Preliminary Trial of Male to Female Sex Reversal by 17β-Estradiol in Combination with Trilostane in Spotted Scat (Scatophagus argus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Experimental Feed Preparation and Culturing Process
2.3. Sampling
2.3.1. Histology Observation
2.3.2. Measurement of Serum Hormone Levels
2.3.3. Gene Expression Analysis
2.3.4. Immunohistochemical (IHC) Analysis
2.4. Data Statistics
3. Results
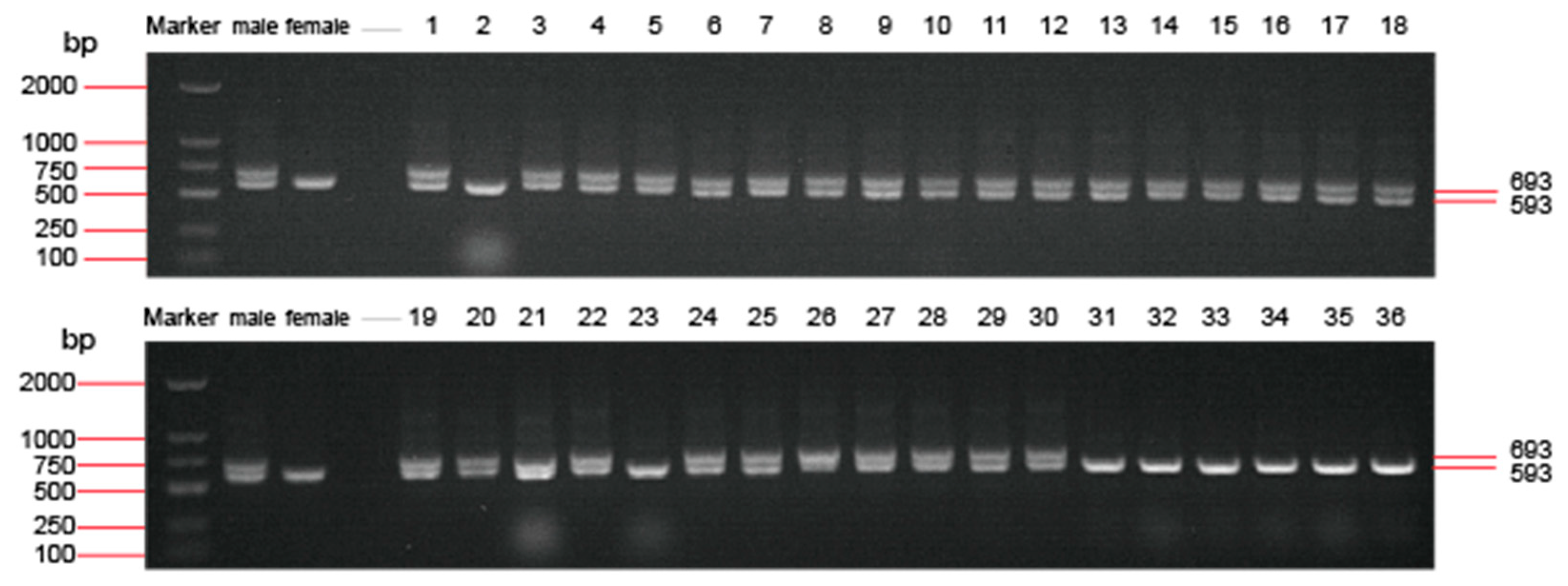
3.1. Genetic and Phenotype Sex Identification of the Treated Spotted Scats
3.2. Changes in Serum Sex Steroid Hormone Levels
3.3. Expression Changes in Sex Differentiation-Related Genes
3.4. Localization Analysis of Key Gene Expression by Immunohistochemical (IHC) Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.Y.; Zhang, M.; Ye, K.; Shen, W.L.; Wu, X.F.; Wang, Z.Y. Effects of 17β-estradiol on the Expression of Genes related to Sex Differentiation of the Large Yellow Croaker. J. Jimei Univ. 2019, 24, 6. [Google Scholar]
- Li, C.Y. The effects on sexual reversion of Xiphophrus helleri by temperature. Jilin Norm. Univ. J. 2001, 1, 70–72. [Google Scholar]
- Robert, H. Devlin and Yoshitaka Nagahama. Sex determination and sex differentiation in fish: An overview of genetic, physiological, and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar]
- Ge, X.P.; Xia, D.Q.; Yu, J.H. Research progress of fish sex determination. J. Fish. Sci. China 2002, 9, 371–374. [Google Scholar]
- Strüssmann, C.A.; Takashima, F.; Toda, K. Sex differentiation and hormonal feminization in pejerrey Odontesthes bonariensis. Aquaculture 1996, 139, 31–45. [Google Scholar] [CrossRef]
- Mei, J.; Gui, J.F. Genetic basis and biotechnological manipulation of sexual dimorphism and sex determination in fish. Sci. China Life Sci. 2015, 58, 124–136. [Google Scholar] [CrossRef]
- Barry, T.P.; Fast, A.W. Biology of the spotted scat (Scatophagus argus) in the Philippines. Asian Fish. Sci. 1992, 5, 163–179. [Google Scholar] [CrossRef]
- Cai, Z.P.; Wang, Y.; Hu, J.W.; Zhang, J.B.; Lin, Y.G. Reproductive biology of Scatophagus argus and artificial induction of spawning. J. Trop. Oceanogr. 2010, 29, 180–185. [Google Scholar]
- Biswas, G.; Sundaray, J.K.; Bhattacharyya, S.B.; Kailasam, M. Evaluation of growth performance and survival of wild collected spotted scat, Scatophagus argus (Linnaeus, 1766) during rearing of fry to marketable size juveniles for aquarium trade at varied stocking densities. J. Indian Soc. Coast. Agric. Res. 2016, 34, 120–126. [Google Scholar]
- Mustapha, U.F.; Jiang, D.N.; Liang, Z.H.; Gu, H.T.; Yang, W.; Chen, H.P.; Deng, S.P.; Wu, T.L.; Tian, C.X.; Zhu, C.H.; et al. Male-specific Dmrt1 is a candidate sex determination gene in spotted scat (Scatophagus argus). Aquaculture 2018, 495, 351–358. [Google Scholar] [CrossRef]
- Mandal, B.; Kailasam, M.; Bera, A.; Sukumaran, K.; Hussain, T.; Makesh, M.; Thiagarajan, G.; Vijayan, K.K. Gonadal recrudescence and annual reproductive hormone pattern of captive female Spotted Scats (Scatophagus argus). Anim. Reprod. Sci. 2020, 213, 106273. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Liu, Z.W.; Liu, N.X.; Zhang, Y.Y.; Zhang, J.B. Histological study on the gonadal development of Scatophagus Arg. J. Fish. China 2013, 37, 5. [Google Scholar] [CrossRef]
- Sun, X.N.; Liu, J.Y.; Feng, G.P.; Zhuang, P.; Wang, Y. Advances in reproductive biology of Scatophagus argus. Fish. Sci. 2022, 47, 283–288. [Google Scholar]
- Piferrer, F. Endocrine sex control strategies for the feminization of teleost fish. Aquaculture 2001, 197, 229–281. [Google Scholar] [CrossRef]
- Mustapha, U.F. Early Gonadal Development, Expression Profile and Regulation of Sex-Related Genes and Hormonal Induction of Sex-Reversal Mechanism in Scatophagus argus. Ph.D. Thesis, Guangdong Ocean University, Zhanjiang, China, 2022, (unpublished material). [Google Scholar]
- Shi, H.J. Functional Study of Dax1, Dax2, Foxj1a and Foxh1 in Gametogenesis in tilapia. Ph.D. Thesis, South-West University, Chongqing, China, 2018. [Google Scholar]
- Zhi, F.; Jiang, D.N.; Mustapha, U.F.; Li, S.X.; Shi, H.J.; Li, G.L.; Zhu, C.H. Expression and regulation of 42Sp50 in spotted scat (Scatophagus argus). Front. Genet. 2022, 13, 964150. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, U.F.; Peng, Y.X.; Huang, Y.Q.; Assan, D.; Zhi, F.; Shi, G.; Huang, Y.; Li, G.L.; Jiang, D.N. Comparative transcriptome analysis of the differentiating gonads in Scatophagus argus. Front. Mar. Sci. 2022, 9, 962534. [Google Scholar] [CrossRef]
- Jiang, D.N.; Mustapha, U.F.; Shi, H.J.; Huang, Y.Q.; Si-Tu, J.X.; Wang, M.; Deng, S.P.; Chen, H.P.; Tian, C.X.; Zhu, C.H.; et al. Expression and transcriptional regulation of gsdf in spotted scat (Scatophagus argus). Comp. Biochem. Physiol. B 2019, 233, 35–45. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.; Zhao, J.; Shi, H.; Zeng, S.; Ye, K.; Jiang, D.; Zhou, L.; Sun, L.; Tao, W.; et al. A tandem duplicate of anti-mullerian hormone with a missense SNP on the y chromosome is essential for male sex determination in Nile tilapia, Oreochromis niloticus. PLoS. Genet. 2015, 11, e1005678. [Google Scholar] [CrossRef]
- Jiang, X.L. Effects of Sex Steroids on Sex Determination and Differentiation of Nile tilapia. Master’s Thesis, South-West University, Chongqing, China, 2014; pp. 21–27. [Google Scholar]
- Li, Y.J.; Wu, L.M.; Wang, L.; Ma, X.; Li, X.J. Molecular cloning and characterization of cyp19a1b gene and the effect of Letrozole on its expression in Carassius auratus. J. Fish. China 2018, 42, 1169–1180. [Google Scholar]
- Hu, P.; Liu, B.; Meng, Z.; Liu, X.F.; Jia, Y.D.; Yang, Z.; Lei, J.L. Recovery of gonadal development in tiger puffer Takifugu rubripes after exposure to 17β-estradiol during early life stages. Chin. J. Oceanol. Limnol. 2017, 35, 613–623. [Google Scholar] [CrossRef]
- Bhanda, R.K.; Nakamura, M.; Kobayashi, T.; Nagahama, Y. Suppression of steroidogenic enzyme expression during androgen-induced sex reversal in Nile tilapia (Oreochromis niloticus). Gen. Comp. Endocrinol. 2006, 145, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Brion, F.; Tyler, C.R.; Palazzi, X.; Laillet, B.; Porcher, J.M.; Garric, J.; Flammarion, P. Impacts of 17β-estradiol, including environmentally relevant concentra-tions, on reproduction after exposure during embryo-larval-, juvenile- and adult-life stages in zebrafish (Danio rerio). Aquat. Toxicol. 2004, 68, 193–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Hu, Q.; Guan, X.M.; Li, F.; Wang, M.K.; Li, S.Z. Feminization induc-tion and hermaphroditism of northern pike (Esox lucius). J. Fish. China 2018, 42, 557–564. [Google Scholar]
- Wang, C.L.; Guan, W.Z.; Li, Y.Q.; Liu, F. Study on 17β-estradiol induced feminization of Pelteobagrus fulvidraco. South China Fish. Sci. 2020, 16, 25–30. [Google Scholar]
- Fuzzen, M.L.M.; Bernier, N.J.; Kraak, G. Differential effects of 17β-estradiol and 11-ketotestosterone on the endocrine stress response in zebrafish (Danio rerio). Gen. Comp. Endocrinol. 2001, 170, 365–373. [Google Scholar] [CrossRef]
- Zhou, F.Q.; Xie, Q.P.; Lou, B.; Meng, X.L.; He, X.; Sun, Y.; Zhan, W.; Chen, R.Y.; Liu, F.; Wang, L.G.; et al. Effect of 17β-estradiol bath on growth and gonadal development of early stage of Larimichthys polyactis. J. Zhejiang Ocean Univ. Nat. Sci. 2018, 37, 302–307. [Google Scholar]
- Carvalho, C.V.A.; Passini, G.; Costa, W.M.; Vieira, B.N.; Cerqueira, V.R. Effect of estradiol-17β on the sex ratio, growth and survival of juvenile common snook (Centropomus undecimalis). Acta. Sci. Anim. Sci. 2014, 36, 239–245. [Google Scholar] [CrossRef]
- Blazquez, M.; Zanuy, S.; Carrillo, M.; Piferrer, F. Structural and functional effects of early exposure to estradiol-17β and 17α-ethynylestradiol on the gonads of the gonochoristic teleost Dicentrarchus labrax. Fish. Physiol. Biochem. 1998, 18, 37–47. [Google Scholar] [CrossRef]
- Chad, N.T.; Daniel, J.S.; Susan, B.F.; Colby, M.R.; Kevin, F.; Javan, M.B.; William, T.S.; Scott, A.B. The effects of estradiol-17β on the sex reversal, survival, and growth of green sunfish Lepomis cyanellus. Aquaculture 2022, 562, 738853. [Google Scholar]
- Ouschan, C.; Lepschy, M.; Zeugswetter, F.; Möstl, E. The influence of trilostane on steroid hormone metabolism in canine adrenal glands and corpora lutea-an in vitro study. Vet. Res. Commun. 2012, 36, 35–40. [Google Scholar] [CrossRef]
- DeQuattro, Z.A.; Hemming, J.D.; Barry, T.P. Effects of androstenedione exposure on fathead minnow (Pimephales promelas) reproduction and embryonic development. Environ. Toxicol. Chem. 2015, 34, 2549–2554. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kajiura-Kobayashi, H.; Nagahama, Y. Induction of XY sex reversal by estrogen involves altered gene expression in a teleost, tilapia. Cytogenet. Genome. Res. 2003, 101, 289–294. [Google Scholar] [CrossRef]
- Nakamura, M.; Bhandari, R.K.; Higa, M. The role estrogens play in sex differentiation and sex changes of fish. Fish. Physiol. Biochem. 2003, 28, 113–117. [Google Scholar] [CrossRef]
- Lee, Y.H.; Wu, G.C.; Du, J.L.; Chang, C.F. Estradiol-17beta induced a reversible sex change in the fingerlings of protandrous black porgy, Acanthopagrus schlegeli Bleeker: The possible roles of luteinizing hormone in sex change. Biol. Reprod. 2004, 71, 1270–1278. [Google Scholar] [CrossRef]
- Wu, G.C.; Tomy, S.; Nakamura, M.; Chang, C.F. Dual roles of cyp19a1a in gonadal sex differentiation and development in the protandrous black porgy, Acanthopagrus schlegeli. Biol. Reprod. 2008, 79, 1111–1120. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, G.; Li, M.; Zhu, F.; Liu, Q.; Naruse, K.; Herpin, A.; Nagahama, Y.; Li, J.; Hong, Y. Autosomal gsdf acts as a male sex initiator in the fish medaka. Sci. Rep. 2016, 6, 19738. [Google Scholar] [CrossRef]
- Hattori, R.S.; Murai, Y.; Oura, M.; Masuda, S.; Majhi, S.K.; Sakamoto, T.; Fernandino, J.I.; Somoza, G.M.; Yokota, M.; Strussmann, C.A. A Y-linked anti-Mullerian hormone duplication takes over a critical role in sex determination. Proc. Natl. Acad. Sci. USA 2012, 109, 2955–2959. [Google Scholar] [CrossRef]
- Inaba, H.; Hara, S.; Horiuchi, M.; Ijiri, S.; Kitano, T. Gonadal expression profiles of sex-specific genes during early sexual differentiation in Japanese eel Anguilla japonica. Fish. Sci. 2021, 87, 203–209. [Google Scholar] [CrossRef]
- Smith, E.K.; Guzmán, J.M.; Luckenbach, J.A. Molecular cloning, characterization, and sexually dimorphic expression of five major sex differentiation-related genes in a Scorpaeniform fish, sablefish (Anoplopoma fimbria). Comp. Biochem. Physiol. B 2013, 165, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, H.; Dong, Y.; Dong, T.; Tian, Z.; Hu, H. Identification and dimorphic expression of sex-related genes during gonadal differentiation in sterlet Acipenser ruthenus, a primitive fish species. Fish. Physiol. Biochem. 2017, 43, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.L.; Desvignes, T.; Bremiller, R.; Wilson, C.; Dillon, D.; High, S.; Draper, B.; Buck, C.L.; Postlethwait, J. Gonadal soma controls ovarian follicle proliferation through Gsdf in zebrafish. Dev. Dyn. 2017, 246, 925–945. [Google Scholar] [CrossRef]
- Kaneko, H.; Ijiri, S.; Kobayashi, T.; Izumi, H.; Kuramochi, Y.; Wang, D.S.; Mizuno, S.; Nagahama, Y. Gonadal soma-derived factor (gsdf), a TGF-beta superfamily gene, induces testis differentiation in the teleost fish Oreochromis niloticus. Mol. Cell. Endocrinol. 2015, 415, 87–99. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, B.; Chen, W.; Ge, W. Anti-Müllerian hormone (AMH/amh) plays dual roles in maintaining gonadal homeostasis and gametogenesis in zebrafish. Mol. Cell. Endocrinol. 2020, 1, 517. [Google Scholar] [CrossRef]
- Bej, D.K.; Miyoshi, K.; Hattori, R.S.; Strüssmann, C.A.; Yamamoto, Y. A duplicated, truncated amh gene is involved in male sex determination in an Old World Silverside. G3 2017, 7, 2489–2495. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Ma, H.; Liu, X.; Shi, H.; Li, M.; Wang, D. Mutation of foxl2 or cyp19a1a results in female to male sex reversal in XX Nile tilapia. Endocrinology 2017, 158, 2634–2647. [Google Scholar] [CrossRef]
- Ijiri, S.; Kaneko, H.; Kobayashi, T.; Wang, D.S.; Sakai, F.; Paul-Prasanth, B.; Nakamura, M.; Nagahama, Y. Sexual dimorphic expression of genes in gonads during early differentiation of a teleost fish, the Nile tilapia, Oreochromis niloticus. Biol. Reprod. 2008, 78, 333–341. [Google Scholar] [CrossRef]
- Hirst, C.E.; Major, A.T.; Smith, C.A. Sex determination and gonadal sex differentiation in the chicken model. Int. J. Dev. Biol. 2018, 62, 149–162. [Google Scholar] [CrossRef]
- Jiang, M.; Jia, S.; Chen, J.; Chen, K.; Ma, W.; Wu, X.; Luo, H.; Li, Y.; Zhu, Z.; Hu, W. Timing of gonadal development and dimorphic expression of sex-related genes in gonads during early sex differentiation in the Yellow River carp. Aquaculture 2020, 518, 734825. [Google Scholar] [CrossRef]
- Wei, H.; Li, W.; Liu, T.; Li, Y.; Liu, L.; Shu, Y.; Zhang, L.J.; Wang, S.; Xing, Q.; Zhang, L.L.; et al. Sexual development of the hermaphroditic scallop Argopecten irradians revealed by morphological, endocrine and molecular analysis. Front. Cell Dev. Biol. 2021, 9, 646754. [Google Scholar] [CrossRef]
- Li, M.; Sun, L.; Wang, D. Roles of estrogens in fish sexual plasticity and sex differentiation. Gen. Comp. Endocrinol. 2019, 277, 9–16. [Google Scholar] [CrossRef]
- Chen, J.L. The Role of Elongation Factor eEF1A1b and 42Sp50 in Gametogenesis in Nile tilapia. Ph.D. Thesis, South-West University, Chongqing, China, 2018. [Google Scholar]




| Gene Name | Primer Sequences (5′-3′) Forward | Primer Sequences (5′-3′) Reverse | NCBI ID |
|---|---|---|---|
| 42sp50 | GCCACCAGTGCGAACCATCAA | TTGCCTGTGCGACGGTCAAG | 124049430 |
| foxl2 | GGCAGAACAGTATCAGACA | CCATCTCCTCCGAACAAG | 124058880 |
| figlα | GATACAGACAGCGATGATG | GGTGCTACTTGAATGATGAA | 124057770 |
| zar1 | ACCACAGAAGAGTGAAGATG | CCTCAACACGATACGGATT | 124061181 |
| zp2 | GAGGATTCAGTGTGGTTCA | CTCTAAGCATTCGGTGTCT | 124059932 |
| cyp19a1a | TGCATCGGCATGAACGAGAGG | TTCCAGGTCATCCAGGTGAGTCT | 124061003 |
| dmrt1 | GAAGGCAGCAAGATCAGGAGGA | CAGCAGCAGGTCAGATGGTTCC | 124057575 |
| amh | TTCCACAGAGACCAGAGAT | TTCAGAAGTTCCAGTCCATT | 124061215 |
| gsdf | GGTCTCTGGCTACTCTGT | GCATCCTGGTCATTGGTC | 124051891 |
| cyp11b2 | GTCTACTGCTCAACAAGGA | GCCAATACGCTCACCATA | 124065384 |
| sox3 | CCGTAGGAAGACCAAGAC | TGTTCATGCTGTGATGCT | 124068361 |
| b2m | GAACTTCCTGGCGCTAAGCA | TATGATGTCCCCATGAGTGACC | 124053526 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.-T.; Shi, G.; Jiang, D.-N.; Li, Y.; Huang, Y.-Q.; Shi, H.-J.; Li, G.-L. Preliminary Trial of Male to Female Sex Reversal by 17β-Estradiol in Combination with Trilostane in Spotted Scat (Scatophagus argus). Fishes 2024, 9, 1. https://doi.org/10.3390/fishes9010001
Jiang Z-T, Shi G, Jiang D-N, Li Y, Huang Y-Q, Shi H-J, Li G-L. Preliminary Trial of Male to Female Sex Reversal by 17β-Estradiol in Combination with Trilostane in Spotted Scat (Scatophagus argus). Fishes. 2024; 9(1):1. https://doi.org/10.3390/fishes9010001
Chicago/Turabian StyleJiang, Zheng-Ting, Gang Shi, Dong-Neng Jiang, Yu Li, Yuan-Qing Huang, Hong-Juan Shi, and Guang-Li Li. 2024. "Preliminary Trial of Male to Female Sex Reversal by 17β-Estradiol in Combination with Trilostane in Spotted Scat (Scatophagus argus)" Fishes 9, no. 1: 1. https://doi.org/10.3390/fishes9010001
APA StyleJiang, Z.-T., Shi, G., Jiang, D.-N., Li, Y., Huang, Y.-Q., Shi, H.-J., & Li, G.-L. (2024). Preliminary Trial of Male to Female Sex Reversal by 17β-Estradiol in Combination with Trilostane in Spotted Scat (Scatophagus argus). Fishes, 9(1), 1. https://doi.org/10.3390/fishes9010001








