aerea algae for 30 days enhanced the production of their fry, the feed conversion rate, and growth metrics (growth rate, specific growth rate) that were optimized.
Guppy growth performance in the current feeding trial significantly changed when meals containing different concentrations of C. aerea. According to Bishat et al. [21], guppy fish fed diets with 10–15% Moringa oleifera showed improved growth performance. According to Sattanathan et al. [19], C. aerea performed similarly to fish administration through the intra-peritoneal cavity of C. aerea extract in terms of various growth parameters and food utilization responses in L. rohita. These findings are consistent with the current research. Studies on ornamental guppies reveal that feeding garlic extract significantly modifies the growth interpretation of guppy fish [32]. The findings of Hindu et al. [33] highlighted the bioactive compounds in the algae, including minerals, polyunsaturated fatty acids, polyphenols, different pigments, and growth hormones, which appear to be the reason for the parameters’ favorable impacts. Labeo rohita’s development and feeding effectiveness were both enhanced by the dietary inclusion of C. aerea’s 75 mg/g methanolic extract, according to Sattanathan et al. [34]. According to the literature mentioned above, C. aerea’s inclusion in the present investigation boosted the performance of growth and utilization of the feed. The benefits of medicinal plants and algae to boost fish health and immunological development have gained popularity recently. A variety of compounds produced by algae cause fish to react immunologically in both specific and non-specific ways [35]. In commercial aquaculture, green algae are employed for many different purposes, such as nutrients, growth promoters, and antibacterial agents. Investigations are also being conducted into their ability to both prevent and treat fish infections [36]. According to the data obtainable at that point, C. aerea supplementation significantly affected the levels of Ig, activities of MPO and lysozyme, as well as ACH50 in the diet. As the concentration of C. aerea algae in the diet increased, the skin mucus immunity responses in the guppy diet increased as well, reaching their maximum values in guppies fed with 4% C. aerea algae concentration.
Oral administration of plant-derived bioactive compounds has been shown to impact reproductive activities, boost immunological responses, and encourage growth in aquatic animals [37,38,39]. Fish sex reversal, delayed maturity, and increased fertility have all been made possible by plant extracts [35,36,40,41]. Hormonal function has been demonstrated by several bioactive chemicals that have been separated from plants. The most active phytoestrogens have estrogenic activity and interact with oestrogen receptors, whereas interactions between isoflavones and phytoestrogens and the androgen and progesterone receptors are less prevalent [42,43]. Earlier studies have shown that plant compounds can mimic sex hormones as agonists or antagonists to nuclear receptors, as well as modulate the biosynthesis of sex hormones by altering the activity and expression of enzymes [44,45,46,47]. An ethanol extract was initially discovered as a possible endocrine disruptor in an investigation on reproductive efficiency and steroid hormone levels in zebrafish treated with the RE isolated from Ruta graveolens L. [48]. Plant extracts have been studied as possible replacements for controlling reproduction to produce monosex populations in Tilapia culture [37]. Marine algal biomass has health benefits beyond enriching nutrition. As a component of functional foods, they contribute to optimal health and reduced health risks or disease prevention [49]. Many proteins, oils, carbohydrates, vitamins, carotenoids, and other nutrients are found in various species of algae [50,51,52]. These bioactive ingredients offer a variety of health benefits to terrestrial and aquatic organisms. For instance, they protect against diseases, prevent nutrient deficiencies, and promote proper growth and development among aquaculture species [53,54]. It is possible that the rich proteins or beta-carotene may artificially enhance the color of ornamental fish.
Research on the impact of plants on the reproductive markers in farmed fish is crucial, particularly when considering long-term use, to prevent unintended detrimental consequences on the fish’s capacity to reproduce. The protein content of macroalgae varies greatly from phylum to phylum [55]. Generally, the protein fraction of brown seaweeds is low (3–15% of dry weight) compared with that of green or red seaweeds (10–47% of dry weight) [56]. The protein in macroalgae contains all essential amino acids; however, variations in their concentrations are known to occur [57]. Recently, much attention has been paid to unraveling the structural, compositional, and sequential properties of bioactive peptides. Bioactive peptides usually contain 3–20 amino acid residues, and their activities are based on their amino acid composition and sequence [58,59]. These peptides are reported to be involved in various biological functions such as antihypertension, immunomodulatory, antithrombotic, antioxidant, anticancer, and antimicrobial activities, in addition to nutrient utilization [59,60]. Contrary to other studies’ findings indicating that the group not receiving algae therapy had a decline in sex hormone levels, our findings demonstrated that adding C. aerea to meals dramatically enhanced the synthesis of sex hormones. Consuming plants and algal extracts may prevent cholesterol from being transferred to the mitochondria in Leydig cells and from being converted to testosterone, preventing the production of steroid hormones. Consuming green algae and herbal plant extracts may also harm Leydig cells and prevent the expression of enzymes involved in the steroidogenic process [61]. Despite this, our study showed that this research is the first to consider how C. aerea algae can affect fish reproduction. The literature lacks the presence of any additional articles on this issue. Undoubtedly, significant issues that call for further study are how these effects function biologically and whether they affect an adult’s capacity for reproduction. The highest carotenoid concentrations were seen in fish that were fed 4% C. aerea algae. Yanar et al. [62] documented the growing impacts of sweet and hot red pepper as a food supplement for rainbow trout fish coloring. Red pepper enhances the coloration of the yellowtail cichlid’s tail, as claimed [63]. Red pepper meal is a natural source of carotenoids for blue streak hap, as stated by Yimaz and Ergun [64].
Fish develop brilliant colors due to red pepper’s optimal level of body tissue carotenoids, according to reports by Yilmaz et al. [65]. Additionally, it appears that the chemical components in this herbal supplement have favorable effects on pigmentation, although studies on the benefits of garlic or onion feed on the pigmentation of fish have not been discovered. For instance, anthocyanin comprises 10% of red onions’ flavonoid concentration [66]. These are naturally occurring plant pigments that give plants and some vegetables their different purple colors (red, blue, and purple). According to the study by Wang et al. [67], allicin isolated from garlic is a poorly understood oxygenated carotenoid. The flavonoids and carotenoids in the herbal additives used in this investigation therefore seem to produce higher coloration than the control group.
Carotenoids are synthesized from geranyldiphosphate by all photosynthetic organisms [68]. Macro- and microalgae, which are important in the production of larval fish because of their nutritive ingredients, can be used as a natural pigment source in fish feeds. The use of algal biomass has been recently investigated regarding its potential as a coloring agent [69,70]. However, the use of synthetic pigment sources is more common because they are easy to obtain [71]. There is no study on the effect of natural and synthetic pigments on the color of guppy fish. Skin coloration is one of the most important marketing criteria in the ornamental fish trade [72]. Dietary additives such as essential fatty acids, alpha-tocopherol, ascorbic acid, and carotenoids influence the reproductive performance and coloring of fish [72]. Enhanced growth performance, health, nutritional performance, and coloration have been declared with dietary small amounts of microalgae inclusion (1–5%) in a range of fish species [72,73]. Spirulina is a microalga commercially used as a supplementary food in human nutrition and finfish diets due to its rich source of protein, indispensable fatty acids, essential amino acids, vitamins, and minerals [72,74,75,76]. The effects of dietary Spirulina on the growth performance and skin coloration have been studied for ornamental finfish species, including red swordtail, Xiphophorus helleri [77], goldfish, Carassius auratus [78,79], yellow tail cichlid, Pseudo tropheusacei [72], and three-spot gourami, Trichopodus trichopterus [80].
5. Conclusions
In conclusion, feeding guppy fish with C. aerea, the only feed that contains two protein sources as well as macro green algal and pigment additives, resulted in an average final weight but also in the highest skin mucosal immunity and body pigments compared to the control group. Additionally, feeding guppy fish with C. aerea algae improved growth, fry generation, and survival rate. The overall findings of this study showed that P. reticulata fry’s production and the concentration of sex hormone levels were enhanced by the mean optimal dosage of C. aerea algae-supplemented food.
Author Contributions
Conceptualization, B.B., W.-C.L. and S.G.; methodology, data curation and formal analysis, S.G., V.T. and S.V.; funding acquisition, B.B. and W.-C.L.; project administration, S.G. and B.B.; writing—original draft, S.G., V.T. and B.B.; interpretation, writing—review and editing, B.B., W.-C.L. and S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The institutional ethical clearance committee from St. Joseph University endorsed all testing procedures. The experimental protocol for this study was reviewed and approved by the Animal Care and Use Institutional Committee (Approval code: SJU/ZOO/142, Approval date: 21 March 2023).
Data Availability Statement
The data presented in this study are available upon request from the corresponding authors.
Acknowledgments
All the authors are thankful to their respective institutes for their support.
Conflicts of Interest
The authors hereby declare that they have no conflicts of interest and have no known competing financial interest or personal relationship that could have appeared to influence the work reported in this paper.
References
- Tlusty, M. The benefits and risks of aquacultural production for the aquarium trade. Aqaculture 2002, 3–4, 203–219. [Google Scholar] [CrossRef]
- Monticini, P. The Ornamental Fish Trade: Production and Commerce of Ornamental Fish: Technical-Managerial and Legislative Aspects; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010; Volume 12, 134p. [Google Scholar]
- Lim, L.C.; Dhert, P.; Sorgeloos, P. Recent developments and improvements in ornamental fish packaging systems for air transport. Aquac. Res. 2003, 34, 923–935. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Ahmadi, A.; Khalili, M.; Raeisi, M.; Van Doan, H.; Caipang, C.M. The study of antioxidant enzymes and immune-related genes expression in common carp (Cyprinuscarpio) fingerlings fed different prebiotics. Aquac. Res. 2017, 48, 5447–5454. [Google Scholar] [CrossRef]
- Awad, E.; Awaad, A. Role of medicinal plants on growth performance and immune status in fish. Fish Shellfish. Immunol. 2017, 67, 40–50. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Esteban, M.A. Beneficial roles of feed additives as immunostimulants in aquaculture: A review. Rev. Aquac. 2018, 10, 950–974. [Google Scholar] [CrossRef]
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Wijesekara, I.; Kim, S.K. Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: Prospects in the pharmaceutical industry. Mar. Drugs 2010, 8, 1080–1093. [Google Scholar] [CrossRef]
- Elena, M.; Francisco, Y.; Erickson, K.L. Mailiohydrin, a Cytotoxic Chamigrene Dibromohydrin from a Phillippine Laurencia Species. J. Nat. Prod. 2001, 64, 790–791. [Google Scholar]
- Selvin, J.; Lipton, A.P. Development of a Rapid Mollusc Foot Adherence Bioassay for Detecting Potent Antifouling Bioactive Compounds. Curr. Sci. 2002, 83, 735–737. [Google Scholar]
- Daniel, N.; Sivaramakrishnan, T.; Saravanan, K.; Shalini, B.; Arunjyoti, B.; Sankar, R.; Dann Roy, S. A review on microalgae as potential fish feed ingredient. J. Andeman. Sci. Assoc. 2016, 1, 140–144. [Google Scholar]
- Younis, E.S.M.; Al-Quffail, A.S.; Al-Asgah, N.A.; Abdel-Warith, A.W.A.; Al-Hafedh, Y.S. Effect of dietary fish meal replacement by red algae, Gracilaria arcuata on growth performance and body composition of Nile tilapia, Oreochromis niloticus. Saud. J. Biol. Sci. 2018, 25, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Perez-Velazquez, M.; Gatlin, I.I.I.; Gonzalez-Felix, M.L.; Garcia-Ortega, A. Partial replacement of fishmeal and fish oil by algal meals in diets of red drum Sciaenopsocellatus. Aquaculture 2018, 487, 41–50. [Google Scholar] [CrossRef]
- Leliaraert, F.; D’hondt, S.; Tyberghein, L.; Verbruggen, H.; De Clerck, O. Atypical development of Chaetomorpha antennina in culture (Cladophorales, Chlorophyta). Physiol. Res. 2011, 9, 91–97. [Google Scholar]
- Novaczek, I. A Guide to the Common Edible and Medicinal Sea Plants of the Pacific Islands; The University of the South Pacific: Suva, Fiji, 2001. [Google Scholar]
- Apaydin-Yagci, M.A.; Turna, I.I. A new record for the algal flora of Turkey: Chaetomorpha crassa (C.Ag.) Kutz. (Cladophoraceae, Chlorophyceae). Turk. J. Bot. 2002, 26, 171–174. [Google Scholar]
- Tsutsui, I.; Songphatkaew, J.; Meeanan, C. Co-culture with Chaetomorpha sp. enhanced growth performance and reduced feed conversion ratio of the giant tiger prawn, Penaeus monodon. Int. Aquat. Res. 2015, 7, 193–199. [Google Scholar] [CrossRef]
- Sattanathan, G.; Thanapal, G.; Swaminathan, S.; Kim, H.; Vijaya, R.; Kim, H.; Balasubramanian, B. Influences of dietary inclusion of algae Chaetomporpha aerea enhanced growth performance, immunity, haematological response and disease resistance of Labeo rohita challenged with Aeromonas hydrophila. Aquac. Rep. 2020, 17, 100353. [Google Scholar] [CrossRef]
- Sattanathan, G.; Tamizhazhagan, V.; Padmapriya, S.; Liu, W.C.; Balasubramanian, B. Effect of Green Algae Chaetomorpha antennina extract on growth, modulate immunity, and defenses against Edwardsiella tarda infection in Labeo rohita. Animals 2021, 10, 2033. [Google Scholar] [CrossRef]
- Song, X.; Xu, Q.; Zhou, Y.; Lin, C.; Yang, H. Growth, feed utilization and energy budgets of the sea cucumber Apostichopus japonicus with different diets containing the green tide macroalgae Chaetomorpha linum and the seagrass Zostera marina. Aquaculture 2017, 470, 157–163. [Google Scholar] [CrossRef]
- Bishat, M.; Kumar, A.; Shah, T.K. Effect of Moringa oleifera leaf powder on skin mucosal immune responses and growth performance of guppy, Poecillia reticulate (Peter, 1860). Aquac. Res. 2020, 51, 4984–4990. [Google Scholar] [CrossRef]
- Sankaran, K.; Gurnani, S. On the variation in the catalytic activity of lysozyme in fishes. Ind. J. Biochem. Biophys. 1972, 9, 162–165. [Google Scholar]
- Yano, T. Assays of hemolytic complement activity. In Techniques in Fish Immunology; Stolen, J.S., Fletcher, T.C., Anderson, D.P., Kaattari, S.L., Rowley, A.F., Eds.; SOS Publications: Fair Haven, NJ, USA, 1992; pp. 131–141. [Google Scholar]
- Quade, M.J.; Roth, J.A. A rapid direct assay to measure degranulation of bovine neutrophil primary granules. Vet. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Siwicki, A.K.; Anderson, D.P.; Rumsey, G.L. Dietary intake of immunostimulants by rainbow trout affects non-specific immunity and protection against furunculosis. Vet. Immunol. Immunopathol. 1994, 41, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Pham, M.A.; Lee, S.M. Effects of dietary paprika and lipid levels on growth and skin pigmentation of Pale Chub (Zacco platypus). Asian-Aust. J. Anim. Sci. 2012, 23, 724–732. [Google Scholar] [CrossRef]
- Feist, G.; Schreck, C.B.; Fitzpatrick, M.S.; Redding, J.M. Sex steroid profiles of coho salmon (Oncorhynchus kisutch) during early development and sexual differentiation. Gen. Comp. Endocrinol. 2009, 80, 299–313. [Google Scholar] [CrossRef]
- Kagawa, H.; Young, G.; Nagahama, Y. In vitro estradiol-17 β and testosterone production by ovarian follicles of the goldfish, Carassius auratus. Gen. Comp. Endocrinol. 1984, 54, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Varijakzhan, D.; Chong, C.M.; Abushelaibi, A.; Lai, K.S.; Lim, S.H.E. Middle Eastern plant extracts: An alternative to modern medicine problems. Molecules 2020, 25, 1126. [Google Scholar] [CrossRef]
- Sharma, A.; Deo, A.D.; Riteshkumar, S.T.; Chanu, T.I.; Das, A. Effect of Withania somnifera (L. Dunal) root as a feed additive on immunological parameters and disease resistance to Aeromonas hydrophila in Labeo rohita (Hamilton) fingerlings. Fish. Shellfish. Immunol. 2010, 29, 508–512. [Google Scholar] [CrossRef]
- Srinivasan, G.; Babu, P.; Murugeswari, V. Effect of neem products and insecticides on the egg parasitoids, Trichogramma spp. (Trichogrammatidae: Hymenoptera). Pestic. Res. J. 2001, 13, 250–253. [Google Scholar]
- Motlagh, A.H.; Paolucci, M.; Lashkarizadeh Bami, M.; Safari, O. Sexual parameters, digestive enzyme activities, and growth performance of guppy (Poecilia reticulata) fed garlic (Allium sativum) extract supplemented diets. J. World Aquac. Soc. 2020, 51, 1087–1097. [Google Scholar] [CrossRef]
- Hindu, S.V.; Chandrasekaran, N.; Mukherjee, A.; Thomas, J. A review on the impact of seaweed polysaccharide on the growth of probiotic bacteria and its application in aquaculture. Aquac. Int. 2018, 27, 227–238. [Google Scholar] [CrossRef]
- Sattanathan, G.; Tamizhazhagan, V.; Raza, N.; Shah, S.Q.A.; Hussain, M.Z.; Kim, K.H. Effects of Green Alga, Chaetomorpha aerea Extract on Non-Specific Immune Responses and Disease Resistance against Edwardsiella tarda Infection in Labeo rohita. Appl. Sci. 2021, 11, 4325. [Google Scholar] [CrossRef]
- Elizondo-Gonzalez, R.; Quiroz-Guzman, E.; Escobedo-Fregoso, C.; Magallon-Servin, P.; Pena-Rodríguez, A. Use of seaweed Ulva lactuca for water bioremediation and as feed additive for white shrimp Litopenaeus vannamei. PeerJ 2018, 5, 44–59. [Google Scholar] [CrossRef]
- Moghanlou, K.S.; Isfahani, E.N.; Dorafshan, S.; Tukmechi, A.; Aramli, M.S. Effects of dietary supplementation with Stachys lavandulifolia Vahl extract on growth performance, hemato-biochemical and innate immunity parameters of rainbow trout (Oncorhynchus mykiss). Anim. Feed Sci. Technol. 2017, 237, 98–105. [Google Scholar] [CrossRef]
- Gabriel, N.N.; Qiang, J.; He, J.; Ma, X.Y.; Kpundeh, M.D. Dietary Aloe vera supplementation on growth performance, some haemato-biochemical parameters and disease resistance against Streptococcus iniae in tilapia. Fish. Shellfish. Immunol. 2015, 44, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Parrillo, L.; Coccia, E.; Volpe, M.G.; Siano, F.; Pagliarulo, C.; Scioscia, E.; Paolucci, M. Olive mill wastewater enriched diet positively affects growth, oxidative and immune status and intestinal microbiota in the crayfish, Astacus leptodactylus. Aquaculture 2017, 473, 161–168. [Google Scholar] [CrossRef]
- Farsani, M.N.; Hoseinifar, S.H.; Rashidian, G.; Farsani, H.G.; Ashouri, G.; Van Doan, H. Dietary effects of Coriandrum sativum extract on growth performance, physiological and innate immune responses and resistance of rainbow trout (Oncorhynchus mykiss) against Yersinia ruckeri. Fish. Shellfish. Immunol. 2019, 91, 233–240. [Google Scholar] [CrossRef]
- Chakraborty, S.B.; Horn, P.; Hancz, C. Application of phytochemicals as growth-promoters and endocrine modulators in fish culture. Rev. Aquac. 2014, 6, 1–19. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Biswas, R.; Sharma, A.; Banerjee, S.; Biswas, S.; Katiyar, C.K. Validation of medicinal herbs for anti-tyrosinase potential. J. Herb. Med. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- Beck, V.; Unterrieder, E.; Krenn, L.; Kubelka, W.; Jungbauer, A. Comparison of hormonal activity (estrogen, androgen and progestin) of standardized plant extracts for large scale use in hormone replacement therapy. J. Steroid Biochem. Mol. Biol. 2003, 84, 259–268. [Google Scholar] [CrossRef]
- Yeganeh, S.; Sotoudeh, A.; Movaffagh, A.N. Effects of Tribulus terrestris extract on growth and reproductive performance of male convict cichlid (Cichlasoma nigrofasciatum). Turk. J. Fish. Aquat. Sci. 2017, 17, 1003–1007. [Google Scholar] [CrossRef]
- Davis, K.B.; Goudie, C.A.; Simco, B.A. Influence of dihydrotestosterone on sex determination in channel catfish and blue catfish: Period of developmental sensitivity. Gen. Comp. Endocrinol. 1992, 86, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Patiňo, R.; Davis, K.B.; Shoore, J.E. Sex differentiation of channel catfish gonads: Normal development and effects of temperature. J. Exp. Zool. 1996, 276, 209–218. [Google Scholar] [CrossRef]
- Tzchori, I.; Degani, G.; Elisha, R. The influence of phytoestrogens and oestradiol-17β on growth and sex determination in the European eel (Anguilla anguilla). Aquacult. Res. 2004, 35, 1213–1219. [Google Scholar] [CrossRef]
- Green, C.C.; Kelly, A.M. Effects of the estrogen mimic genistein as a dietary component on sex differentiation and ethoxyresorufin-O-deethylase (EROD) activity in channel catfish (Ictalurus punctatus). Fish. Physiol. Biochem. 2009, 35, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Forsatkar, M.N.; HedayatiRad, M.; Luchiari, A.C. “Not tonight zebrafish”: The effects of Ruta graveolens on reproduction. Pharm. Biol. 2018, 56, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.I.; Constantinides, S.V.; Bhandari, S.; Frongillo, E.A.; Schreinemachers, P.; Wertheim-Heck, S. Actions in global nutrition initiatives to promote sustainable healthy diets. Glob. Food Sec. 2021, 31, 100585. [Google Scholar] [CrossRef]
- Idenyi, J.N.; Eya, J.C.; Ogbonna, J.C. Characterization of strains of Chlorella from Abakaliki, Nigeria, for the production of high-value products under variable temperatures. J. Appl. Phycol. 2021, 33, 275–285. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, L.; Tredici, R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal. Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Moheimani, N.R.; Vadiveloo, A.; Ayre, J.M.; Pluske, J.R. Nutritional profile and in vitro digestibility of microalgae grown in anaerobically digested piggery effluent. Algal. Res. 2018, 35, 362–369. [Google Scholar] [CrossRef]
- Shah, M.R.; Lutzu, G.A.; Alam, M.D.; Pallab Sarker, M.A.; Chowdhury, K.; Parsaeimehr, A.; Liang, Y.; Daroch, M. Microalgae in aquafeeds for a sustainable aquaculture industry. J. Appl. Phycol. 2018, 30, 197–213. [Google Scholar] [CrossRef]
- Nethravathy, M.U.; Jitendra, G.; Sandeep, N.; Mudliar, A.; Shekh, Y. Recent advances in microalgal bioactives for food, feed, and healthcare products: Commercial potential, market space, and sustainability. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1882–1897. [Google Scholar]
- Mabeau, S.; Fleurence, J. Seaweed in food products: Biochemical and nutritional aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Galland-Irmouli, A.-V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.-P.; Villaume, C.; Guéant, J.-L. Nutritional value of proteins from edible seaweed palmaria palmata (dulse). J. Nutr. Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Pihlanto-Leppälä, A. Bioactive peptides derived from bovine whey proteins: Opioid and aceinhibitory peptides. Trends Food Sci. Technol. 2000, 11, 347–356. [Google Scholar] [CrossRef]
- Mayer, A.M.; Gustafson, K.R. Marine pharmacology in 2000: Antitumor and cytotoxic compounds. Int. J. Cancer 2003, 105, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Hajiuon, B. Effects of garlic (Allium sativum L.) hydroalcoholic extract on estrogen, progesterone and testosterone levels in rats exposed to cell phone radiation. Zahedan J. Res. Med. Sci. 2014, 16, 20–25. [Google Scholar]
- Yanar, M.; Buyukcapar, H.M.; Yanar, Y. Effects of hot and sweet red peppers (Capsicum annum) as feed supplements on pigmentation, sensory properties and weight gain of rainbow trout (Onchorhynchus mykiss). Ann. Anim. Sci. 2016, 16, 825–834. [Google Scholar] [CrossRef]
- Yigit, N.O.; Bahadır Koca, S.; Özmen, Ö.; Didinen, B.I.; Metin, S. The effects of dietary administration with high level red pepper (Capsicum annuum) on growth performance, coloration, histology and protection against Aeromonas sobria in yellow tail cichlid, Pseudotropheusacei. Acta Aquat. Turc. 2019, 15, 340–346. [Google Scholar] [CrossRef]
- Yimaz, S.; Ergun, S. Effect of red pepper (Capsicum annum) on pigmentation blue streak hap (Labidpchromis caeruleus). Isr. J. Aquac.-Bamidgeh 2011, 63, 633–638. [Google Scholar]
- Yilmaz, S.; Ergun, S.; Soytas, N. Enhancement of growth performance, and pigmentation in red Oreochromis mossambicus associated with dietary intake Astaxanthin, Paprikla, or Capsicum. Isr. J. Aquac.-Bamidgeh 2013, 65, 1–7. [Google Scholar]
- Silmestad, R.; Fossen, T.; Vagen, I.M. Onions: A Source of unique dietary flavonoids. J. Agric. Food Chem. 2007, 55, 10067–10080. [Google Scholar] [CrossRef]
- Wang, X. Allicin attentunates lipopolysaccharides induced acute lung injury in neonatal rates via the PI3K/Akt pathway. Mol. Med. Rep. 2018, 17, 6777–6783. [Google Scholar] [PubMed]
- Giuliano, G.; Aquilani, R.; Dharmapuri, S. Metabolic engineering of plant carotenoids. Trends Plant Sci. 2000, 5, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Jha, A.K.; Pal, A.K.; Venktaeshwarlu, G. Use of natural carotenoids for pigmentation in fishes. Nat. Prod. Radiance 2007, 6, 46–49. [Google Scholar]
- Raymundo, A.; Gouveida, L.; Batista, A.P.; Empis, J.; Sousa, I. Fat mimetic capacity of Chlorella vulgaris biomas in oil-in-water food emulsions stabilized by pea protein. Food Res. Int. 2005, 38, 961–965. [Google Scholar] [CrossRef]
- Sales, K.; Janseens, G.P. Nutrient requirements of ornamental fish. Aquat. Living Resour. 2003, 16, 533–540. [Google Scholar] [CrossRef]
- Guroy, B.; Şahin, I.; Mantoğlu, S.; Kayalı, S. Spirulina as a natural carotenoid source on growth, pigmentation and reproductive performance of yellow tail cichlid Pseudotropheusacei. Aquac. Int. 2012, 20, 869–878. [Google Scholar] [CrossRef]
- Güroy, D.; Güroy, B.; Merrifield, D.L.; Ergün, S.; Tekinay, A.A.; Yiğit, M. Effect of dietary Ulva and Spirulina on weight loss and body composition of rainbow trout, Oncorhynchus mykiss (Walbaum), during a starvation period. J Anim. Physiol. Anim. Nutr. (Berl.) 2011, 95, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Nakagawa, H.; Montgomery, W.L. Algae. In Dietary Supplements for the Health and Quality of Cultured Fish; Nakagawa, H., Sato, M., Gatlin, D.M., Eds.; Cabi International: Cambridge, UK, 2007; pp. 133–167. [Google Scholar]
- Sattanathan, G.; Liu, W.L.; Padmapriya, S.S.; Pushparaj, K.; Sureshkumar, S.; Lee, J.W.; Balasubramanian, B.; Kim, I.H. Effects of Dietary Blend of Algae Extract Supplementation on Growth, Biochemical, Haemato-Immunological Response, and Immune Gene Expression in Labeo rohita with Aeromonas hydrophila Post-Challenges. Fishes 2023, 8, 7. [Google Scholar] [CrossRef]
- James, R.; Sampath, K.; Thangarathinam, R.; Vasudevan, I. Effect of dietary Spirulina level on growth, fertility, coloration and leucocyte count in red swordtail, Xiphophorus helleri. Isr. J. Aquac.-Bamidgeh 2006, 58, 97–104. [Google Scholar] [CrossRef]
- James, R.; Vasudhevan, I.; Sampath, K. Interaction of Spirulina with different levels of vitamin E on growth, reproduction, and coloration in goldfish (Carassius auratus). Isr. J. Aquac.-Bamidgeh 2009, 61, 330–338. [Google Scholar] [CrossRef]
- Vasudhevan, I.; James, R. Effect of optimum Spirulina along with different levels of vitamin C incorporated diets on growth, reproduction and coloration in goldfish Carassius auratus (Linnaeus, 1758). Indian J. Fish. 2011, 58, 101–106. [Google Scholar]
- Khanzadeh, M.; Fereidouni, A.E.; Berenjestanaki, S.S. Effects of partial replacement of fish meal with Spirulina platensis meal in practical diets on growth, survival, body composition, and reproductive performance of three-spot gourami (Trichopodustrichopterus) (Pallas, 1770). Aquac. Int. 2016, 24, 69–84. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).