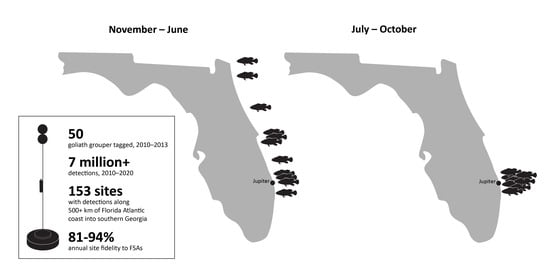
Spawning Migrations of the Atlantic Goliath Grouper along the Florida Atlantic Coast
Abstract
1. Introduction
2. Materials and Methods
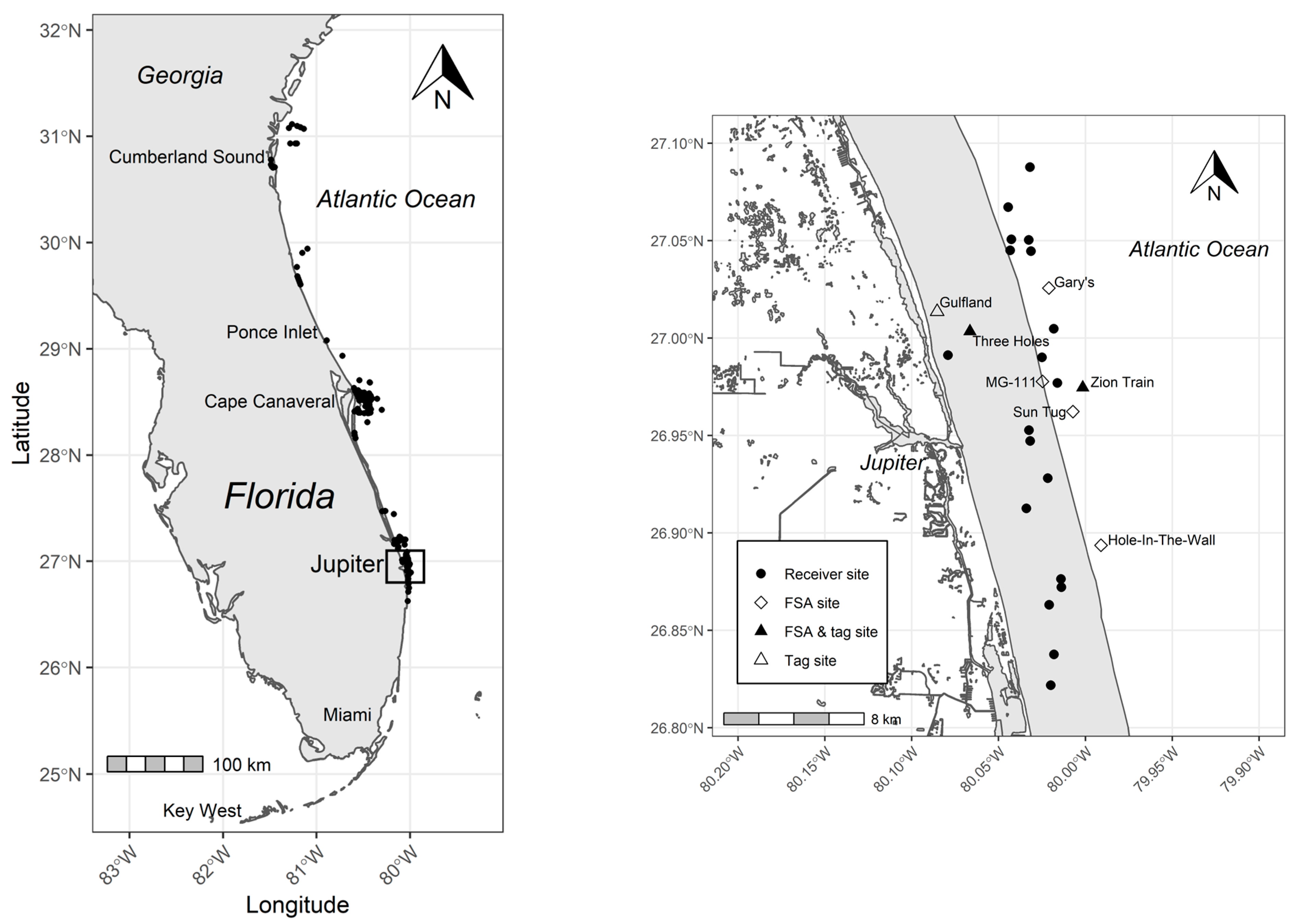
2.1. Fish Tagging
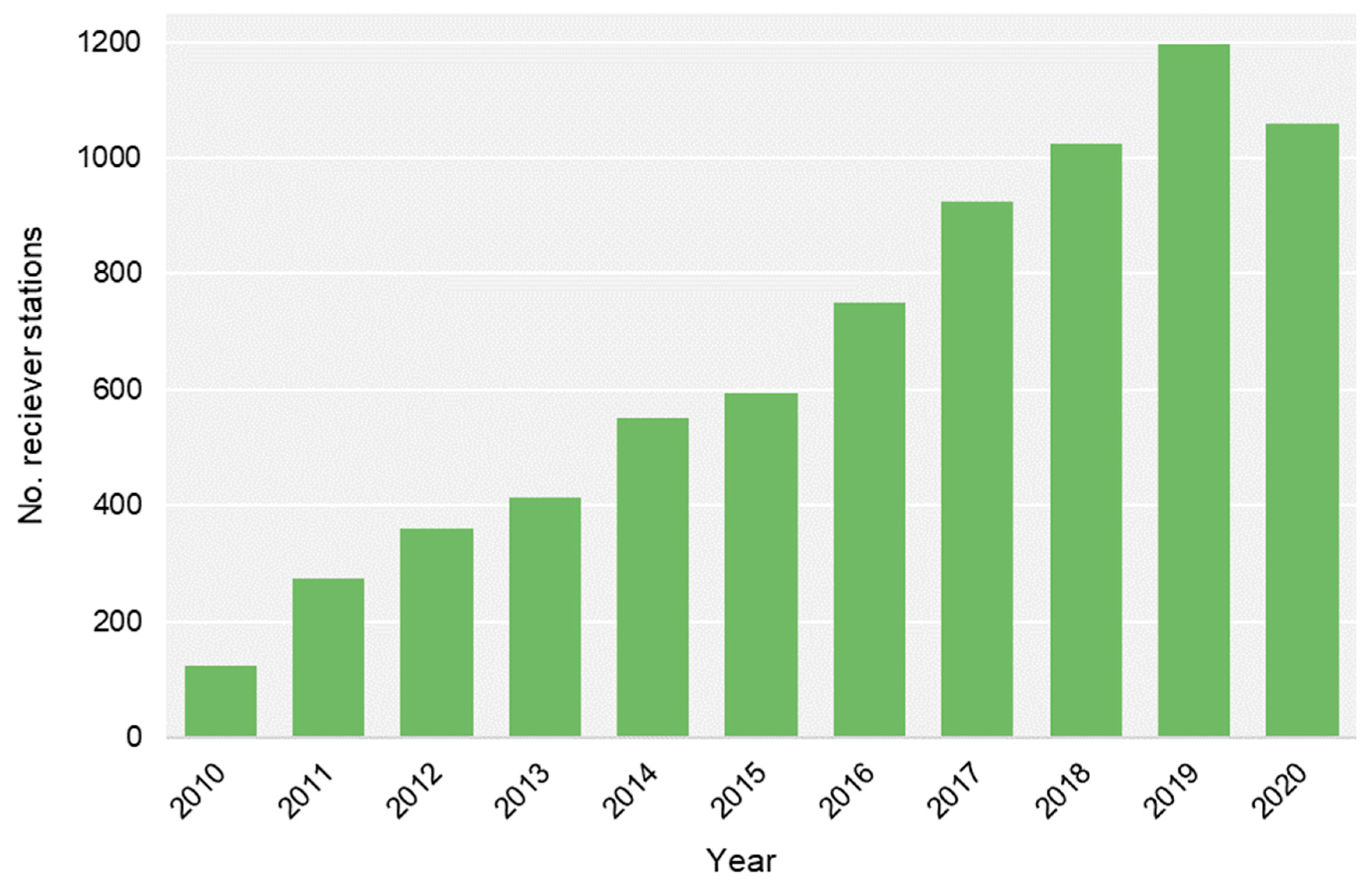
2.2. Acoustic Tracking and Monitoring
2.3. Data Analysis
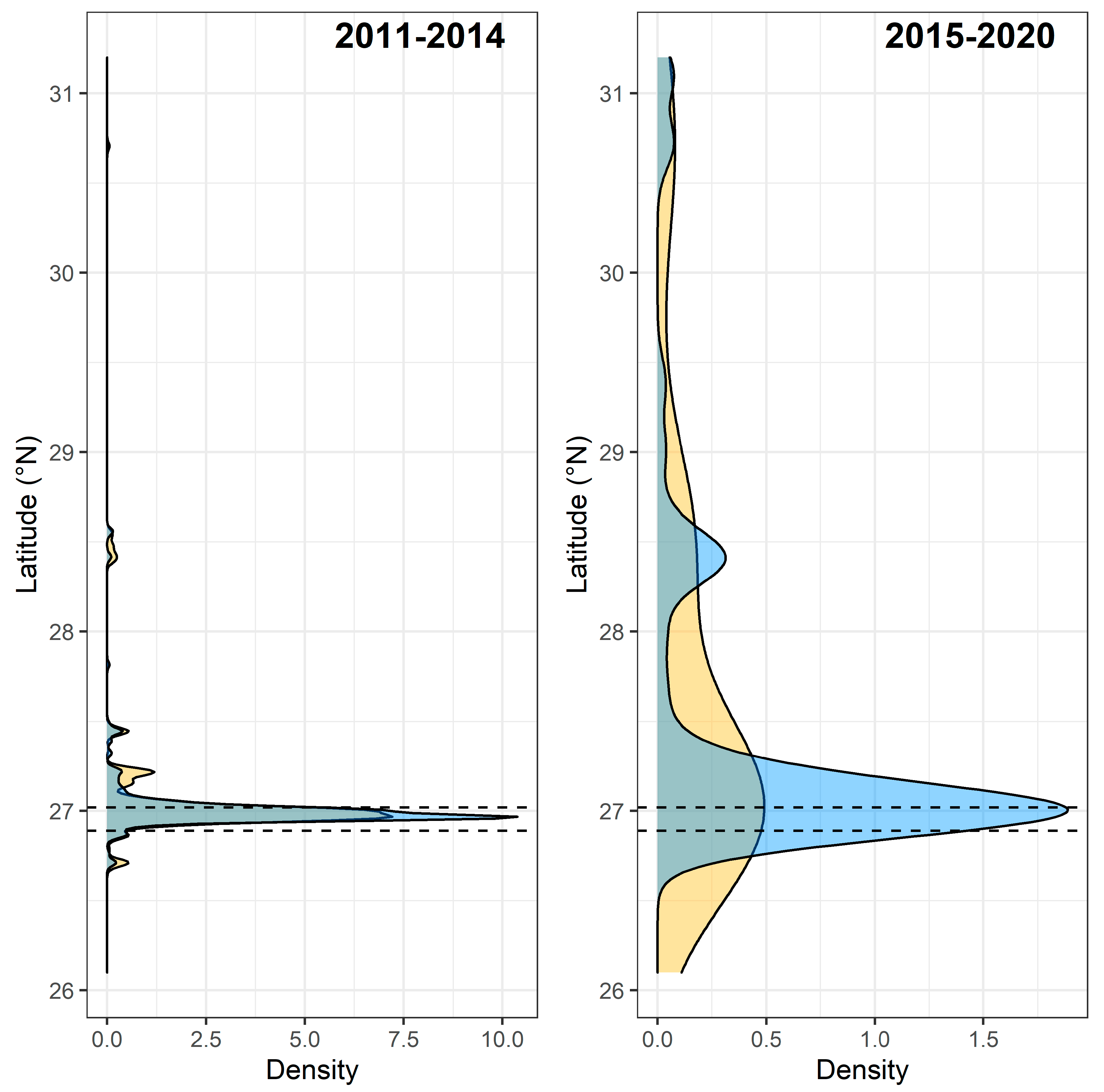
3. Results
3.1. Fish Capture and Tagging
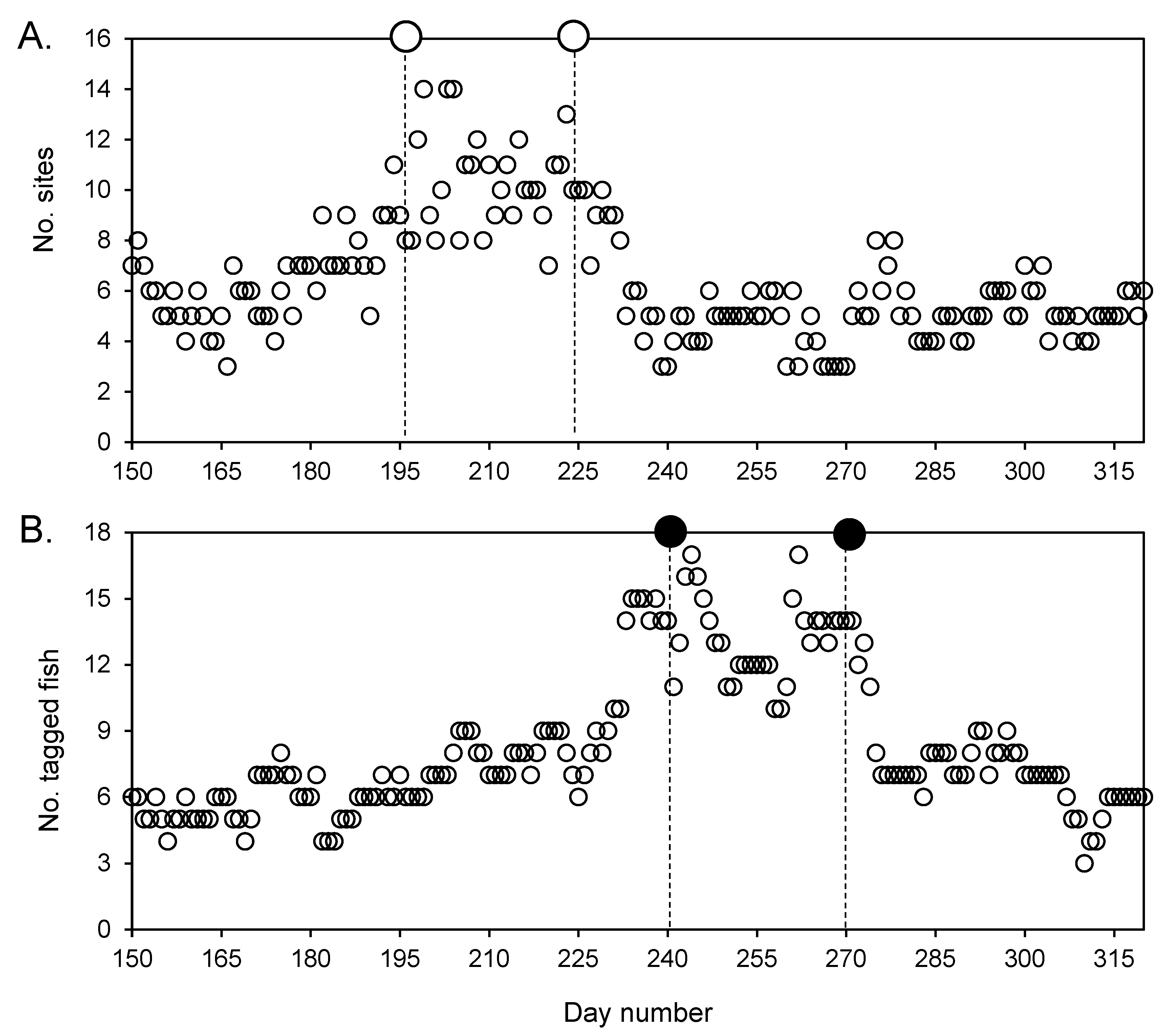
3.2. General Detection Patterns
- (1)
- Fish #058 (180 cm TL, female): July 2011—moved 222 km between Ponce Inlet and an artificial reef near the St. Lucie Inlet in 9 days (~25 km d−1);
- (2)
- Fish #060 (186 cm TL, female): (a) July 2011—moved 252 km between Ponce Inlet and a natural reef site near the Jupiter Inlet in 22 days (~11.5 km d−1); (b) August 2012—moved 438 km between a site inside Cumberland Sound (near the Florida—Georgia state border) and the spawning site MG-111 in 10 days (~44 km d−1);
- (3)
- Fish #417 (194 cm TL, female): (a) August 2013—moved 175 km between Cape Canaveral and the Sun Tug spawning site over 12 days (~14.5 km d−1); (b) late July to early August 2014—moved 184 km between Cape Canaveral and the Sun Tug spawning site over 13 days (~14 km d−1); (c) late July to early August 2015 moved 419 km between Cumberland Sound to sites near the St Lucie Inlet in 15 days (~28 km d−1); (d) August 2016—moved 424 km from Cumberland Sound to an artificial reef near the St. Lucie Inlet over 13 days (~32.6 km d−1); (e) July to August 2017—moved 430 km between Cumberland Sound and the St Lucie Inlet in 21 days (~20.4 km d−1). Note that although we were no longer monitoring the six focal FSA sites after July 2015 and thus cannot confirm that fish #417 returned to one of these FSAs, this individual was detected at other sites located within 10 km of confirmed FSA sites during both the 2015 and 2016 spawning seasons.
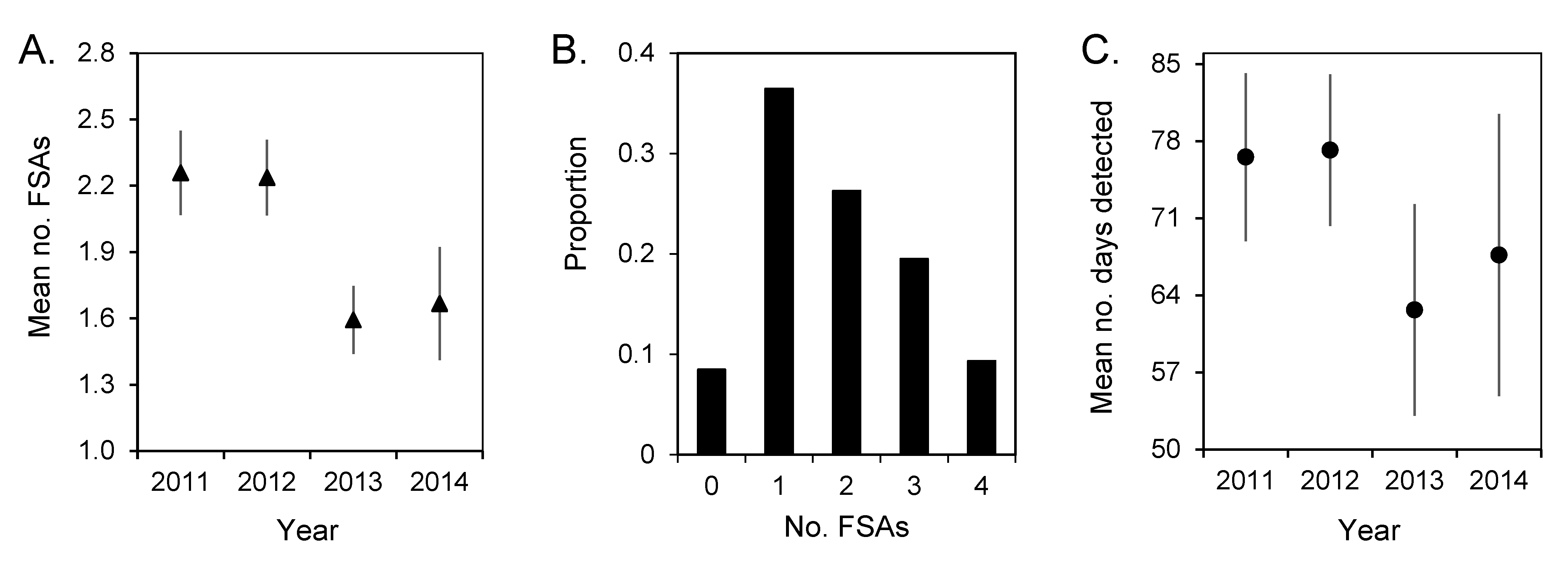
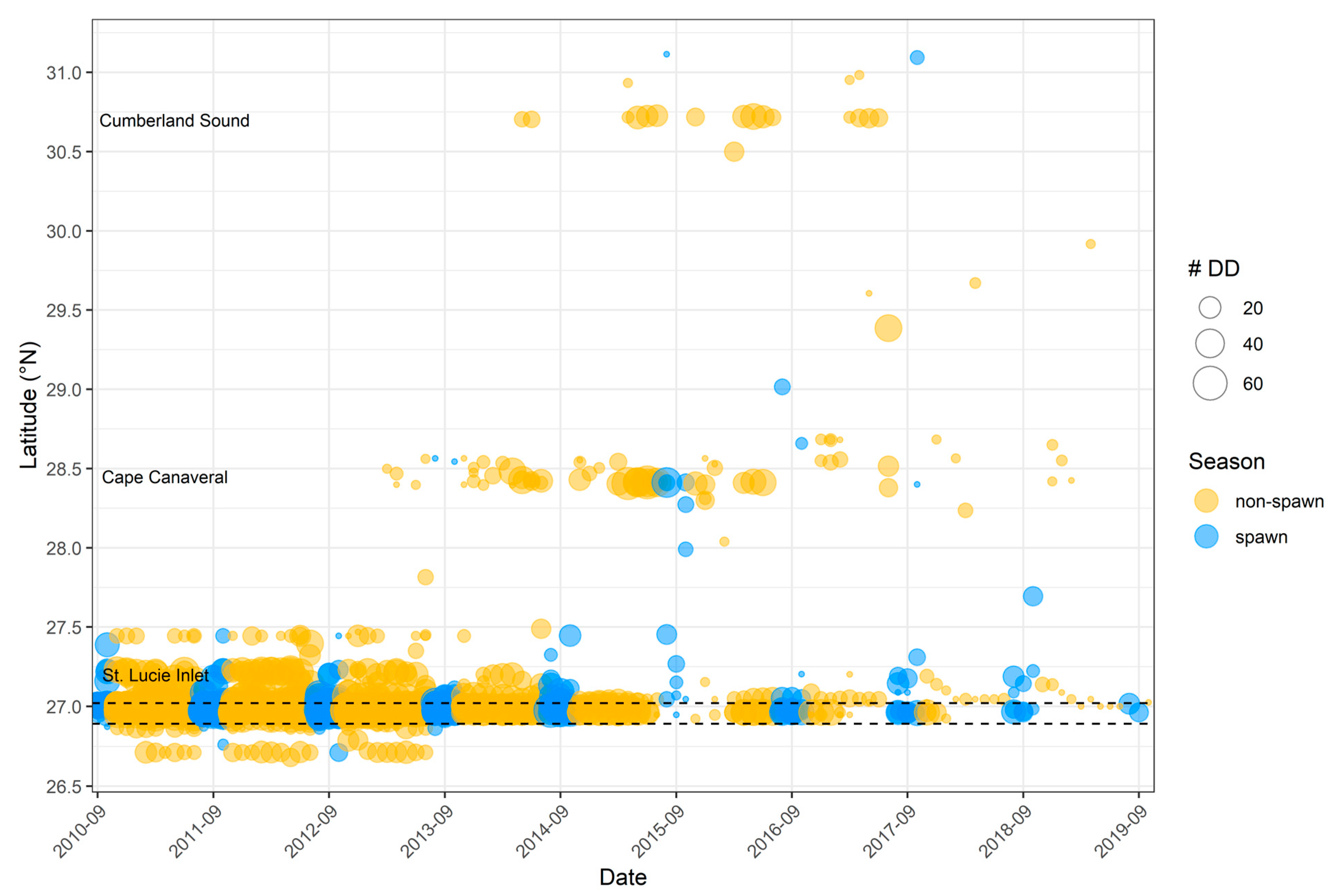
3.3. Spawning Site Fidelity
3.4. Aggregation Formation
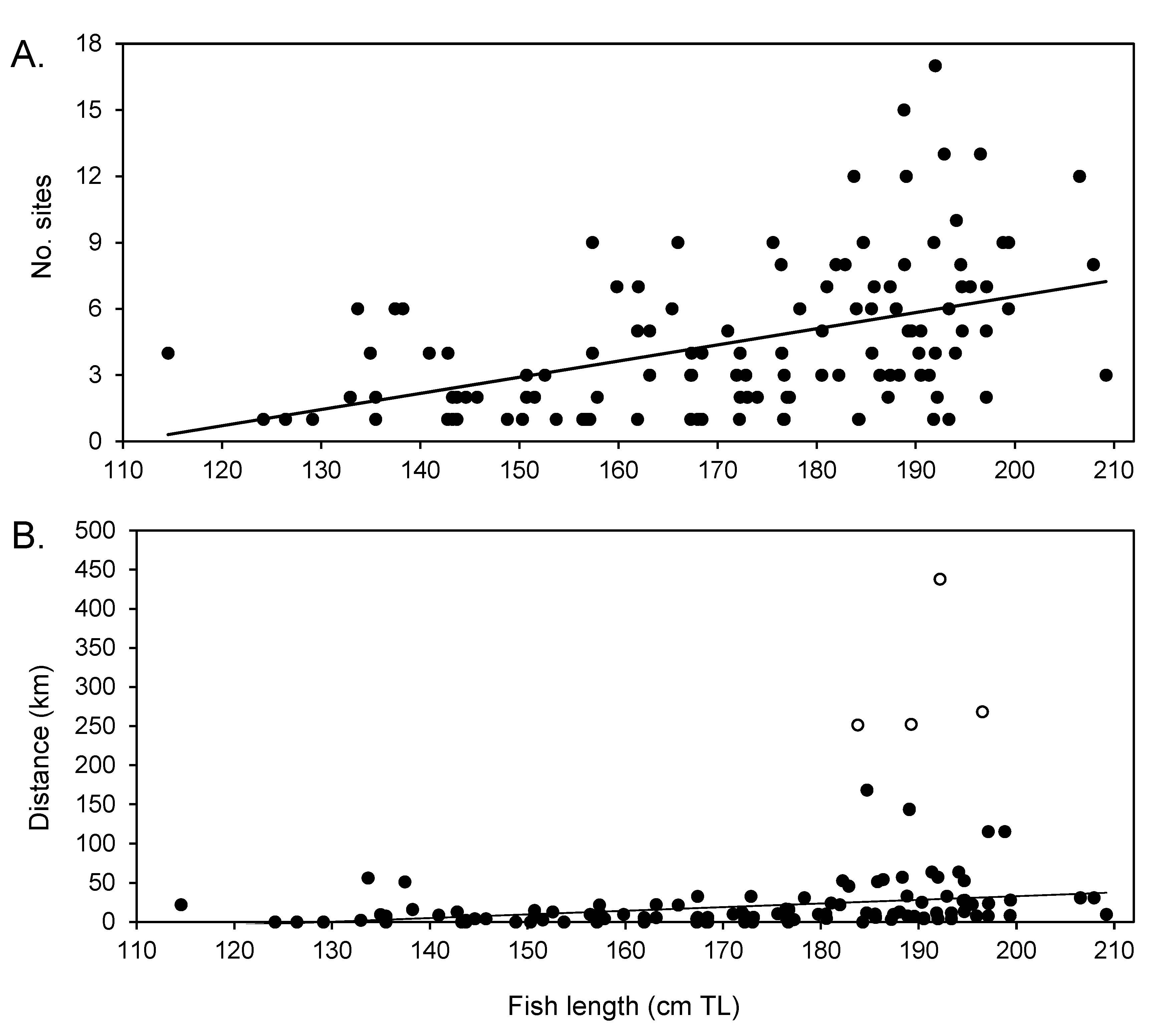
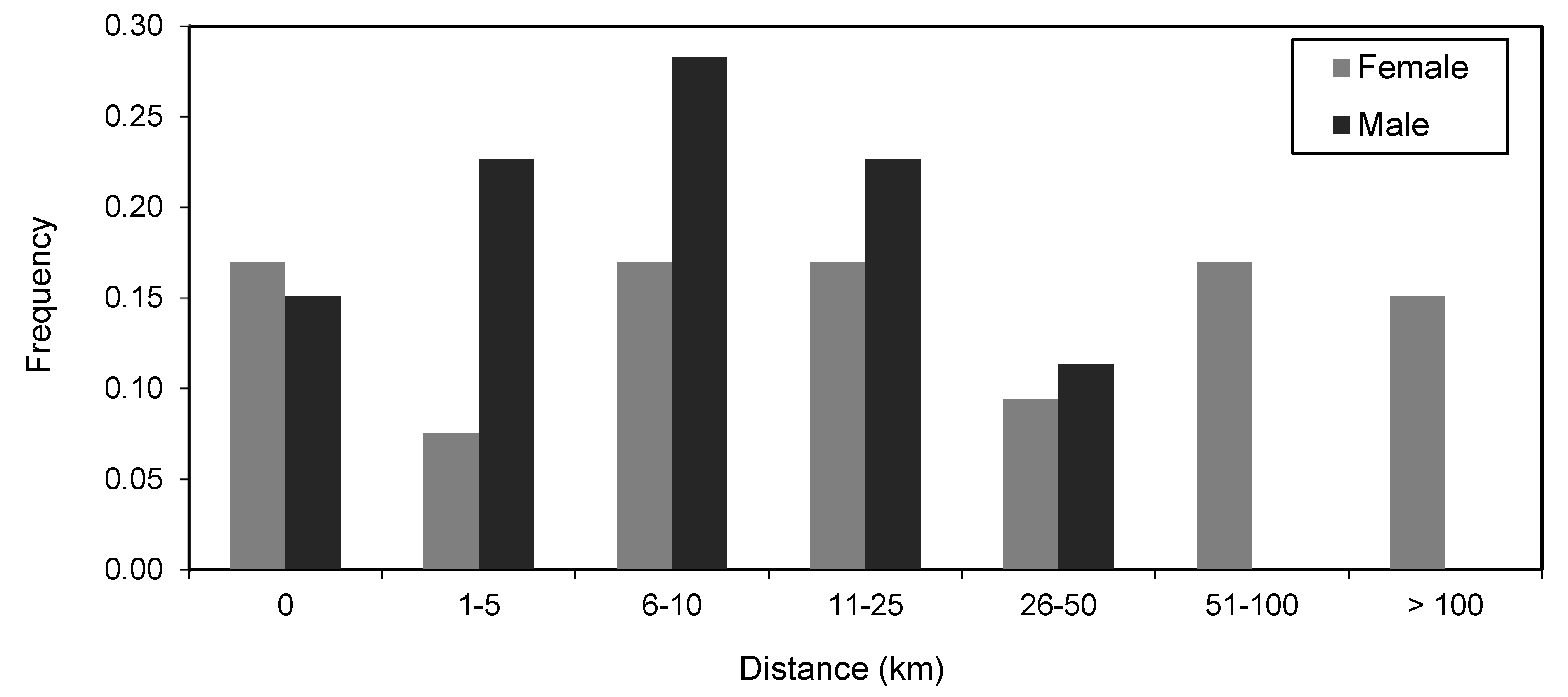
3.5. Size and Sex Differences in Movement Patterns
3.6. Catchment Area and Kernel Density Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Investigating Attrition Rate of Detected Fish

References
- Colin, P.L. Preliminary investigations of reproductive activity of the jewfish, Epinephelus itajara (Pisces: Serranidae). Proc. Gulf Carib. Fish. Inst. 1990, 43, 138–147. [Google Scholar]
- Koenig, C.; Coleman, F.C.; Eklund, A.M.; Schull, J.; Ueland, J.S. Mangroves as essential nursery habitat for goliath grouper (Epinephelus itajara). Bull. Mar. Sci. 2007, 80, 567–586. [Google Scholar]
- Koenig, C.C.; Coleman, F.C.; Kingon, K. Pattern of recovery of the Goliath Grouper Epinephelus itajara population in the southeastern U.S. Bull. Mar. Sci. 2011, 87, 891–911. [Google Scholar] [CrossRef]
- Collins, A.B.; Barbieri, L.R.; McBride, R.S.; McCoy, E.D.; Motta, P.J. Reef relief and volume are predictors of Atlantic goliath grouper presence and abundance in the eastern Gulf of Mexico. Bull. Mar. Sci. 2015, 91, 399–418. [Google Scholar] [CrossRef]
- Koenig, C.C.; Bueno, L.S.; Coleman, F.C.; Cusick, J.A.; Ellis, R.D.; Kingon, K.; Locascio, J.V.; Malinowski, C.; Murie, D.J.; Stallings, C.D. Diel, lunar, and seasonal spawning patterns of the Atlantic Goliath Grouper, Epinephelus itajara, off Florida, United States. Bull. Mar. Sci. 2017, 93, 391–406. [Google Scholar] [CrossRef]
- Domeier, M.L.; Colin, P.L. Tropical reef fish spawning aggregations: Defined and reviewed. Bull. Mar. Sci. 1997, 60, 698–726. [Google Scholar]
- Domeier, M.L. Revisiting spawning aggregations: Definitions and challenges. In Reef fish Spawning Aggregations: Biology, Research and Management; Sadovy de Mitcheson, Y., Colin, P.L., Eds.; Fish and Fisheries Series 35; Springer Science + Business Media: Berlin, Germany, 2012; pp. 1–20. [Google Scholar]
- Sadovy de Mitcheson, Y.; Cornish, A.; Domeier, M.L.; Colin, P.L.; Russell, M.; Lindeman, K. A global baseline for spawning aggregation of reef fishes. Conserv. Biol. 2008, 22, 1233–1244. [Google Scholar] [PubMed]
- Nemeth, R.S. Ecosystem aspects of species that aggregate to spawn. In Reef Fish Spawning Aggregations: Biology, Research and Management; Sadovy de Mitcheson, Y., Colin, P.L., Eds.; Fish and Fisheries Series 35; Springer Science + Business Media: Berlin, Germany, 2012; pp. 21–56. [Google Scholar]
- Aguilar-Perera, A. Disappearance of a Nassau grouper spawning aggregation off the southern Mexican Caribbean coast. Mar. Ecol. Prog. Ser. 2006, 327, 289–296. [Google Scholar]
- Erisman, B.; Heyman, W.D.; Kobara, S.; Ezer, T.; Pittman, S.; Nemeth, R.S. Fish spawning aggregations: Where well-placed management actions can yield big benefits for fisheries and conservation. Fish Fish. 2017, 18, 128–144. [Google Scholar] [CrossRef]
- Ellis, R.D.; Koenig, C.C.; Coleman, F.C. Spawning-related movement patterns of goliath grouper (Epinephelus itajara) off the Atlantic coast of Florida. Proc. Gulf Carib. Fish. Inst. 2014, 66, 395–400. [Google Scholar]
- Levitan, D.R.; Fogarty, N.D.; Jara, J.; Lotterhos, K.E.; Knowlton, N. Genetic, spatial, and temporal components of precise spawning synchrony in reef building corals of the Montastraea annularis species complex. Evolution 2011, 65, 1254–1270. [Google Scholar] [PubMed]
- Lin, C.H.; Takahashi, S.; Mulla, A.J.; Nozawa, Y. Moonrise timing is key for synchronized spawning in coral Dipsastraea speciosa. Proc. Natl. Acad. Sci. USA 2021, 118, e2101985. [Google Scholar] [CrossRef] [PubMed]
- Shima, J.S.; Osenberg, C.W.; Alonzo, S.H.; Noonburg, E.G.; Mitterwallner, P.; Swearer, S.E. Reproductive phenology across the lunar cycle: Parental decisions, offspring responses, and consequences for reef fish. Ecology 2020, 10, e03086. [Google Scholar]
- Mann, D.A.; Locascio, J.V.; Coleman, F.C.; Koenig, C.C. Goliath grouper (Epinephelus itajara) sound production and movement patterns on aggregation sites. Endanger. Species Res. 2009, 7, 229–236. [Google Scholar]
- Lara, M.R.; Schull, J.; Jones, D.L.; Allman, R. Early life history stages of goliath grouper Epinephelus itajara (Pisces: Epinephelidae) from Ten Thousand Islands, Florida. Endanger. Species Res. 2009, 7, 221–228. [Google Scholar] [CrossRef]
- Koenig, C.C.; Coleman, F.C.; Malinowski, C.R. Atlantic Goliath Grouper of Florida: To Fish or Not to Fish. Fisheries 2020, 45, 20–32. [Google Scholar] [CrossRef]
- Brusher, J.H.; Schull, J. Non-lethal age determination for juvenile goliath grouper (Epinephelus itajara) from southwest Florida. Endanger. Species Res. 2009, 7, 205–212. [Google Scholar]
- Sadovy, Y.; Eklund, A.-M. Synopsis of Biological Data on the Nassau Grouper, Epinephelus striatus (Bloch, 1792), and the Jewfish, E. itajara (Lichtenstein, 1822); Technical Report National Marine Fisheries Service (NMFS): Silver Spring, MD, USA; National Oceanic and Atmospheric Administration (NOAA): Washington, DC, USA, 1999; Volume 146, p. 68. [Google Scholar]
- SEDAR. SEDAR 47 Stock Assessment Report South Atlantic U.S. Goliath Grouper; SEDAR: North Charleston, SC, USA, 2016; p. 206. [Google Scholar]
- SEDAR. SEDAR 23 Stock Assessment Report South Atlantic and Gulf of Mexico Goliath Grouper; SEDAR: North Charleston, SC, USA, 2011; p. 248. [Google Scholar]
- Tringali, M.D. Reproductive success dynamics could limit precision in close-kin mark-recapture abundance estimation for Atlantic Goliath Grouper (Epinephelus itajara). Fishes 2023, 8, 254. [Google Scholar] [CrossRef]
- Carroll, J.L.; Ellis, R.D.; Collins, A.B.; Murie, D.J. Dorsal Fin Spines and Rays for Nonlethal Ageing of Goliath Grouper Epinephelus itajara. Fishes 2023, 8, 239. [Google Scholar] [CrossRef]
- Murie, D.J.; Parkyn, D.C.; Koenig, C.C.; Coleman, F.C.; Malinowski, C.R.; Cusick, J.A.; Ellis, R.D. Age, Growth, and Functional Gonochorism with a Twist of Diandric Protogyny in Goliath Grouper in the Southeastern USA. Fishes, 2023; in review. [Google Scholar]
- Namami, A.; Kawabata, Y.; Sato, T.; Yamaguchi, T.; Kawabe, R.; Soyano, K. Spawning migration and returning behavior of white-streaked grouper Epinephelus ongus determined by acoustic telemetry. Mar. Biol. 2013, 161, 669–680. [Google Scholar] [CrossRef]
- Dahlgren, C.E.; Buch, K.; Rechisky, E.; Hixon, M.A. Multiyear Tracking of Nassau Grouper Spawning Migrations. Mar. Coast. Fish. 2016, 8, 522–535. [Google Scholar] [CrossRef]
- Sleugh, T.; McCoy, C.M.; Pattengill-Semmens, C.V.; Johnson, B.C.; Heppell, S.A.; Waterhouse, L.; Stock, B.C.; Semmens, B.X. Migratory behavior of aggregating male Tiger Grouper (Mycteroperca tigris) in Little Cayman, Cayman Islands. Environ. Biol. Fishes 2023, 106, 1195–1206. [Google Scholar] [CrossRef]
- Nemeth, R.S.; Kadison, E.; Jossart, J.; Shivji, M.; Wetherbee, B.; Matley, J. Acoustic telemetry provides insights for improving conservation and management at a spawning aggregation site of the endangered Nassau grouper (Epinephelus striatus). Front. Mar. Sci. 2023, 10, 1154689. [Google Scholar] [CrossRef]
- Young, J.M.; Bowers, M.E.; Reyier, E.A.; Morley, D.; Ault, E.R.; Pye, J.D.; Gallagher, R.M.; Ellis, R.D. The FACT Network: Philosophy, evolution, and management of a collaborative coastal tracking network. Mar. Coast. Fish. 2020, 12, 258–271. [Google Scholar] [CrossRef]
- Currier, R.; Kirkpatrick, B.; Simoniello, C.; Lowerre-Barbieri, S.; Bickford, J. iTAG: Developing a Cloud Based, Collaborative Animal Tracking Network in the Gulf of Mexico. In Proceedings of the OCEANS 2015—MTS/IEEE Washington, Washington, DC, USA, 19–22 October 2015; pp. 1–3. [Google Scholar] [CrossRef]
- Bangley, C.W.; Whoriskey, F.G.; Young, J.M.; Ogburn, M.B. Networked Animal Telemetry in the Northwest Atlantic and Caribbean Waters. Mar. Coast. Fish. 2020, 12, 339–347. [Google Scholar] [CrossRef]
- Kessel, S.T.; Hussey, N.E.; Webber, D.M.W.; Gruber, S.H.; Young, J.M.; Smale, M.J.; Fish, A.T. Close proximity detection interference with acoustic telemetry: The importance of considering tag power output in low ambient noise environments. Anim. Biotelem. 2015, 3, 5. [Google Scholar] [CrossRef]
- R-Core-Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Pincock, D.G. False Detections: What They Are and How to Remove Them. Document DOC-004691, InnovaSea Inc., AMIRIX Systems: Halifax, NS, Canada, 2012; p. 14. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.A.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2016, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar]
- Koenig, C.; Coleman, F.; Malinowski, C.; Ellis, R.; Murie, D.; Parkyn, D.; Friess, C.; Tzadik, O. Regional Age Structure, Reproductive Biology and Trophic Patterns of Adult Goliath Grouper in Florida; NOAA Marine Fisheries Initiative (MARFIN) Project Final Report: Washington, DC, USA, 2016; p. 222. [Google Scholar]
- Bullock, L.H.; Murphy, M.D.; Godcharles, M.F.; Mitchell, M.E. Age, growth, and reproduction of jewfish Epinephelus itajara in the eastern Gulf of Mexico. Fish. Bull. 1992, 90, 243–249. [Google Scholar]
- Chapman, B.B.; Brönmark, C.; Nilsson, J.Å.; Hansson, L.A. The ecology and evolution of partial migration. Oikos 2011, 120, 1764–1775. [Google Scholar] [CrossRef]
- Chapman, B.B.; Skov, C.; Hulthén, K.; Brodersen, J.; Nilsson, P.A.; Hansson, L.A.; Brönmark, C. Partial migration in fishes: Definitions, methodologies and taxonomic distribution. J. Fish Biol. 2012, 81, 479–499. [Google Scholar] [CrossRef]
- Chapman, B.B.; Hulthén, K.; Brodersen, J.; Nilsson, P.A.; Skov, C.; Hansson, L.A.; Brönmark, C. Partial migration in fishes: Causes and consequences. J. Fish Biol. 2012, 81, 456–478. [Google Scholar] [CrossRef]
- TinHan, T.; Erisman, B.; Aburto-Oropeza, O.; Weaver, A.; Vazquez-Arce, D.; Lowe, C.G. Residency and seasonal movements in Lutjanus argentiventris and Mycteroperca rosacea at Los Islotes Reserve, Gulf of California. Mar. Ecol. Prog. Ser. 2014, 501, 191–206. [Google Scholar] [CrossRef]
- Rowell, T.J.; Nemeth, R.S.; Schärer, M.T.; Appeldoorn, R.S. Fish sound production and acoustic telemetry reveal behaviors and spatial patterns associated with spawning aggregations of two Caribbean groupers. Mar. Ecol. Prog. Ser. 2015, 518, 239–254. [Google Scholar] [CrossRef]
- Molloy, P.P.; Cote, I.M.; Reynolds, D.J. Why spawn in aggregations. In Reef Fish Spawning Aggregations: Biology, Research and Management; Sadovy de Mitcheson, Y., Colin, P.L., Eds.; Fish and Fisheries Series 35; Springer Science + Business Media: Berlin, Germany, 2012; pp. 57–83. [Google Scholar]
- Jonsson, B.; Jonsson, N. Partial migration: Niche shift versus sexual maturation in fishes. Rev. Fish Biol. Fish. 1993, 3, 348–365. [Google Scholar] [CrossRef]
- Coleman, F.C.; Koenig, C.C.; Collins, L.A. Reproductive styles of shallow-water grouper (Pisces: Serranidae) in the eastern Gulf of Mexico and the consequences of fishing spawning aggregations. Environ. Biol. Fishes 1996, 47, 129–141. [Google Scholar] [CrossRef]
- Ellis, R.D.; Powers, J.E. Gag grouper, marine reserves, and density-dependent sex change in the Gulf of Mexico. Fish. Res. 2012, 115, 89–98. [Google Scholar] [CrossRef]
- FWC. Goliath Harvest Program. Available online: https://myfwc.com/fishing/saltwater/recreational/goliath/#harvest (accessed on 21 May 2023).
- Coleman, F.C.; Nunes, J.A.C.C.; Bertoncini, A.A.; Bueno, L.S.; Freitas, M.O.; Borgonha, M.; Leite, J.R.; Lima-Júnior, M.J.C.A.; Ferreira, B.; Bentes, B.; et al. Controversial opening of a limited fishery for Atlantic Goliath Grouper in the United States: Implications for population recovery. Mar. Pol. 2023, 155, 105752. [Google Scholar] [CrossRef]
- Smith, N.P. Temporal and spatial characteristics of summer upwelling along Florida’s Atlantic shelf. J. Phys. Oceanogr. 1983, 13, 1709–1715. [Google Scholar] [CrossRef]
- Whaylen, L.; Bush, P.; Johnson, B.; Luke, K.; McCoy, C.; Heppell, S.; Semmens, B.; Boardman, M. Aggregation dynamics and lessons learned from five years of monitoring at a Nassau grouper (Epinephelus striatus) spawning aggregation in Little Cayman, Cayman Islands, BWI. Proc. Gulf Carib. Fish. Inst. 2006, 57, 1–14. [Google Scholar]
- Stock, B.C.; Mullen, A.D.; Jaffe, J.S.; Candelmo, A.; Heppell, S.A.; Pattengill-Semmens, C.V.; McCoy, C.M.; Johnson, B.C.; Semmens, B.X. Protected fish spawning aggregations as self-replenishing reservoirs for regional recovery. Proc. Roy. Soc. B 2023, 290, 20230551. [Google Scholar] [CrossRef]
- FWC. CORRECTION: FWC Suspended Discussions on Goliath Grouper Spawning Aggregation Site Protections. Available online: https://myfwc.com/news/all-news/goliath-com-523/ (accessed on 21 May 2023).
- Sadovy de Mitcheson, Y. Mainstreaming fish spawning aggregation into fishery management calls for a precautionary approach. BioScience 2016, 66, 295–306. [Google Scholar] [CrossRef]
- Malinowski, C.; Coleman, F.; Koenig, C.; Locascio, J.V.; Murie, D.J. Are Atlantic goliath grouper, Epinephelus itajara, establishing more northerly spawning sites? Evidence from the northeast Gulf of Mexico. Bull. Mar. Sci. 2019, 95, 371–391. [Google Scholar] [CrossRef]



| Year | ||||
|---|---|---|---|---|
| 2011 | 2012 | 2013 | 2014 | |
| No. fish detected | 34 | 34 | 26 | 13 |
| No. fish detected at FSA site | 31 | 32 | 21 | 12 |
| % of fish detected at FSA site | 91.2% | 94.1% | 80.8% | 92.3% |
| Year | Zion Train | Three Holes | MG-111 | Sun Tug | Gary’s | Hole-in-the-Wall |
|---|---|---|---|---|---|---|
| 2011 | 1 | 3 | 4 | 2 | 5 | 6 |
| 2012 | 1 | 4 | 2 | 3 | 6 | 5 |
| 2013 | 1 | 2 | 4 | 4 | 3 | 6 |
| 2014 | 1 | 2 | 4 | 3 | 4 | 6 |
| Year | No. Fish | Mean Arrival Date ± SE | Latest Arrival Date |
|---|---|---|---|
| 2011 | 19 | August 8 ± 4.5 | 20 September |
| 2012 | 25 | August 2 ± 5.6 | 3 October |
| 2013 | 14 | August 16 ± 7.7 | 14 October |
| 2014 | 8 | August 11 ± 9.2 | 14 September |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellis, R.D.; Koenig, C.C.; Locascio, J.V.; Malinowski, C.R.; Coleman, F.C. Spawning Migrations of the Atlantic Goliath Grouper along the Florida Atlantic Coast. Fishes 2023, 8, 398. https://doi.org/10.3390/fishes8080398
Ellis RD, Koenig CC, Locascio JV, Malinowski CR, Coleman FC. Spawning Migrations of the Atlantic Goliath Grouper along the Florida Atlantic Coast. Fishes. 2023; 8(8):398. https://doi.org/10.3390/fishes8080398
Chicago/Turabian StyleEllis, Robert D., Christopher C. Koenig, James V. Locascio, Christopher R. Malinowski, and Felicia C. Coleman. 2023. "Spawning Migrations of the Atlantic Goliath Grouper along the Florida Atlantic Coast" Fishes 8, no. 8: 398. https://doi.org/10.3390/fishes8080398
APA StyleEllis, R. D., Koenig, C. C., Locascio, J. V., Malinowski, C. R., & Coleman, F. C. (2023). Spawning Migrations of the Atlantic Goliath Grouper along the Florida Atlantic Coast. Fishes, 8(8), 398. https://doi.org/10.3390/fishes8080398