The Migratory Biology and Feeding Habits of Downstream-Migrating Juvenile Chum Salmon Oncorhynchus keta in the Amur River of Northeast China
Abstract
:1. Introduction
2. Materials and Methods
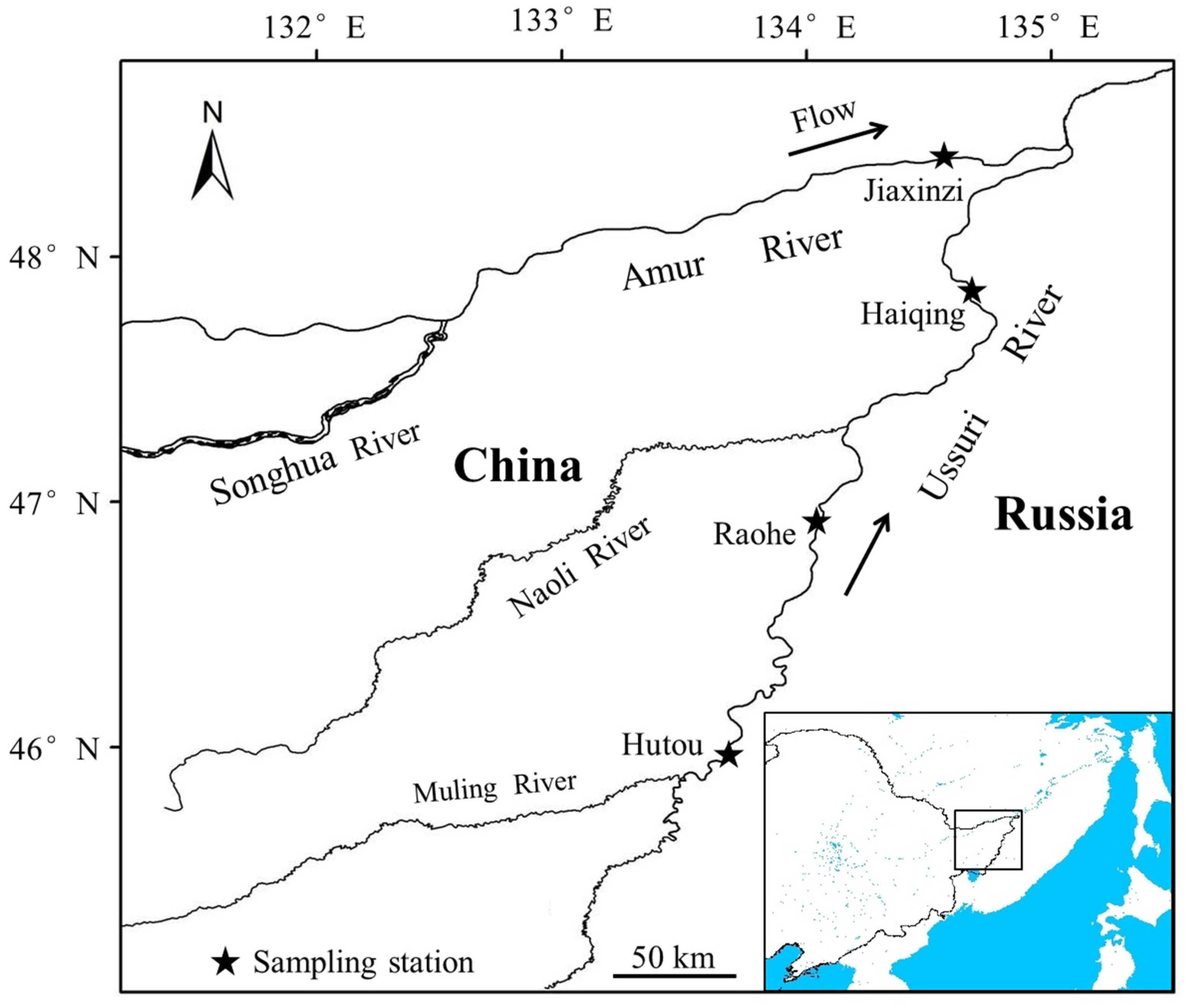
2.1. Study Area
2.2. Fish Sampling
2.3. Sample Processing and Data Analysis
3. Results
3.1. Capture of Juvenile Chum Salmon
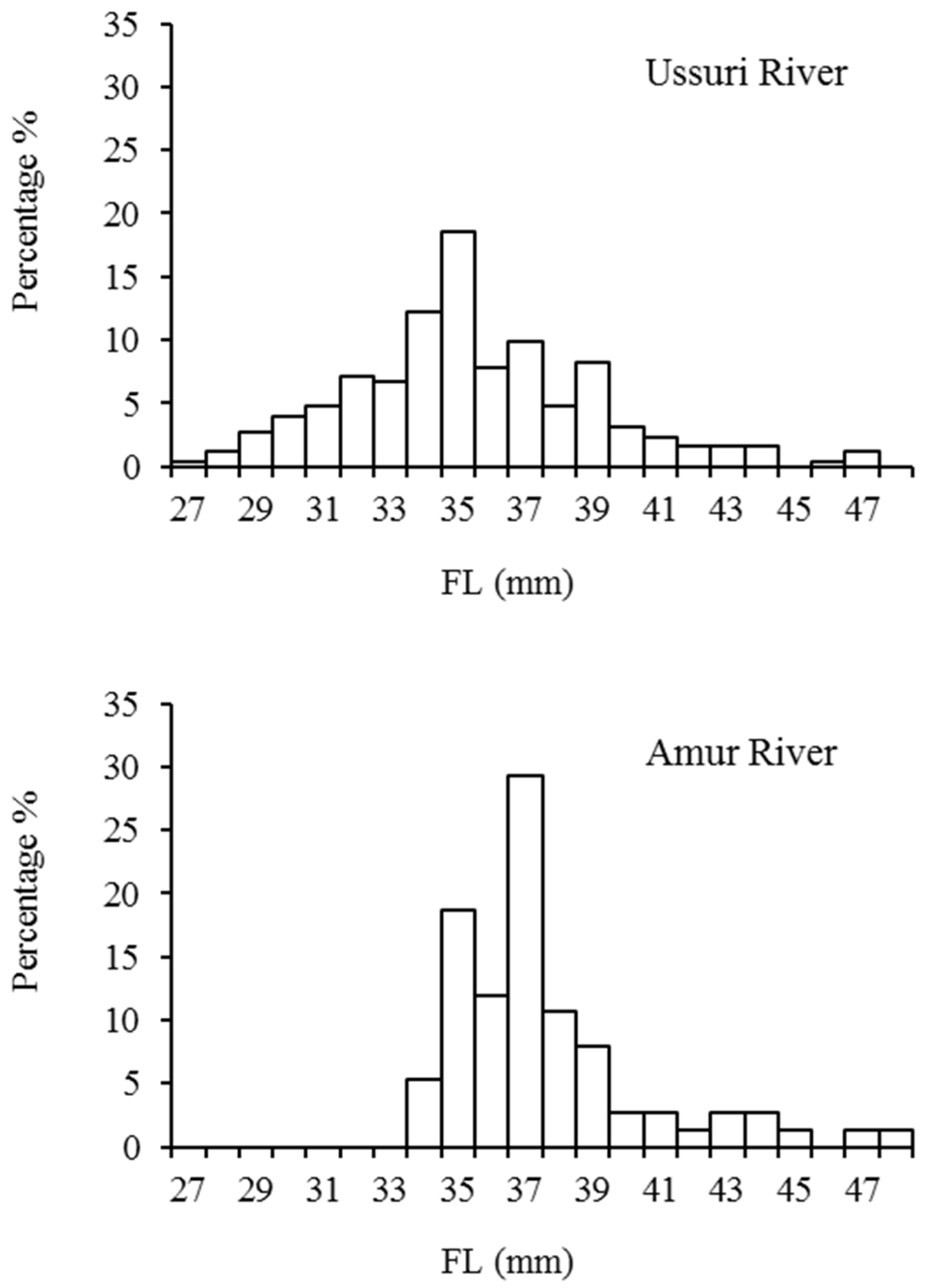
3.2. Size of Samples
3.3. Feeding Habits
4. Discussion
4.1. Timing of Juvenile Chum Salmon Migration in the Amur River System
4.2. Size and Feeding Habits of Juvenile Chum Salmon
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salo, E.O. Life history of chum salmon (Oncorhynchus keta). In Pacific Salmon Life Histories; Groot, C., Margolis, L., Eds.; University of British Columbia Press: Vancouver, WA, Canada, 1991; pp. 232–309. [Google Scholar]
- Zhang, J.M. Ichthyology of Heilongjiang Province; Heilongjiang Science and Technology Press: Harbin, China, 1995. [Google Scholar]
- Chen, J.P.; Sun, D.J.; Dong, C.Z.; Liang, B.; Wu, W.H.; Zhang, S.Y. Genetic analysis of four wild chum salmon Oncorhynchus keta populations in China based on microsatellite markers. Environmental Biol. Fishes. 2005, 73, 181–188. [Google Scholar] [CrossRef]
- Wang, J.L.; Gao, Y.W.; Liu, W.; Zhang, H.Y.; David, L.D. The life history and populations of chum salmon (Oncorhynchus keta) in China: An otolith isotopic investigation. Appl. Geochem. 2021, 127, 104903. [Google Scholar] [CrossRef]
- Dong, C.Z.; Li, G.G. A preliminary study on the population structures of salmonoids migration in Suifen River. J. Fish. China 1989, 13, 124–132. [Google Scholar]
- Han, Y.; Wang, Y.; Fan, Z.T.S.; Liu, M. A survey on the resources of chum salmon (Oncorhynchus keta Walbaum) in Heilongjiang waters. Chin. J. Fish. 2002, 15, 24–34. [Google Scholar]
- Wang, J.L.; Tang, F.J.; Zhu, Z.; Pan, Z.Q.; Liu, W. Characteristics and analysis of colony structure of breeding migratory salmon in autumn Wusuli river. Hunan Agric. Sci. 2011, 21, 120–123. [Google Scholar]
- Wang, J.L.; Liu, E.; Li, P.L.; Tang, F.J.; Lu, W.; Yang, J.; Jiang, T. Evidence of return of chum salmon released from Tangwang River by strontium marking method. ACTA Oceanol. Sin. 2021, 40, 183–187. [Google Scholar] [CrossRef]
- Han, Y.; Fan, Z.T.; Wang, Y.S.; Yin, H.F. Population structures of chum salmon (Oncorhynchus keta Walbaum) in Heilongjiang waters. J. Northeast. Agric. Univ. 2004, 35, 25–29. [Google Scholar]
- Wang, J.L.; Liu, W.; Tang, F.J. Analysis of biological traits of Chum salmon (Oncorhynchus keta Walbaum) in the Amur River, China. J. Fish. Sci. China 2013, 20, 93–100. [Google Scholar] [CrossRef]
- Yamada, Y.; Sasaki, K.; Yamane, K.; Yatsuya, M.; Shimizu, Y.; Nagakura, Y.; Kurokawa, T.; Nikaido, H. The utilization of cold-water zooplankton as prey for chum salmon fry (Oncorhynchus keta) in Yamada Bay, Iwate, Pacific coast of northern Japan. Reg. Stud. Mar. Sci. 2019, 29, 100633. [Google Scholar]
- Wang, J.L.; Liu, W.; Yang, W.B.; Li, P.L.; Tang, F.J.; Lu, W.Q.; Yang, J.; Jiang, T. Analysis of life history and population identification of Chum salmon (Oncorhynchus keta) based on otolith microchemistry characteristics. Period. Ocean. Univ. China 2021, 51, 51–59. [Google Scholar]
- Beamish, R.J.; Mahnken, C. A critical size and period hypothesis to explain natural regulation of salmon abundance and the linkage to climate and climate change. Prog. Oceanogr. 2001, 49, 423–437. [Google Scholar] [CrossRef]
- Healey, M.C. Timing and relative intensity of size-selective mortality of juvenile chum salmon (Oncorhynchus keta) during early sea life. Can. J. Fish. Aquat. Sci. 1982, 39, 952–957. [Google Scholar] [CrossRef]
- Tucker, S.; Hipfner, J.M.; Trudel, M. Size and condition dependent predation: A seabird disproportionately targets substandard individual juvenile salmon. Ecology 2016, 97, 461–471. [Google Scholar] [CrossRef]
- Terazaki, M.; Iwata, M. Feeding habits of chum salmon Oncorhynchus keta collected from Otsuchi Bay. Nippon. Suisan Gakkaishi 1983, 49, 1187–1193. [Google Scholar] [CrossRef]
- Ban, M.; Hasegawa, H.; Ezure, M. Effects of starvation and refeeding on physiological condition of juvenile chum salmon Oncorhynchus keta. Sci. Rep. Hokkaido Salmon Hatch. 1996, 50, 117–123. [Google Scholar]
- Seki, J. Study of characteristic of feeding habitat of juvenile chum salmon and their food environment in the Pacific coastal waters, central part of Hokkaido. Bull. Natl. Salmon Resour. Cent. 2005, 7, 1–104. [Google Scholar]
- Bradley, P.T.; Evans, M.D.; Seitz, A.C. Characterizing the juvenile fish community in turbid Alaskan rivers to assess potential interactions with hydrokinetic devices. Trans. Am. Fish. Soc. 2015, 144, 1058–1069. [Google Scholar] [CrossRef]
- Cortés, E. A critical review of methods of studying fish feeding based on analysis of stomach contents: Application to elasmobranch fishes. Can. J. Fish. Aquat. Sci. 1997, 54, 726–738. [Google Scholar] [CrossRef]
- Mohan, M.V.; Sankaran, T.M. Two new indices for stomach content analysis of fishes. J. Fish Biol. 1988, 33, 289–292. [Google Scholar] [CrossRef]
- Jump, S.; Courtney, M.B.; Seitz, A.C. Vertical distribution of juvenile salmon in a large turbid river. J. Fish Wildl. Manag. 2019, 19, 575–581. [Google Scholar] [CrossRef]
- Bax, N.J. Early marine mortality of marked juvenile chum salmon (Oncorhynchus keta) released into Hood Canal, Puget Sound, Washington, in 1980. Can. J. Fish. Aquat. Sci. 1983, 40, 426–435. [Google Scholar] [CrossRef]
- Hasegawa, K.; Honda, K.; Yoshiyama, T.; Suzuki, K.; Fukui, S. Small biased body size of salmon fry preyed upon by piscivorous fish in riverine and marine habitats. Can. J. Fish. Aquat. Sci. 2021, 78, 631–638. [Google Scholar] [CrossRef]
- Randy, J.B.; Catherine, B.; Jeffery, L.M. Population trends for Chinook and summer chum salmon in two Yukon River tributaries in Alaska. J. Fish Wildl. Manag. 2020, 11, 377–400. [Google Scholar]
- Quinn, T.P. The Behavior and Ecology of Pacific Salmon and Trout; American Fisheries Society: Bethesda, MD, USA, 2005; 378p. [Google Scholar]
- Sogard, S.M. Size selective mortality in the juvenile stage of teleost fishes. Bull. Mar. Sci. 1997, 60, 1129–1157. [Google Scholar]
- Kaczynski, W.V.; Feller, R.J.; Clayton, J.; Gerke, R.J. Trophic analysis of juvenile pink and chum salmon (Oncorhynchus gorbuscha and O. keta) in Puget Sound. J. Fish. Res. Board Can. 1973, 30, 1003–1008. [Google Scholar] [CrossRef]
- Kaeriyama, M. Ecological study on early life of the chum salmon Oncorhynchus keta (Walbaum). Sci. Rep. Hokkaido Salmon Hatch. 1986, 40, 31–92. [Google Scholar]
- Irie, T. Ecological studies on the migration of juvenile chum salmon, Oncorhynchus keta, during early ocean life. Bull. Seikai Natl. Fish. Inst. 1990, 68, 1–142. [Google Scholar]
- Brodeur, R.D.; Pearcy, W.G. Tropic relations of juvenile Pacific salmon off the Oregon and Washington coast. Fish. Bull. 1990, 88, 617–636. [Google Scholar]
- Suzuki, T.; Fukuwaka, M.; Shimizu, I.; Seki, J.; Kaeriyama, M.; Mayama, H. Feeding selectivity of juvenile chum salmon in the Japan Sea coast of northern Honshu. Sci. Rep. Hokkaido Salmon Hatch. 1994, 48, 11–16. [Google Scholar]
- Okada, S.; Taniguchi, A. Size relationship between salmon juveniles in shore waters and their prey animals. Bull. Fish. Sci. Hokkaido Univ. 1971, 22, 30–36. [Google Scholar]
- Bailey, E.J.; Wing, B.L.; Mattson, C.R. Zooplankton abundance and feeding habits of fry of pink salmon, Oncorhynchus gorbuscha, and chum salmon, Oncorhynchus keta, in Traitors Cove, Alaska, with speculations on the carrying capacity of the area. Fish. Bull. 1975, 73, 846–861. [Google Scholar]
- Volk, E.C.; Wissmar, R.C.; Simenstad, C.A.; Eggers, D.M. Relationship between Otolith Microstructure and the Growth of Juvenile Chum Salmon (Oncorhynchus keta) under Different Prey Rations. Can. J. Fish. Aquat. Sci. 1984, 41, 126–133. [Google Scholar] [CrossRef]
- Thorpe, J.E. Salmonid fishes and the estuarine environment. Estuaries 1994, 17, 76–93. [Google Scholar] [CrossRef]

| River | Sampling Station | Mean CPUE | Mean FL (mm) | Range of CPUE | Rang of FL (mm) |
|---|---|---|---|---|---|
| Amur River | Jiaxinzi | 0.140 ± 0.213 | 37.1 ± 2.9 | 0–0.952 | 33.5–47.5 |
| Ussuri River | Hutou | 0.234 ± 0.178 | 30.3 ± 1.9 | 0.046–0.734 | 26.9–36.1 |
| Raohe | 0.209 ± 0.167 | 35.4 ± 2.9 | 0.051–0.746 | 29.7–46.2 | |
| Haiqing | 0.315 ± 0.290 | 36.6 ± 3.4 | 0–1.260 | 31.0–46.6 | |
| Pooled | 0.255 ± 0.227 | 34.9 ± 3.7 | 0–1.260 | 26.9–46.6 |
| Prey Category | Frequency Percentage F/% | Percentage by Number N/% | Percentage by Weight W/% | Index of Relative Importance IRI | Percentage Index of Relative Importance IRI/% | Index of Preponderance Ip |
|---|---|---|---|---|---|---|
| Fish | 3.17 | 0.19 | 1.15 | 4.25 | 0.03 | 0.05 |
| Aquatic insect | 93.65 | 33.49 | 91.82 | 11735.74 | 92.36 | 94.41 |
| Ephemeroptera | 80.95 | 22.83 | 68.91 | 7426.89 | 58.45 | 79.84 |
| Tendipes | 19.05 | 2.08 | 3.76 | 111.13 | 0.87 | 1.02 |
| Psychodidae | 4.76 | 0.38 | 0.57 | 4.51 | 0.04 | 0.04 |
| Unidentified insect | 50.79 | 8.21 | 18.58 | 1360.69 | 10.71 | 13.51 |
| Copepoda | 58.73 | 63.96 | 6.85 | 4158.72 | 32.73 | 5.47 |
| Cyclopoidea | 57.14 | 56.13 | 6.62 | 3586.12 | 28.22 | 5.42 |
| Harpacticoida | 22.22 | 4.15 | 0.05 | 93.36 | 0.73 | 0.02 |
| Calanoida | 14.29 | 3.68 | 0.17 | 55.04 | 0.43 | 0.04 |
| Cladocera | 25.40 | 2.36 | 0.18 | 64.42 | 0.51 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, P.; Liu, W.; Lu, W.; Tang, F. The Migratory Biology and Feeding Habits of Downstream-Migrating Juvenile Chum Salmon Oncorhynchus keta in the Amur River of Northeast China. Fishes 2023, 8, 458. https://doi.org/10.3390/fishes8090458
Wang J, Li P, Liu W, Lu W, Tang F. The Migratory Biology and Feeding Habits of Downstream-Migrating Juvenile Chum Salmon Oncorhynchus keta in the Amur River of Northeast China. Fishes. 2023; 8(9):458. https://doi.org/10.3390/fishes8090458
Chicago/Turabian StyleWang, Jilong, Peilun Li, Wei Liu, Wanqiao Lu, and Fujiang Tang. 2023. "The Migratory Biology and Feeding Habits of Downstream-Migrating Juvenile Chum Salmon Oncorhynchus keta in the Amur River of Northeast China" Fishes 8, no. 9: 458. https://doi.org/10.3390/fishes8090458
APA StyleWang, J., Li, P., Liu, W., Lu, W., & Tang, F. (2023). The Migratory Biology and Feeding Habits of Downstream-Migrating Juvenile Chum Salmon Oncorhynchus keta in the Amur River of Northeast China. Fishes, 8(9), 458. https://doi.org/10.3390/fishes8090458




