Abstract
The aim of this study was to evaluate the effect of supplementing feed with a functional mixture of immunomodulators, including β-glucans, nucleotides, ascorbic acid, and alpha-tocopherol, associated with a diet with lower levels of animal protein (11.5%) and higher levels of soybean meal (43.5%), on the zootechnical performance, health, hematological and immunological parameters, intestinal morphology, centesimal composition, and intestinal microbiome of juvenile Nile tilapia (initial weight 1.88 g ± 0.25 g, mean ± standard deviation). Two isocaloric and isoproteic diets (35% crude protein) were formulated, one with the inclusion of the immunostimulant functional mixture (40 kg·t−1), composed of 150 mg·kg−1 of nucleotides, 1000 mg·kg−1 of β-glucans, 1000 mg·kg−1 of ascorbic acid (vitamin C), and 20 mg·kg−1 of alpha-tocopherol (vitamin E), and another without. The combined supplementation of nucleotides, β-glucans, ascorbic acid, and alpha-tocopherol resulted in a 59.95% increase in final weight, 64% weight gain, 66% daily gain, a 21.31% decrease in feed conversion rate, and double the retention of body protein. Supplementation also improved intestinal morphology and modulated the intestinal microbiome, increasing Chao-1 diversity. Transmission electron microscopy confirmed that fish fed with both diets exhibited intact intestinal mucosal membranes. Supplementation did not alter the hematological and immunological parameters, suggesting that there was no overstimulation of the fish’s immune system. This work allows us to evaluate the effect of reducing the use of animal protein in the diets of fish, along with the effects of nucleotides, β-glucans, ascorbic acid, and alpha-tocopherol. Together, these compounds can provide fish with the necessary tools to achieve optimal health and growth.
Key Contribution:
A functional mixture of immunomodulators, including β-glucans, nucleotides, ascorbic acid, and alpha-tocopherol, associated with a diet with lower levels of animal protein and higher levels of soybean meal, improved the growth and intestinal morphology, and modulated the intestinal microbiome, of Nile tilapia Juveniles.
1. Introduction
Functional feeds represent an emerging standard in the development of fish and crustacean diets [1], encouraging the use of immune-stimulating compounds as preventive strategies to limit and/or control fish diseases [2]. Among the immune-stimulating compounds, food additives such as β-glucans and nucleotides stand out, being commonly added to functional diets to improve the immunity [3,4] and zootechnical performance of animals [5,6].
Although the use of food additives has been widely studied, the mechanisms by which these additives alter the metabolism and health of fish are still not well understood [7]. Furthermore, while researchers have reported that the individual use of β-glucans, nucleotides, and vitamins C and E can improve the health and zootechnical performance of Nile tilapia [8,9,10,11], knowledge about their combined use is scarce.
The effects of β-glucans in the diet have been extensively investigated in fish [12,13], with β-glucans being the most widely used immune-stimulant in aquaculture. β-glucans are carbohydrates composed of glucose molecules, which are the main structural components of fungal, bacterial, plant, and algal cell walls [14]. These compounds differ in molecular weight and branching degree depending on their source [14,15]. These polysaccharides can be recognized by the immune systems of different vertebrate species and can promote a healthier gut due to their prebiotic effects [15].
Another molecule of interest in the formulation of functional diets is nucleotides. Nucleotides are the basic building blocks for the synthesis of deoxyribonucleic acid (DNA), ribonucleic acid (RNA), adenosine triphosphate (ATP), and the key coenzymes involved in essential metabolic processes [8]. Nucleotides have recently shown promise as a dietary supplement to enhance immunity and disease resistance in fish and shrimp production systems [12]. Under normal conditions, nucleotide production occurs through the salvage pathway (recycling from dead cells) or de novo synthesis from amino acids [12]. De novo production occurs in most cells, but some cells, such as intestinal cells, hepatocytes, and immune cells, have limited synthesis capacity and require an exogenous supply, especially under conditions such as tissue injury, liver dysfunction, diseases, the reproductive period, and the rapid growth stage [12,16]. Nucleotides have also been tested for developing functional feeds with reduced amounts of fishmeal. These molecules have been used as functional additives in alternative protein-based diets, where nucleotides have been found to reduce the negative effects of alternative proteins, improving the efficiency of their utilization and leading to improved zootechnical indices and health in aquatic organisms [12].
Vitamins C and E are important antioxidant additives; they reduce oxidative stress in animals, and their combined use increases antioxidant potential [17]. In most aquatic species, especially fish, vitamin C is not biosynthesized due to the absence of the final enzyme, L-gulonolactone oxidase, in the biosynthetic pathway [18]. Since vitamin C is an essential micronutrient, it must be included in the diet [19].
It is worth noting that the above-mentioned food additives contribute positively to environmental and economic sustainability. This is essential for increasing global aquaculture production, especially in intensive production systems, since health and functionality are directly correlated with the economic performance of aquaculture farms [1].
The present study aims to evaluate the effect of the combination of β-glucans, nucleotide molecules, ascorbic acid, and alpha-tocopherol on the zootechnical performance, hemato-immunological parameters, and intestinal health of Nile tilapia juveniles (O. niloticus).
2. Materials and Methods
Fish management procedures followed protocol number 8409161118, which was approved by the Animal Use Ethics Committee of the Federal University of Santa Catarina (CEUA, UFSC).
2.1. Experimental Diets
The diets were formulated to meet the nutritional requirements of Nile tilapia, following the Nutrient Requirements of Fish and Shrimp [20] guidelines (Table 1). An isocaloric and an isoproteic diet were formulated, and the effects of the dietary inclusion or exclusion of the functional immunostimulant mixture (Rovimax®, DSM, Heerlen, the Netherlands) were evaluated. The boosting immunostimulant contained 150 mg·kg−1 nucleotides, 1000 mg·kg−1 β-glucans, 1000 mg·kg−1 of ascorbic acid, and 20 mg·kg−1 of alpha-tocopherol (Table 1).

Table 1.
Centesimal formulation and composition (in dry matter) of control diet and that supplemented with functional immunostimulant mixture.
The dose of the functional immunostimulant mixture feed additive (40 kg·t−1) was established according to the manufacturer’s recommendation. Therefore, the concentration of each compound in the feed was equivalent to 6 mg·kg−1 of nucleotides and 40 mg·kg−1 of β-glucans, along with 40 mg·kg−1 of ascorbic acid and 0.8 mg·kg−1 of alpha-tocopherol. The vitamins present in the functional immunostimulant mixture were thermoprotected.
The feed for each diet was formed through extrusion into 2 mm pellets. A horizontal mixer (Inbramaq, Ribeirão Preto, Brazil) was used to mix the dry components, and extrusion was performed using a single-screw extruder (MX40, Inbramaq, Ribeirão Preto, Brazil). The extrusion conditions were previously tested and adjusted to a temperature of 85 °C at the barrel head and 24% humidity using deionized water. After extrusion, the feed was dried in an oven at 50 °C for 4 h, followed by packaging and storage at −20 °C until further use.
2.2. Fish and Experimental Conditions
The experimental fish used in this study were male Nile tilapia (Oreochromis niloticus) juveniles, which were sexually reversed and belonged to the GIFT lineage. These fish were obtained from the Santa Catarina Agricultural Research and Rural Extension Company (EPAGRI).
To acclimate the fish in preparation for the trial, they were initially kept in two 100 L tanks connected to a recirculating aquaculture system (RAS) for a period of 21 days. During the acclimatization period, the fish were fed a control diet. Subsequently, 120 fish were redistributed randomly among the experimental units (100 L tanks), with 20 fish (initial weight 1.88 ± 0.25 g, mean ± standard deviation) assigned to each; each tank received one dietary treatment with three replications per treatment, totaling six experimental units. The amount of feed given was weighed daily, and the individual feed consumption was recorded for each tank. The fish were fed to apparent satiety four times a day for a period of 50 days.
All the tanks were connected to an RAS system with a continuous flow rate of 0.55 L/min. The system was equipped with mechanical and biological filters, as well as an ultraviolet disinfection system. The temperature and dissolved oxygen levels of the water were monitored daily, while other water quality parameters were monitored on a weekly basis. Measurements were taken using a digital oximeter (for temperature and oxygen), a multiparametric sensor (for pH), and a colorimetric kit (for total ammonia, nitrite, and alkalinity). The recorded water quality parameters remained within the ideal range for Nile tilapia [21,22], with the following mean ± standard deviation values: temperature = 27.67 ± 0.35 °C, dissolved oxygen = 8.45 ± 0.74 mg·L−1, pH = 7.87 ± 0.12, total ammonia = 0.14 ± 0.09 mg·L−1, nitrite = 0.01 ± 0.00 mg·L−1, and alkalinity = 40 ± 0.00 mg CaCO3·L−1.
2.3. Sampling Procedure
At the end of the 50-day supplementation period, all the fish in each tank were individually weighed to assess their zootechnical parameters. Three fish per tank were used to perform the proximate analysis. Blood was collected from nine fish per tank for hematological and immunological analyses. For the immunological analyses, three pools per tank were created, totaling nine pools per treatment. The same fish used for blood collection were also used for tissue collection for histology (three fish per tank), metagenomics (three fish per tank), and transmission electron microscopy (three fish per tank), totaling nine fish per treatment for each type of analysis.
2.4. Growth Performance and Survival
At the conclusion of the feeding trial, the following parameters were calculated. using data on live weight and feed consumption to evaluate the growth and feed utilization of the fish:
Weight gain (g) = final weight (g) − initial weight (g)
Daily weight gain (g) = (final weight (g) − initial weight (g))/duration of the feeding period
Feed conversion ratio = quantity of feed offered/weight gain
Protein retention (PR, %) = [(final body weight × final body protein) −
(initial body weight × initial body protein)]/protein intake
(initial body weight × initial body protein)]/protein intake
Survival (%) = (final number of fish/initial number of fish) × 100
2.5. Proximate Analysis
A proximate composition analysis of feed and carcass samples was conducted at the Nutrition Laboratory (LabNutri/UFSC) of the Federal University of Santa Catarina (UFSC), following the standard method of the Association of Official Analytical Chemists [23]. Triplicate samples of feed per diet and triplicate samples of carcass per tank, totaling three samples of feed and nine samples of carcass per diet, were homogenized, freeze-dried, and analyzed to determine the content of crude protein, moisture, crude lipid, ash, and nitrogen-free extract.
The moisture content of the samples was determined gravimetrically by drying them in an oven at 105 °C for 1 h until a constant weight was reached, and the percentage of moisture was calculated. The crude protein content (Nx6.25) was analyzed in the dried samples following the Kjeldahl method (Method No. 978.04) after acid digestion [23]. Crude fat was determined using the Soxhlet extraction method with petroleum ether as the solvent (60–80 °C) for 16 h (Method No. 930.09) [23]. The ash content was determined by incinerating the samples in a muffle furnace at 550 °C (Method No. 930.05) [23]. Crude fiber (CF) was determined through acid (sulfuric acid, 1.25% w/v) and base (sodium hydroxide, 1.25% w/v) digestion of defatted samples (Method No. 975.03). The nitrogen-free extract (NFE) was calculated using the difference method as per the AOAC [23] guidelines.
The dietary energy values were calculated using the caloric values 23.9, 39.8, and 17.6 MJ·g−1 for protein, lipids, and NFE, respectively, based on the recommendations of the Nutrient Requirements of Fish and Shrimp [20].
2.6. Hematological Parameters
At the conclusion of the feeding experiment, 9 animals per experimental unit, 27 per treatment, were anesthetized with Eugenol Vetec® (Darmstadt, Germany) (75 mg·L−1), and blood was collected by puncturing the caudal vessel, using a syringe containing ethylenediaminetetraacetic acid (10% EDTA) solution for different hematological analyses.
A portion of the blood was used to determine the levels of hematocrit, using the microhematocrit method [24]. Another portion of the blood was used for a total erythrocyte count, which was performed using a Neubauer chamber after dilution (1:200) in modified Dacie fluid [24]. The hemoglobin concentration was determined using the cyanmethemoglobin method, and hematimetric equations were applied to determine the mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), and mean corpuscular hemoglobin (MCH) [24].
2.7. Immunological Parameters
After the blood samples were collected, a pooled sample consisting of three samples from each experimental unit, nine per treatment, was allowed to rest for 1 h. Subsequently, the pool was centrifuged at 1400× g for 15 min at 4 °C to obtain blood plasma, which was stored at −20 °C for further immunological analysis.
The total plasma protein concentration was measured using a Total Protein kit (Biotécnica, Varginha, MG, Brazil), and the total immunoglobulin concentration (mg·mL−1) was measured following the method described by Amar et al. [25].
The plasma agglutinating activity was assessed in a microplate with 96 U-bottom wells. The plasma was diluted with phosphate-buffered saline (PBS) at a 1:1 ratio (50 μL PBS: 50 μL plasma solution) in the first well. Subsequently, the plasma was serially diluted into the other wells at a ratio of 1:2. Then, 50 μL of inactivated Streptococcus agalactiae S13 serotype Ib [26] at a concentration of 1 × 108 colony-forming units (CFU) mL−1 was added to all wells. The microplate was then incubated at 25 °C for 18 h in a humid chamber. The titer was determined as the reciprocal of the last dilution showing observable agglutination, where aggregates could be seen at the bottom of the well with the naked eye [27].
Furthermore, the minimum inhibitory concentration (MIC) titer was determined in a 96-well flat-bottom microplate. Initially, 150 µL of brain–heart infusion broth (BHI) was added to the first well, while 100 µL was added to the remaining wells. Then, 50 µL of plasma was added to the first well, and serial dilution by a factor of two was performed until the last well was reached. Subsequently, 20 µL of the Streptococcus agalactiae bacterial suspension (1 × 108 CFU mL−1) diluted in BHI was added. The microplate was incubated at 28 °C for 24 h. The lowest inhibitory concentration was determined as the last dilution of plasma where bacterial growth was completely inhibited [27].
2.8. Histological Analysis
After 50 days of feeding, three fish per experimental unit (9 per treatment) were anesthetized using eugenol solution (75 mg·L−1) and euthanized via medullary sectioning to obtain histological samples. Fragments of the liver, spleen, and median intestine were fixed in 10% buffered formalin. Subsequently, the tissues were dehydrated using a series of ethanol solutions, clarified in xylene, and finally, embedded in paraffin at 60 °C. Fragments 3–5 μm in thickness (PAT-MR10 microtome) were stained with Harris hematoxylin–eosin. After staining, the slides were mounted on Entellan® medium and analyzed under an Axio Imager A.2 (DIC) phase-interference contrast microscope (Zeiss, Gottingen, Germany).
During morphological analysis of the median intestine, the following parameters were measured in each histological section: the number of intestinal folds, length (µm) and width (µm) of the intestinal folds, number of goblet cells, total area (µm2), and total perimeter (µm). The length, width, perimeter, and area of the villi were measured using Zen Pro software 2.3 (Zeiss, Oberkochen, Germany).
For the histological analysis of all organs, using the method described by Schwaiger et al. [28] with minor modifications [29], the following values were assigned to histological alterations based on the degree of alteration intensity: 0 (no alteration), 1 (slight alteration, corresponding to <25% of the tissue area), 2 (moderate alteration, 25–50% of the tissue area), and 3 (severe alteration, >50% of the tissue area).
Furthermore, the following organ–cell alterations were considered in the liver: the cordonal appearance of the hepatocytes; uniform size of the cells and nuclei; intact acid and zymogen granules in the pancreas; balloon-like appearance in the hepatocytes; pancreas and sinusoid congestion; eosinophilic and lymphocytic infiltrates; hepatocyte hypertrophy; loose melanomacrophages; and macrosteatosis, microsteatosis, and necrosis. In the spleen, alterations in the integrity of the white and red pulps and the centers of melanomacrophages, loose melanomacrophages, and necrosis were evaluated. In the median intestine, histological alterations were evaluated as eosinophilic infiltrate and loose melanomacrophages.
2.9. Transmission Electron Microscopy (TEM)
To assess the integrity of intestinal cells and microvilli, samples of the intestinal tract were fixed in a solution containing 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2) with 0.2 M sucrose. They were then post-fixed in 1% osmium tetroxide for 4 h. Dehydration was performed using a series of graded acetone concentrations, and the samples were subsequently embedded in Spurr’s resin [30]. Ultrathin sections were prepared using an ultramicrotome (Leica, Reicheit Ultracut S, Vienna, Austria) and contrasted with uranyl acetate and lead citrate. Photomicrographs were taken using a TEM JEM 1011 (JEOL, Tokyo, Japan) operating at 80 kV.
2.10. Statistical Analysis
All the collected data were assessed for normality using the Shapiro–Wilk test, and the homogeneity of variance was examined using the Levene test. Subsequently, the data underwent a t-test. The statistical analyses were conducted using Statistica 10.0 software (Statsoft Inc., Tulsa, OK, USA). Whenever necessary, data transformations were applied. A significance level of 5% was considered for all tests.
2.11. Metagenomic Analysis
At the conclusion of the experiments, three fish from each tank were anesthetized with Eugenol® (75 mg·L−1) and euthanized via spinal cord section. Subsequently, each fish was aseptically opened with a sterilized scalpel to extract the midgut, and the samples were individually stored in RNAse- and DNAse-free cryotubes and stored in liquid nitrogen until further analysis.
For the extraction of deoxyribonucleic acid (DNA) from bacteria in the collected material, 200 mg of the portion comprising the fish midgut (pools of five fish per experimental unit) was used. The QIAamp® Fast DNA Stool Mini kit (QIOGEN, Hilden, Germany) was utilized following the supplier’s specifications. Subsequently, the extracted DNA was quantified using a NanoDrop™ 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and maintained at a concentration above 100 μg μL−1.
After DNA extraction, the samples were sent to Neoprospecta® for metagenomic analysis. For this purpose, amplification of the 16S ribosomal RNA (rRNA) gene region from the preserved V3 and V4 regions was performed.
For sequencing, Illumina SBS technology was employed, which marks nucleotides with fluorescence when they bind to their complementary strand in each cycle. Sequences with noise were removed, and the remaining representative reads from the clusters were grouped using the fast length adjustment of short reads (FLASH) algorithm. The reads with 100% identity (ID) were clustered into a single file using CD-HIT-DUP. Operational Taxonomy Units (OTUs) were collected using a quality filter to ensure 97% ID at the species level. The minimum alignment for sequencing was set at 300 bp, with 100 k reads per sample. The sequences were analyzed using Quantitative Insights into Microbial Ecology (QIIME).
A bioinformatic analysis was performed using the QIIME program, following the methodology of Langille et al. [31], where the created OTUs were related to their corresponding taxa through the “assign_taxonomy.py” tool and compared with the Greengenes database http://qiime.org (URL accessed on 28 November 2023). To estimate bacterial richness and diversity, an alpha rarefaction analysis was performed and the Sequencing Coverage, Chao1, and Shannon indices calculated using the “alpha_diversity.py” tool. The Sequencing Coverage index (C = 1 − (S/n)), where S is the number of unique OTUs and n is the number of individuals in the sample, was calculated to express how much the sample represents the environment in relative terms. The Shannon diversity index, which considers both the number and evenness of species distribution; the Chao1 richness index, which estimates the Chao1 richness for an OTU definition; and the Inverse Simpson index (), where s is the number of OTUs and pi is the proportion of the community represented by OTU i, were calculated. A Venn diagram was designed to determine unique bacterial OTUs and those shared between treatments using the InteractiVenn program https://www.interactivenn.net (URL accessed on 28 November 2023). A heat map for the phylum was generated using Heatmapper [32].
3. Results
3.1. Growth Performance, Survival, and Proximate Composition
In our study, the inclusion levels of nucleotides and β-glucans for 50 days were able to improve the final weight by 59.95%, the weight gain by 64%, the daily gain by 66%, and food conversion by 21.31%, and double the body protein retention of Nile tilapia juveniles.
Fish that received the diet supplemented with the functional immunostimulant mixture showed higher values (p < 0.05) compared to those fed with the control diet (Table 2). No statistical difference was found between the survival values.

Table 2.
Zootechnical parameters of tilapia juvenile fed for 50 days with control diet and that supplemented with functional immunostimulant mixture (40 g·kg−1).
Regarding body composition, only mineral matter, at 2.21 ± 0.184 (p = 0.0376), was affected by the diet supplemented with the functional immunostimulant mixture at the end of the 50-day supplementation period (Table 3).

Table 3.
Body composition (g·kg−1 dry weight) of tilapia juvenile fed for 50 days with control diet and that supplemented with functional immunostimulant mixture (40 g·kg−1).
3.2. Hematological and Immunological Parameters
The hematological and immunological parameters were not affected by the diet supplemented with the functional immunostimulant mixture at the end of the 50-day supplementation period (Table 4).

Table 4.
Hematological and immunological parameters of Nile tilapia juveniles fed for 50 days with control diet and that supplemented with functional immunostimulant mixture (40 g·kg−1).
3.3. Histological Analysis
After the 50-day period of supplementation with the functional immunostimulant mixture, the length (37.88 ± 4.20 µm, p = 0.0481) and width (17.29 ± 2.89 µm, p = 0.0380) of the intestinal folds and the number of goblet cells (501.55 ± 83.82, p = 0.0389) increased (Figure 1 and Table 5). However, no significant statistical differences were observed in the intestine, spleen, and liver histology among the different treatments (Table 5 and Table 6).
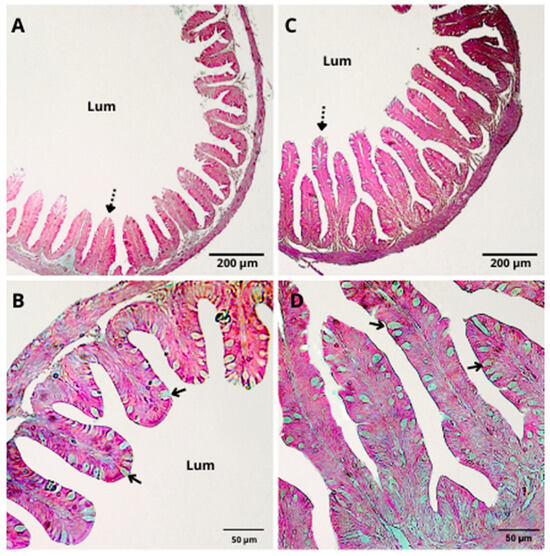
Figure 1.
H&E staining of the intestinal epithelium of Nile tilapia juveniles after 50 days of feeding with a control diet (A,B) or a diet supplemented with a functional immunostimulant mixture (40 g·kg−1) (C,D). Dashed arrows indicate intestinal folds; solid arrows indicate goblet cells; Lum: luminal surface. (A,C) The bar is equivalent to 200 µm; (B,D) the bar is equivalent to 50 µm.

Table 5.
Morphology and intensity of histological changes in intestine of Nile tilapia juveniles fed for 50 days with control diet and that supplemented with functional immunostimulant mixture (40 g·kg−1).

Table 6.
Intensity of histological changes in liver and spleen tissue of Nile tilapia juveniles fed for 50 days with control diet and that supplemented with functional immunostimulant mixture (40 g·kg−1).
Transmission Electron Microscopy (TEM)
The TEM confirmed that animals fed both diets showed total integrity of the intestinal mucous membrane. The intestinal epithelium was found to be intact, with well-defined cells, showing neither vacuoles nor intercellular spaces (Figure 2).
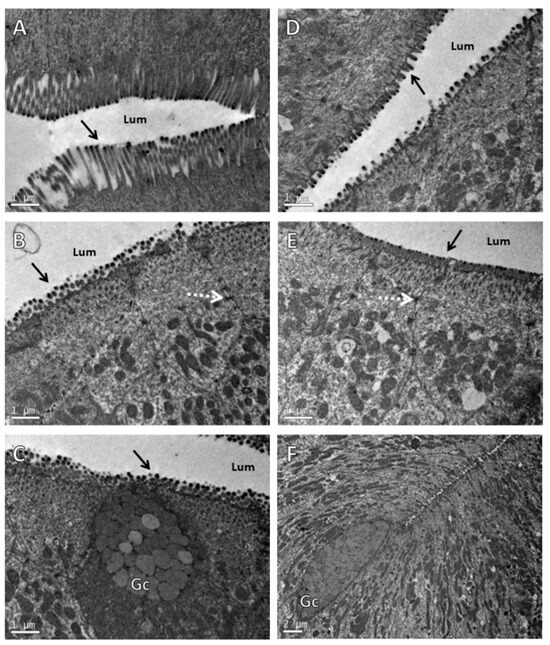
Figure 2.
Transmission electron microscopy of intestinal epithelium of Nile tilapia juveniles fed for 50 days with control diet (A–C) and that supplemented with functional immunostimulant mixture (40 g·kg−1) (D–F). Arrows shows microvilli; dashed arrow indicates tight junction; Lum: luminal surface; Gc: cell containing mucous secretory granules typical of intestinal goblet cells. (A–D) The bar is equivalent to 1 µm. (F) The bar is equivalent to 2 µm.
3.4. Metagenomic Analysis
A total of 49,532 bacterial 16S rRNA reads were generated from the Nile tilapia intestinal samples, with sequence libraries ranging from 16,628 to 32,904 reads. Based on the total number of OTU reads, it was observed that all samples had a coverage greater than 99.5% (Table 7). In total, 39 different species belonging to 20 genera and 4 phyla were identified across all samples.

Table 7.
Operational Taxonomy Units (OTU) at the species level; Chao1, Shannon, and Inverse Simpson indices; and Good’s Coverage estimated in intestinal microbiome samples of Nile tilapia fed for 50 Days with control and immunomodulator functional blend-supplemented diets (40 g·kg−1).
The Chao richness and alpha diversity indices (Shannon and Simpson) are presented in Table 7. The Shannon and Simpson index values were higher in the control group. Chao richness, on the other hand, was higher in the supplemented group than in the control group.
The analysis of the heat map (Figure 3) revealed a higher abundance of the Fusobacteria phylum in both groups.
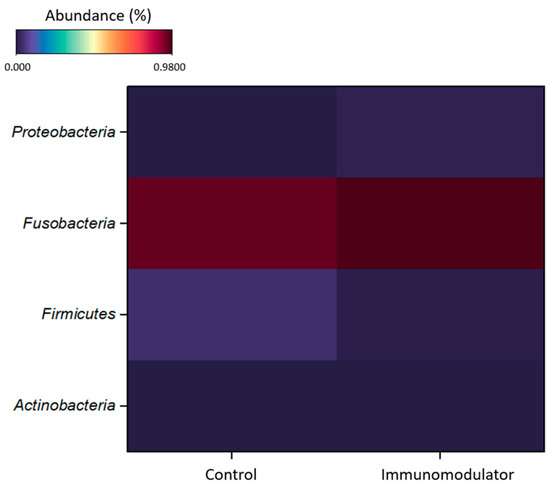
Figure 3.
Heat map, at phylum level, of Nile tilapia (Oreochromis niloticus) intestinal microbiome after 50 days of feeding with control diet and diet supplemented with functional blend of immunomodulators (40 g·kg−1).
The Venn diagram shown in Figure 4 presents the number of Operational Taxonomic Units (OTUs) at the species level that are unique or shared between treatments. A total of 39 OTUs were identified. The control group exhibited the lowest number of OTUs (11), while the supplemented group showed a higher number of OTUs (29). Seven bacterial species were shared between the control group and the group receiving supplementation (Acinetobacter johnsonii, Cetobacterium somerae, Clostridium ruminantium, Paenibacillus cellulosilyticus, Paenibacillus kobensis, Plesiomonas shigelloides, and Serratia liquefaciens).
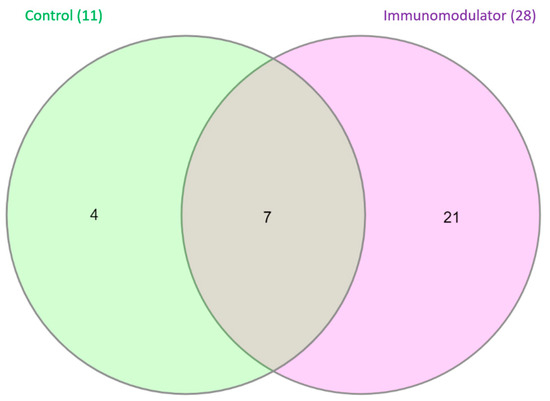
Figure 4.
Representation of Operational Taxonomic Units (OTUs) at species level in Nile tilapia (Oreochromis niloticus) after 50 days of feeding with control diet and diet supplemented with functional blend of immunomodulators (40 g·kg−1).
The diversity index revealed that the most abundant genus was Cetobacterium in both treatments. The control group exhibited lower species diversity than the other treatment groups (Figure 5). In the control group, the genera Cetobacterium (95%) and Clostridium (4.4%) were the most abundant (Figure 5). In contrast, in the group that received supplementation, the most abundant genera were Cetobacterium (97%), Clostridium (0.69%), Aeromonas (0.68%), and Plesiomonas (0.41%). These differences are more noticeable when analyzing the diversity index, except for the genus Cetobacterium (Figure 5B).
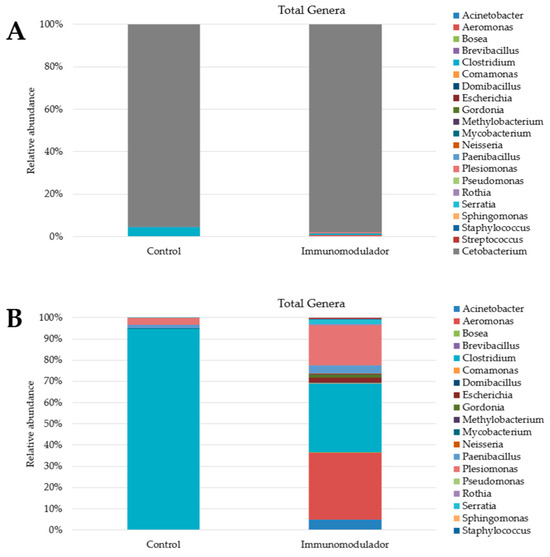
Figure 5.
(A) Diversity index, at genus level, of Nile tilapia (Oreochromis niloticus) intestinal microbiome after 50 days of feeding with control diet and diet supplemented with functional blend of immunomodulators (40 g·kg−1). (B) Diversity index, at genus level, of Nile tilapia (Oreochromis niloticus) intestinal microbiome after 50 days of feeding with control diet and diet supplemented with functional blend of immunomodulators (40 g·kg−1), except for the genus Cetobacterium.
4. Discussion
Longer periods of immunomodulator administration have been associated with the overstimulation and consequent exhaustion of the immune system [9,33,34]. Supplementation periods exceeding 45 days are considered long [33]. No adverse effects on fish health and growth were observed in our study with a 50-day supplemented diet. Fish fed the supplemented diet showed a better weight gain, and no statistical differences in survival rates were found. The inclusion level of β-glucans we determined is considered low (0.004%) according to other authors who detected higher inclusion levels: 0.1% in Nile tilapia juveniles (fed for 45 days) [33]; 0.5% in Piaractus mesopotamicus juveniles (fed for 10 days) [35]; and levels up to 0.05% in Oncorhynchus mykiss juveniles (fed for 6 weeks) [8].
The improved zootechnical performance observed in fish receiving the supplemented diet can also be attributed to nucleotide inclusion. Nucleotides have been studied as functional ingredients in alternative protein diets [12,36,37]. Nucleotide inclusion can mitigate the harmful effects of diets formulated with large amounts of soybean meal [38]. Nucleotides supplemented at 120–240 mg·kg−1 for 10 weeks in a low-fishmeal (6%) and high-soybean meal (56%) diet improved immune responses and survival after challenge with Streptococcus iniae [37].
In our study, the dietary inclusion of an immunomodulator functional mixture (40 kg·ton−1) associated with a diet with a relatively low amount of animal protein (11.5%) and a higher amount of soybean meal (43.5%) was able to improve the zootechnical performance and health of fish. An increase in the amount of mineral matter in the proximate body composition of fish receiving the supplemented diet occurred, because this diet contained higher levels of mineral matter compared to the control diet.
Prolonged and excessive doses of β-glucans can result in inadequate immune responses, likely due to the activation of stress response mechanisms [33,35,39]. Stress responses generally involve changes in defense systems (e.g., heat shock proteins, antioxidant systems, immunity) and, more importantly, in energy metabolism. Fish exposed to stressful mechanisms tend to show increased energy consumption and antioxidant defenses, and as the energy demand for maintenance increases, the energy available for growth, reproduction, activity, and the storage of reserves decreases. This can result in a temporary reduction in body mass, growth, and/or reproduction [40].
A strategy to avoid stress responses following supplementation with immunomodulators is to add vitamins E and C along with the low inclusion of β-glucans and nucleotides. Vitamin C and vitamin E can act synergistically as antioxidants. Vitamin C has also been proposed to be beneficial for stimulating the immune response and reducing oxidative damage in tissues [41]. Vitamin E is involved in the immune response, and one of its main physiological functions is to protect membranes against oxidative damage [42].
Research that observed the effect of the dietary inclusion of fermented extracts from Saccharomyces cerevisiae, corresponding to inclusion levels of 252 mg·kg−1 of nucleotides, 940 mg·kg−1 of β-glucans, and 568 mg·kg−1 of MOS, was able to enhance the nutrition, immunity, and intestinal histomorphology of Nile tilapia breeders [16]. In line with these findings, in the current study, the intestinal histomorphology of Nile tilapia juveniles was improved by the diet supplemented with immunomodulators. The hematological and immunological parameters did not show statistical differences, probably due to lower supplementation doses (6 mg·kg−1 of nucleotides and 40 mg·kg−1 of β-glucans). This suggests that the fish were able to allocate their energy resources more efficiently, without the need for prolonged periods of compromise to immune system stimulation.
These findings are consistent with those described by other authors, where European sea bass (Dicentrarchus labrax) fed a diet supplemented with 0.15% and 0.3% nucleotides showed a trend of improved growth performance [38]. Furthermore, the dietary provision of nucleotides positively influenced the functional topography of the gastrointestinal tract of European sea bass, both at the macro and ultrastructural levels. In our study, transmission electron microscopy (TEM) confirmed that animals fed both diets showed complete integrity of the intestinal mucosal membrane.
We identified an improvement in intestinal health that likely reflected a significant enhancement in zootechnical performance and protein retention levels. Intestinal morphology reflects the association of the health status of fish with their ability to assimilate nutrients and with immune function [43]. These factors may have contributed to the improved zootechnical indices in fish supplemented with the immunomodulator mixture, as an increase in the length of the intestinal folds, the fold width, and the number of goblet cells was observed in the supplemented group. An increase in the surface area of the intestinal folds indicates an improvement in intestinal health and an increase in nutrient absorption capacity [44,45].
An increase in the number of goblet cells results in greater mucus production. The mucus from the fish epithelium plays several roles; for example, it acts as an integral component of the innate immune mechanism and as a mechanical barrier that hinders the entry of pathogenic bacteria, and it contains various antimicrobial components of the innate immune response, such as lysozymes, immunoglobulins, complement system proteins, and lectins [45]. These functions can help establish a beneficial bacterial microbiome in the immune system for better nutrient absorption, which, in turn, may lead to improved zootechnical rates in groups supplemented with an immunomodulator mixture. The improved optimization of feed nutrients also enhanced the protein retention efficiency, consequently reducing the release of residual nutrients (ammonia and phosphorus) into the environment [46].
The intestinal microbiome of fish is composed of microorganisms living in association with their hosts, including commensal, symbiotic, and pathogenic communities; they play important physiological roles in the host’s overall health and immunity, providing a nutrient-rich environment for the development and maintenance of these microorganisms [47,48]. In this work, a coverage exceeding 99.5% of the samples indicates that microbial communities were highly represented in the metagenomic analysis, thereby reinforcing the reliability of our results.
Cetobacterium somerae (a Fusobacteriaceae) had the highest number of reads and proportional abundance in our study. The genus Cetobacterium has characteristics such as amino acid production, involvement in various metabolic activities, and vitamin synthesis, playing an important role in fish nutrition [49,50]. C. somerae is a native bacterium in the intestinal tract of cultivated freshwater fish and is highly efficient at producing vitamin B12, an essential micronutrient [51,52]. In the supplemented group, the increase in the relative abundance of the genus Cetobacterium, especially the species C. somerae, may have contributed to maintaining intestinal health. On the other hand, the relative abundance of the genus Cetobacterium in the supplemented group determined that the Shannon and Simpson indices were higher in the control group.
These results suggest that supplementing fish diets with a functional mixture of immunomodulators, including nucleotides and β-glucans, along with antioxidants such as vitamins C and E, may improve intestinal health, the Chao-1 richness index, the efficiency of zootechnical performance, and protein retention, even in diets with low animal protein content and high soybean meal content. However, further research is needed to fully understand the long-term effects and best supplementation practices in different fish species and cultivation conditions.
Additional studies are still needed, but our work allows us to evaluate the effect of reducing the use of animal protein in the diets of fish, along with the effects of nucleotides, β-glucans, ascorbic acid, and alpha-tocopherol. Together, these compounds can provide fish with the necessary tools to achieve optimal health and growth.
5. Conclusions
Supplementation with nucleotides, β-glucans, ascorbic acid, and alpha-tocopherol resulted in enhanced growth and improved intestinal morphology, leading to better nutrient utilization, an increased Chao-1 richness index, and improved feed conversion and protein retention in Nile tilapia juveniles. Supplementation did not cause alterations in the hematological and immunological parameters of the fish, suggesting that they did not need to mobilize energy for the maintenance of prolonged immune stimulation.
Author Contributions
Conceptualization, L.S.d.S.; formal analysis, L.S.d.S.; investigation, L.S.d.S.; methodology, D.M.F.; data curation, T.S. and E.Y.; writing—original draft preparation, L.S.d.S.; writing—review and editing, G.T.J.; supervision, J.L.P.M.; project administration, J.L.P.M.; funding acquisition, J.L.P.M. and M.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development (CNPq), which provided financial support and a research grant to M.L. Martins (CNPq 305869/2014–0) and J.L.P. Mouriño (CNPq 301524/2017–3), Coordination for the Improvement of Higher Education Personnel (CAPES) for awarding a Ph.D. scholarship to L. S. Sá and providing financial support (Finance Code 001).
Institutional Review Board Statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All animal procedures were approved by the Ethics Committee on Animal Use (CEUA/UFSC 6882170516).
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank the staff of the Central Laboratory of Electron Microscopy (LCME), Federal University of Santa Catarina, Florianopolis, Santa Catarina, Brazil, for the use of their transmission electron microscope.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zuberi, A.; Kamran, M.; Younus, N.; Abdel-Tawwab, M. Editorial: Functional Feed Additives: Current Trends. Front. Aquac. 2024, 3, 1385508. [Google Scholar] [CrossRef]
- Dimitroglou, A.; Merrifield, D.L.; Carnevali, O.; Picchietti, S.; Avella, M.; Daniels, C.; Güroy, D.; Davies, S.J. Microbial Manipulations to Improve Fish Health and Production—A Mediterranean Perspective. Fish Shellfish Immunol. 2011, 30, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Aramli, M.S.; Kamangar, B.; Nazari, R.M. Effects of Dietary β-Glucan on the Growth and Innate Immune Response of Juvenile Persian Sturgeon, Acipenser persicus. Fish Shellfish Immunol. 2015, 47, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Cornet, V.; Khuyen, T.D.; Mandiki, S.N.M.; Betoulle, S.; Bossier, P.; Reyes-López, F.E.; Tort, L.; Kestemont, P. GAS1: A New β-Glucan Immunostimulant Candidate to Increase Rainbow Trout (Oncorhynchus mykiss) Resistance to Bacterial Infections with Aeromonas salmonicida achromogenes. Front. Immunol. 2021, 12, 693613. [Google Scholar] [CrossRef] [PubMed]
- Vijayaram, S.; Ringø, E.; Zuorro, A.; van Doan, H.; Sun, Y. Beneficial Roles of Nutrients as Immunostimulants in Aquaculture: A Review. Aquac. Fish 2023, in press. [CrossRef]
- Yang, G.; Zhang, Z.; Kumar, V. Editorial: Functional Feed Additives and Intestinal Health in Aquatic Animals. Front. Physiol. 2024, 15, 1385046. [Google Scholar] [CrossRef] [PubMed]
- Elkatatny, N.M.; El Nahas, A.F.; Helal, M.A.; Fahmy, H.A.; Tanekhy, M. The Impacts of Seasonal Variation on the Immune Status of Nile Tilapia Larvae and Their Response to Different Immunostimulants Feed Additives. Fish Shellfish Immunol. 2020, 96, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Menanteau-Ledouble, S.; Skov, J.; Lukassen, M.B.; Rolle-Kampczyk, U.; Haange, S.-B.; Dalsgaard, I.; von Bergen, M.; Nielsen, J.L. Modulation of Gut Microbiota, Blood Metabolites, and Disease Resistance by Dietary β-Glucan in Rainbow Trout (Oncorhynchus mykiss). Anim. Microbiome 2022, 4, 58. [Google Scholar] [CrossRef]
- Machuca, C.; Méndez-Martínez, Y.; Reyes-Becerril, M.; Angulo, C. Yeast β-Glucans as Fish Immunomodulators: A Review. Animals 2022, 12, 2154. [Google Scholar] [CrossRef]
- Medagoda, N.; Chotikachinda, R.; Hasanthi, M.; Lee, K.J. Dietary Supplementation of a Mixture of Nucleotides, β-Glucan and Vitamins C and E Improved the Growth and Health Performance of Olive Flounder, Paralichthys olivaceus. Fishes 2023, 8, 302. [Google Scholar] [CrossRef]
- Abdo, S.E.; Gewaily, M.S.; Abo-Al-Ela, H.G.; Almeer, R.; Soliman, A.A.; Elkomy, A.H.; Dawood, M.A.O.; Nunes, B. Vitamin C Rescues Inflammation, Immunosuppression, and Histopathological Alterations Induced by Chlorpyrifos in Nile Tilapia. Environ. Sci. Pollut. Res. Int. 2021, 28, 28750–28763. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Koshio, S.; Kestemont, P. Recent Advances of Nucleotide Nutrition Research in Aquaculture: A Review. Rev. Aquac. 2020, 12, 1028–1053. [Google Scholar] [CrossRef]
- Rodrigues, M.V.; Zanuzzo, F.S.; Koch, J.F.A.; de Oliveira, C.A.F.; Sima, P.; Vetvicka, V. Development of Fish Immunity and the Role of β-Glucan in Immune Responses. Molecules 2020, 25, 5378. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary Modulation of Immune Function by β-Glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, M.; Li, N.; Dong, Z.; Cai, L.; Wu, B.; Xie, J.; Liu, L.; Ren, L.; Shi, B. New Insights into β-Glucan-Enhanced Immunity in Largemouth Bass Micropterus salmoides by Transcriptome and Intestinal Microbial Composition. Front. Immunol. 2022, 13, 1086103. [Google Scholar] [CrossRef]
- Abu-Elala, N.M.; Ali, T.E.S.; Ragaa, N.M.; Ali, S.E.; Abd-Elsalam, R.M.; Younis, N.A.; Abdel-Moneam, D.A.; Hamdien, A.H.; Bonato, M.; Dawood, M.A.O. Analysis of the Productivity, Immunity, and Health Performance of Nile Tilapia (Oreochromis niloticus) Broodstock-Fed Dietary Fermented Extracts Sourced from Saccharomyces cerevisiae (Hilyses): A Field Trial. Animals 2021, 11, 815. [Google Scholar] [CrossRef]
- Gao, J.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; Edward, R.; Mamauag, P. Interactive Effects of Vitamin C and E Supplementation on Growth Performance, Fatty Acid Composition and Reduction of Oxidative Stress in Juvenile Japanese Fl Ounder Paralichthys olivaceus Fed Dietary Oxidized Fi Sh Oil. Aquaculture 2014, 422–423, 84–90. [Google Scholar] [CrossRef]
- Abo-Al-Ela, H.G.; El-Nahas, A.F.; Mahmoud, S.; Ibrahim, E.M. Vitamin C Modulates the Immunotoxic Effect of 17α-Methyltestosterone in Nile Tilapia. Biochemistry 2017, 56, 2042–2050. [Google Scholar] [CrossRef]
- Trichet, V.V.; Santigosa, E.; Cochin, E.; Gabaudan, J. The Effect of Vitamin C on Urinary Excretion. In Dietary Nutrients, Additives, and Fish Health; Lee, C.S., Lim, C., Gatlin, D.M., III, Webster, C.D., Eds.; John Wiley & Sons, Ltd.: Haboken, NJ, USA, 2015; pp. 151–171. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-16338-5. [Google Scholar]
- El-Sherif, M.S.; El-Feky, A.M.I. Performance of Nile Tilapia (Oreochromis niloticus) Fingerlings. I. Effect of PH. Int. J. Agric. Biol. 2009, 11, 297–300. [Google Scholar]
- Tran-Duy, A.; Schrama, J.W.; van Dam, A.A.; Verreth, J.A.J. Effects of Oxygen Concentration and Body Weight on Maximum Feed Intake, Growth and Hematological Parameters of Nile Tilapia, Oreochromis niloticus. Aquaculture 2008, 275, 152–162. [Google Scholar] [CrossRef]
- AOAC Association of Official Analytical Chemists. Official Methods of Analysis of the AOAC International, 22nd ed.; George, W.L., Jr., Ed.; Oxford University Press: Oxford, UK, 2022; ISBN 0197610137978-0197610138. [Google Scholar]
- Blaxhall, P.C.; Daisley, K.W. Routine Haematological Methods for Use with Fish Blood. J. Fish Biol. 2018, 5, 771–781. [Google Scholar] [CrossRef]
- Amar, E.C.; Kiron, V.; Satoh, S.; Okamoto, N. Effects of Dietary b-carotene on the immune response of rainbow trout Oncorhynchus mykiss. Fish. Sci. 2000, 66, 1068–1075. [Google Scholar] [CrossRef]
- Facimoto, C.T.; Chideroli, R.T.; Gonçalves, D.D.; do Carmo, A.O.; Kalaphotakis, E.; Pereira, U.d.P. Whole-Genome Sequence of Streptococcus agalactiae Strain S13, Isolated from a Fish Eye from a Nile Tilapia Farm in Southern Brazil. Genome Announc. 2017, 5, e00917-17. [Google Scholar] [CrossRef]
- Silva, B.C.; Martins, M.L.; Jatobá, A.; Buglione Neto, C.C.; Vieira, F.N.; Pereira, G.V.; Jerônimo, G.T.; Seiffert, W.Q.; Mouriño, J.L.P. Hematological and Immunological Responses of Nile Tilapia after Polyvalent Vaccine Administration by Different Routes. Pesqui. Veterinária Bras. 2009, 29, 874–880. [Google Scholar] [CrossRef]
- Schwaiger, J.; Wanke, R.; Adam, S.; Pawert, M.; Hönnen, W.; Triebskorn, R. The Use of Histopathological Indicators to Evaluate Contaminant-Related Stress in Fish. J. Aquat. Ecosyst. Stress Recovery 1997, 6, 75–86. [Google Scholar] [CrossRef]
- Brum, A.; Cardoso, L.; Chagas, E.C.; Chaves, F.C.M.; Mouriño, J.L.P.; Martins, M.L. Histological Changes in Nile Tilapia Fed Essential Oils of Clove Basil and Ginger after Challenge with Streptococcus agalactiae. Aquaculture 2018, 490, 98–107. [Google Scholar] [CrossRef]
- Schmidt, É.C.; dos Santos, R.; Horta, P.A.; Maraschin, M.; Bouzon, Z.L. Effects of UVB Radiation on the Agarophyte Gracilaria domingensis (Rhodophyta, Gracilariales): Changes in Cell Organization, Growth and Photosynthetic Performance. Micron 2010, 41, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive Functional Profiling of Microbial Communities Using 16S RRNA Marker Gene Sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-Enabled Heat Mapping for All. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Koch, J.F.A.; de Oliveira, C.A.F.; Zanuzzo, F.S. Dietary β-Glucan (MacroGard®) Improves Innate Immune Responses and Disease Resistance in Nile Tilapia Regardless of the Administration Period. Fish Shellfish Immunol. 2021, 112, 56–63. [Google Scholar] [CrossRef]
- Sabioni, R.E.; Zanuzzo, F.S.; Gimbo, R.Y.; Urbinati, E.C. β-Glucan Enhances Respiratory Activity of Leukocytes Suppressed by Stress and Modulates Blood Glucose Levels in Pacu (Piaractus mesopotamicus). Fish Physiol. Biochem. 2020, 46, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Reda, R.M.; Selim, K.M.; Mahmoud, R.; El-Araby, I.E. Effect of Dietary Yeast Nucleotide on Antioxidant Activity, Non-Specific Immunity, Intestinal Cytokines, and Disease Resistance in Nile Tilapia. Fish Shellfish Immunol. 2018, 80, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Shiau, S.Y.; Gabaudan, J.; Lin, Y.H. Dietary Nucleotide Supplementation Enhances Immune Responses and Survival to Streptococcus Iniae in Hybrid Tilapia Fed Diet Containing Low Fish Meal. Aquac. Rep. 2015, 2, 77–81. [Google Scholar] [CrossRef]
- Bowyer, P.H.; El-Haroun, E.R.; Hassaan, M.; Salim, H.; Davies, S.J. Dietary Nucleotides Enhance Growth Performance, Feed Efficiency and Intestinal Functional Topography in European Seabass (Dicentrarchus labrax). Aquac. Res. 2019, 50, 1921–1930. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, M.; Pereiro, P.; Reyes-López, F.E.; Tort, L.; Figueras, A.; Novoa, B. Analysis of the Long-Lived Responses Induced by Immunostimulants and Their Effects on a Viral Infection in Zebrafish (Danio rerio). Front. Immunol. 2018, 9, 388727. [Google Scholar] [CrossRef] [PubMed]
- Gandar, A.; Laffaille, P.; Canlet, C.; Tremblay-Franco, M.; Gautier, R.; Perrault, A.; Gress, L.; Mormède, P.; Tapie, N.; Budzinski, H.; et al. Adaptive Response under Multiple Stress Exposure in Fish: From the Molecular to Individual Level. Chemosphere 2017, 188, 60–72. [Google Scholar] [CrossRef]
- Xu, C.M.; Yu, H.R.; Li, L.Y.; Li, M.; Qiu, X.Y.; Fan, X.Q.; Fan, Y.L.; Shan, L.L. Effects of Dietary Vitamin C on the Growth Performance, Biochemical Parameters, and Antioxidant Activity of Coho Salmon Oncorhynchus kisutch (Walbaum, 1792) Postsmolts. Genet. Res. 2022, 2022, 6866578. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M.; Jiménez, J.I.; Saleh, R.; Hernández-Cruz, C.M.; Domínguez, D.; Zamorano, M.J.; Hamre, K. Interaction between Taurine, Vitamin E and Vitamin C in Microdiets for Gilthead Seabream (Sparus aurata) Larvae. Aquaculture 2019, 498, 246–253. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef]
- Vu, V.; Muthuramalingam, K.; Singh, V.; Choi, C.; Kim, Y.M.; Unno, T.; Cho, M. Schizophyllum Commune-Derived β-Glucan Improves Intestinal Health Demonstrating Protective Effects against Constipation and Common Metabolic Disorders. Appl. Biol. Chem. 2022, 65, 9. [Google Scholar] [CrossRef]
- Subramanian, S.; MacKinnon, S.L.; Ross, N.W. A Comparative Study on Innate Immune Parameters in the Epidermal Mucus of Various Fish Species. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 148, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Elvy, J.E.; Symonds, J.E.; Hilton, Z.; Walker, S.P.; Tremblay, L.A.; Casanovas, P.; Herbert, N.A. The Relationship of Feed Intake, Growth, Nutrient Retention, and Oxygen Consumption to Feed Conversion Ratio of Farmed Saltwater Chinook Salmon (Oncorhynchus tshawytscha). Aquaculture 2022, 554, 738184. [Google Scholar] [CrossRef]
- Carballo, C.; Pinto, P.I.S.; Mateus, A.P.; Berbel, C.; Guerreiro, C.C.; Martinez-Blanch, J.F.; Codoñer, F.M.; Mantecon, L.; Power, D.M.; Manchado, M. Yeast β-Glucans and Microalgal Extracts Modulate the Immune Response and Gut Microbiome in Senegalese Sole (Solea senegalensis). Fish Shellfish Immunol. 2019, 92, 31–39. [Google Scholar] [CrossRef] [PubMed]
- de Souza, F.P.; de Lima, E.C.S.; Pandolfi, V.C.F.; Leite, N.G.; Furlan-Murari, P.J.; Leal, C.N.S.; Mainardi, R.M.; Suphoronski, S.A.; Favero, L.M.; Koch, J.F.A.; et al. Effect of β-Glucan in Water on Growth Performance, Blood Status and Intestinal Microbiota in Tilapia under Hypoxia. Aquac. Rep. 2020, 17, 100369. [Google Scholar] [CrossRef]
- Ofek, T.; Lalzar, M.; Laviad-Shitrit, S.; Izhaki, I.; Halpern, M. Comparative Study of Intestinal Microbiota Composition of Six Edible Fish Species. Front. Microbiol. 2021, 12, 760266. [Google Scholar] [CrossRef]
- Zhao, N.; Guo, J.; Zhang, B.; Liu, K.; Liu, Y.; Shen, Y.; Li, J. Heterogeneity of the Tissue-Specific Mucosal Microbiome of Normal Grass Carp (Ctenopharyngodon idella). Mar. Biotechnol. 2022, 24, 366–379. [Google Scholar] [CrossRef]
- Salger, S.A.; Reza, J.; Dec, C.A.; Wahab, M.A.; Baltzegar, D.A.; Mur, A.T.; Borsk, R.J. Enhanced Biodiversity of Gut Flora and Feed Efficiency in Pond Cultured Tilapia under Reduced Frequency Feeding Strategies. PLoS ONE 2020, 15, e0236100. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, Z.; Yi, M.; Liu, Z.; Ke, X.; Gao, F.; Cao, J.; Wang, M.; Chen, G.; Lu, M. Characterization of the Core Gut Microbiota of Nile Tilapia (Oreochromis niloticus): Indication of a Putative Novel Cetobacterium Species and Analysis of Its Potential Function on Nutrition. Arch. Microbiol. 2022, 204, 690. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).