Abstract
To investigate the age composition, growth pattern, mortality, and exploitation rate of Triplophysa scleroptera in the upper reaches of the Yellow River, we measured the total length (L) and body weight (W) of 347 individuals based on three sampling surveys from 2022 to 2023. The otoliths were used in this investigation to determine age. The total length of the collected samples ranged from 5.69 cm to 18.40 cm, body weight ranged from 1.65 g to 50.22 g, and the age ranged from 1 to 7 years old. The relationship of total length and body weight was for the total samples, and the growth pattern observed in the study belongs to the isometric type. The von Bertalanffy growth equation revealed that the fish had an asymptotic total length L∞ of 37.536 cm, and the growth coefficient K was 0.064 yr−1. Total instantaneous mortality rate (Z) of total samples calculated by the age-based catch curve method was 1.092 yr−1. The average instantaneous rate of the natural mortality (M), estimated by three different methods, for the total samples was 0.237 yr−1. The instantaneous rate of fishing mortality (F) for the total samples was calculated as 0.855 yr−1, and the exploitation rate (E) was determined as 0.783. As a whole, compared with other Triplophysa fishes, the growth rate of T. scleroptera in the upper reaches of the Yellow River is relatively slow, and the population of T. scleroptera has been overexploited. It is crucial to establish some effective management strategies to protect this species.
Key Contribution:
This study estimates the age, growth, and mortality of T. scleroptera and points out that the population of T. scleroptera has been overexploited in the upper reaches of the Yellow River.
1. Introduction
The Qinghai–Tibet Plateau, also known as “the roof of the world”, is rich in biodiversity and a relatively unique area with many endemic species [1]. The native fish found in the Qinghai–Tibet region belong to three orders: Cypriniformes, Siluriformes and Salmoniformes [1,2]. Fish of the genus Triplophysa are indeed found throughout the Qinghai–Tibet Plateau and its surrounding regions, and it is the largest and most diversified genus in the subfamily Noemacheilinae, which belongs to the family Nemacheilidae (Cypriniformes) [3]. These fishes are evolved to exist in high-altitude, cold-water environments and can be found in a variety of aquatic settings including rivers, streams, and lakes in the area. They exhibit a wide range of adaptations and have evolved unique characteristics to survive in the harsh conditions of the plateau, which has important ecological value [1,2,3,4]. Among the 122 species of Triplophysa fish published in the “Red List of Vertebrate Animals in China” [5], 23 species are classified as extinct, critically endangered, endangered, vulnerable, or near-threatened, accounting for more than 18.85% of the total species. Additionally, 21 species are identified as “non-endangered”. However, there are still 78 species of Triplophysa fish for which data are lacking, making evaluation challenging. This highlights the need to enhance monitoring and investigation efforts for Triplophysa fish germplasm resources, despite the relatively abundant populations of some species.
Triplophysa scleroptera is one of the unique fish species in the Qinghai–Tibet Plateau, only distributed in the upper Yellow River and Qinghai Lake in China [6]. T. scleroptera, an omnivorous fish, primarily consumes zoobenthos, such as chironomid larvae and Gammarid, and also consumes a smaller amount of algae and vascular plants, which plays a crucial role in the water ecosystem [7]. Additionally, it provides food for predatory fishes, which affects the dynamics of the entire food chain [7]. Currently, there are limited reports available on T. scleroptera, which primarily concentrate on issues like geographical distribution, feeding habits, sexual maturity, respiratory physiology, and other related themes [6,7,8]. T. scleroptera, adapted to the extreme climate of the plateau, has special biological characteristics and physiological mechanisms. It has an important role in maintaining biodiversity and ecosystem stability in the cold and vulnerable plateau ecological environment. Although T. scleroptera is listed as a “non-endangered” fish species in the “Red List of Vertebrate Animals in China” [5], there is little information on its age structure, population growth, mortality, and resource status in the upper reaches of the Yellow River, which is its primary habitat. Furthermore, its meat is very delectable and well-liked by consumers, which adds to its high fishery utilization value. Therefore, continued research and data collection on T. scleroptera are crucial in order to create effective management strategies and guarantee the populations’ long-term survival.
Age, growth, and mortality are indispensable biological parameters that provide fundamental data for comprehending the fundamental biological traits and population dynamics of species [9,10]. Among them, fish age is an important biological variable, which is essential for calculating the growth parameters and mortality. The von Bertalanffy equation, which is frequently used and offers useful insights through its asymptotic body length L∞ and growth coefficient K, presents a year-by-year picture of fish growth dynamics. By estimating the rates of natural mortality and fishing mortality, we can evaluate the dynamics of fish populations and gauge their sustainable resource utilization. On the whole, these parameters play a significant role in fishery management by helping to determine the health of a population, estimate sustainable harvest levels, and develop appropriate conservation measures [11,12]. In view of this, we investigated the population resources of the T. scleroptera in the upper reaches of the Yellow River from 2022 to 2023, and analyzed individual biological characteristics such as age, growth and mortality. The primary objectives of this study were: (1) estimating the age and growth parameters of T. scleroptera through otolith sections and comparing its growth traits with other Triplophysa fishes; (2) estimating the mortality rates of T. scleroptera and assessing its resource utilization status; (3) discussing the implications of our findings for the conservation management of the T. scleroptera population.
2. Materials and Methods
2.1. Study Area and Sample Collection
This study was conducted in the upper reaches of the Yellow River, which spans from the Guide section in Qinghai Province to the Jingyuan section in Gansu Province (Figure 1). The survey sections are located in the canyon type terrain of the upper Yellow River, which is known as a concentrated distribution area of hydropower stations [13]. Recently, the distribution area of certain fish species in the upper reaches of the Yellow River has been gradually shrinking due to the reduction in water resources, water pollution, and water conservancy construction [13,14]. As a result, their populations have experienced a sharp decline, and some species have even become endangered or extinct.
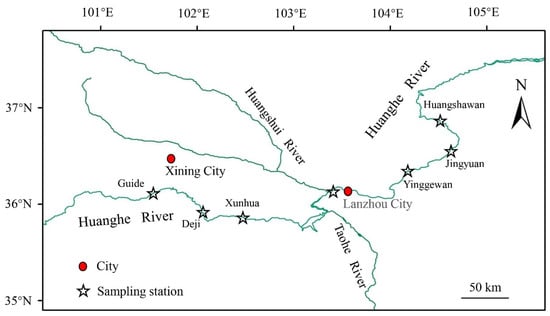
Figure 1.
Sampling locations of T. scleroptera in the upper reaches of the Yellow River, China.
In July–August 2022, February–March 2023 and May 2023, a total of 347 T. scleroptera specimens (Figure 2) were collected from the upper reaches of the Yellow River using cage nets (measuring 15 cm in length, 40 cm in width, and 40 cm in height) and gillnets with mesh sizes ranging from 1 cm to 4 cm. Under fresh sample conditions, routine biological measurements were performed on the fish body, including accurate recordings of their total length (L) to 0.01 cm and body weight (W) to 0.01 g. Subsequently, a biological anatomy was performed to distinguish between males and females based on the gonadal morphology. The sagitta otoliths were removed from the inner ear sac of the croaker using tweezers. After the surface connective tissue was removed, otoliths were preserved in labeled tubes containing a 95% ethanol solution. During the survey, water temperature was measured by portable water quality analyzer (HACH, Loveland, CO, USA). All samples were conducted in accordance with the guidelines of Heilongjiang River Fisheries Research Institute of CAFS Application for Laboratory Animal Welfare and Ethical Review (Issue No.: 20220413-001).

Figure 2.
T. scleroptera collected from the upper reaches of Yellow River.
2.2. Age Estimation
In this study, the left otoliths were used for age estimation, and the right otoliths were used as a backup. First, the otoliths were embedded in transparent nail polish and polished using 1500–2000 grit sandpaper until the growth center became clearly visible. During the polishing process, the otoliths were observed under an optical microscope. The otolith sections were then washed with anhydrous ethanol, made transparent with xylene, and sealed with neutral gum. Finally, the annual rings were observed and counted under an optical microscope to determine the characteristics of each ring. Additionally, the age of each otolith was identified through blind examination, following the method described by Li et al. [15].
2.3. Length–Weight Relationship
The length–weight relationship was calculated using the power equation: , where W is the body weight (g), L is the total length (cm), a is the condition factor and b is the allometric growth factor [16,17]. A t-test with a significance level of 0.05 was performed to determine whether the acquired b value significantly differed from the value “3” used to evaluate the allometry in growth [18]. The statistical analyses were carried out using Microsoft Excel 2016 and SPSS Statistics 19.0.
2.4. Estimation of Growth Equation Parameters
The von Bertalanffy growth equation [19] was used to describe the growth characteristics of T. scleroptera. The length growth equation is , where Lt is the total length at t age (cm); L∞ is the asymptotic total length (cm); K is growth coefficient (yr−1); t is the age of the sample (yr); and t0 is the theoretical initial age at which the total length is zero (yr). The growth characteristic index (φ) was calculated using the formula: . Additionally, the residual sum of squares (ARSS) was used to statistically compare the fitted growth curves between sexes [20]. This measure helps assess the discrepancy between the observed data and the predicted values based on the growth curves for each sex, providing insight into potential differences in growth patterns.
2.5. Total Mortality, Fishing Mortality and Exploitation Rate
To estimate the total instantaneous mortality rate (Z), an age-based catch curve analysis was used [21]. Catch curves were constructed by plotting the natural logarithm of the number of sampled fishes in each age group against their respective age group. Only age groups that were fully recruited to the fishing gear were considered for estimating Z. The estimation of Z involved fitting a linear regression equation of the form “” to the right limb of the catch curve, and the absolute value of the slope (m) of this regression equation represents the value of Z [22].
The instantaneous rate of natural mortality (M) can be obtained by three different methods: (1) the length-based empirical relationship proposed by Pauly [23] is , where T represents the annual habitat temperature (°C) of the water where the fish stocks reside, and the values of L∞ and K are the asymptotic length and average curvature of the von Bertalanffy growth equation, respectively. (2) The age-based method proposed by Zhan [24] is , where tm is the observed maximum age in years. (3) The age-based method proposed by Ralston [25] is , where K represents the growth coefficient from the von Bertalanffy growth equation.
The instantaneous rate of fishing mortality (F) can be obtained by subtracting the instantaneous rate of natural mortality coefficient (M) from the total instantaneous mortality coefficient (Z). Mathematically, this is expressed as: . Furthermore, the population exploitation rate (E) can be calculated by dividing the fishing mortality (F) by the total instantaneous mortality (Z): . These formulas provide a way to assess the impact of fishing on the population by comparing the fishing mortality to the overall mortality rate. The exploitation rate (E) represents the proportion of the total mortality that is attributed to fishing operations [26].
3. Result
3.1. Population Structure
During the survey, a total of 347 T. scleroptera specimens were collected. Among them, 173 were identified as females, 167 as males, and 7 had an unknown sex. The sexual proportion of the population was approximately 1: 0.97 (female: male). The total length of the collected samples ranged from 5.69 cm to 18.40 cm, with an average length of 10.95 ± 2.16 cm (Figure 3). The majority of the samples, accounting for 84.15% of the total, had a total length between 8.0 cm and 14.0 cm. The body weight distribution of the population samples ranged from 1.65 g to 50.22 g, with an average weight of 11.71 ± 7.14 g (Figure 2). The dominant body weight category in the population was below 20 g, which accounted for 90.78% of the total samples.
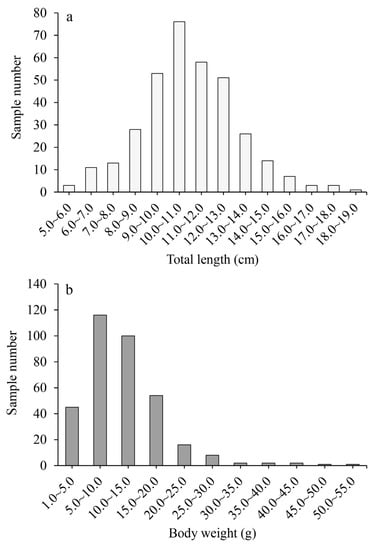
Figure 3.
The distribution of total length (a) and body weight (b) of T. scleroptera.
3.2. Age Distribution
Left otoliths were used in this study for age estimation of T. scleroptera. The growth rings were analyzed through the observation of otolith grinding slices (Figure 4). The results showed that the age range of T. scleroptera individuals was between 1 and 7 years (Table 1). Among them, age-2, age-3, and age-4 individuals were the most abundant, accounting for 84.73% of the total. Additionally, the proportion of age-5, age-6, and age-7 individuals was comparatively low, indicating a simplified age structure within the T. scleroptera population.
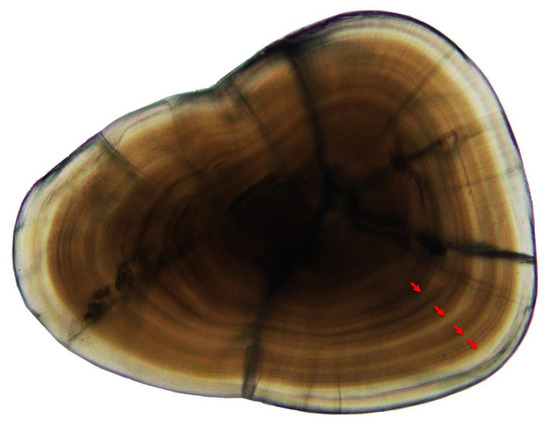
Figure 4.
Sagitta otolith section of T. scleroptera (L = 14.53 cm, W = 23.62 g, age-5). Note: the red arrows indicate the otolith ring pattern consists of four light and dark rings, so the age of the sample is 4+ years old (age-5).

Table 1.
Numbers of samples and total length (L) and weight (W) in different ages of T. scleroptera in the upper reaches of the Yellow River.
3.3. Length–Weight Relationship
Based on the regression analysis conducted on the relationship between the total length (L) and body weight (W) of T. scleroptera in the upper Yellow River, the fitting formula was (R2 = 0.951) (Figure 5). Further analysis involved conducting a t-test to assess the significance of the regression coefficient (b value). The results showed that there was no significant difference (t-test, t = 1.155, p = 0.124) between the obtained b value (2.942) and the theoretical value of isometric growth (3.00). This implies that the length–weight relationship of T. scleroptera in the upper reaches of the Yellow River follows an isometric growth pattern, in which the total length and body weight increase in proportion to each other.
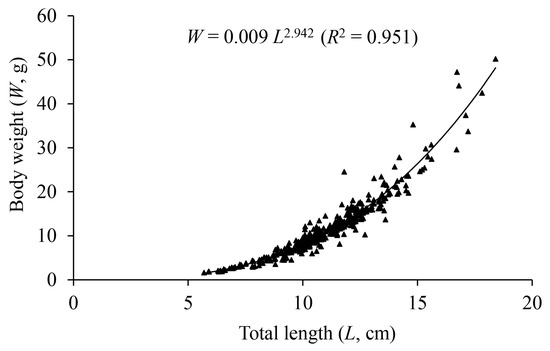
Figure 5.
Length–weight relationship of T. scleroptera.
3.4. Growth Equation
The von Bertalanffy growth equations were used to model the growth of female and male T. scleroptera. For females, the equation was , and for males, the equation was . The ARSS test was conducted to evaluate the significance of growth differences between the sexes, and the results showed that there was no significant difference (ARSS test, F = 0.029, p = 1.029) in growth between female and male T. scleroptera. Therefore, the length growth equation of total samples was: (Figure 6). Additionally, the growth characteristic index (φ) for T. scleroptera was determined to be 1.955, and Table 2 lists the age range, growth parameters and other indicators of several Triplophysa fishes [15,17,27,28,29,30,31,32,33].
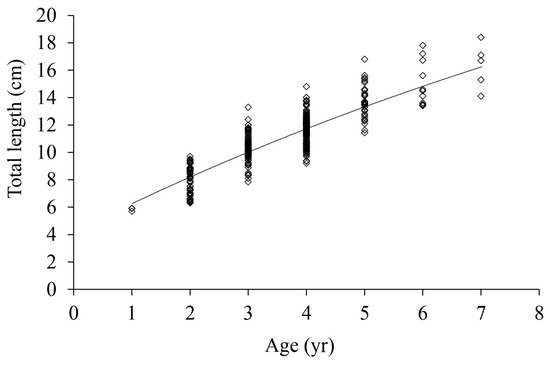
Figure 6.
Von Bertalanffy growth curve fitted to total length-at-age for T. scleroptera from samples captured.

Table 2.
Comparison of growth characters of several Triplophysa fishes in different studies.
3.5. Mortality and Exploitation Rate
Based on the results of age identification, it seems that the age-1, age-2 and age-3 groups were not fully recruited due to the poor effect of the survey net mesh. As a result, when evaluating the total instantaneous mortality rate (Z), the data of the age-1, age-2 and age-3 groups were excluded, following the methodology of Beverton and Holt [22]. Using age-based catch curve analysis, the Z value for the total samples was determined to be 1.092 yr−1 (Figure 7). Additionally, the average water temperature (T) in the investigation area of the upper Yellow River was recorded as 12.5 °C. Based on Pauly’s method [23], the instantaneous rate of natural mortality (M) for the total samples was estimated as 0.192 yr−1. However, when applying the other two methods, the M values for the total samples were reported as 0.368 yr−1 [24] and 0.151 yr−1 [25], respectively. Taking the average of these three M values, the estimated M for the total samples was 0.237 yr−1. Consequently, the instantaneous rate of fishing mortality (F) for the total samples was calculated as 0.855 yr−1, and the exploitation rate (E) was determined as 0.783.
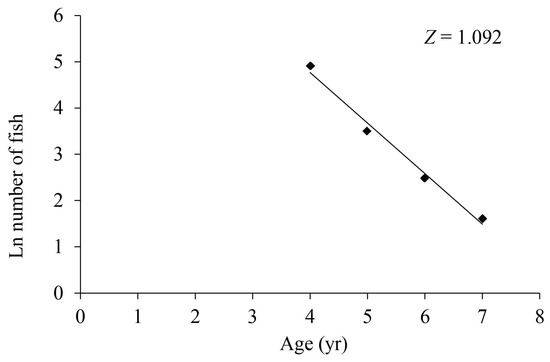
Figure 7.
Catch curve based on observed age for T. scleroptera samples. Note: Z represents the total instantaneous mortality rate.
4. Discussion
Accurate determination of fish age is essential for researchers examining growth characteristics, life history, and population dynamics. However, inaccurate age estimations can significantly impede the development and utilization of fishery resources [29,34]. With the growth of fish, growth marks are laid down on their calcified structures such as scales, vertebrae, opercula and otoliths, and the age of the fish can be estimated according to these marks [35]. Campana and Thorrold [36] and Duan et al. [37] pointed out that the otoliths possess distinct characteristics compared to other age identification materials such as scales, vertebrae, and opercula. Even in the later stages of a fish’s life, it is recognized that otoliths have a resistance to reabsorption and an ability to continuously grow. Consequently, otoliths are considered an ideal material for recording the life history of fish as well as monitoring changes in the surrounding environment. Chen et al. [38] indicated that otoliths were found to be more accurate than scales and fins in age estimation of Gymnocypris selincuoensis, especially in estimating the age of slow-growing and relatively long-lived populations. Zeng and Tang [39] conducted a comparative study on the age estimation materials of two esquamate Triplophysa fishes, and the results showed that there were no obvious annual ring markers in fin rays and operculum, and the age discrimination ability and estimation rate of vertebrae were not as good as those of otoliths. So, otoliths are a suitable material for identifying Triplophysa fishes and have been used widely by numerous researchers [15,17,27,28,29,30,31,32,33]. Based on the aforementioned studies, otoliths were the chosen material in this study for age estimation. Specifically, for T. scleroptera, the otolith ring pattern consists of alternating clear dark and bright areas, making it suitable for precise age estimation. In the investigated area, the age distribution of T. scleroptera varied from 1 to 7 years old, and the proportion of individuals belonging to age-5, age-6, and age-7 was relatively low. In comparison to other species within the same genus, the age structure of T. scleroptera population was similar to T. stewarti (1–6) [17], T. bombylons (1–6) [30], T. siluroides (2–6) [31], and T. tenuis (1–6) [32], higher than that of T. anterodorsalis (1–5) [29], and lower than that of T. orientalis (3–9) [15], T. stenura (3–14) [28], and T. yarkandensis (1–11) [33]. This shows that the age structure of the T. scleroptera population is simple and tends to be younger. In addition, the numbers of age-1, age-2 and age-3 fish were not fully recruited in the collected samples of T. scleroptera, which may be related to the mesh size of the fishing net.
The growth characteristics of fish serve as a visual representation of the development of fish populations. These growth patterns are influenced by both genetic factors inherent to the species and the intricate natural environment they inhabit [40,41]. Additionally, populations of the same species residing in different geographical locations or experiencing varying growth environments may exhibit distinct interpopulation variations [42]. The slope b of length–weight relationship, growth coefficient K, growth characteristic index (φ) are the key parameters to evaluate the growth potential of fish population [43,44]. According to Onikura and Nakajima [45], the b value reflects the growth pattern of fish, and when b is close to “3”, it is isometric growth, otherwise, it is allometric growth. In this study, the slope b of length–weight relationship was 2.942, which is obviously lower than Xie’s [46] reported b value (3.33) for T. scleroptera in the Longyangxia Reservoir of the Yellow River. This indicates that gonad maturity, age structure, stomach fullness, and food abundance, can lead to different b values even within the same species [46]. Compared with several fishes of the same genus, it is similar to T. markehenensis (2.952) [27], T. stenura (2.976) [28], and T. anterodorsalis (3.012) [29], indicating that these species exhibit an isometric growth pattern. However, the slope b for T. bombylons (2.240) [30], T. yarkandensis (2.510) [47], T. tenuis (2.530) [47], T. naziri (2.430) [48], and T. kashmirensis (2.510) [48] were significantly less than “3”, indicating that these species exhibit a negative allometric growth pattern. On the contrary, the T. strauchii (3.280) [47] and T. brahui (3.120) [48] were significantly higher than “3”, indicating that these species exhibit a positive allometric growth pattern. This demonstrates that the geographical location and environmental conditions also exert an influence on the variation in growth characteristic parameter b among populations of the genus Triplophysa.
In this study, the growth coefficient K of T. scleroptera was 0.064 yr−1. Compared with other Triplophysa fishes, the K value of T. scleroptera is similar to T. stenura (0.059 yr−1) [28] and T. bombifrons (0.050 yr−1) [30], but significantly lower than T. stewarti (0.168 yr−1) [17], T. markehenensis (0.159 yr−1) [27], T. anterodorsalis (0.260 yr−1) [29], T. siluroides (0.334 yr−1) [31], T. tenuis (0.640 yr−1) [32], and T. yarkandensis (♀ 0.112 yr−1, ♂ 0.157 yr−1) [33]. In addition to habitat environmental factors, the difference in the K value in Triplophysa fishes may also be attributed to the size of the collected individuals as the maximum and minimum total length within the samples greatly influence the estimation of growth coefficient K [30,31,32,33]. The growth characteristic index (φ) combines the values of L∞ and K, which is positively correlated with the growth rate and can be used to compare the growth performance of fish belonging to the same genus [49,50]. Currently, reports show that the φ value of Triplophysa fishes had a wide range of 1.404 to 4.268 [15,17,27,28,29,30,31,32,33]. The φ value in our study was 1.955, higher than T. stewarti (1.510) [17], T. markehenensis (1.404) [27], T. stenura (1.556) [28], T. anterodorsalis (1.404) [29], and T. siluroides (1.551) [31], lower than T. bombifrons (2.302) [30], T. tenuis (2.558) [32], T. yarkandensis (♀ 2.269, ♂ 2.227) [33], etc. As a whole, the growth rate of T. scleroptera in the upper reaches of the Yellow River in Triplophysa fishes is slow, which may be due to the low water temperature, high sediment content of the water body and the low abundance of food organisms in the investigated area [51,52].
The exploitation rate (E) of fish is an important parameter in fishery management [44]. The reasonable exploitation intensity of fish should be less than 0.5, less than this intensity is mild development, and higher than this intensity is overexploitation, which will have a negative impact on the sustainability of fish stocks [53]. In the present study, we used three methods to derive a relatively certain range for the real value of instantaneous rate of natural mortality (M) [23,24,25]. The current exploitation rate (E) of T. scleroptera has been over 0.5 (E = 0.783), higher than Gymnocypris firmispinatus, a small-size Schizothoracinae fish, in the Anning River [54], and several prey fish of Hemiculter leucisculus, Acheilognathus macropterus, Rhodeus sericeus, and Pseudorasbora parva in the lower reaches of the Songhua River [55], suggesting that the population in the upper reaches of the Yellow River has been overexploited under the current fishing intensity. Nowadays, with the current increase in commercial fishing intensity and the reduction in mesh size, the small and middle-sized fish has gradually become the target of fishing and made into fishmeal for profit [55]. Ma et al. [54] indicated that the populations of slow-growing fish would be difficult to restore if their resources were overexploited. Due to the fragile habitat, fewer resources, simple age structure, and slower growth of T. scleroptera in the upper reaches of the Yellow River, it will be difficult to recover when its resource is excessively destroyed.
To ensure the protection of T. scleroptera, it is crucial to establish some effective management strategies. Here are a few possible strategies: (1) Prohibition of illegal nets: it is important to prohibit the use of illegal fishing gear such as cage nets and smaller mesh nets. These nets can capture T. scleroptera and other vulnerable species, which causes a drop in their populations. (2) Spawning season protection: implementing fishing prohibitions during the spawning season of T. scleroptera can promote sustainable reproduction. This closed fishing season can prevent excessive exploitation during a critical period of reproductive activity. (3) Regular monitoring: regular monitoring of T. scleroptera populations and other aquatic biological resources in the area is essential. This monitoring can provide valuable insights into population dynamics, habitat conditions, and potential dangers. It will help in identifying any changes or declines and allow for quick conservation measures. By integrating these strategies, effective protection and conservation of T. scleroptera can be achieved, ensuring its long-term sustainability.
5. Conclusions
It was concluded that T. scleroptera seem to be a small-size fish with relatively short longevity, when compared to other Triplophysa fishes. The growth rate of T. scleroptera in the upper reaches of the Yellow River is relatively slow. Our results also indicated that the population of T. scleroptera has been overexploited and that it is crucial to establish some effective management strategies to protect this species.
Author Contributions
Methodology, P.L. and J.W.; formal analysis, P.L., J.L. and J.W.; investigation, P.L., J.L., T.W. and J.W.; writing—original draft, P.L.; writing—review and editing, P.L., J.L., T.W. and J.W.; project administration, J.W.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Project of Yellow River Fisheries Resources and Environment Investigation from the MARA, P. R. China: HHDC-2022-02; the investigation on alien invasive aquatic animals in the Yellow River: ZF2022512304; and the China Academy of Fishery Sciences: 2020TD07.
Institutional Review Board Statement
All samples were conducted in accordance with the guidelines of Heilongjiang River Fisheries Research Institute of CAFS Application for Laboratory Animal Welfare and Ethical Review (Issue No.: 20220413-001).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, T.; Zhang, Y.P.; Yang, Z.Y.; Liu, Z.; Du, Y.Y. DNA barcoding reveals cryptic diversity in the underestimated genus Triplophysa (Cypriniformes: Cobitidae, Nemacheilinae) from the northeastern Qinghai-Tibet Plateau. Bmc. Evol. Biol. 2020, 20, 151. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Liu, N.F.; Yang, J.X. A brief review of Triplophysa (Cypriniformes: Balitoridae) species from the Tarim Basin in Xinjiang, China, with description of a new species. Zootaxa 2007, 1605, 47–58. [Google Scholar] [CrossRef]
- Hu, J.X.; Liu, M.D.; He, D.K. Phylogeography of Triplophysa stenura (Nemacheilidae): Responded to the Mid-Pleistocene Climate Transition in the Qinghai-Tibetan Plateau. Zool. Stud. 2020, 30, e67. [Google Scholar]
- Wang, Y.; Shen, Y.J.; Feng, C.G.; Zhao, K.; Song, Z.B.; Zhang, Y.P.; Yang, L.D.; He, S.P. Mitogenomic perspectives on the origin of Tibetan loaches and their adaptation to high altitude. Sci. Rep. 2016, 6, 29690. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.G.; Jiang, J.P.; Wang, Y.Z.; Zhang, E.; Zhang, Y.Z.; Li, L.L.; Xie, F.; Cai, B.; Cao, L.; Zheng, G.M.; et al. Red List of China’s Vertebrates. Biodivers. Sci. 2016, 24, 500–551. (In Chinese) [Google Scholar]
- Ding, R.H. The Fishes of Sichuan, China; Sichuan Publishing House of Science and Technology: Chengdu, China, 1994; pp. 73–75. (In Chinese) [Google Scholar]
- Qi, W.H.; Guo, Y.S.; Wang, H.; He, M.; Chen, Y.L.; Yu, T.L. Study on biology of Triplophysa scleroptera in Xiaman Nature Reserve, Sichuan. Sichuan. J. Zool. 2008, 27, 1157–1162. (In Chinese) [Google Scholar]
- Zhu, S.H.; Yan, S.; Chen, Z.L. Study on the relationship between oxygen consumption rate and asphyxiation piont and body mass of Triplophysa scleroptera. J. Qinghai. Univ. 2020, 38, 34–38. (In Chinese) [Google Scholar]
- Jackson, G.D. Advances in defining the life histories of myopsid squid. Mar. Freshwater. Res. 2004, 55, 357–365. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, W.; Li, P.L.; Tang, F.J.; Lu, W.Q. Estimation of Coregonus ussuriensis age, growth, and maturation in China’s Amur River. PeerJ. 2022, 10, e12817. [Google Scholar] [CrossRef]
- Labbaci, A.; Chaoui, L.; Kara, M.H. Age, growth and reproduction of the Mediterranean killifish Aphanius fasciatus Nardo, 1827 in Mellah Lagoon (Eastern Algeria). Environ. Biol. Fish. 2019, 102, 663–674. [Google Scholar] [CrossRef]
- Andres, M.J.; Slack, W.T.; Peterson, M.S.; Kimmel, K.D.; Lewis, B.R.; Grammer, P.O. Growth estimation of western population segment gulf sturgeon using length-at-age and mark–recapture data. T Am. Fish. Soc. 2019, 148, 176–190. [Google Scholar] [CrossRef]
- Fan, J.J.; Zhao, G.J.; Mu, X.M.; Lu, A.; Tian, P.; Gao, P.; Sun, W.Y. Effects of cascading reservoirs on streamflow and sediment load with machine learning reconstructed time series in the upper Yellow River basin. Catena 2023, 225, 107008. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Y.P.; Guan, L.H.; Du, Y.Y.; Lou, Z.Y.; Jiao, W.L. Current freshwater fish resources and the application of DNA barcoding in species identification in Gansu Province. Biodivers. Sci. 2015, 23, 306–313. (In Chinese) [Google Scholar] [CrossRef]
- Li, L.T.; Yang, X.F.; Yang, R.B.; Fan, Q.X.; Wei, K.J.; Jiang, H. Age structure and growth characteristics of Triplophysa orientalis in the middle of the Yarlung Tsangpo River, Tibet. J. Huazhong Agric. Univ. 2016, 35, 117–123. (In Chinese) [Google Scholar]
- Froese, R.; Tsikliras, A.C.; Stergiou, K.I. Editorial note on weight–length relations of fishes. Acta. Ichthyol. Piscat. 2011, 41, 261–263. [Google Scholar] [CrossRef]
- Tian, N.N.; Yang, R.B.; Tan, B.Z.; Zeng, X.L.; He, L.Q.; Xu, Z.L.; Zhu, Z.; Liu, H.P.; Yang, X.F. Age, growth, and reproductive characteristics of Triplophysa stewarti in Lake Chugutso, Tibet. J. Fish. Sci. China 2022, 29, 1013–1021, (In Chinese with English abstract). [Google Scholar]
- Ricker, W.E. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Board Can. 1975, 191, 1–382. [Google Scholar]
- von Bertalanffy, L. A quantitative theory of organic growth (inquiries on growth laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Chen, Y.; Jackson, D.A.; Harvey, H.H. A comparison of von Bertalanffy and polynomial functions in the modeling fish growth data. Can. J. Fish. Aquat. Sci. 1992, 49, 1228–1235. [Google Scholar] [CrossRef]
- Pauly, D.; Moreau, J.; Abad, N. Comparison of age-structured and length-converted catch curves of brown trout Salmo trutta in two French rivers. Fish. Res. 1995, 22, 197–204. [Google Scholar] [CrossRef]
- Beverton, R.J.H.; Holt, S.J. On the Dynamics of Exploited Fish Populations; Chapman and Hall: London, UK, 1957; p. 533. [Google Scholar]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. J. Conserv. Inter. Explor. Marit. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Zhan, B.Y.; Lou, D.C.; Zhong, J.S. An assessment of the filefish population and rational exploitation of the resource. J. Fish. China 1986, 10, 409–418. (In Chinese) [Google Scholar]
- Ralston, S. Mortality rates of snappers and groupers. In Tropical Snappers and Grouper: Biology and Fisheries Management; Polovina, J.J., Ralston, S., Eds.; Westview Press: Boulder, CO, USA, 1987; pp. 375–404. [Google Scholar]
- Gray, C.A.; Barnes, L.M.; Robbins, W.D.; Van Der Meulen, D.E.; Ochwada–Doyle, F.A.; Kendall, B.W. Length–and age–based demographics of exploited populations of stout whiting, Sillago robusta Stead, 1908. J. Appl. Ichthyol. 2017, 33, 1073–1082. [Google Scholar] [CrossRef]
- Zhang, X.F.; He, C.L.; Song, Z.B. Age and Growth of Triplophysa markehenensis from the Markehe River in Upper Reaches of the Dadu River. Chin. J. Zool. 2010, 45, 11–20. (In Chinese) [Google Scholar]
- Deng, H.T.; Yue, X.J.; Chen, D.Q.; Tian, H.W.; Liu, S.P. Growth characteristics and feed habit of Triplophysa atenura in Nujiang River. Freshw. Fish. 2010, 40, 26–33. (In Chinese) [Google Scholar]
- Wang, C.; Liang, Y.Q. Age and growth of Triplophysa anterodorsalis Zhu & Cao, 1989 in the Heishui River, China. J. Appl. Ichthyol. 2017, 33, 1215–1217. [Google Scholar]
- Yao, N.; Wang, M.; Gong, Y.R.; Ju, M.H.; Wang, S.; Chen, S.A.; Song, Y. Biological characteristics of Triplophysa bombifrons in Tarim River Basin. Jiangsu Agr. Sci. 2018, 46, 146–149. (In Chinese) [Google Scholar]
- Yao, N.; Ma, L.; Jing, S.S.; Chen, S.A. Growth characteristics of Triplophysa siluroides (Herzenstein) in Beichuanhe Basin of Qinghai. J. Inn. Mong. Agr. Univ. 2019, 40, 5–10. (In Chinese) [Google Scholar]
- Jin, S.S.; Wang, X.Y.; Lin, X.; Chen, S.A.; Liu, M.C.; Xie, C.X. Age and Growth of Triplophysa tenuis in Kaidu River, Xinjiang. Xinjiang Agr. Sci. 2020, 57, 181–189. (In Chinese) [Google Scholar]
- Zhao, J.F.; Qiu, L.H.; Zhou, Q.; Zhou, X.Y.; Shen, J.Z. Age structure and growth characteristics of Triplophysa yarkandensis in the Qarqan River, Xinjiang. J. Huazhong Agr. Univ. 2023, 42. Available online: https://kns.cnki.net/kcms2/detail/42.1181.S.20230801.1115.002.html (accessed on 1 August 2023). (In Chinese).
- Campana, S.E. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish. Biol. 2001, 59, 197–242. [Google Scholar] [CrossRef]
- Phelps, Q.E.; Edwards, K.R.; Willis, D.W. Precision of five structures for estimating age of common carp. N. Am. J. Fish. Manag. 2007, 27, 103–105. [Google Scholar] [CrossRef]
- Campana, S.E.; Thorrold, S.R. Otoliths, increments, andelements: Keys to a comprehensive understanding of fish populations. Can. J. Fish. Aquat. Sci. 2001, 58, 30–38. [Google Scholar] [CrossRef]
- Duan, Y.J.; Xie, C.X.; Zhou, X.J.; Ma, S.S.; Huo, B. Age and growth characteristics of Schizopygopsis younghusbandi Regan, 1905 in the Yarlung Tsangpo River in Tibet, China. J. Appl. Ichthyol. 2014, 30, 948–954. [Google Scholar] [CrossRef]
- Chen, Y.F.; He, D.K.; Duan, Z.H. Annuli characters of selincuo schizothoracine fish (Gymnocypris selincuoensis) in Selincuo Lake, Qinghai–Tibetan Plateau. Acta. Zool. Sin. 2002, 48, 384–392, (In Chinese with English abstract). [Google Scholar]
- Zeng, L.; Tang, W.Q. Discussion on age determination methods for two esquamate Triplophysa fishes. Chin. J. Zool. 2010, 45, 94–103, (In Chinese with English abstract). [Google Scholar]
- Tsagarakis, K.; Basusta, A.; Basusta, N.; Biandolino, F.; Bostanci, D.; Buz, K.; Djodjo, Z.; Dulcic, J.; Gokoglu, M.; Gucu, A.; et al. New Fisheries-related data from the Mediterranean Sea (October 2015). Mediterr. Mar. Sci. 2015, 16, 703–713. [Google Scholar] [CrossRef]
- Yedier, S.; Kontaş, S.; Bostancı, D. Marmara Denizi’nde Yaşayan Pagellus acarne (Risso, 1827)’nin Kondisyon Faktörü, Boy-Boy ve Boy-Ağırlık İlişkileri. J. Anatol. Environ. Anim. Sci. 2019, 4, 82–88. [Google Scholar]
- Szymon, S.; Berg, F. Varying relationships between fish length and scale size under changing environmental conditions—Multidecadal perspective in Atlantic herring. Ecol. Indic. 2022, 134, 108494. [Google Scholar]
- Froese, R. Cube law, condition factor and weight–length relationships: History, meta–analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, C.; Tian, Y.J.; Watanabe, Y. Age, growth, and mortality rate of the yellow goosefish Lophius litulon (Jordan, 1902) in the Yellow Sea. J. Oceanol. Limnol. 2021, 39, 732–740. [Google Scholar] [CrossRef]
- Onikura, N.; Nakajima, J. Age, growth and habitat use of the topmouth gudgeon, Pseudorasbora parva in irrigation ditches on northwestern Kyushu Island, Japan. J. Appl. Ichthyol. 2013, 29, 186–192. [Google Scholar] [CrossRef]
- Xie, J.Y. Length-weight and length-length relationships of four endemic fish species from the upper Yellow River in the Tibetan Plateau, China. J. Appl. Ichthyol. 2015, 31, 958–960. [Google Scholar] [CrossRef]
- Chen, S.; Xie, C.; Li, D.; Yao, N.; Ding, H.; Zhang, Z. Length–weight relationships of five Triplophysa species from the northwest of China. J. Appl. Ichthyol. 2017, 33, 1234–1236. [Google Scholar] [CrossRef]
- Naveed, A.; Muhammad, F.K. Length–weight relationships of four Triplophysa species from northern, Pakistan. J. Appl. Ichthyol. 2018, 34, 1223–1224. [Google Scholar]
- Munro, J.; Pauly, D. A simple method for comparing the growth of fishes and invertebrates. Fishbyte 1983, 1, 5–6. [Google Scholar]
- Pauly, D.; Moreau, J.; Prein, M. A comparison of overall growth performance of tilapia in open waters and aquaculture. In Proceedings of the ICLARM Conference Proceedings of the Second International Symposium on Tilapia in Aquaculture, Bangkok, Thailand, 16–20 March 1987; pp. 469–479. [Google Scholar]
- Stearns, S.C. The Evolution of life history traits: A critique of the theory and a review of the data. Annu. Rev. Ecol. Evol. S. 1977, 8, 145–171. [Google Scholar] [CrossRef]
- Mims, M.C.; Olden, J.D. Life history theory predicts fish assemblage response to hydrologic regimes. Ecology 2012, 93, 35–45. [Google Scholar] [CrossRef]
- Li, X.Q.; Chen, Y.F. Age structure, growth and mortality estimates of an endemic Ptychobarbus dipogon (Regan, 1905) (Cyprinidae: Schizothoracinae) in the Lhasa River, Tibet. Environ. Biol. Fish. 2009, 86, 97–105. [Google Scholar] [CrossRef]
- Ma, B.S.; Nie, Y.Y.; Wei, K.J.; Xu, B.; Zhu, X.Y.; Xu, J. Estimates on age, growth, and mortality of Gymnocypris firmispinatus (Cyprinidae: Schizothoracinae) in the Anning River, China. J. Oceanol. Limnol. 2019, 37, 736–744. [Google Scholar] [CrossRef]
- Lu, W.Q.; Li, P.L.; Ma, B.; Huo, T.B.; Yin, Z.Q.; Tang, F.J.; Wang, J.L. Assessment of fishery management parameters for major prey fish species in the lower reaches of the Songhua River. Front. Mar. Sci. 2023, 10, 1166634. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).