Reproductive Conditioning of the Peruvian Scallop Argopecten purpuratus in Different Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Biological Material
2.2. Experimental Design
2.2.1. Natural Maturation of A. purpuratus Broodstock in Marine Conditions
2.2.2. Conditioning of Broodstock in the Hatchery
2.2.3. Spawning, Fertilization, Early Larval Development and Sampling of Treatments
- The gonadosomatic index (GSI) was calculated by using the following formula: GSI = (Gonad Weight/Total Body Weight) × 100;
- Percentage of spawning assessed using the fertility calculation method: Spawning% = (N° of fertilized eggs/N° of spawning broodstock)
- The number of oocytes per individual was counted in a Sedgewick-Rafter counting chamber: N° of oocytes = (N° of oocyte cells counted/Area of the counting chamber)×gram of gonadal tissue. According to the protocol used by [35]
- Percentage of fertilized oocytes, which were counted with the help of a 1 mL (50 × 20 × 1 mm) Sedgewick-Rafter counting camera using a Leica MZ10 stereoscope. The percentage of fertilized eggs was calculated with the following formula:Percentage of Fertilized Eggs = (N° of Fertilized Eggs/(N° of Fertilized Eggs + N° of Unfertilized Eggs) × 100
- Larval survival (a). The survival of the D larvae of A. purpuratus was determined 48 h after fertilization by taking samples of 20 mL from each tank and using the volumetric method: Larval survival = (N° of surviving larvae/N° of initial larvae) × 100
2.3. Feeding of the Broodstock
2.4. Size Measurements and Survival
2.5. Proximal Biochemical Composition
2.6. Statistical Analyses
3. Results
3.1. Development of Gonadal Maturation
3.2. Oocyte Evaluation
3.3. Straight-Hinge Larvae (“D” Larvae) Evaluation
3.4. Breeding Stock Conditioning in Hatchery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Molina, R.; Cerda, R.; González, E.; Hurtado, F. Simulation model of the scallop (Argopecten purpuratus) farming in northern Chile: Some applications in the decision making process. Lat. Am. J. Aquat. Res. 2012, 40, 679–693. [Google Scholar] [CrossRef]
- Stotz, W. When aquaculture restores and replaces an overfished stock: Is the conservation of the species assured? The case of the scallop Argopecten purpuratus in northern Chile. Aquac. Int. 2000, 8, 237–247. [Google Scholar] [CrossRef]
- Bakit, J.; Álvarez, G.; Díaz, P.A.; Uribe, E.; Sfeir, R.; Villasante, S.; Bas, T.G.; Lira, G.; Pérez, H.; Hurtado, A.; et al. Disentangling Environmental, Economic, and Technological Factors Driving Scallop (Argopecten purpuratus) Aquaculture in Chile. Fishes 2022, 7, 380. [Google Scholar] [CrossRef]
- Bakit, J.; Hurtado, A.; Márquez Porras, R.; Villasante, S. Navigating transformations from artisanal fishers to entrepreneurial scallop farmers in Chile. Front. Mar. Sci. 2023, 10. [Google Scholar] [CrossRef]
- Avendaño, M.; Cantillánez, M.; Riascos, J.M. The decreasing availability of settlement surfaces affects the transition from larvae to early recruitment of the scallop Argopecten purpuratus through El Niño and La Niña episodes. Front. Mar. Sci. 2019, 6, 630. [Google Scholar] [CrossRef]
- Merino, G.; Uribe, E.; Soria, G.; Von-Brand, E. A comparison of larval production of the northern scallop, Argopecten purpuratus, in closed and recirculating culture systems. Aquac. Eng. 2009, 40, 95–103. [Google Scholar] [CrossRef]
- Soria, G.; Merino, G.; Uribe, E.; Von Brand, E. Effect of increasing salinity on weight-specific filtration rate of juvenile scallop Argopecten purpuratus reared at two temperatures: Is any effect related to ammonia buildup? J. Shellfish. Res. 2011, 30, 279–286. [Google Scholar] [CrossRef]
- Velasco, L.; Barros, J. Potential for hatchery broodstock conditioning of the Caribbean scallops Argopecten nucleus and Nodipecten nodosus. Aquaculture 2007, 272, 767–773. [Google Scholar] [CrossRef]
- Utting, S.; Millican, P. Techniques for the hatchery conditioning of bivalve broodstocks and the subsequent effect on egg quality and larval viability. Aquaculture 1997, 155, 45–54. [Google Scholar] [CrossRef]
- Berntsson, K.P.J.; Wangberg, S.; Carlsson, A. Effects of broodstock diets on fatty acid composition, survival and growth rates in larvae of the European flat oyster, Ostrea edulis. Aquaculture 1997, 154, 139–153. [Google Scholar] [CrossRef]
- Benavides, C.K.; Borrell, J.M.; García, C.R.; Gómez, K.K.; Gonzales, A.K. Design and Management of a Bivalve Cultivation System in Sechura Bay; University of Piura: Piura, Perú, 2018; pp. 34–35. Available online: https://hdl.handle.net/11042/3618 (accessed on 10 December 2023).
- Fonseca, P. Improving Larval Survival in Mollusk Cultures by Improving Microalgae Cultivation Techniques and Introducing New Microalgae Species. Ph.D. Thesis, University of Santiago de Compostela, Santiago de Compostela, España, 2016. Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=125679 (accessed on 10 December 2023).
- Olivares, J. Temporal variation of oceanographic conditions in Herradura de Guayacá Bay, Chile. Biota 1988, 4, 89–106. [Google Scholar]
- Uribe, E.; Blanco, J. Capacity of aquatic systems to sustain the culture of pectinids: The case of Argopecten purpuratus in Tongoy Bay, Chile. Los Moluscos Pectínidos Iberoam. Cienc. Acuic. Cap 2001, 12, 233–248. [Google Scholar]
- Pernet, F.; Tremblay, R.; Comeau, L.; Guderley, H. Temperature adaptation in two bivalve species from different thermal habitats: Energetics and remodelling of membrane lipids. J. Exp. Biol. 2007, 210, 2999–3014. [Google Scholar] [CrossRef] [PubMed]
- Ascencio, L.A.; Enriquez, M.; Martínez, I.; Aldana, D. Effect of temperature and salinity on the reproductive cycle of female and male Crassostrea virginica (Bivalvia Ostreidae). Trop. Biol. Mag. 2016, 64, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Martinez, G.; Perez, H. Effect of different temperature regimes on reproductive conditioning in the scallop Argopecten purpuratus. Aquaculture 2003, 228, 153–167. [Google Scholar] [CrossRef]
- Lardies, M.A.; Benitez, S.; Osores, S.; Vargas, C.A.; Duarte, C.; Lohrmann, K.B.; Lagos, N.A. Physiological and histopathological impacts of increased carbon dioxide and temperature on the scallops Argopecten purpuratus cultured under upwelling influences in northern Chile. Aquaculture 2017, 479, 455–466. [Google Scholar] [CrossRef]
- Martinez, G.; Brokordt, K.; Aguilera, C.; Soto, V.; Guderley, H. Effect of diet and temperature upon muscle metabolic capacities and biochemical composition of gonad and muscle in Argopecten purpuratus Lamarck 1819. J. Exp. Mar. Biol. Ecol. 2000, 247, 29–49. [Google Scholar] [CrossRef]
- Marshall, R.; McKinley, S.; Pearce, C.M. Effects of nutrition on larval growth and survival in bivalves. Rev. Aquac. 2010, 2, 33–55. [Google Scholar] [CrossRef]
- Helm, M.; Holland, D.; Stephenson, R. The effect of supplementary algal feeding of a hatchery breeding stock of Ostrea edulis L. on larval vigour. J. Mar. Biol. Assoc. UK 1973, 53, 673–684. [Google Scholar] [CrossRef]
- Caers, M.; Utting, S.; Coutteau, P.; Millican, P.; Sorgeloos, P. Impact of the supplementation of a docosahexaenoic acid rich emulsion on the reproductive output of oyster broodstock, Crassostrea gigas. Mar. Biol. 2002, 140, 1157–1166. [Google Scholar]
- Courtens, N.N.V.; Gajardo, M.H.G.; Sorgeloos, P. Effect of lipid emulsions on production and fatty acid composition. Mar. Biol. 2003, 143, 327–338. [Google Scholar]
- Hendriks, I.E.; van Duren, L.A.; Herman, P.M. Effect of dietary polyunsaturated fatty acids on reproductive output and larval growth of bivalves. J. Exp. Mar. Biol. Ecol. 2003, 296, 199–213. [Google Scholar] [CrossRef]
- Martinez, G.; Mettifogo, L. Mobilization of energy from adductor muscle for gametogenesis of the scallop, Argopecten purpuratus Lamarck. J. Shellfish. Res. 1998, 17, 113–116. [Google Scholar]
- Brokordt, K.B.; Guderley, H.E. Energetic requirements during gonad maturation and spawning in scallops: Sex differences in Chlamys islandica (Muller 1776). J. Shellfish. Res. 2004, 23, 25–33. [Google Scholar]
- Brown, M.R.; Jeffrey, S.W.; Volkman, J.K.; Dunstan, G.A. Nutritional properties of microalgae for mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Martinez, G.; Torres, M.; Uribe, E.; Diaz, M. Biochemical composition of broodstock and early juvenile Chilean scallops, Argopecten purpuratus Lamarck, held in two different environments. J. Shellfish. Res. 1992, 11, 307. [Google Scholar]
- Uriarte, I.; Farías, A.; Hernandez, J.; Schäfer, C.; Sorgeloos, P. Reproductive conditioning of Chilean scallop (Argopecten purpuratus) and the Pacific oyster (Crassostrea gigas): Effects of enriched diets. Aquaculture 2004, 230, 349–357. [Google Scholar] [CrossRef]
- Andriopoulos, V.; Gkioni, M.D.; Koutra, E.; Mastropetros, S.G.; Lamari, F.N.; Hatziantoniou, S.; Kornaros, M. Total phenolic content, biomass composition and antioxidant activity of selected marine microalgae species with potential as aquaculture feed. Antioxidantes 2022, 11, 1320. [Google Scholar] [CrossRef]
- Blanco-Llamero, C.; Señoráns, F.J. Biobased solvents for pressurized liquid extraction of nannochloropsis gaditana Omega-3 lipids. Mar. Drugs 2021, 19, 107. [Google Scholar] [CrossRef]
- Gallager, S.M.; Mann, R. Growth and survival of larvae of Mercenaria mercenaria (L.) and Crassostrea virginica (Gmelin) relative to broodstock conditioning and lipid content of eggs. Aquaculture 1986, 56, 105–121. [Google Scholar] [CrossRef]
- Sargent, J.R.; Tocher, D.R.; Bell, J.G. The lipids. In Fish Nutrition; Springer: Berlin/Heidelberg, Germany, 2003; pp. 181–257. [Google Scholar]
- Pérez, H.; Brokordt, K.; Martínez, G.; Guderley, H. Effect of spawning on force production during escape responses of the scallop Argopecten purpuratus. Mar. Biol. 2009, 156, 1585–1593. [Google Scholar] [CrossRef]
- Dionicio-Acedo, J.; Cabrera-Simon, A.; Rosado-Salazar, M.; Aguirre-Velarde, A. Optimización del tratamiento térmico para la inducción al desove de Argopecten purpuratus (Mollusca: Bivalvia). Rev. Biol. Mar. Oceanogr. 2021, 56, 145–150. [Google Scholar] [CrossRef]
- Castañeda, V.; Zevallos, S.; Ayerbe, R.; Castillo, C.R. Experiences in Controlled Systems for Obtaining Fan Scallop Argopecten purpuratus Seeds (Lamarck, 1819). Ilo, Moquegua. Technical Report, Instituto del Mar del Perú. 2008. Available online: https://repositorio.imarpe.gob.pe/bitstream/20.500.12958/2176/1/INF%2038%284%29-6.pdf (accessed on 10 December 2023).
- Méndez, S.; Alvarez, Y.; Sosa, L.E.; Vizcarra, Y.G. Cellular concentration and dry biomass in three species of marine microalgae: Chlorella vulgaris, Nannochloropsis oculata, and Tetraselmis striata. Rev. Investig. Altoandinas 2020, 22, 155–160. [Google Scholar] [CrossRef]
- Mann, R.; Gallager, S.M. Physiological and biochemical energetics of larvae of Teredo navalis L. and Bankia gouldi (Bartsch) (Bivalvia: Teredinidae). J. Exp. Mar. Biol. Ecol. 1985, 85, 211–228. [Google Scholar] [CrossRef]
- Dorsey, T.E.; McDonald, P.W.; Roels, O.A. A heated biuret-Folin protein assay which gives equal absorbance with different proteins. Anal. Biochem. 1977, 78, 156–164. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Reza, M.A. Implementation of Prostaglandin Analysis in Oyster Tissues and Comparison of Prostaglandin e2 after Sampling Stress in Pleasure Oyster (Crassostrea corteziensis) and Japanese Oyster (C. Gigas) and during Spawning of C. Gigas. Master’s Thesis, Master of Science in the Use, Management and Preservation of Natural Resources, Northwest Biological Research Centre, La Paz, México, 2009. Available online: https://cibnor.repositorioinstitucional.mx/jspui/bitstream/1001/272/1/reza_m.pdf (accessed on 10 December 2023).
- Farías, A.; Uriarte, I.; Varas, P. Study of the nutritional requirements of the northern scallop Argopecten purpuratus (Lamarck, 1819) during the reproductive conditioning. Oceanogr. Lit. Rev. 1998, 8, 1447. [Google Scholar]
- Caers, M.; Coutteau, P.; Cure, K.; Morales, V.; Gajardo, G.; Sorgeloos, P. The Chilean scallop Argopecten purpuratus (Lamarck, 1819): II. Manipulation of the fatty acid composition and lipid content of the eggs via lipid supplementation of the broodstock diet. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1999, 123, 97–103. [Google Scholar] [CrossRef]
- Caers, M.; Coutteau, P.; Sorgeloos, P.; Gajardo, G. Impact of algal diets and emulsions on the fatty acid composition and content of selected tissues of adult broodstock of the Chilean scallop Argopecten pupuratus (Lamarck, 1819). Aquaculture 2003, 217, 437–452. [Google Scholar] [CrossRef]
- Pérez, H.M.; Brokordt, K.; Gallardo, A.; Vidal, I.; Guderley, H. A diet rich in polyunsaturated fatty acids improves the capacity for HSP70 synthesis in adult scallop Argopecten purpuratus and their offspring. Mar. Biol. 2016, 163, 193. [Google Scholar] [CrossRef]
- Palma-Fleming, H.; Navarro, J.M.; Peña, E.; Martínez, G. Effect of three conditioning diets on the fatty acid composition of gonads and muscle of Argopecten purpuratus. N. Z. J. Mar. Freshw. Res. 2002, 36, 605–620. [Google Scholar] [CrossRef][Green Version]
- Farıas, A.; Bell, J.; Uriarte, I.; Sargent, J. Polyunsaturated fatty acids in total lipid and phospholipids of Chilean scallop Argopecten purpuratus (L.) larvae: Effects of diet and temperature. Aquaculture 2003, 228, 289–305. [Google Scholar] [CrossRef]
- Rojas, I.; Cárcamo, C.B.; Defranchi, Y.; Jeno, K.; Rengel, J.; Araya, M.; Tarnok, M.E.; Aguilar, L.; Álvarez, G.; Schmitt, P.; et al. A Diet Rich in HUFAs Enhances the Energetic and Immune Response Capacities of Larvae of the Scallop Argopecten purpuratus. Animals 2023, 13, 1416. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.; Bourne, N.; Ginther, N. Biochemical and energy changes during embryogenesis in the rock scallop Crassadoma gigantea. Mar. Biol. 1990, 106, 239–244. [Google Scholar] [CrossRef]
- Cerpa, J.; González, F.; Becerra, J.; Lépez, I. Lipid and polypeptide profiles in the female portion of gonads from diet-conditioned broodstock of North Chilean scallops Argopecten purpuratus. North Am. J. Aquac. 2003, 65, 1–7. [Google Scholar] [CrossRef]
- Contreras, R.; Pacheco, E.; Puebla, C. Desarrollo embrionario y larval temprano de Gari solida (Gray, 1828) (Bivalvia Psammobiidae). Lat. Am. J. Aquat. Res. 2014, 42, 283–288. [Google Scholar] [CrossRef]
- Whyte, J.; Bourne, N.; Ginther, N. Depletion of nutrient reserves during embryogenesis in the scallop Patinopecten yessoensis (Jay). J. Exp. Mar. Biol. Ecol. 1991, 149, 67–79. [Google Scholar] [CrossRef]
- Nevejan, N.; Saez, I.; Gajardo, G.; Sorgeloos, P. Supplementation of EPA and DHA emulsions to a Dunaliella tertiolecta diet: Effect on growth and lipid composition of scallop larvae, Argopecten purpuratus (Lamarck, 1819). Aquaculture 2003, 217, 613–632. [Google Scholar] [CrossRef]
- Volkman, J.K.; Jeffrey, S.W.; Nichols, P.D.; Rogers, G.I.; Garland, C.D. Fatty acid and lipid composition of 10 species of microalgae used in mariculture Exp. Mar. Biol. Ecol. 1989, 128, 219–240. [Google Scholar] [CrossRef]

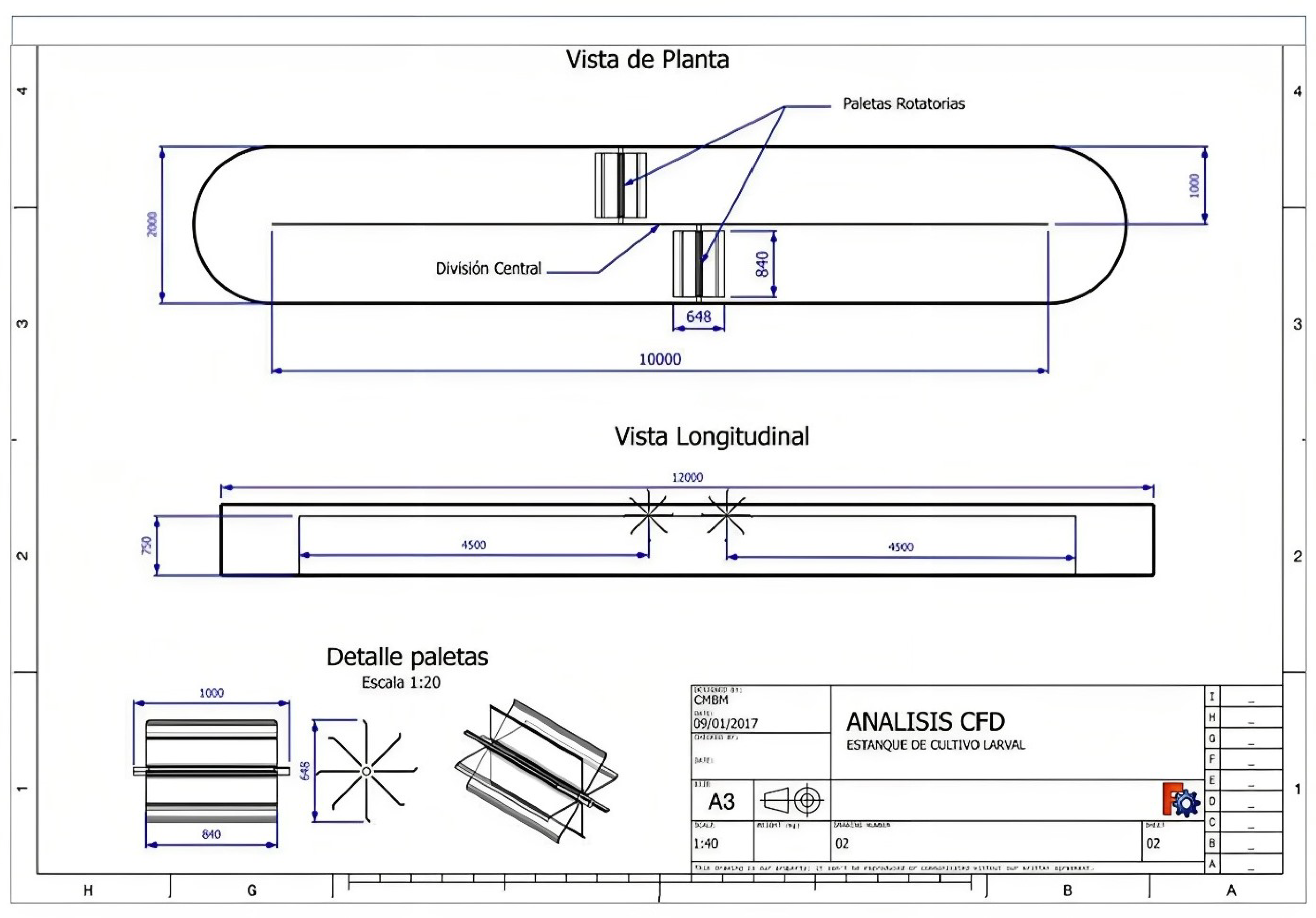
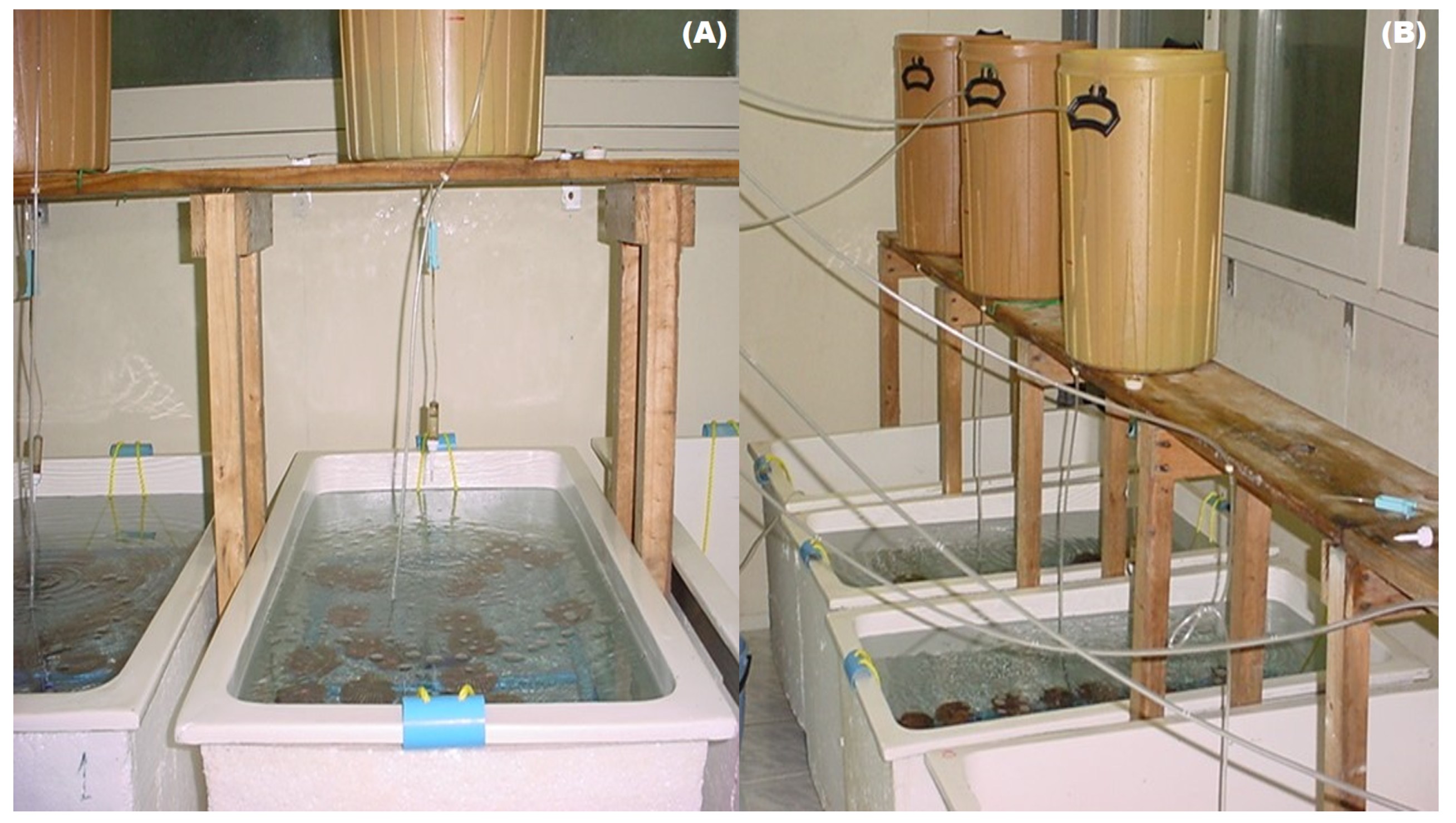

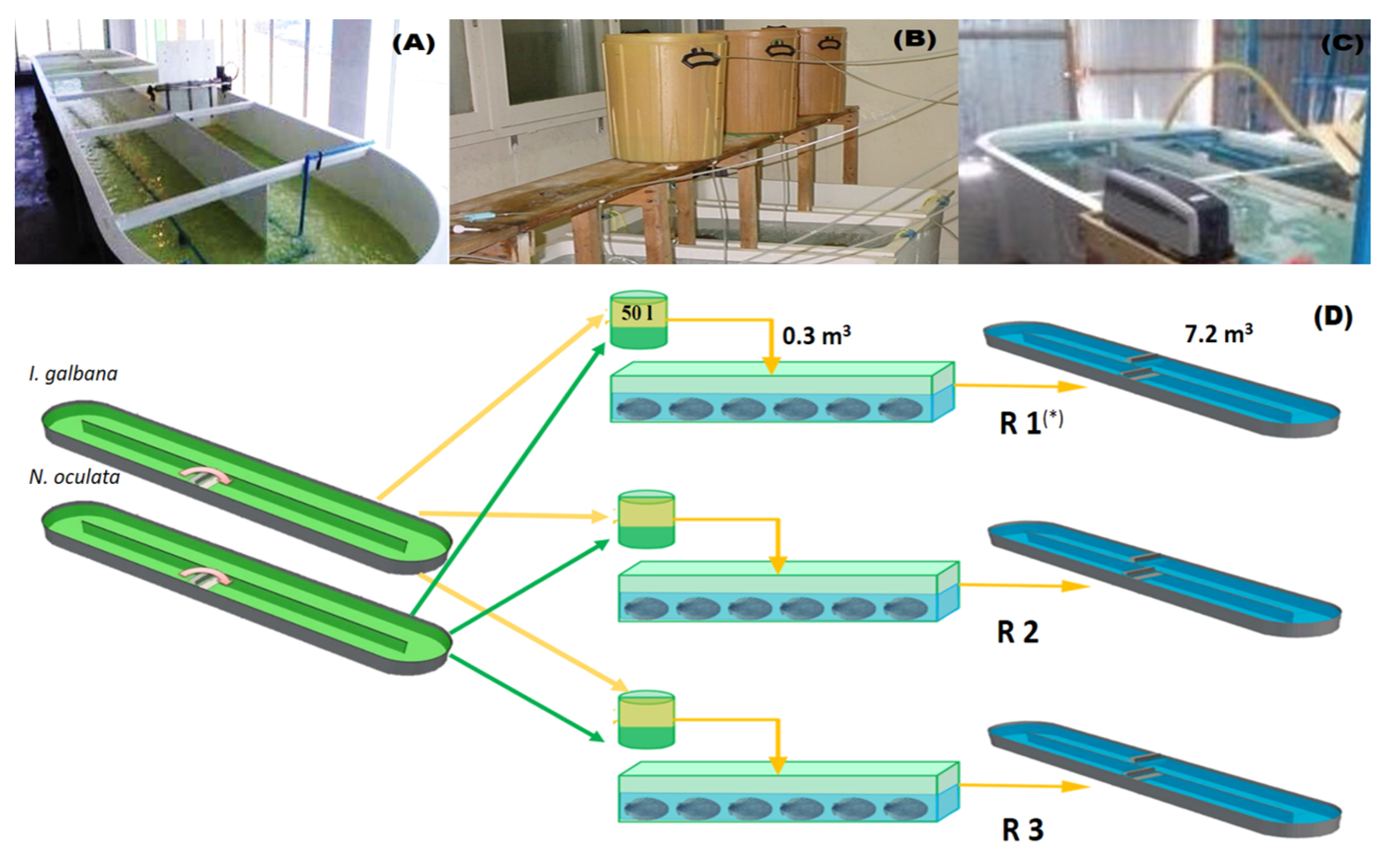
| Treatments | GSI | Spawning (%) | Number of Oocytes/ Ind. |
|---|---|---|---|
| Environment | 27 ± 2.9 | 88 | 1,654,343 |
| Hatchery | 25 ± 2.5 | 82 | 1,534,576 |
| Treatments | Fertilized Eggs (%) | Oocyte Size () |
|---|---|---|
| Environment | 93.2 ± 3.3 | 65.9 ± 1.7 * |
| Hatchery | 91.7 ± 2.8 | 61.4 ± 1.2 |
| Treatments | Proteins
(mg g Dry Mass) | Lipids
(mg g Dry Mass) | Carbohydrates (mg g Dry Mass) |
|---|---|---|---|
| Environment | 356.7 ± 93.8 | 98.9 ± 8.3 * | 39.4 ± 6.3 |
| Hatchery | 342.5 ± 85.7 | 41.6 ± 6.7 | 36.7 ± 7.1 |
| Treatments | Larval Size () | Larval Survival (%) |
|---|---|---|
| Environment | 95.8 ± 3.1 * | 52.1 ± 2.5 |
| Hatchery | 91.2 ± 2.7 | 49.5 ± 3.3 |
| Treatments | Proteins(mg g Dry Mass) | Lipids
(mg g Dry Mass) | Carbohydrates (mg g Dry Mass) |
|---|---|---|---|
| Environment | 256.5 ± 21.1 * | 25.2 ± 3.1 * | 13.1 ± 3.3 |
| Hatchery | 198.8 ± 17.4 | 13.5 ± 1.9 | 11.3 ± 3.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crisóstomo, R.O.; Pepe-Victoriano, R.; Méndez-Ancca, S.; Zambrano-Cabanillas, A.W.; Marín-Machuca, O.; Perez, H.M.; Yana-Mamani, V.; Ruiz-Choque, M. Reproductive Conditioning of the Peruvian Scallop Argopecten purpuratus in Different Environments. Fishes 2024, 9, 9. https://doi.org/10.3390/fishes9010009
Crisóstomo RO, Pepe-Victoriano R, Méndez-Ancca S, Zambrano-Cabanillas AW, Marín-Machuca O, Perez HM, Yana-Mamani V, Ruiz-Choque M. Reproductive Conditioning of the Peruvian Scallop Argopecten purpuratus in Different Environments. Fishes. 2024; 9(1):9. https://doi.org/10.3390/fishes9010009
Chicago/Turabian StyleCrisóstomo, Rafael Octavio, Renzo Pepe-Victoriano, Sheda Méndez-Ancca, Abel Walter Zambrano-Cabanillas, Olegario Marín-Machuca, Hernan Mauricio Perez, Víctor Yana-Mamani, and Mario Ruiz-Choque. 2024. "Reproductive Conditioning of the Peruvian Scallop Argopecten purpuratus in Different Environments" Fishes 9, no. 1: 9. https://doi.org/10.3390/fishes9010009
APA StyleCrisóstomo, R. O., Pepe-Victoriano, R., Méndez-Ancca, S., Zambrano-Cabanillas, A. W., Marín-Machuca, O., Perez, H. M., Yana-Mamani, V., & Ruiz-Choque, M. (2024). Reproductive Conditioning of the Peruvian Scallop Argopecten purpuratus in Different Environments. Fishes, 9(1), 9. https://doi.org/10.3390/fishes9010009








