Expected Climate Change in the High Arctic—Good or Bad for Arctic Charr?
Abstract
1. Introduction
2. Materials and Methods
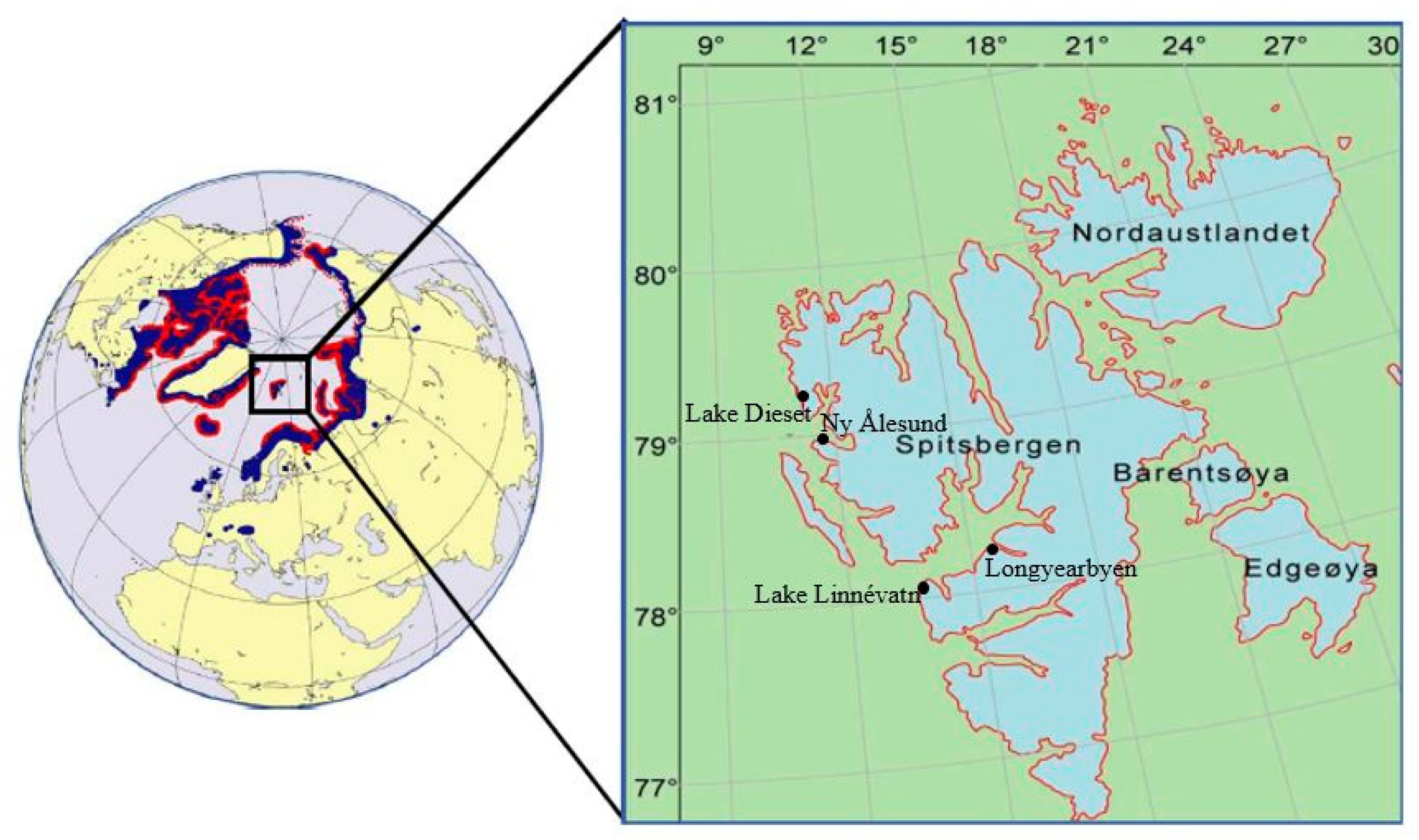
2.1. Study Sites
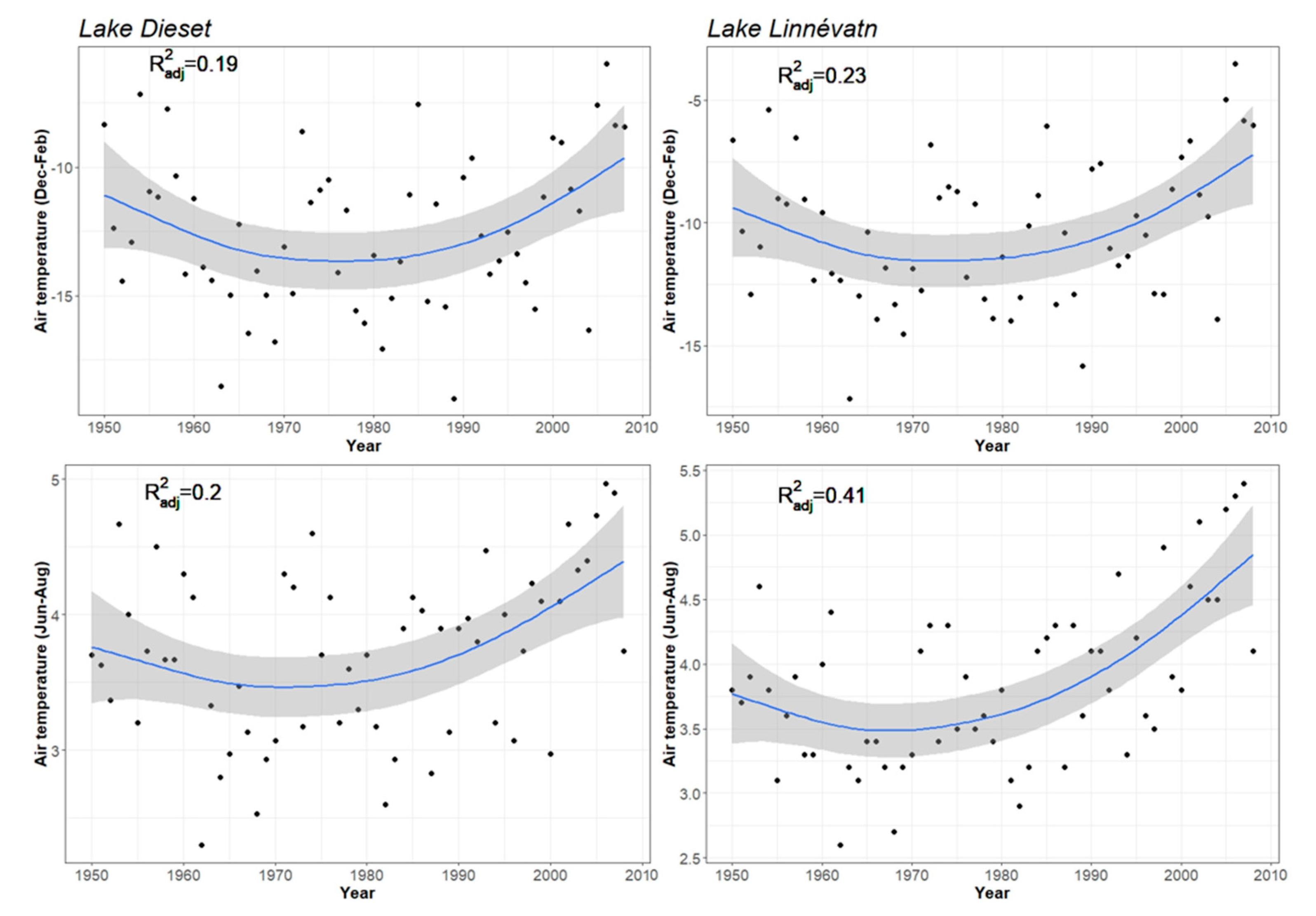
2.2. Meteorological Data
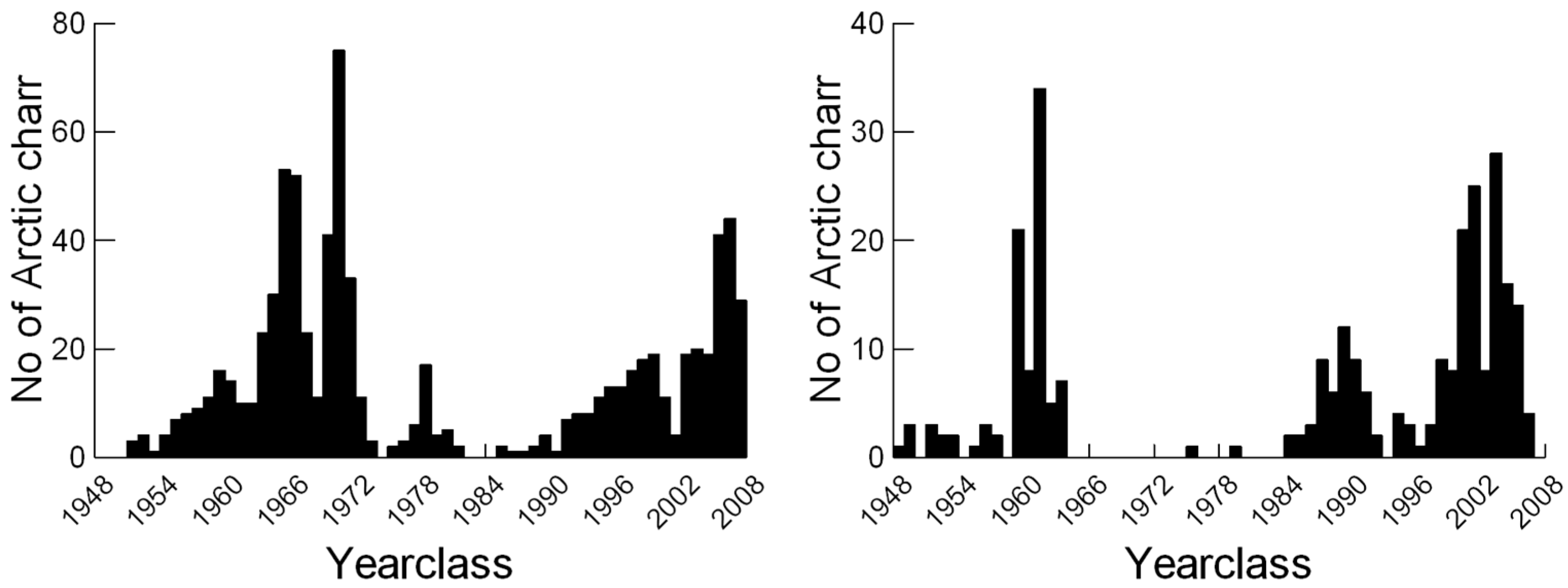
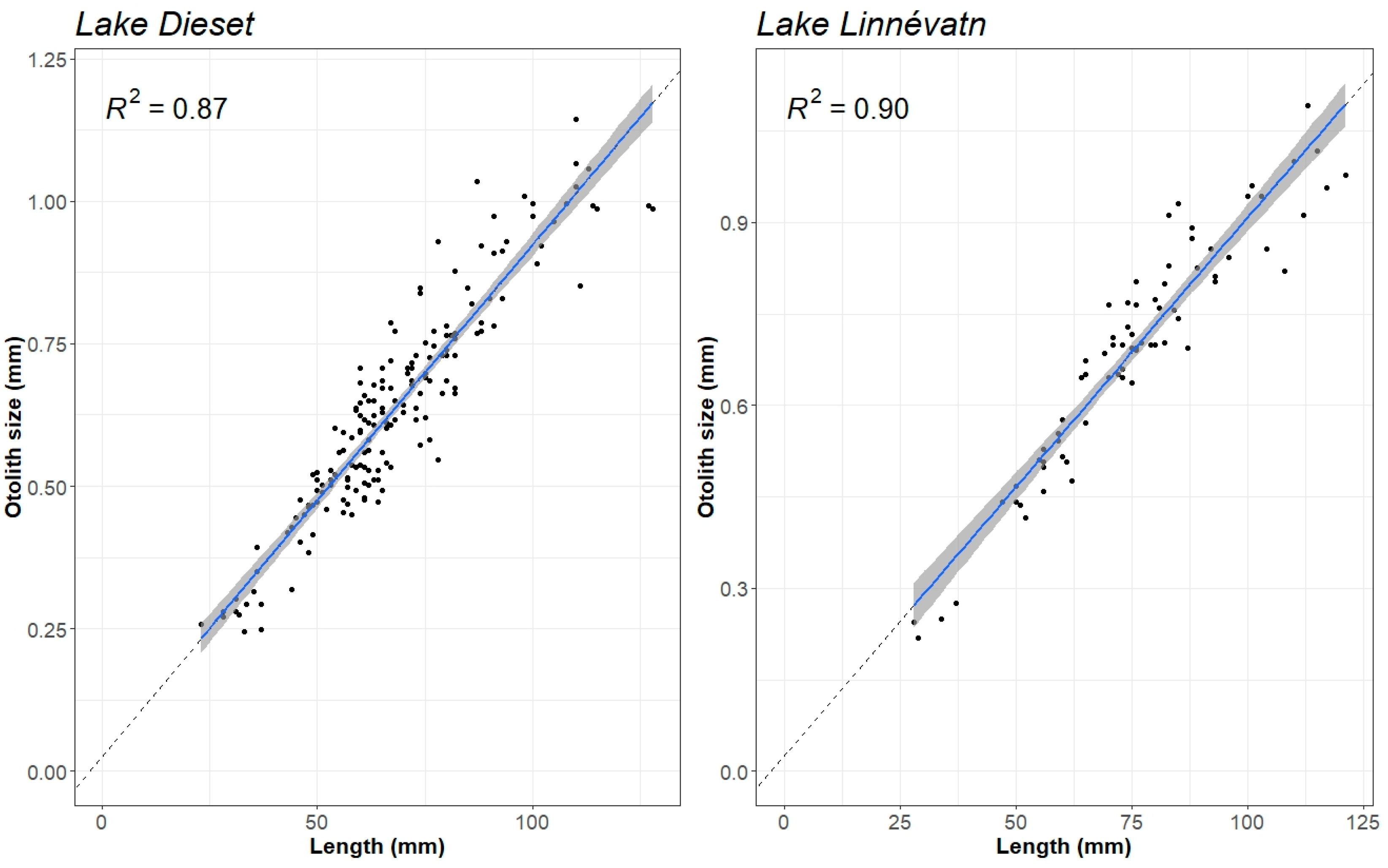
2.3. Fish Data and Otolith Analyses
2.4. Statistics
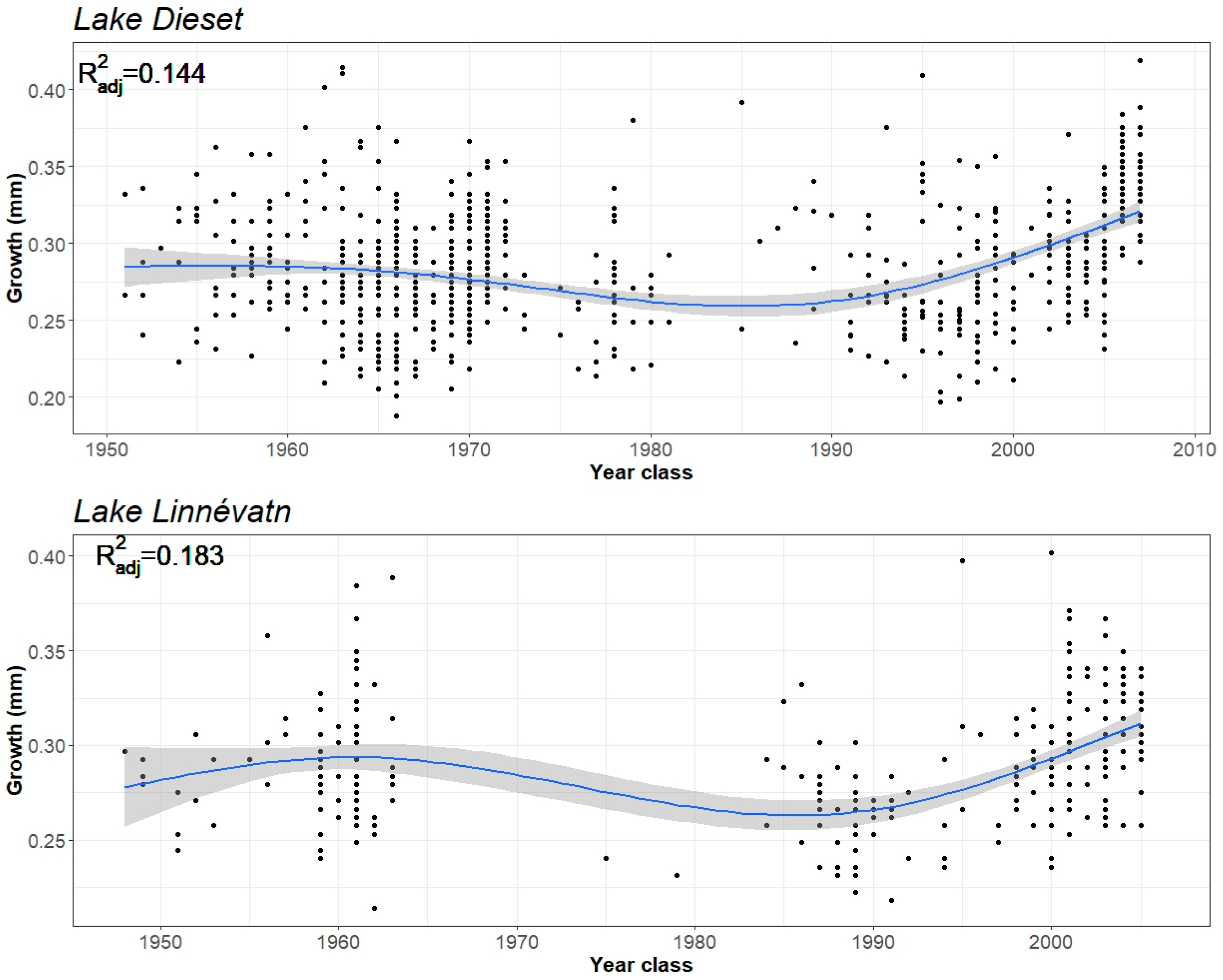
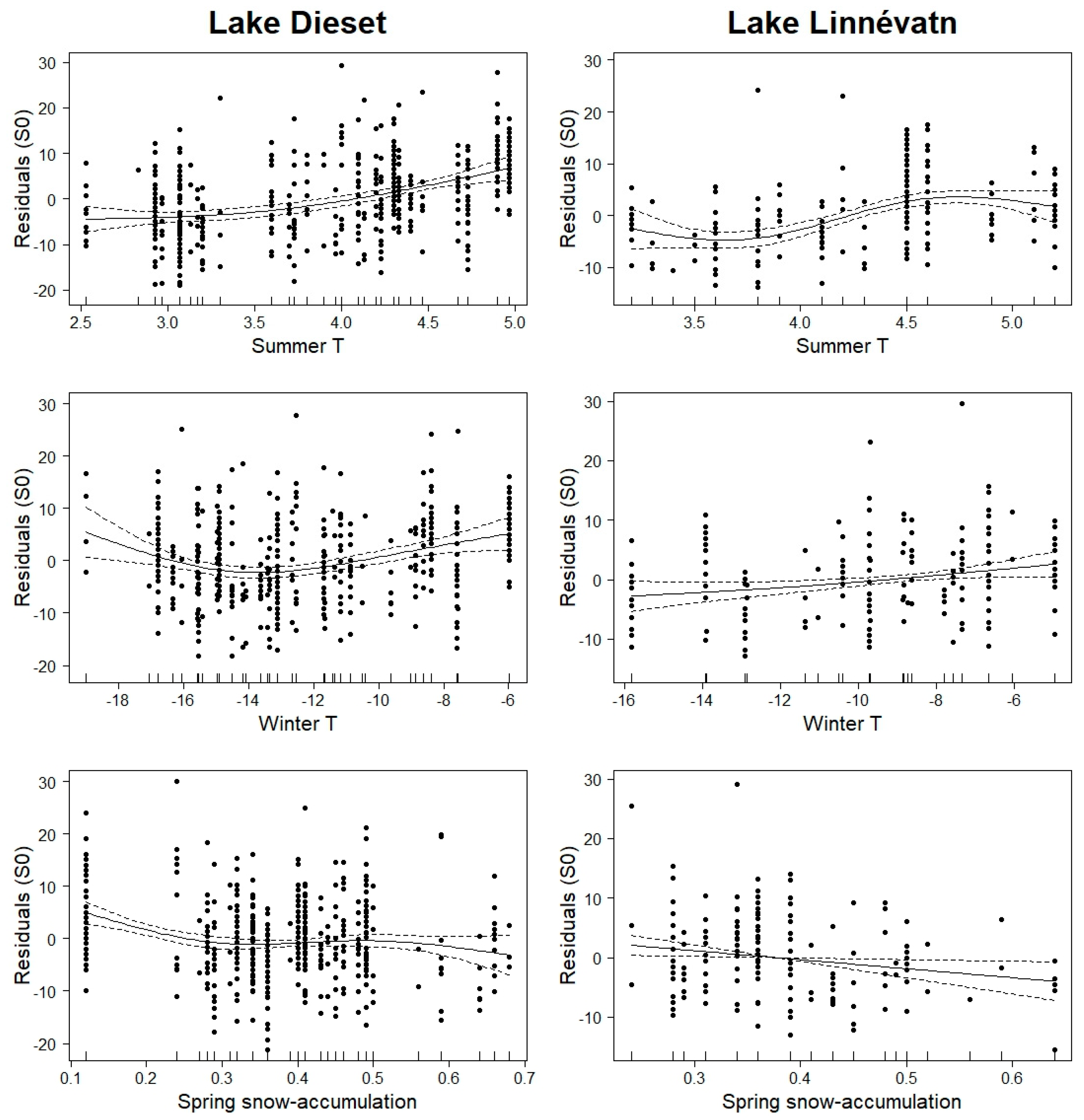
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, L. The Arctic Charr, Salvelinus alpinus; Balon, E.K., Ed.; W. Junk Publishers: Hague, The Netherlands, 1980. [Google Scholar]
- Svenning, M.-A.; Gullestad, N. Adaptations to Stochastic Environmental Variations: The Effects of Seasonal Temperatures on the Migratory Window of Svalbard Arctic Charr. Environ. Biol. Fish 2002, 64, 165–174. [Google Scholar] [CrossRef]
- Hammar, J. Freshwater Ecosystems of Polar Regions: Vulnerable Resources. Ambio 1989, 18, 6–22. [Google Scholar]
- Svenning, M.-A.; Klemetsen, A.; Olsen, T. Habitat and Food Choice of Arctic Charr in Linnévatn on Spitsbergen, Svalbard: The First Year-Round Investigation in a High Arctic Lake. Ecol. Freshw. Fish 2007, 16, 70–77. [Google Scholar] [CrossRef]
- Christoffersen, K.S.; Amsinck, S.L.; Landkildehus, F.; Lauridsen, T.L.; Jeppesen, E. Lake Flora and Fauna in Relation to Ice-Melt, Water Temperature and Chemistry at Zackenberg. Adv. Ecol. Res. 2008, 40, 371–389. [Google Scholar] [CrossRef]
- Førland, E.J.; Benestad, R.E.; Hanssen-Bauer, I.; Haugen, J.E.; Skaugen, T.E. Temperature and Precipitation Development at Svalbard 1900–2100. Adv. Meteorol. 2011, 2011, 893790. [Google Scholar] [CrossRef]
- Hammar, J. Natural Selection at the Edge of Life: Allelic Polymorphism and Recruitment in High Latitude Arctic Char (Salvelinus alpinus) Generated and Maintained by Environmental Extremes. Diversity 2023, 15, 74. [Google Scholar] [CrossRef]
- Jobling, M. The Influence of Environmental Manipulations on Inter- and Intra-Individual Variation in Food Acquisition and Growth Performance of Arctic Charr, Salvelinus alpinus. J. Fish. Biol. 1994, 44, 1069–1087. [Google Scholar] [CrossRef]
- Kotowych, N.; Smalås, A.; Amundsen, P.A.; Primicerio, R. Climate Warming Accelerates Somatic Growth of an Arctic Fish Species in High-Latitude Lakes. Sci. Rep. 2023, 13, 16749. [Google Scholar] [CrossRef]
- Huang, M.; Ding, L.; Wang, J.; Ding, C.; Tao, J. The Impacts of Climate Change on Fish Growth: A Summary of Conducted Studies and Current Knowledge. Ecol. Indic. 2021, 121, 106976. [Google Scholar] [CrossRef]
- Jobling, M. Influence of Body Weight and Temperature on Growth Rates of Arctic Charr, Salvelinus alpinus (L.). J. Fish Biol. 1983, 22, 471–475. [Google Scholar] [CrossRef]
- Jensen, K.W. On the Dynamics and Exploitation of the Population of Brown Trout, Salmo Trutta, in Lake Øvre Heimdalsvatn, Southern Norway. Rep. Inst. Freshw. Res. Drottningholm 1977, 56, 18–69. [Google Scholar]
- Kristensen, D.M.; Jørgensen, T.R.; Larsen, R.K.; Forchhammer, M.C.; Christoffersen, K.S. Inter-Annual Growth of Arctic Charr (Salvelinus alpinus L.) in Relation to Climate Variation. BMC Ecol. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Isaksen, K.; Nordli, Ø.; Ivanov, B.; Køltzow, M.A.; Aaboe, S.; Gjelten, H.M.; Mezghani, A.; Eastwood, S.; Førland, E.; Benestad, R.E.; et al. Exceptional Warming over the Barents Area. Sci. Rep. 2022, 12, 9371. [Google Scholar] [CrossRef] [PubMed]
- Rantanen, M.; Karpechko, A.Y.; Lipponen, A.; Nordling, K.; Hyvärinen, O.; Ruosteenoja, K.; Laaksonen, A. The Arctic Has Warmed Nearly Four Times Faster than the Globe since 1979. Commun. Earth Environ. 2022, 3, 168. [Google Scholar] [CrossRef]
- Borgstrøm, R. Annual Growth of Brown Trout in Alpine Lakes Is Highly Influenced by Spring Snow Depth and Ice-out Day. Fauna Nor. 2023, 42, 37–46. [Google Scholar] [CrossRef]
- Fang, X.; Stefan, H.G. Potential Climate Warming Effects on Ice Covers of Small Lakes in the Contiguous U.S. Cold Reg. Sci. Technol. 1998, 27, 119–140. [Google Scholar] [CrossRef]
- Duguay, C.R.; Prowse, T.D.; Bonsal, B.R.; Brown, R.D.; Lacroix, M.P.; Ménard, P. Recent Trends in Canadian Lake Ice Cover. Hydrol. Process 2006, 20, 781–801. [Google Scholar] [CrossRef]
- Stefan, H.G.; Fang, X.; Hondzo, M. Simulated Climate Change Effects on Year-Round Water Temperatures in Temperate Zone Lakes. Clim. Change 1998, 40, 547–576. [Google Scholar] [CrossRef]
- Wrona, F.J.; Prowse, T.D.; Reist, J.; Hobbie, J.E.; Levesque, L.M.; Vincent, W.F. Climate Change Effects on Aquatic Biota, Ecosystem Structure and Function. Ambio 2006, 35, 359–369. [Google Scholar] [CrossRef]
- Nordli, Ø.; Hanssen-Bauer, I.; Førland, E.J. Homogeneity Analyses of Temperature and Precipitation Series from Svalbard and Jan Mayen. Klima 1996, 16, 41. [Google Scholar]
- Nordli, Ø.; Przybylak, R.; Ogilvie, A.E.J.; Isaksen, K. Long-Term Temperature Trends and Variability on Spitsbergen: The Extended Svalbard Airport Temperature Series, 1898–2012. Polar Res 2014, 33, 21349. [Google Scholar] [CrossRef]
- Hisdal, V.S. Svalbard Nature History. In Polarhåndbok nr 11; Norsk Polarinstitutt: Tromsø, Norway, 1998. [Google Scholar]
- Hanssen-Bauer, I. Climate in Svalbard 2100—A Knowledge Base for Climate Adaptation; NCCS Report No. 1/2019; Norwegian Centre for Climate Services (NCCS): Oslo, Norway, 2019; p. 207. [Google Scholar]
- Bøyum, A.; Kjensmo, J. Physiography of Lake Linnévatn Western Spitsbergen. Int. Ver. Für Theor. Angew. Limnol. Verhandlungen 1978, 20, 609–614. [Google Scholar] [CrossRef]
- Gulseth, O.A. Seawater Tolerance, Migratory Behavior and Growth of Charr, Salvelinus alpinus, with Emphasis on the High Arctic Dieset Charr on Spitsbergen, Svalbard. Ph.D. Thesis, Department of Zoology, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, 2000. [Google Scholar]
- Anon Norwegian Polar Institute. Austre Brøggerbreen and Midtre Lovenbreen Mass Balance; Environmental Monitoring of Svalbard and Jan Mayen (MOSJ): Tromsø, Norway, 2023. [Google Scholar]
- Svenning, M.-A.; Smith-Nilsen, A.M.; Jobling, M. Sea Water Migration of Arctic Charr (Salvelinus alpinus). Correlation between Freshwater Growth and Seaward Migration, Based on Back-Calculation from Otoliths. Nord. J. Freshw. Res. 1992, 67, 18–26. [Google Scholar]
- Kristoffersen, K.; Klemetsen, A. Age Determination of Arctic Charr (Salvelinus alpinus) from Surface and Cross Section of Otoliths Related to Otolith Growth. Nord. J. Freshw. Res. 1991, 66, 98–107. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman Hall/CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Bustnes, J.O.; Yoccoz, N.G.; Bangjord, G.; Polder, A.; Skaare, J.U. Temporal Trends (1986–2004) of Organochlorines and Brominated Flame Retardants in Tawny Owl Eggs from Northern Europe. Environ. Sci. Technol. 2007, 41, 8491–8497. [Google Scholar] [CrossRef]
- Mysterud, A.; Stenseth, N.C.; Yoccoz, N.G.; Langvatn, R.; Steinheim, G. Nonlinear Effects of Large-Scale Climatic Variability on Wild and Domestic Herbivores. Nature 2001, 410, 1096–1099. [Google Scholar] [CrossRef]
- Godiksen, J.A.; Svenning, M.A.; Sinnatamby, R.N.; Dempson, J.B.; Borgstrøm, R.; Power, M. Stable Isotope-Based Determinations of the Average Temperatures Experienced by Young-of-the-Year Svalbard Arctic Charr (Salvelinus alpinus (L.)). Polar Biol. 2011, 34, 591–596. [Google Scholar] [CrossRef]
- Gulseth, O.A.; Nilssen, K.J. Growth Benefit from Habitat Change by Juvenile High-Arctic Char. Trans. Am. Fish. Soc. 1999, 128, 593–602. [Google Scholar] [CrossRef]
- Nordeng, H. On the Biology of Char (Salmo Alpinus L.) in Salangen, North Norway. 1. Age and Spawning Frequency Determined from Scales and Otoliths. Nytt Mag. Zool. 1961, 10, 67–123. [Google Scholar]
- Power, G. Fish Population Structure in Arctic Lakes. J. Fish. Res. Bd. Can. 1978, 35, 53–59. [Google Scholar] [CrossRef]
- Reay, P.J. The Seasonal Pattern of otolith Growth and Its Application to Back-Calculation Studies in Ammodytes Tobianus L. J. Cons. Int. Explor. Mer. 1972, 34, 485–504. [Google Scholar] [CrossRef]
- Casselman, J.M. Growth and Relative Size of Calcified Structure of Fish. Trans. Amer. Fish. Soc. 1990, 119, 673–688. [Google Scholar] [CrossRef]
- Elliott, J.M. The Energetics of Feeding, Metabolism and Growth of Brown Trout (Salmo trutta L.) in Relation to Body Weight, Water Temperature and Ration Size. J. Anim. Ecol. 1976, 45, 923. [Google Scholar] [CrossRef]
- Ruosteenoja, K. The Date of Break-up of Lake Ice as a Climate Index. Geophysica 1986, 22, 89–99. [Google Scholar]
- Weyhenmeyer, G.A.; Meili, M.M.; Livingstone, D.M. Nonlinear Temperature Response of Lake Ice Breakup. Geophys. Res. Lett. 2004, 31, L07203. [Google Scholar] [CrossRef]
- Higgins, S.N.; Desjardins, C.M.; Drouin, H.; Hrenchuk, L.E.; Sanden, J.J. The Role of Climate and Lake Size in Regulating the Ice Phenology of Boreal Lakes. J. Geophys. Res. Biogeosci. 2021, 126, e2020JG005898. [Google Scholar] [CrossRef]
- Tuttle, S.E.; Roof, S.R.; Retelle, M.J.; Werner, A.; Gunn, G.E.; Bunting, E.L. Evaluation of satellite-derived estimates of lake ice cover timing on Linnévatnet, Kapp Linné, Svalbard using in-situ data. Remote Sens. 2022, 14, 1311. [Google Scholar] [CrossRef]
- Rizzi, P.; Graziano, P.; Dallara, A. A Capacity Approach to Territorial Resilience: The Case of European Regions. Ann. Reg. Sci. 2018, 60, 285–328. [Google Scholar] [CrossRef]
- L’Abée-Lund, J.H.; Vøllestad, A.; Brittain, J.E.; Kvambekk, S.; Solvang, T. Geographic Variation and Temporal Trends in Ice Phenology in Norwegian Lakes during the Period 1890–2020. Cryosphere 2021, 15, 2333–2356. [Google Scholar] [CrossRef]
- Kainz, M.J.; Ptacnik, R.; Rasconi, S.; Hager, H.H. Irregular Changes in Lake Surface Water Temperature and Ice Cover in Subalpine Lake Lunz, Austria. Inland. Waters 2017, 7, 27–33. [Google Scholar] [CrossRef]
- Lopez, L.S.; Hewitt, B.A.; Sharma, S. Reaching a Breaking Point: How Is Climate Change Influencing the Timing of Ice Breakup in Lakes across the Northern Hemisphere? Limnol. Oceanogr. 2019, 64, 2621–2631. [Google Scholar] [CrossRef]
- Caldwell, P.V.; Kennen, J.G.; Hain, E.F.; Nelson, S.A.; Sun, G.; McNulty, S.G. Hydrologic Modeling for Flow-Ecology Science in the Southeastern United States and Puerto Rico; e-Gen; US Department of Agriculture Forest Service, Southern Research Station: Asheville, NC, USA, 2020; p. 77. [Google Scholar]
- Rigler, F.H. Limnology in the High Arctic: A Case Study of Char Lake. Verh. Internat. Verein. Limnol. 1978, 20, 127–140. [Google Scholar] [CrossRef]
- Jonsson, B. Thermal Effects on Ecological Traits of Salmonids. Fishes 2023, 8, 337. [Google Scholar] [CrossRef]
- Amundsen, P.A.; Klemetsen, A. Diet, Gastric Evacuation Rates and Food Consumption in a Stunted Population of Arctic Charr, Salvelinus alpinus L., in Takvatn, Northern Norway. J. Fish. Biol. 1988, 33, 697–709. [Google Scholar] [CrossRef]
- Berge, J.; Heggland, K.; Lønne, O.J.; Cottier, F.; Hop, H.; Gabrielsen, G.W.; Nøttestad, L.; Misund, O.A. First Records of Atlantic Mackerel (Scomber scombrus) from the Svalbard Archipelago, Norway, with Possible Explanations for the Extension of Its Distribution. Arctic 2015, 68, 54–61. [Google Scholar] [CrossRef]
- Svenning, M.-A.; Aas, M.; Borgstrøm, R. First Records of Three-Spined Stickleback Gasterosteus Aculeatus in Svalbard Freshwaters: An Effect of Climate Change? Polar Biol. 2015, 38, 1937–1940. [Google Scholar] [CrossRef]
- van Pelt, W.; Schuler, T.V.; Pohjola, V.; Pettersson, R. Accelerating Future Mass Loss of Svalbard Glaciers from a Multi-Model Ensemble. J. Glaciol. 2021, 67, 485–499. [Google Scholar] [CrossRef]
- Geyman, E.; van Pelt, W.; Maloof, A.; Aas, H.F.; Kohler, J. Historical Record of Glacier Change on Svalbard Predicts Doubling of 21st Century Mass Loss. Nature 2022, 601, 374–379. [Google Scholar] [CrossRef]

| Lake | Lake Dieset | Lake Linnévatn |
|---|---|---|
| Year of Birth | 14.4 (67.1) [N = 772] | 18.3 (47.6) [N =285] |
| Summer T | 19.6 (63.0) [N = 772] | 10.9 (51.8) [N =285] |
| Winter T | 16.0 (65.8) [N = 772] | 1.9 (57.0) [N = 285] |
| Snow | 0.00 (77.4) [N = 504] | 3.9 (59.0) [N = 190] |
| Summer T + Winter T | 20.1 (62.7) [N = 772] | 10.6 (52.2) [N = 285] |
| Summer T + Snow | 30.3 (54.6) [N = 504] | 22.5 (48.4) [N = 190] |
| Winter T + Snow | 23.9 (59.4) [N = 504] | 11.6 (55.1) [N = 190] |
| Summer T + Winter T + Snow | 31.6 (53.9) [N = 504] | 24.9 (47.1) [N = 190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svenning, M.A.; Bjørvik, E.T.; Godiksen, J.A.; Hammar, J.; Kohler, J.; Borgstrøm, R.; Yoccoz, N.G. Expected Climate Change in the High Arctic—Good or Bad for Arctic Charr? Fishes 2024, 9, 8. https://doi.org/10.3390/fishes9010008
Svenning MA, Bjørvik ET, Godiksen JA, Hammar J, Kohler J, Borgstrøm R, Yoccoz NG. Expected Climate Change in the High Arctic—Good or Bad for Arctic Charr? Fishes. 2024; 9(1):8. https://doi.org/10.3390/fishes9010008
Chicago/Turabian StyleSvenning, Martin A., Eigil T. Bjørvik, Jane A. Godiksen, Johan Hammar, Jack Kohler, Reidar Borgstrøm, and Nigel G. Yoccoz. 2024. "Expected Climate Change in the High Arctic—Good or Bad for Arctic Charr?" Fishes 9, no. 1: 8. https://doi.org/10.3390/fishes9010008
APA StyleSvenning, M. A., Bjørvik, E. T., Godiksen, J. A., Hammar, J., Kohler, J., Borgstrøm, R., & Yoccoz, N. G. (2024). Expected Climate Change in the High Arctic—Good or Bad for Arctic Charr? Fishes, 9(1), 8. https://doi.org/10.3390/fishes9010008





