Current and Historical Genetic Variability of Native Brown Trout Populations in a Southern Alpine Ecosystem: Implications for Future Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Museum Vouchers: Collection Strategy, Laboratory Procedures, and Data Analyses of Voucher Samples
2.2. Current Trout Population: Sampling Strategy, Tissue Collection, Laboratory and Data Analyses
| CR mtDNA Variable Positions | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Source | Year | Haplotype | 15,757 | 15,777 | 15,790 | 15,810 | 15,842 | 15,860 | 15,864 | 15,926 | 15,927 | 16,011 | 16,053 | 16,054 | 16,067 |
| AF273086 | ATcs-1 | C | - | C | G | T | A | C | G | C | G | C | T | T | ||
| AY836330 | ADcs-1 | . | . | . | . | . | . | . | C | . | . | T | C | C | ||
| AY836350 | MEcs-1 | . | . | . | A | . | C | . | . | . | . | T | C | C | ||
| JQ582461 | MA2c | . | A | . | . | C | . | . | A | T | . | T | C | C | ||
| SZ01 | Torbole | 1821 | ADcs-1 | . | . | . | . | . | . | . | C | . | . | T | C | C |
| SZ02 | Torbole | 1884 | Adriatic ± | . | . | . | . | . | . | . | / | / | / | T | C | C |
| SZ03 | Torbole | 1884 | AD_SZ3 * | . | . | . | . | . | . | . | C | . | A | T | C | C |
| SZ04 | Torbole | 1884 | Adriatic ± | . | . | . | . | . | . | . | C | . | . | T | C | C |
| SZ05 | Fish farm | 1892 | ATcs-1 | . | . | . | . | . | . | . | . | . | . | . | . | . |
| SZ06 | Fish farm | 1892 | ATcs-1 | . | . | . | . | . | . | . | . | . | . | . | . | . |
| SZ07 | Fish farm | 1892 | ADcs-1 | . | . | . | . | . | . | . | C | . | . | T | C | C |
| SZ08 | Fish farm | 1892 | Ma2c | . | A | . | . | C | . | . | A | T | . | T | C | C |
| SZ09 | Fish farm | 1892 | Ma2c | . | A | . | . | C | . | . | A | T | . | T | C | C |
| SZ10 | Fish farm | 1892 | ADcs-1 | . | . | . | . | . | . | C | . | . | T | C | C | |
| SZ11 | Rovereto | - | Ma2c | . | A | . | . | C | . | . | A | T | . | T | C | C |
| SZ12 | Rovereto | - | MA_SZ12 * | T | A | T | . | C | . | T | A | T | . | T | C | C |
| SZ13 | Torbole | 1884 | Ma2c | . | A | . | . | C | . | . | A | T | . | T | C | C |
| SZ15 | Caffaro | 1900 | AD/MA ± | / | / | / | / | / | / | / | / | / | . | T | C | C |
| SZ18 | Serio | 1858 | ADcs-1 | . | . | . | . | . | . | . | C | . | . | T | C | C |
| SZ19 | Serio | 1858 | AD/MA± | / | / | / | / | / | / | / | / | . | . | T | C | C |
3. Results
3.1. Taxonomic Attribution of Museum Vouchers
3.2. Current Trout Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Dearing, A.J.; Battarbee, R.W.; Dikau, R.; Larocque, I.; Oldfield, F. Human–environment interactions: Learning from the past. Reg. Environ. Change 2006, 6, 1–16. [Google Scholar] [CrossRef]
- Copp, G.H.; Bianco, P.G.; Bogutskaya, N.G.; Erős, T.; Falka, I.; Ferreira, M.T.; Fox, M.G.; Freyhof, J.; Gozlan, R.E.; Grabowska, J.O.; et al. To be, or not to be, a non-native freshwater fish? J. Appl. Ichthyol. 2005, 21, 242–262. [Google Scholar] [CrossRef]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Kottelat, Cornol, Switzerland and Freyhof: Berlin, Germany, 2007. [Google Scholar]
- Bernatchez, L.; Guyomard, R.; Bonhomme, F. DNA sequence variation of the mitochondrial control region among geographically and morphologically remote European brown trout Salmo trutta populations. Mol. Ecol. 1992, 1, 3. [Google Scholar] [CrossRef]
- Bernatchez, L. The Evolutionary History of Brown Trout (Salmo trutta L.) Inferred from Phylogeographic, Nested Clade, and Mismatch Analyses of Mitochondrial DNA Variation. Evolution 2001, 55, 351–379. [Google Scholar]
- Snoj, A.; Marić, S.; Sušnik, B.S.; Berrebi, P.; Janjani, S.; Schöffmann, J. Phylogeographic structure and demographic patterns of brown trout in North-West Africa. Mol. Phylogenet. Evol. 2011, 61, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Splendiani, A.; Ruggeri, P.; Giovannotti, M.; Pesaresi, S.; Occhipinti, G.; Fioravanti, T.; Lorenzoni, M.; Nisi Cerioni, P.; Caputo Barucchi, V. Alien brown trout invasion of the Italian peninsula: The role of geological, climate and anthropogenic factors. Biol. Inv. 2016, 18, 2029–2044. [Google Scholar] [CrossRef]
- Stefani, F.; Anzani, A.; Marieni, A. Echoes from the past: A genetic trace of native brown trout in the Italina Alps. Environ. Biol. Fishes 2019, 102, 1327–1335. [Google Scholar] [CrossRef]
- Giuffra, E.; Bernatchez, L.; Guyomard, R. Mitochondrial control region and protein coding genes sequence variation among phenotypic forms of brown trout Salmo trutta from northern Italy. Mol. Ecol. 1994, 3, 161–171. [Google Scholar] [CrossRef]
- Splendiani, A.; Giovannotti, M.; Cerioni, P.N.; Caniglia, M.L.; Caputo, V. Phylogeographic inferences on the native brown trout mtDNA variation in central Italy. Ital. J. Zool. 2006, 73, 179–189. [Google Scholar] [CrossRef]
- Splendiani, A.; Fioravanti, T.; Giovannotti, M.; Olivieri, L.; Ruggeri, P.; Nisi Cerioni, P.; Vanni, S.; Enrichetti, F.; Caputo Barucchi, V. Museum samples could help to reconstruct the original distribution of Salmo trutta complex in Italy. J. Fish Biol. 2017, 90, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Galli, P.; Stefani, F.; Benzoni, F.; Crosa, G.; Zullini, A. New records of alien monogeneans from Lepomis gibbosus and Silurus glanis in Italy. Parassitologia 2003, 45, 147–150. [Google Scholar]
- Splendiani, A.; Palmas, F.; Sabatini, A.; Caputo Barucchi, V. The name of the trout: Considerations on the taxonomic status of the Salmo trutta L., 1758 complex (Osteichthyes: Salmonidae) in Italy. Eur. Zool. J. 2019, 86, 432–442. [Google Scholar] [CrossRef]
- Meraner, A.; Baric, S.; Pelster, B.; Dalla Via, J. Microsatellite DNA data point to extensive but incomplete admixture in a marble and brown trout hybridisation zone. Conserv. Genet. 2010, 11, 985–998. [Google Scholar] [CrossRef]
- Gratton, P.; Allegrucci, G.; Sbordoni, V.; Gandolfi, A. The evolutionary jigsaw puzzle of the surviving trout (Salmo trutta L. complex) diversity in the Italian region. A multilocus Bayesian approach. Mol. Phylogenet. Evol. 2014, 79, 292–304. [Google Scholar] [CrossRef] [PubMed]
- ISPRA. Tabella Specie Ittiche Autoctone—Richiesta di Modifica Status non Autoctonia Trota Mediterranea (Salmo ghigii); ISPRA: Rome, Italy, 2021. [Google Scholar]
- Jonsson, B.; Jonsson, N. Ecology of Atlantic Salmon and Brown Trout: Habitat as a Template for Life Histories; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Budy, P.; Thiede, G.P.; Lobón-Cerviá, J.; Fernandez, G.G.; McHugh, P.; McIntosh, A.; Vøllestad, L.A.; Becares, E.; Jellyman, P. Limitation and facilitation of one of the world’s most invasive fish: An intercontinental comparison. Ecology 2013, 94, 356–367. [Google Scholar] [CrossRef]
- Splendiani, A.; Ruggeri, P.; Giovannotti, M.; Caputo Barucchi, V. Role of environmental factors in the spread of domestic trout in Mediterranean streams. Freshw. Biol. 2013, 58, 2089–2101. [Google Scholar] [CrossRef]
- Wandeler, P.; Hoeck, P.E.A.; Keller, L.F. Back to the future: Museum specimens in population genetics. Trends Ecol. Evol. 2007, 22, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Boessenkool, S.; Star, B.; Scofield, R.P.; Seddon, P.J.; Waters, J.M. Lost in translation or deliberate falsification? Genetic analyses reveal erroneous museum data for historic penguin specimens. Proc. R. Soc. B Biol. Sci. 2009, 277, 1057–1064. [Google Scholar] [CrossRef]
- Hansen, M.M.; Fraser, D.J.; Meier, K.; Mensberg, K.-L.D. Sixty years of anthropogenic pressure: A spatio-temporal genetic analysis of brown trout populations subject to stocking and population declines. Mol. Ecol. 2009, 18, 2549–2562. [Google Scholar] [CrossRef]
- Nielsen, E.E.; Hansen, M.M.; Loeschcke, V. Analysis of Applications DNA from Old Scale Samples: Technical Aspects, and Perspectives for Conservation. Hereditas 1999, 130, 265–276. [Google Scholar] [CrossRef]
- Palandačić, A.; Kruckenhauser, L.; Ahnelt, H.; Mikschi, E. European minnows through time: Museum collections aid genetic assessment of species introductions in freshwater fishes (Cyprinidae: Phoxinus species complex). Heredity 2020, 124, 410–422. [Google Scholar] [CrossRef]
- Su, B.; Wang, Y.-x.; Lan, H.; Wang, W.; Zhang, Y. Phylogenetic Study of Complete Cytochrome b Genes in Musk Deer (Genus Moschus) Using Museum Samples. Mol. Phylogenet. Evol. 1999, 12, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.K.; Pääbo, S.; Villablanca, F.X.; Wilson, F.X. Spatial and temporal continuity of kangaroo rat populations shown by sequencing mitochondrial DNA from museum specimens. J. Mol. Evol. 1990, 31, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Lynch Alfaro, J.W.; Boubli, J.P.; Olson, L.E.; Di Fiore, A.; Wilson, B.; Gutiérrez-Espeleta, G.A.; Chiou, K.L.; Schulte, M.; Neitzel, S.; Ross, V.; et al. Explosive Pleistocene range expansion leads to widespread Amazonian sympatry between robust and gracile capuchin monkeys. J. Biog. 2012, 39, 272–288. [Google Scholar] [CrossRef]
- McMeel, O.M.; Hoey, E.M.; Ferguson, A. Partial nucleotide sequences, and routine typing by polymerase chain reaction–restriction fragment length polymorphism, of the brown trout (Salmo trutta) lactate dehydrogenase, LDH-C1*90 and *100 alleles. Mol. Ecol. 2001, 10, 29–34. [Google Scholar] [CrossRef]
- Splendiani, A.; Berrebi, P.; Tougard, C.; Righi, T.; Reynaud, N.; Fioravanti, T.; Lo Conte, P.; Delmastro, G.B.; Baltieri, M.; Ciuffardi, L.; et al. The role of the south-western Alps as a unidirectional corridor for Mediterranean brown trout (Salmo trutta complex) lineages. Biol. J. Linn. Soc. 2021, 131, 909–926. [Google Scholar] [CrossRef]
- Sabatini, A.; Cannas, R.; Follesa, M.C.; Palmas, F.; Manunza, A.; Matta, G.; Pendugiu, A.A.; Serra, P.; Cau, A. Genetic characterization and artificial reproduction attempt of endemic Sardinian trout Salmo trutta L., 1758 (Osteichthyes, Salmonidae): Experiences in captivity. Ital. J. Zool. 2011, 78, 20–26. [Google Scholar] [CrossRef]
- Cortey, M.; Pla, C.; García-Marín, J.-L. Historical biogeography of Mediterranean trout. Mol. Phylogenet. Evol. 2004, 33, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Meraner, A.; Gratton, P.; Baraldi, F.; Gandolfi, A. Nothing but a trace left? Autochthony and conservation status of Northern Adriatic Salmo trutta inferred from PCR multiplexing, mtDNA control region sequencing and microsatellite analysis. Hydrobiology 2013, 702, 201–213. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Aljanabi, S.M.; Martinez, I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef] [PubMed]
- Padula, A.; Greco, C.; Talarico, L.; Caniglia, R.; Antognazza, C.M.; D’Antoni, S.; Lorenzoni, M.; Vanetti, I.; Zaccara, S.; Mucci, N. A rapid and reliable detection procedure of Atlantic trout introgression at the diagnostic lactate dehydrogenase chain-1 gene. Aquac. Fish 2023, 3, 388–392. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Librado, P.; Rozas, J. DnaSP: A software for comprehensive analysis of DNA polymorphism data. Bioinformation 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Hasegawa, M.; Kishino, H.; Yano, T.-a. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Swofford, D.L.; Sullivan, J. Chapter 7: Phylogeny inference based on parsimony and other methods using PAUP*. In The Phylogenetic Handbook: A Practical Approach to DNA and Protein Phylogeny; Cambridge University Press: Cambridge, UK, 2003; pp. 160–206. [Google Scholar]
- Zwickl, D.J. Genetic Algorithm Approaches for the Phylogenetic Analysis of Large Biological Sequence Datasets under the Maximum Likelihood Criterion. Ph.D. Thesis, The University of Texas at Austin, Austin, TX, USA, 2006. [Google Scholar]
- Ronquist, F.; Teslenko, M.; Van der Mark, P.; Ayres, D.L.; Darling, A.A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice Across a Large Model Space. System. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 20 February 2023).
- Clement, M.; Posada, D.; Crandall, K. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1660. [Google Scholar] [CrossRef]
- Cortey, M.; García-Marín, J.L. Evidence for phylogeographically informative sequence variation in the mitochondrial control region of Atlantic brown trout. J. Fish Biol. 2002, 60, 1058–1063. [Google Scholar] [CrossRef]
- Kanjuh, T.; Marić, A.; Piria, M.; Špelić, I.; Maguire, I.; Simonović, P. Diversity of brown trout, Salmo trutta (Actinopterygii: Salmoniformes: Salmonidae), in the Danube River basin of Croatia revealed by mitochondrial DNA. Acta Ichthyol. Piscat. 2020, 50, 291–300. [Google Scholar] [CrossRef]
- Zimmermann, J.; Hajibabaei, M.; Blackburn, D.C.; Hanken, J.; Cantin, E.; Posfai, J.; Evans, T.C. DNA damage in preserved specimens and tissue samples: A molecular assessment. Front. Zool. 2008, 5, 1. [Google Scholar] [CrossRef]
- Hall, L.M.; Willcox, D.S.; Jones, D.S. Association of enzyme inhibition with methods of museum skin preparation. Biotechniques 1997, 22, 928–934. [Google Scholar] [CrossRef]
- Crivelli, A.J. Are fish introductions a threat to endemic freshwater fishes in the northern Mediterrenean region? Biol. Cons. 1995, 72, 311–319. [Google Scholar] [CrossRef]
- Jensen, E.L.; Díez-del-Molino, D.; Gilbert, M.T.P.; Bertola, L.D.; Borges, F.; Cubric-Curik, V.; de Navascués, M.; Frandsen, P.; Heuertz, M.; Hvilsom, C. Ancient and historical DNA in conservation policy. Trends Ecol. Evol. 2022, 37, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Splendiani, A.; Fioravanti, T.; Ruggeri, P.; Giovannotti, M.; Carosi, A.; Marconi, M.; Lorenzoni, M.; Righi, T.; Nisi Cerioni, P.; Caputo Barucchi, V. Life history and genetic characterisation of sea trout Salmo trutta in the Adriatic Sea. Fresh Biol. 2020, 65, 460–473. [Google Scholar] [CrossRef]
- Cortey, M.; Vera, M.; Pla, C.; GarcÍA-MarÍN, J.-L. Northern and Southern expansions of Atlantic brown trout (Salmo trutta) populations during the Pleistocene. Biol. J. Linn. Soc. 2009, 97, 904–917. [Google Scholar] [CrossRef]
- Berrebi, P.; Caputo Barucchi, V.; Splendiani, A.; Muracciole, S.; Sabatini, A.; Palmas, F.; Tougard, C.; Arculeo, M.; Marić, S. Brown trout (Salmo trutta L.) high genetic diversity around the Tyrrhenian Sea as revealed by nuclear and mitochondrial markers. Hydrobiologia 2019, 826, 209–231. [Google Scholar] [CrossRef]
- Marić, S.; Sušnik Bajec, S.; Schöffmann, J.; Kostov, V.; Snoj, A. Phylogeography of stream-dwelling trout in the Republic of Macedonia and a molecular genetic basis for revision of the taxonomy proposed by S. Karaman. Hydrobiologia 2017, 785, 249–260. [Google Scholar] [CrossRef]
- Sušnik, S.; Snoj, A.; Wilson, I.F.; Mrdak, D.; Weiss, S. Historical demography of brown trout (Salmo trutta) in the Adriatic drainage including the putative S. letnica endemic to Lake Ohrid. Mol. Phylogenetics Evol. 2007, 44, 63–76. [Google Scholar] [CrossRef]
- Delling, B.; Sabatini, A.; Muracciole, S.; Tougard, C.; Berrebi, P. Morphologic and genetic characterisation of Corsican and Sardinian trout with comments on Salmo taxonomy. Knowl. Manag. Aquat. Ecosyst. 2020, 21, 421. [Google Scholar] [CrossRef]
- Jadan, M.; Strunjak-Perović, I.; Topić Popović, N.; Čož-Rakovac, R. Three major phylogenetic lineages of brown trout (Salmo trutta Linnaeus, 1758) in the Krka River system (Croatia) revealed by complete mitochondrial DNA control region sequencing. J. Appl. Ichthyol. 2015, 31, 192–196. [Google Scholar] [CrossRef]
- Vera, M.; Sourinejad, I.; Bouza, C.; Vilas, R.; Pino-Querido, A.; Kalbassi, M.R.; Martínez, P. Phylogeography, genetic structure, and conservation of the endangered Caspian brown trout, Salmo trutta caspius (Kessler, 1877), from Iran. Hydrobiologia 2011, 664, 51–67. [Google Scholar] [CrossRef]
- Meraner, A.; Baric, S.; Pelster, B.; Dalla Via, J. Trout (Salmo trutta) mitochondrial DNA polymorphism in the centre of the marble trout distribution area. Hydrobiologia 2007, 579, 337–349. [Google Scholar] [CrossRef]
- Segherloo, I.H.; Freyhof, J.; Berrebi, P.; Ferchaud, A.-L.; Geiger, M.; Laroche, J.; Levin, B.A.; Normandeau, E.; Bernatchez, L. A genomic perspective on an old question: Salmo trouts or Salmo trutta (Teleostei: Salmonidae)? Mol. Phylogenetics Evol. 2021, 162, 107204. [Google Scholar] [CrossRef]
- Duftner, N.; Weiss, S.; Medgyesy, N.; Sturmbauer, C. Enhanced phylogeographic information about Austrian brown trout populations derived from complete mitochondrial control region sequences. J. Fish Biol. 2003, 62, 427–435. [Google Scholar] [CrossRef]
- Weiss, S.; Schlötterer, C.; Waidbacher, H.; Jungwirth, M. Haplotype (mtDNA) diversity of brown trout Salmo trutta in tributaries of the Austrian Danube: Massive introgression of Atlantic basin fish—By man or nature? Mol. Ecol. 2001, 10, 1241–1246. [Google Scholar] [CrossRef]
- Machado-Schiaffino, G.; Griffiths, A.; Bright, D.; Stevens, J.; Garcia-Vazquez, E. Salmo salar Isolate Leguer2 Control Region, Partial Sequence; Mitochondrial. GenBank Database, 2009. Available online: https://www.ncbi.nlm.nih.gov/nuccore/GQ376149 (accessed on 18 January 2023).
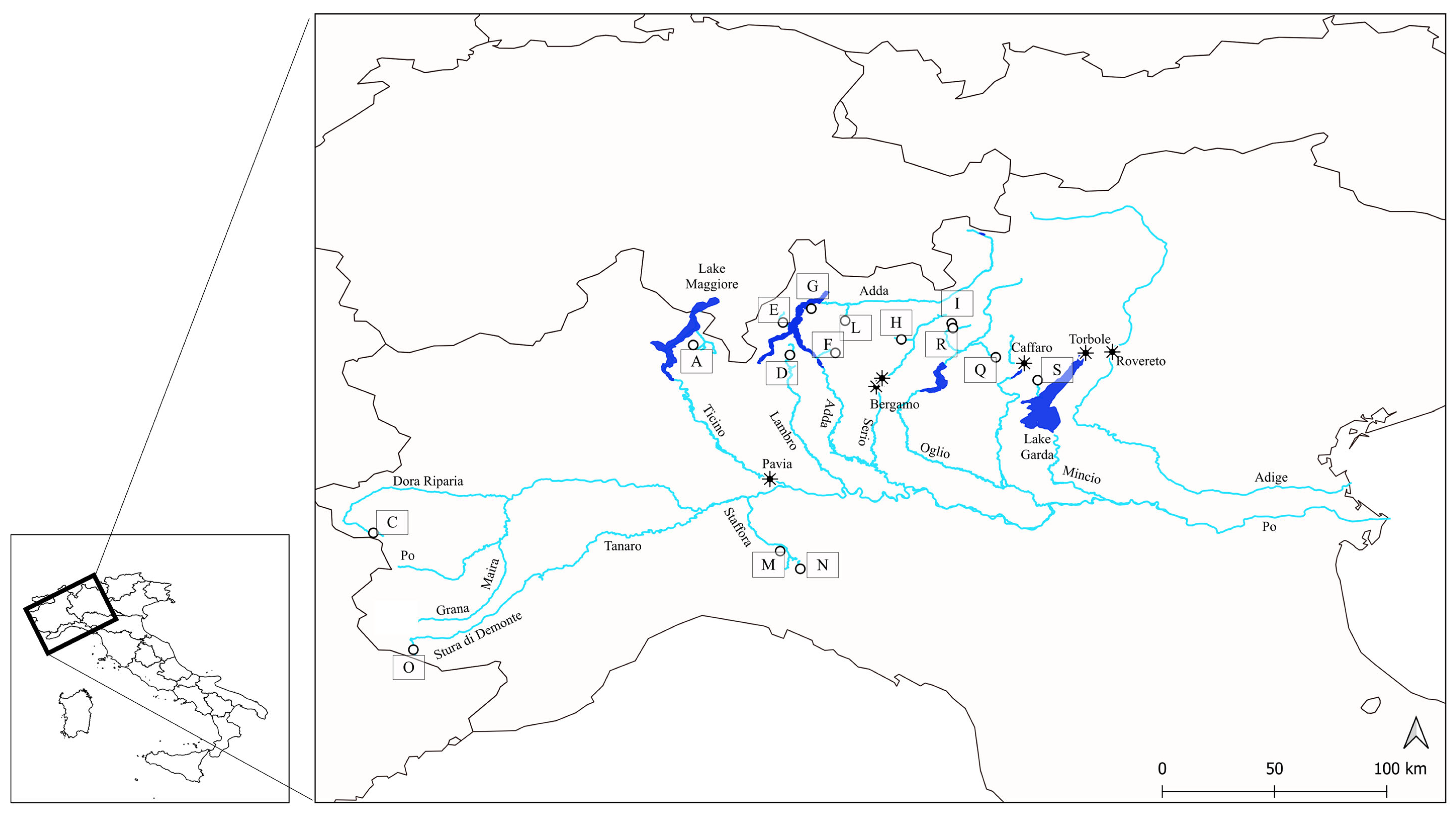
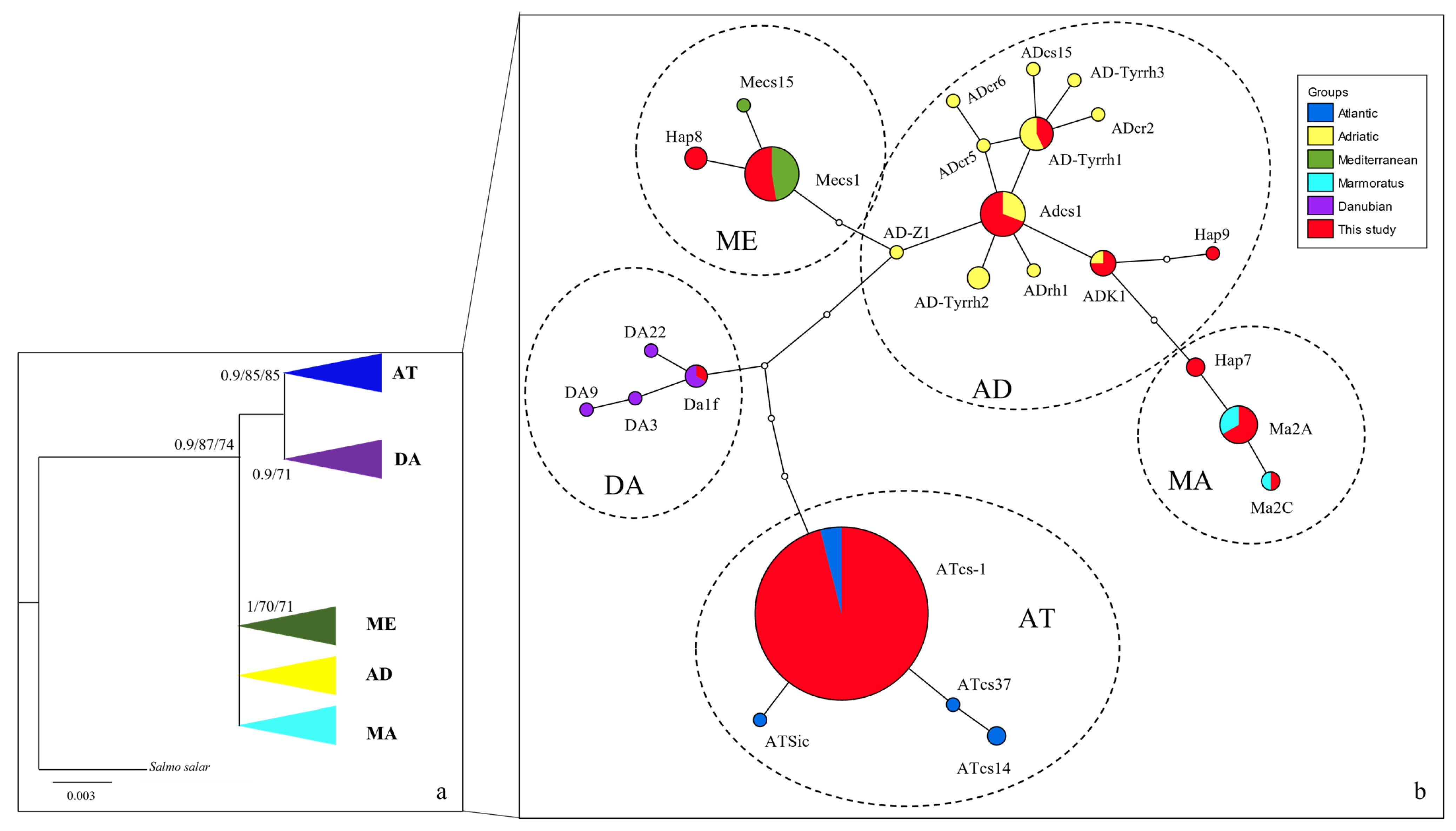
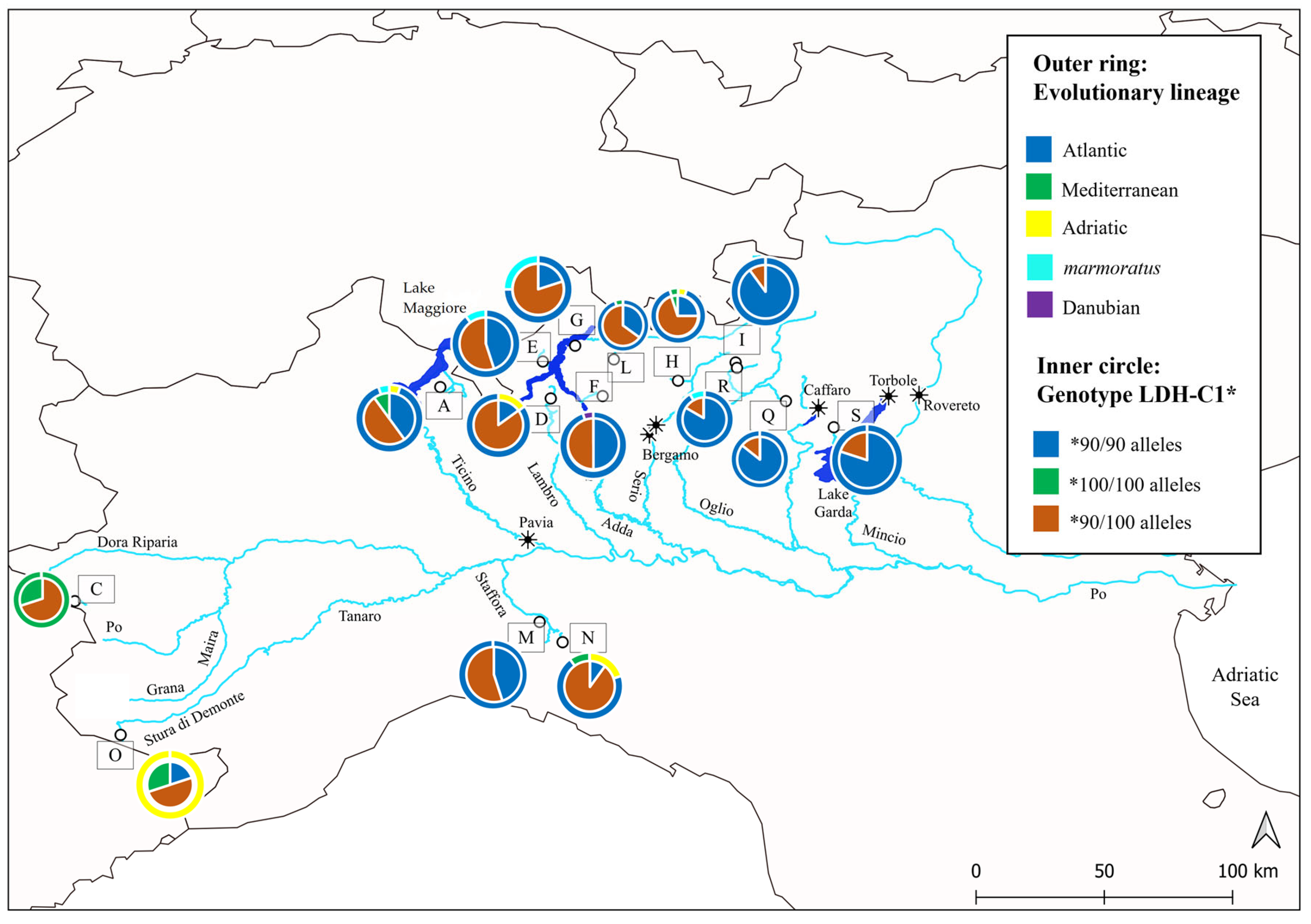
| Museum | Collector | Cat_Num | River Drainage | Locality | Year | ID | Method |
|---|---|---|---|---|---|---|---|
| Vienna | unknown | NMW_96796 | Lake Garda | Torbole | 1821 | SZ01 * | [31] |
| Steindachner | NMW_65951 | Lake Garda | Torbole | 1884 | SZ02 | Three pieces | |
| Steindachner | NMW_65952 | Lake Garda | Torbole | 1884 | SZ03 | [31] | |
| Steindachner | NMW_65954 | Lake Garda | Torbole | 1884 | SZ04 | Three pieces | |
| Sturany | NMW_65415 | Lake Garda | Fish farm | 1892 | SZ05 | [31] | |
| Sturany | NMW_66083_1 | Lake Garda | Fish farm | 1892 | SZ06 | [31] | |
| Sturany | NMW_66083_2 | Lake Garda | Fish farm | 1892 | SZ07 | [31] | |
| Sturany | NMW_66489 | Lake Garda | Fish farm | 1892 | SZ08 | [31] | |
| Sturany | NMW_66490_1 | Lake Garda | Fish farm | 1892 | SZ09 | [31] | |
| Sturany | NMW_66490_2 | Lake Garda | Fish farm | 1892 | SZ10 | [31] | |
| unknown | NMW_67946 | Adige | Rovereto | End 1800 | SZ11 | [31] | |
| unknown | NMW_90252 | Adige | Rovereto | End 1800 | SZ12 | [31] | |
| Steindachner | NMW_59654 | Lake Garda | Torbole | 1884 | SZ13 | [31] | |
| Brescia | unknown | MCSNB 57 | Caffaro | - | Beginning 1900 | SZ14 | Failed |
| unknown | MCSNB 13 | Caffaro | - | Beginning 1900 | SZ15 | [31] | |
| Pavia | unknown | MSNPV CP0433—Ex 435/593 | Serio | Bergamo | End 1800 | SZ16 | Failed |
| unknown | MSNPV CP0435—Ex 438/604 | Ticino | Pavia | 1877 | SZ17 | Failed | |
| Milano | unknown | MSNM Pi 3139_B | Serio | - | 1858 | SZ18 | [31] |
| unknown | MSNM Pi 3139_A | Serio | - | 1858 | SZ19 | [31] |
| Haplotype Distribution | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lineages | AT | AD | Ma | DA | ME | ||||||||||||
| ATcs1 | AD-Thyrr1 | ADK1 | ADcs1 | Ma2C | Ma2A | Da1f | Mecs1 | Genotype | |||||||||
| ID | Stream | Basin | N | Hap1 | Hap2 | Hap4 | Hap11 | Hap9 * | Hap3 | Hap5 | Hap7 * | Hap6 | Hap8 * | Hap10 | ATL | Hyb | MED |
| A | Marianna ± | Ticino | 20 | 17 | 1 | 1 | 8 | 10 | 2 | ||||||||
| C | Ripa ¥ | Dora Riparia | 10 | 10 | - | 7 | 3 | ||||||||||
| D | Valle Rezzago | Lambro | 20 | 17 | 3 | 3 | 17 | - | |||||||||
| E | Senagra | Adda | 20 | 18 | 2 | 9 | 11 | - | |||||||||
| F | Caldone | Adda | 20 | 19 | 1 | 10 | 10 | - | |||||||||
| G | Valle Merla | Adda | 20 | 15 | 3 | 2 | 4 | 16 | - | ||||||||
| H | Acqualina | Adda | 20 | 18 | 1 | 1 | 5 | 14 | 1 | ||||||||
| I | Vò | Oglio | 10 | 10 | 9 | 1 | - | ||||||||||
| L | Valle della Pietra | Adda | 20 | 19 | 1 | 7 | 13 | - | |||||||||
| M | Lella | Staffora | 20 | 20 | 9 | 11 | - | ||||||||||
| N | Avagnone | Trebbia | 10 | 7 | 1 | 1 | 1 | 1 | 9 | - | |||||||
| O | Rio Freddo ¥ | Tanaro | 10 | 10 | 2 | 5 | 3 | ||||||||||
| Q | Grigna | Oglio | 7 | 7 | 6 | 1 | - | ||||||||||
| R | Allione | Oglio | 12 | 11 | 1 | 10 | 2 | - | |||||||||
| S | Valle di Vesta | Mincio | 5 | 5 | 4 | 1 | - | ||||||||||
| 224 | 183 | 3 | 3 | 10 | 1 | 1 | 6 | 2 | 1 | 3 | 10 | 87 | 128 | 9 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antognazza, C.M.; Palandaćić, A.; Delmastro, G.B.; Crosa, G.; Zaccara, S. Current and Historical Genetic Variability of Native Brown Trout Populations in a Southern Alpine Ecosystem: Implications for Future Management. Fishes 2023, 8, 411. https://doi.org/10.3390/fishes8080411
Antognazza CM, Palandaćić A, Delmastro GB, Crosa G, Zaccara S. Current and Historical Genetic Variability of Native Brown Trout Populations in a Southern Alpine Ecosystem: Implications for Future Management. Fishes. 2023; 8(8):411. https://doi.org/10.3390/fishes8080411
Chicago/Turabian StyleAntognazza, Caterina M., Anja Palandaćić, Giovanni B. Delmastro, Giuseppe Crosa, and Serena Zaccara. 2023. "Current and Historical Genetic Variability of Native Brown Trout Populations in a Southern Alpine Ecosystem: Implications for Future Management" Fishes 8, no. 8: 411. https://doi.org/10.3390/fishes8080411
APA StyleAntognazza, C. M., Palandaćić, A., Delmastro, G. B., Crosa, G., & Zaccara, S. (2023). Current and Historical Genetic Variability of Native Brown Trout Populations in a Southern Alpine Ecosystem: Implications for Future Management. Fishes, 8(8), 411. https://doi.org/10.3390/fishes8080411






