Age, Growth, and Functional Gonochorism with a Twist of Diandric Protogyny in Goliath Grouper from the Atlantic Coast of Florida
Abstract
1. Introduction
2. Materials and Methods
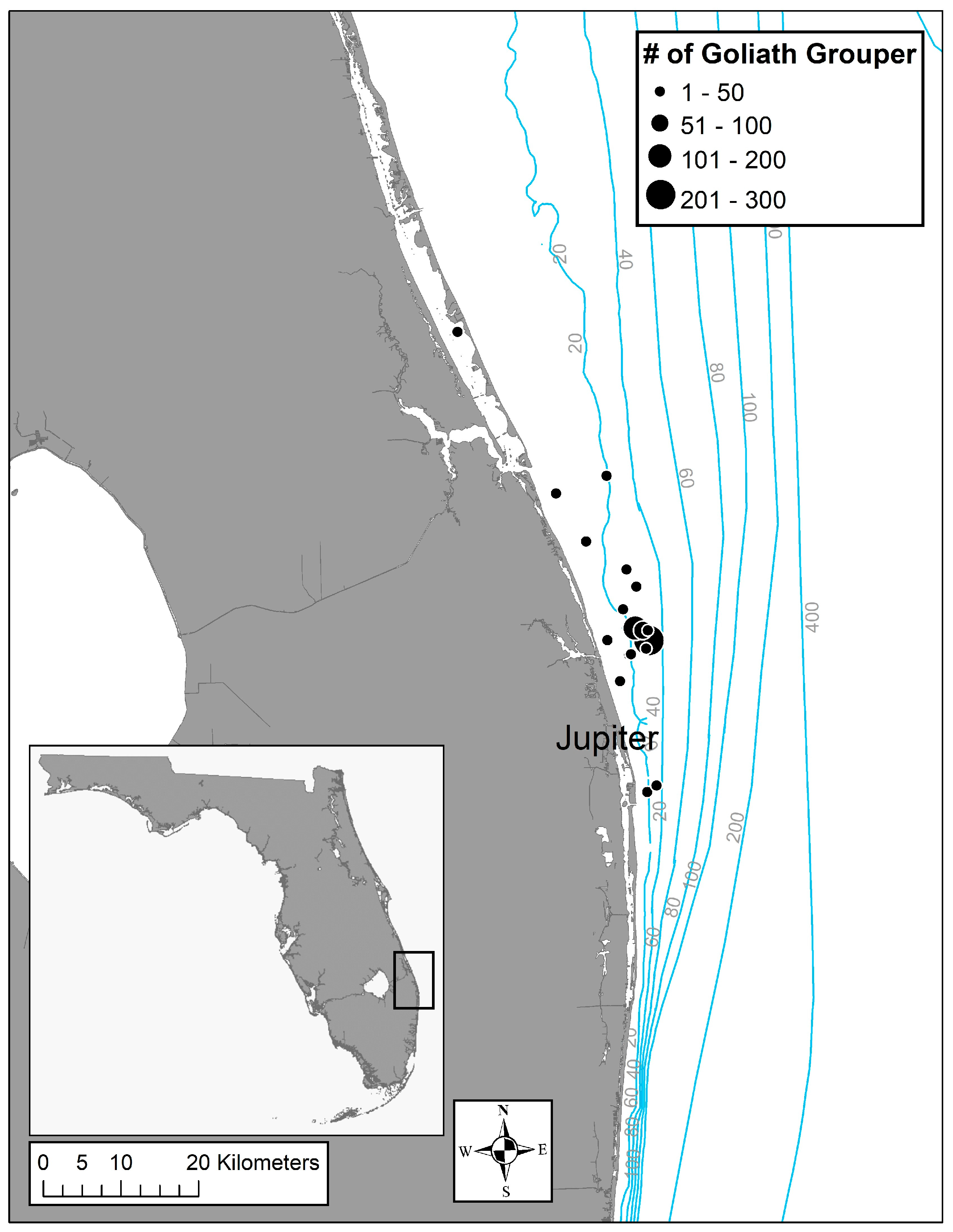
2.1. Fish Sampling
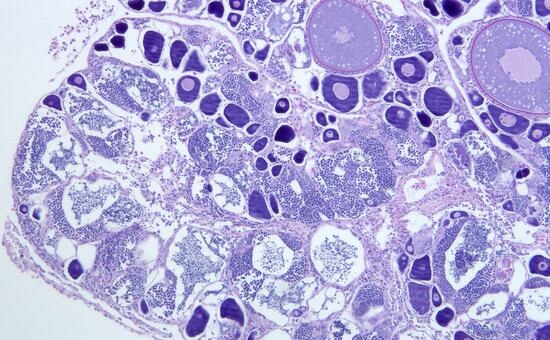
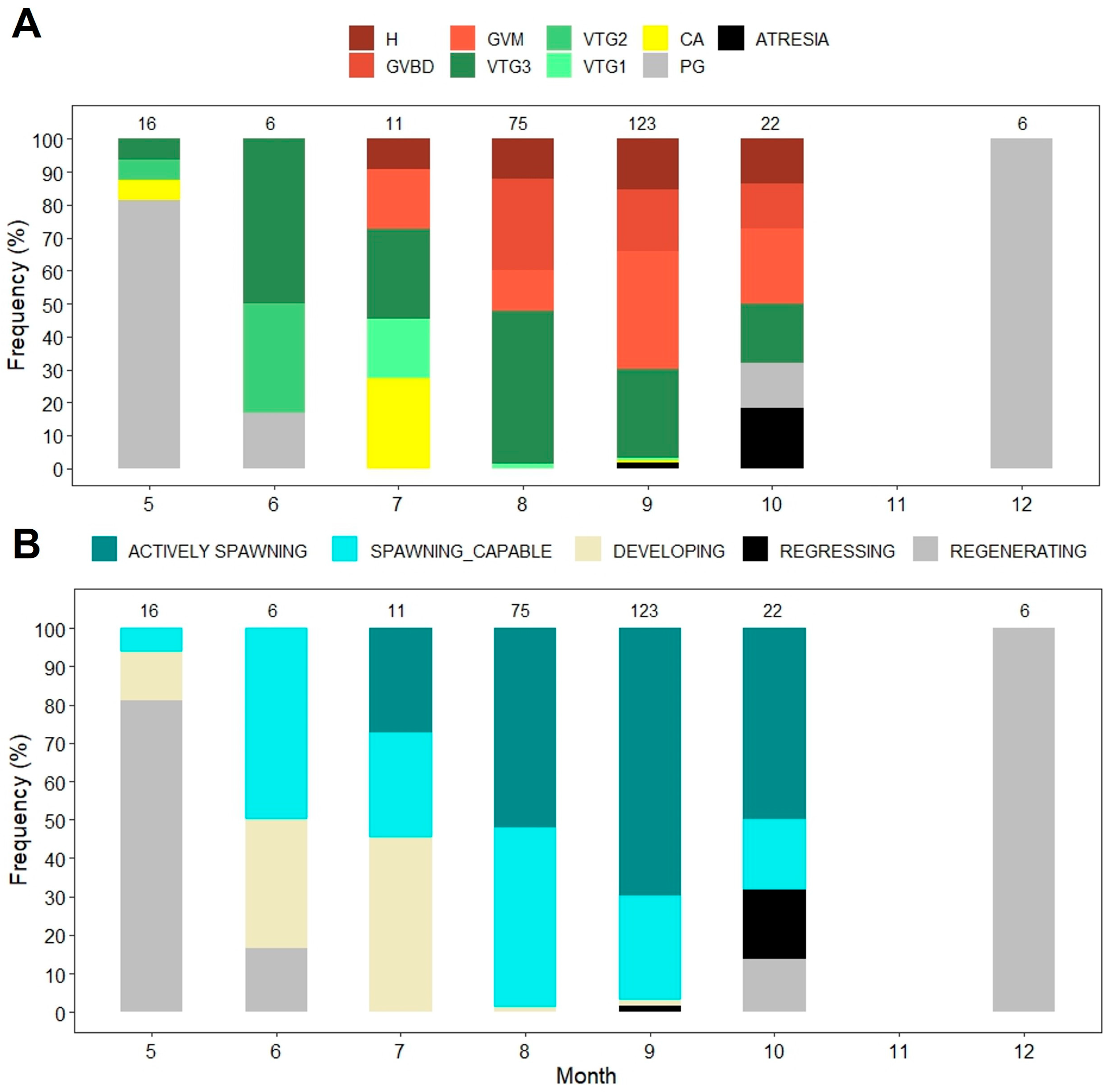
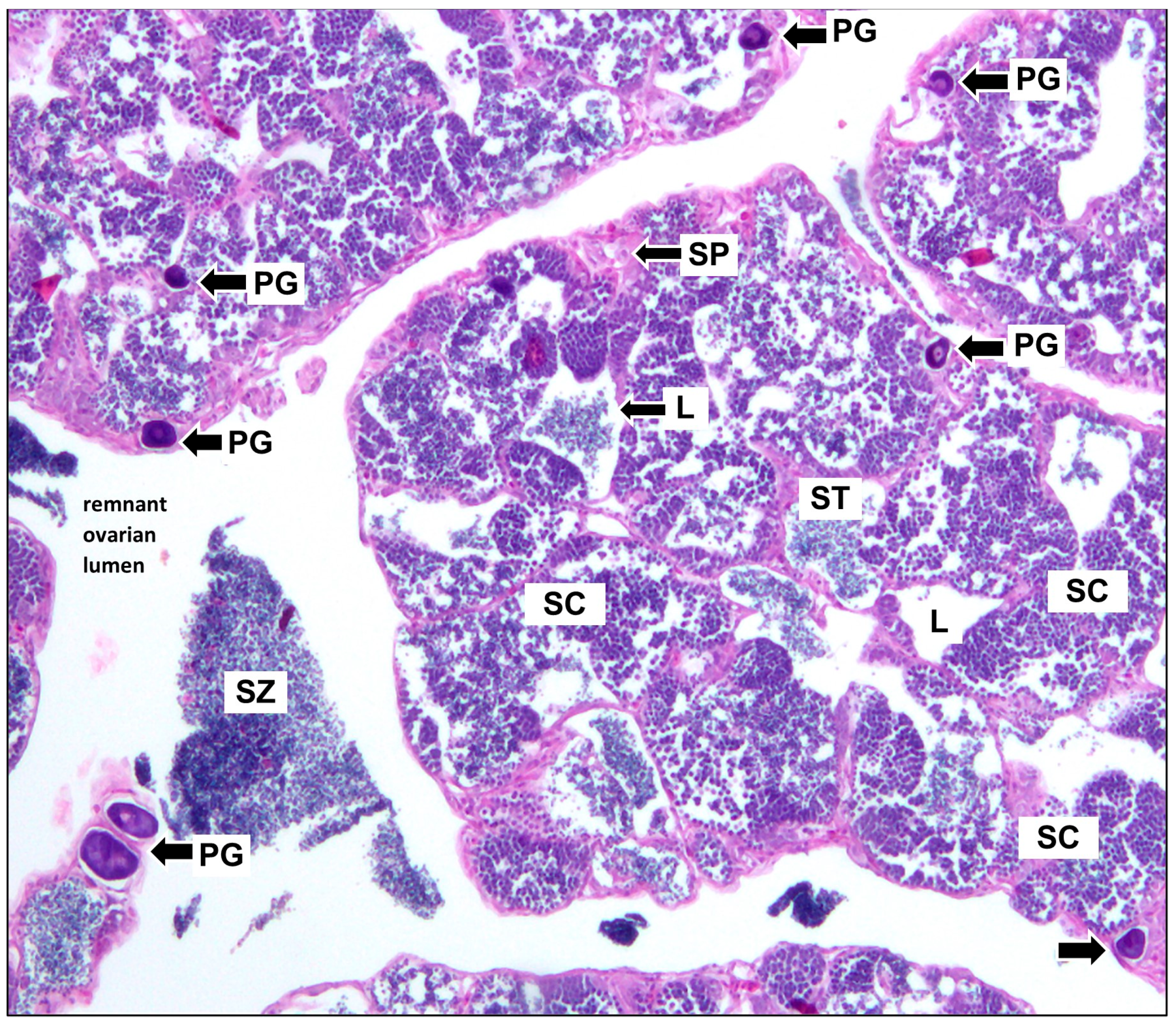
2.2. Reproduction
2.3. Ageing Goliath Grouper Using Dorsal Fin Rays
2.4. Growth
3. Results
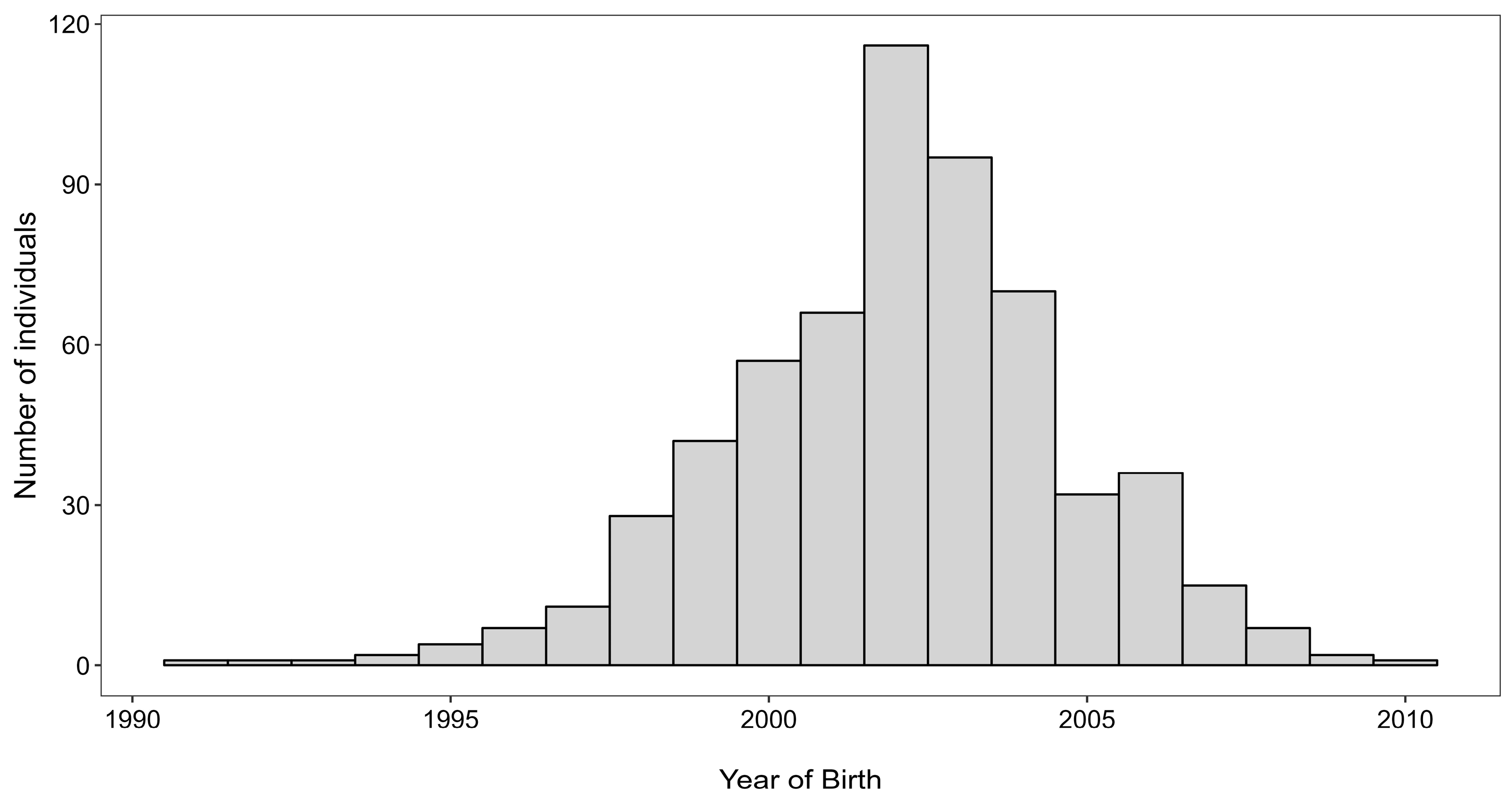
3.1. Fish Samples
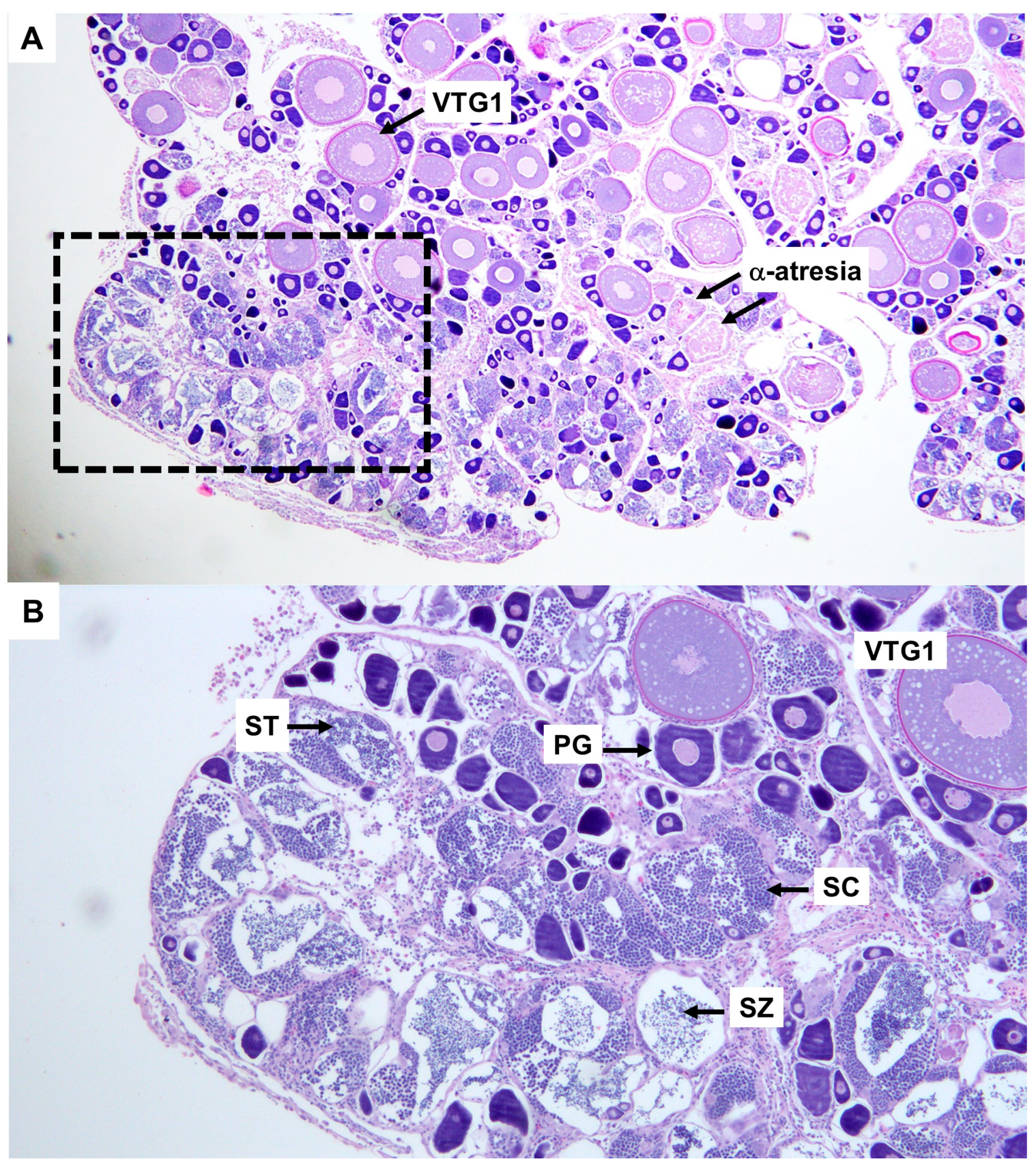
3.2. Reproduction
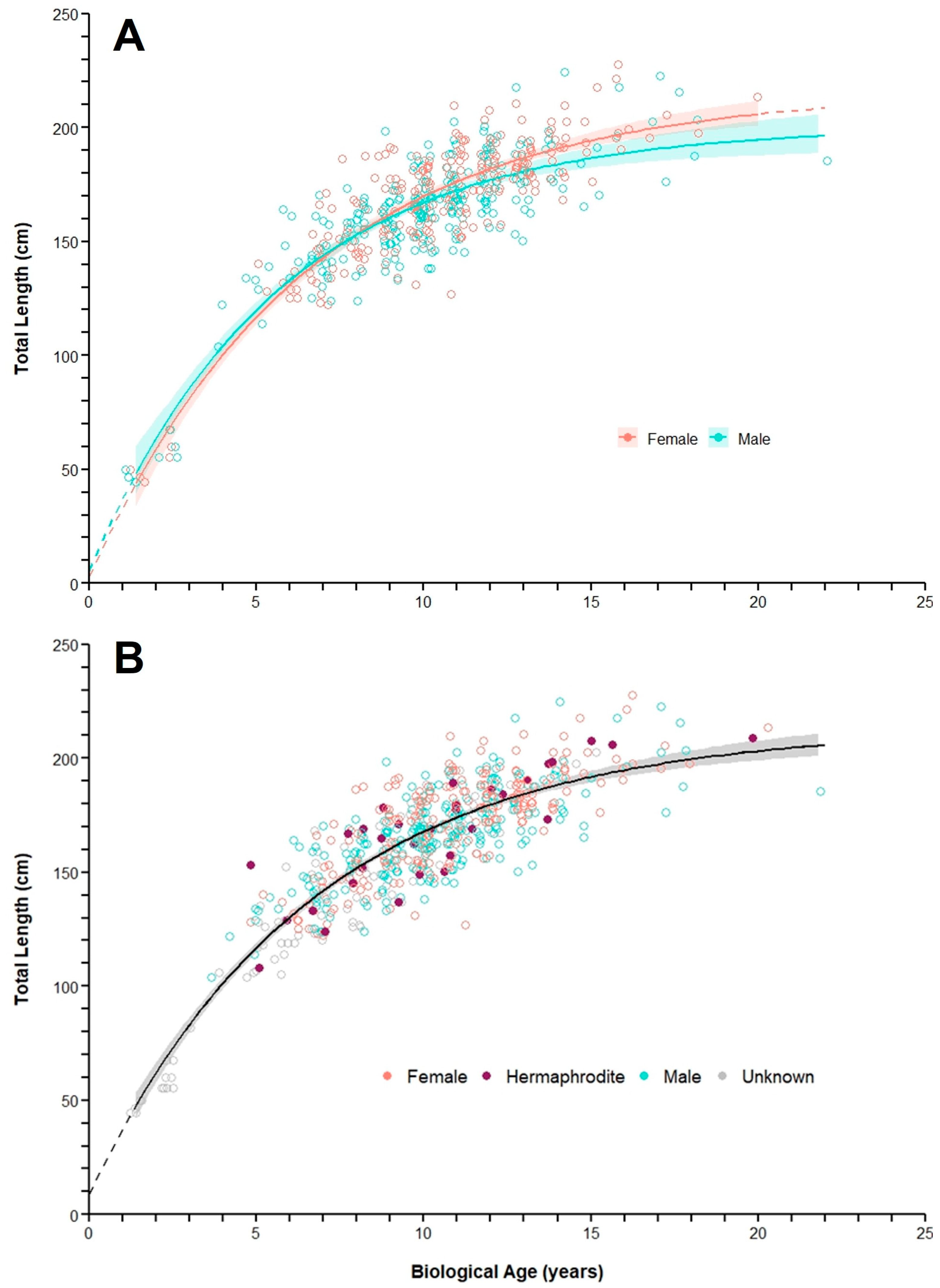
3.3. Sex-Specific Age
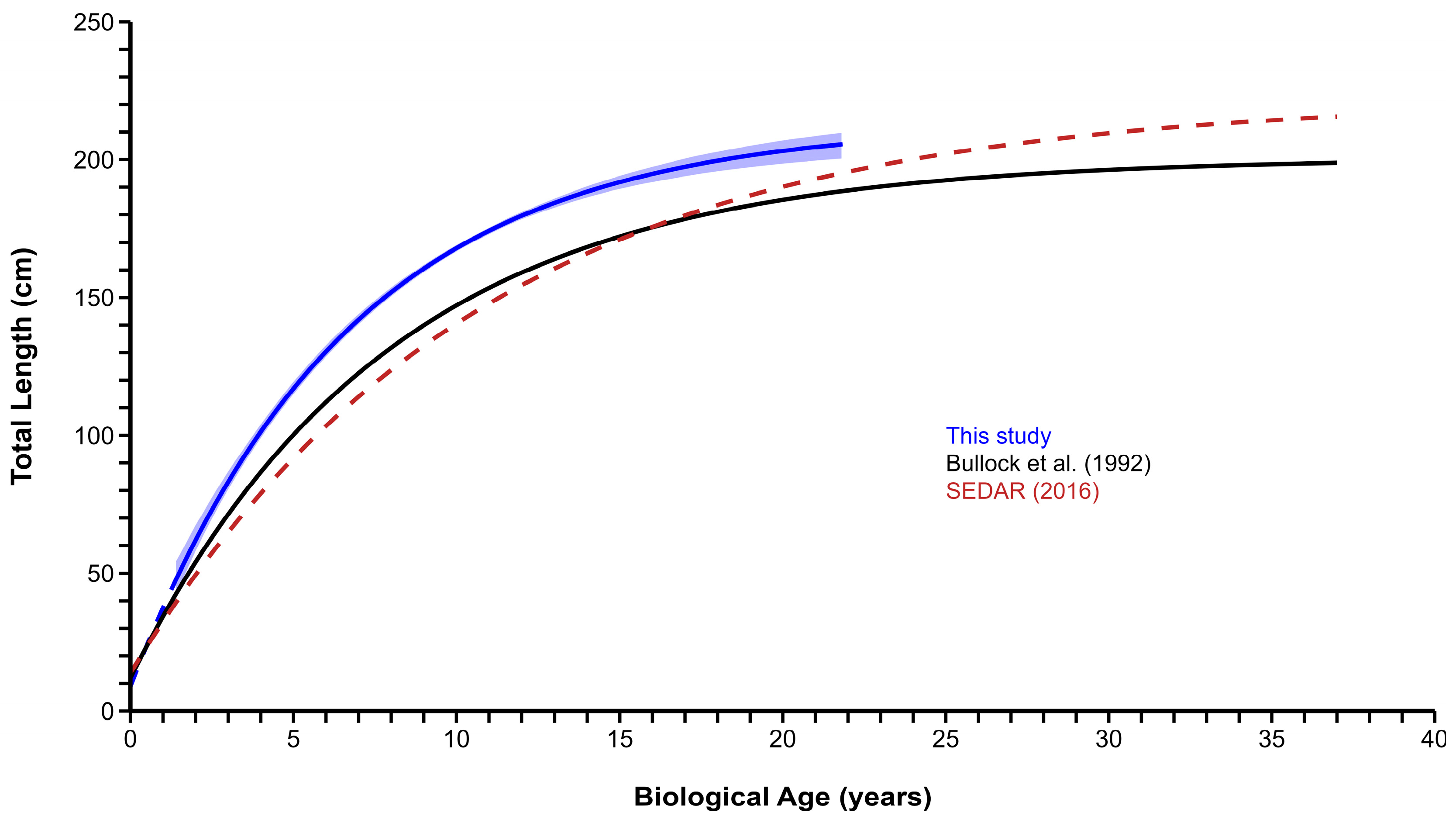
3.4. Growth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sadovy de Mitcheson, Y.; Craig, M.T.; Bertoncini, A.A.; Carpenter, K.E.; Cheung, W.W.L.; Choat, J.H.; Cornish, A.S.; Fennessy, S.T.; Ferreira, B.P.; Heemstra, P.C.; et al. Fishing groupers towards extinction: A global assessment of threats and extinction risks in a billion dollar fishery. Fish Fish. 2013, 14, 119–136. [Google Scholar] [CrossRef]
- Coleman, F.C.; Koenig, C.C.; Collins, L.A. Reproductive styles of shallow-water grouper (Pisces: Serranidae) in the eastern Gulf of Mexico and the consequences of fishing spawning aggregations. Environ. Biol. Fish. 1996, 47, 129–141. [Google Scholar] [CrossRef]
- Sadovy, Y.; Eklund, A.M. Synopsis of Biological Information on the Nassau Grouper, Epinephelus Striatus (Bloch 1792), and the Jewfish, E. itajara (Lichtenstein 1822); NMFS 146; U.S. Department of Commerce: Seattle, WA, USA, 1999; pp. 1–65. [Google Scholar]
- Coleman, F.; Koenig, C.; Huntsman, G.; Musick, J.; Eklund, A.; McGovern, J.; Chapman, R.; Sedberry, G.; Grimes, C. Long-lived Reef Fishes: The Grouper-Snapper Complex. Fisheries 2000, 25, 14–21. [Google Scholar] [CrossRef]
- Morris, A.; Roberts, C.; Hawkins, J. The threatened status of groupers (Epinephelinae). Biodivers. Conserv. 2000, 9, 919–942. [Google Scholar] [CrossRef]
- Sadovy de Mitcheson, Y.; Colin, P.L.; Lindfield, S.J.; Bukurrou, A. A decade of monitoring an Indo-Pacific grouper spawning aggregation: Benefits of protection and importance of survey design. Front. Mar. Sci. 2020, 7, 571878. [Google Scholar] [CrossRef]
- Heemstra, P.C.; Randall, J.E. FAO Species Catalogue, Volume 16. Groupers of the World (Family Serranidae, Subfamily Epinepheline). An Annotated and Illustrated Catalogue of the Grouper, Rockcod, Hind, Coral Grouper and Lyretail Species Known to Date; FAO: Rome, Italy, 1993. [Google Scholar]
- Bullock, L.H.; Murphy, M.D.; Godcharles, M.F.; Mitchell, M.E. Age, growth, and reproduction of jewfish Epinephelus itajara in the eastern Gulf of Mexico. Fish B-Noaa 1992, 90, 243–249. [Google Scholar]
- Bueno, L.S.; Bertoncini, A.A.; Koenig, C.C.; Coleman, F.C.; Freitas, M.O.; Leite, J.R.; De Souza, T.F.; Hostim-Silva, M. Evidence for spawning aggregations of the endangered Atlantic goliath grouper Epinephelus itajara in southern Brazil. J. Fish Biol. 2016, 89, 876–889. [Google Scholar] [CrossRef]
- Koenig, C.C.; Bueno, L.S.; Coleman, F.C.; Cusick, J.A.; Ellis, R.D.; Kingon, K.; Locascio, J.V.; Malinowski, C.; Murie, D.J.; Stallings, C.D. Diel, lunar, and seasonal spawning patterns of the Atlantic goliath grouper, Epinephelus itajara, off Florida, United States. Bull. Mar. Sci. 2017, 93, 391–406. [Google Scholar] [CrossRef]
- Sadovy, Y.; Dormeier, M. Are aggregation-fisheries sustainable? Reef fish fisheries as a case study. Coral Reefs 2005, 24, 254–262. [Google Scholar] [CrossRef]
- Frias-Torres, S. Habitat use of juvenile goliath grouper Epinephelus itajara in the Florida Keys, USA. Endanger. Species Res. 2006, 2, 1–6. [Google Scholar] [CrossRef]
- Koenig, C.C.; Coleman, F.C.; Eklund, A.M.; Schull, J.; Ueland, J. Mangroves as essential nursery habitat for goliath grouper (Epinephelus itajara). Bull. Mar. Sci. 2007, 80, 567–585. [Google Scholar]
- Gulf of Mexico Fishery Management Council (GMFMC). Amendment 2 to the Reef Fish Fishery Management Plan; Gulf of Mexico Fishery Management Council (GMFMC): Tampa, FL, USA, 1990. [Google Scholar]
- South Atlantic Fishery Management Council (SAFMC). Amendment Number 4, Regulatory Impact Review, Initial Regulatory Flexibility Analysis and Environmental Assessment for the Fishery Management Plan for the Snapper Grouper Fishery of the South Atlantic Region; South Atlantic Fishery Management Council: Charleston, SC, USA, 1990; p. 200. [Google Scholar]
- IUCN. IUCN Red List of Threatened Animals; IUCN: Gland, Switzerland, 1996. [Google Scholar]
- Bertoncini, A.A.; Aguilar-Perera, A.; Barreiros, J.; Craig, M.T.; Ferreira, B.P.; Koenig, C. The IUCN Red List of Threatened Species 2018: Epinephelus itajara; IUCN: Gland, Switzerland, 2018; p. e.T195409A46957794. [Google Scholar]
- Locatelli, A.C.P.; Bastos, R.F.; Oliveira, M.A.; Ferreira, B.P. Scientometric analysis and literature synthesis of 60 years of science on the Atlantic goliath grouper (Epinephelus itajara). J. Fish. Biol. 2023, 102, 740–756. [Google Scholar] [CrossRef]
- Barreiros, J.P.; Coleman, F.C. West African Goliath Grouper: Where Are They between Senegal and Angola? Fishes 2023, 8, 318. [Google Scholar] [CrossRef]
- Koenig, C.C.; Coleman, F.C.; Malinowski, C.R. Atlantic Goliath Grouper of Florida: To Fish or Not to Fish. Fisheries 2020, 45, 20–32. [Google Scholar] [CrossRef]
- Cass-Calay, S.; Schmidt, T. Standardized Catch Rates of Juvenile Goliath Grouper, Epinephelus itajara, from the Everglades National Park Creel Survey, 1973–1999; SFD-2003-0016; National Marine Fisheries Service, Sustainable Fisheries Division: Miami, FL, USA, 2003. [Google Scholar]
- Porch, C.E.; Eklund, A.M.; Scott, G.P. A catch-free stock assessment model with application to goliath grouper (Epinephelus itajara) off southern Florida. Fish B-Noaa 2006, 104, 89–101. [Google Scholar]
- Koenig, C.C.; Coleman, F.C.; Kingon, K. Pattern of recovery of the Goliath Grouper Epinephelus itajara population in the southeastern US. Bull. Mar. Sci. 2011, 87, 891–911. [Google Scholar] [CrossRef]
- SEDAR. SEDAR 47 Stock Assessment Report: Southeastern U.S. Goliath Grouper; SEDAR: North Charleston, SC, USA, 2016; p. 206. [Google Scholar]
- Coleman, F.C.; Nunes, J.A.C.C.; Bertoncini, Á.A.; Bueno, L.S.; Freitas, M.O.; Borgonha, M.; Leite, J.R.; Lima-Júnior, M.J.C.A.; Ferreira, B.; Bentes, B.; et al. Controversial opening of a limited fishery for Atlantic Goliath Grouper in the United States: Implications for population recovery. Mar. Policy 2023, 155, 105752. [Google Scholar] [CrossRef]
- Kingsley, M.C.S. The Goliath Grouper in Southern Florida: Assessment Review and Advisory Report. Report Prepared for the South Atlantic Fishery Management Council, the Gulf of Mexico Fishery Management Council, and the National Marine Fisheries Service; REEF: Carlsbad, CA, USA, 2004; p. 17. [Google Scholar]
- Murie, D.J.; Parkyn, D.C.; Koenig, C.C.; Coleman, F.C.; Schull, J.; Frias-Torres, S. Evaluation of finrays as a non-lethal ageing method for protected goliath grouper Epinephelus itajara. Endanger. Species Res. 2009, 7, 213–220. [Google Scholar] [CrossRef][Green Version]
- Collins, A.B.; Barbieri, L.R. Behavior, Habitat, and Abundance of the Goliath Grouper, Epinephelus itajara, in the Central Eastern Gulf of Mexico; NOAA Award Number NA07NMF4540085; Fish and Wildlife Research Institute, Florida Fish & Wildlife Conservation Commission: St. Petersburg, FL, USA, 2010; p. 44. [Google Scholar]
- Collins, A.B.; Barbieri, L.R.; McBride, R.S.; McCoy, E.D.; Motta, P.J. Reef relief and volume are predictors of Atlantic goliath grouper presence and abundance in the eastern Gulf of Mexico. Bull. Mar. Sci. 2015, 91, 399–418. [Google Scholar] [CrossRef]
- Malinowski, C.R.; Perrault, J.R.; Coleman, F.C.; Koenig, C.C.; Stilwell, J.M.; Cray, C.; Stacy, N.I. The iconic Atlantic Goliath Grouper (Epinephelus itajara): A comprehensive assessment of health indices in the southeastern United States population. Front. Vet. Sci. 2020, 7, 635. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, C.R.; Stacy, N.I.; Coleman, F.C.; Cusick, J.A.; Dugan, C.M.; Koenig, C.C.; Ragbeer, N.K.; Perrault, J.R. Mercury offloading in gametes and potential adverse effects of high mercury concentrations in blood and tissues of Atlantic Goliath Grouper Epinephelus itajara in the southeastern United States. Sci. Total Environ. 2021, 779, 146437. [Google Scholar] [CrossRef] [PubMed]
- Malinowski, C.; Coleman, F.; Koenig, C.; Locascio, J.; Murie, D. Are Atlantic goliath grouper, Epinephelus itajara, establishing more northerly spawning sites? Evidence from the northeast Gulf of Mexico. Bull. Mar. Sci. 2019, 95, 371–391. [Google Scholar] [CrossRef]
- Armsworth, P.R. Effects of fishing on a protogynous hermaphrodite. Can. J. Fish. Aquat. Sci. 2001, 58, 568–578. [Google Scholar] [CrossRef]
- Rhodes, K.; Taylor, B.; McIlwain, J. Detailed demographic analysis of an Epinephelus polyphekadion spawning aggregation and fishery. Mar. Ecol. Prog. Ser. 2011, 421, 183–198. [Google Scholar] [CrossRef][Green Version]
- Freitas, M.O.; Abilhoa, V.; Giglio, V.J.; Hostim-Silva, M.; de Moura, R.L.; Francini-Filho, R.B.; Minte-Vera, C.V. Diet and reproduction of the goliath grouper, Epinephelus itajara (Actinopterygii: Perciformes: Serranidae), in eastern Brazil. Acta Ichthyol. Piscat. 2015, 45, 1–11. [Google Scholar] [CrossRef]
- Smith, C.L. A revision of the American groupers: Epinephelus and allied genera. Am. Mus. Nat. Hist. 1971, 146, 69–241. [Google Scholar]
- Alonzo, S.H.; Ish, T.; Key, M.; Maccall, A.D.; Mangel, M. The importance of incorporating protogynous sex change into stock assessments. Bull. Mar. Sci. 2008, 83, 163–179. [Google Scholar]
- Lowerre-Barbieri, S.; Menendez, H.; Bickford, J.; Switzer, T.S.; Barbieri, L.; Koenig, C. Testing assumptions about sex change and spatial management in the protogynous gag grouper, Mycteroperca microlepis. Mar. Ecol. Prog. Ser. 2020, 639, 199–214. [Google Scholar] [CrossRef]
- Munday, P.L.; Kuwamura, T.; Kroon, F.J. Bidirectional sex change in marine fishes. In Reproduction and Sexuality in Marine Fishes: Patterns and Processes; Cole, K., Ed.; University of California Press: Berkeley, CA, USA, 2010. [Google Scholar]
- Haddon, M. Modelling and Quantitative Methods in Fisheries; Chapman & Hall/CRC Press: New York, NY, USA, 2001. [Google Scholar]
- Hood, P.B.; Godcharles, M.F.; Barco, R.S. Age, growth, reproduction, and the feeding ecology of Black Sea Bass, Centropristis striata (Pisces: Serranidae), in the eastern Gulf of Mexico. Bull. Mar. Sci. 1994, 54, 24–37. [Google Scholar]
- Collins, A.B.; Barbieri, L. An Evaluation of the Effects of Catch and Release Angling on Survival and Behavior of Goliath Grouper (Epinephelus itajara) with Additional Investigation into Residence and Long-Term Movement Patterns; NOAA Award Number NA10NMF4330115; Fish and Wildlife Research Institute, Florida Fish & Wildlife Conservation Commission: St. Petersburg, FL, USA, 2014; p. 52. [Google Scholar]
- Koenig, C.; Coleman, F.; Malinowski, C.; Ellis, R.D.; Murie, D.; Parkyn, D.C.; Friess, C.; Tzadik, O.E. Regional age structure, reproductive biology and trophic patterns of adult Goliath Grouper in Florida; NOAA Marine Fisheries Initiative (MARFIN) Project Final Report: Washington, DC, USA, 2016; p. 222. [Google Scholar]
- Wallace, R.A.; Selman, K. Cellular and dynamic aspects of oocyte growth in teleosts. Am. Zool. 1981, 21, 325–343. [Google Scholar] [CrossRef]
- Hunter, J.R.; Macewicz, B.J.; Lo, N.C.; Kimbrell, C.A. Fecundity, spawning, and maturity of female Dover Sole Microstomus pacificus, with an evaluation of assumptions and precision. Fish B-Noaa 1992, 90, 101–128. [Google Scholar]
- Brown-Peterson, N.J.; Wyanski, D.M.; Saborido-Rey, F.; Macewicz, B.J.; Lowerre-Barbieri, S.K. A standardized terminology for describing reproductive development in fishes. Mar. Coast. Fish. 2011, 3, 52–70. [Google Scholar] [CrossRef]
- Carroll, J.L.; Ellis, R.D.; Collins, A.B.; Murie, D.J. Dorsal fin spines and rays for nonlethal ageing of Goliath Grouper Epinephelus itajara. Fishes 2023, 8, 239. [Google Scholar] [CrossRef]
- VanderKooy, S.; Carroll, J.; Elzey, S.; Gilmore, J.; Kipp, J. (Eds.) A practical Handbook for Determining the Ages of Gulf of Mexico and Atlantic Coast Fishes, 3rd ed.; Gulf States Marine Fisheries Commission and the Atlantic States Marine Fisheries Commission: Ocean Springs, MS, USA, 2020. [Google Scholar]
- Beamish, R.J.; Fournier, D.A. A method for comparing the precision of a set of age determinations. J. Fish. Res. Board Can. 1981, 34, 982–983. [Google Scholar] [CrossRef]
- Quist, M.C.; Pegg, M.A.; DeVries, D.R. Age and growth. In Fisheries Techniques, 3rd ed.; Zale, A.V., Parrish, D.L., Sutton, T.M., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 677–731. [Google Scholar]
- Ogle, D.H.; Doll, J.C.; Wheeler, A.P.; Dinno, A. FSA: Simple Fisheries Stock Assessment Methods, R Package Version 0.9.4. 2023. Available online: https://CRAN.R-project.org/package=FSA (accessed on 3 February 2023).
- Baty, F.; Ritz, C.; Charles, S.; Brutsche, M.; Flandrois, J.; Delignette-Muller, M. A toolbox for nonlinear regression in R: The package nlstools. J. Stat. Softw. 2015, 66, 1–21. [Google Scholar] [CrossRef]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In International Symposium on Information Theory; Petrov, B.N., Csaki, F., Eds.; Akademiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c), R Package Version 2.3.2. 2023. Available online: https://cran.r-project.org/package=AICcmodavg (accessed on 3 February 2023).
- Nelson, G.A. Fishery Science Methods and Models, R-Package Version 1.12-0. 2023. Available online: https://cran.r-project.org/package=fishmethods (accessed on 3 February 2023).
- Brusher, J.H.; Schull, J. Non-lethal age determination for juvenile goliath grouper (Epinephelus itajara) from southwest Florida. Endanger Species Res. 2009, 7, 205–212. [Google Scholar] [CrossRef]
- O’Hop, J.; Munyandorero, J. SEDAR 47 Stock Assessment Report for Goliath Grouper of the South Atlantic and Gulf of Mexico, 2016; FWC Report IHR2016-001, May 23, 2016; Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute: St. Petersburg, FL, USA, 2016; p. 109. [Google Scholar]
- Sadovy, Y.; Shapiro, D.Y. Criteria for the diagnosis of hermaphroditism in fishes. Copeia 1987, 1987, 136–156. [Google Scholar] [CrossRef]
- Fennessy, S.T.; Sadovy, Y. Reproductive biology of a diandric protogynous hermaphrodite, the serranid Epinephelus andersoni. Mar. Freshw. Res. 2002, 53, 147–158. [Google Scholar] [CrossRef]
- Liu, M.; Sadovy, Y. The influence of social factors on juvenile sexual differentiation in a diandric, protogynous grouper Epinephelus coioides. Ichthyol. Res. 2011, 58, 84–89. [Google Scholar] [CrossRef]
- Palma, P.; Takemura, A.; Libunao, G.X.; Superio, J.; de Jesus-Ayson, E.G.; Ayson, F.; Nocillado, J.; Dennis, L.; Chan, J.; Thai, T.Q.; et al. Reproductive development of the threatened giant grouper Epinephelus lanceolatus. Aquaculture 2019, 509, 1–7. [Google Scholar] [CrossRef]
- Craig, M.T.; Hastings, P.A. A molecular phylogeny of the groupers of the subfamily Epinephelinae (Serranidae) with a revised classification of the Epinephelini. Ichthyol. Res. 2007, 54, 1–17. [Google Scholar] [CrossRef]
- Erisman, B.; Rosales-Casián, J.; Hastings, P. Evidence of gonochorism in a grouper, Mycteroperca rosacea, from the Gulf of California, Mexico. Environ. Biol. Fish 2008, 82, 23–33. [Google Scholar] [CrossRef]
- Sadovy, Y.; Colin, P.L. Sexual development and sexuality in the Nassau Grouper. J. Fish Biol. 1995, 46, 961–976. [Google Scholar] [CrossRef]
- Pears, R.J.; Choat, J.H.; Mapstone, B.D.; Begg, G.A. Demography of a large grouper, Epinephelus fuscoguttatus, from Australia’s Great Barrier Reef: Implications for fishery management. Mar. Ecol. Prog. Ser. 2006, 307, 259–272. [Google Scholar] [CrossRef]
- Collins, M.R.; Waltz, C.W.; Roumillat, W.A.; Stubbs, D.L. Contribution to the life history and reproductive biology of gag, Mycteroperca microlepis (Serranidae), in the South Atlantic Bight. Fish B-Noaa 1987, 85, 648–653. [Google Scholar]
- Liu, M.; Sadovy, Y. Early gonadal development and primary males in the protogynous epinepheline, Cephalopholis boenak. J. Fish Biol. 2004, 65, 987–1002. [Google Scholar] [CrossRef]
- Warner, R.R.; Robertson, D.R. Sexual Patterns in the Labroid Fishes of the Western Caribbean, I: The Wrasses (Labridae); Smithsonian Institution Press: Washington, DC, USA, 1978; Volume 254. [Google Scholar]
- Yoda, M.; Yoneda, M. Assessment of reproductive potential in multiple-spawning fish with indeterminate fecundity: A case study of yellow sea bream Dentex hypselosomus in the East China Sea. J. Fish Biol. 2009, 74, 2338–2354. [Google Scholar] [CrossRef]
- Ferreri, R.; Barra, M.; Gargano, A.; Aronica, S.; Bonanno, A.; Genovese, S.; Rumolo, P.; Basilone, G. New evaluation of postovulatory follicle degeneration at high-temperature regimes refines criteria for the identification of spawning cohorts in the European Anchovy (Engraulis encrasicolus). Animals 2021, 11, 529. [Google Scholar] [CrossRef]
- Mackie, M.C. Socially controlled sex-change in the half-moon grouper, Epinephelus rivulatus, at Ningaloo Reef, Western Australia. Coral Reefs 2003, 22, 133–142. [Google Scholar] [CrossRef]
- Kline, R.J.; Khan, I.A.; Holt, G.J. Behavior, color change and time for sexual inversion in the protogynous grouper (Ephinephelus adscensionis). PLoS ONE 2011, 6, e19576. [Google Scholar] [CrossRef]
- Kulaw, D.H.; Cowan, J.H., Jr.; Jackson, M.W. Temporal and spatial comparisons of the reproductive biology of northern Gulf of Mexico (USA) red snapper (Lutjanus campechanus) collected a decade apart. PLoS ONE 2017, 12, e0172360. [Google Scholar] [CrossRef]
- Hixon, M.A.; Johnson, D.W.; Sogard, S.M. BOFFFFs: On the importance of conserving old-growth age structure in fishery populations. ICES J. Mar. Sci. 2013, 71, 2171–2185. [Google Scholar] [CrossRef]
- Barneche, D.R.; Robertson, D.R.; White, C.R.; Marshall, D.J. Fish reproductive-energy output increases disproportionately with body size. Science 2018, 360, 642–645. [Google Scholar] [CrossRef]
- Campana, S.E. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 2001, 59, 197–242. [Google Scholar] [CrossRef]





| Model | K | AICc | ΔAICc | AICc Weight | Log Likelihood |
|---|---|---|---|---|---|
| von Bertalanffy | 4 | 4898.12 | 0.00 | 0.99 | −2445.02 |
| Gompertz | 4 | 4913.61 | 15.49 | 0.01 | −2452.77 |
| Logistic | 4 | 4932.48 | 34.36 | 0.00 | −2462.21 |
| Tests | Hypothesis | Chi-Square | df | p-Value |
|---|---|---|---|---|
| H0 vs H1 | L∞M = L∞F | 1.94 | 1 | 0.164 |
| H0 vs H2 | kM = kF | 0.24 | 1 | 0.624 |
| H0 vs H3 | t0M = t0F | 0.38 | 1 | 0.538 |
| H0 vs H4 | L∞M = L∞F; kM = kF; t0M = t0F | 8.67 | 1 | 0.034 |
| Sex | n | L∞ (cm) | k | t0 | |||
|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | ||
| Females | 243 | 215.85 | 206.54–227.37 | 0.153 | 0.131–0.177 | −0.089 | −0.449–0.375 |
| Males | 251 | 200.32 | 190.33–214.82 | 0.177 | 0.138–0.214 | −0.157 | −0.989–0.295 |
| All Fish | 607 | 213.22 | 206.27–222.89 | 0.150 | 0.132–0.169 | −0.283 | −0.676–0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murie, D.J.; Parkyn, D.C.; Koenig, C.C.; Coleman, F.C.; Malinowski, C.R.; Cusick, J.A.; Ellis, R.D. Age, Growth, and Functional Gonochorism with a Twist of Diandric Protogyny in Goliath Grouper from the Atlantic Coast of Florida. Fishes 2023, 8, 412. https://doi.org/10.3390/fishes8080412
Murie DJ, Parkyn DC, Koenig CC, Coleman FC, Malinowski CR, Cusick JA, Ellis RD. Age, Growth, and Functional Gonochorism with a Twist of Diandric Protogyny in Goliath Grouper from the Atlantic Coast of Florida. Fishes. 2023; 8(8):412. https://doi.org/10.3390/fishes8080412
Chicago/Turabian StyleMurie, Debra J., Daryl C. Parkyn, Christopher C. Koenig, Felicia C. Coleman, Christopher R. Malinowski, Jessica A. Cusick, and Robert D. Ellis. 2023. "Age, Growth, and Functional Gonochorism with a Twist of Diandric Protogyny in Goliath Grouper from the Atlantic Coast of Florida" Fishes 8, no. 8: 412. https://doi.org/10.3390/fishes8080412
APA StyleMurie, D. J., Parkyn, D. C., Koenig, C. C., Coleman, F. C., Malinowski, C. R., Cusick, J. A., & Ellis, R. D. (2023). Age, Growth, and Functional Gonochorism with a Twist of Diandric Protogyny in Goliath Grouper from the Atlantic Coast of Florida. Fishes, 8(8), 412. https://doi.org/10.3390/fishes8080412