Handling Effects on Histological Identification of Female Reproductive Status: Examples from Tropical Deepwater Snappers
Abstract
:1. Introduction
2. Materials and Methods
3. Results
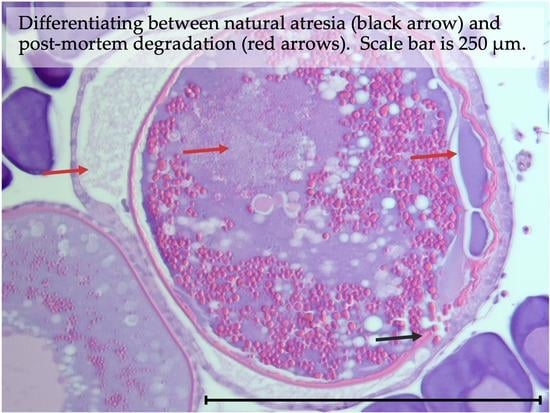
3.1. Natural Ovarian Atresia
3.2. Reproductive Phases of Females Examined
3.3. Primary Growth Oocytes
3.4. Cortical Alveolar Oocytes
3.5. Vitellogenic Oocytes
3.6. Oocyte Maturation
3.7. Post-Ovulatory Follicles
3.8. Atretic Oocytes and Indicators of Prior Spawning
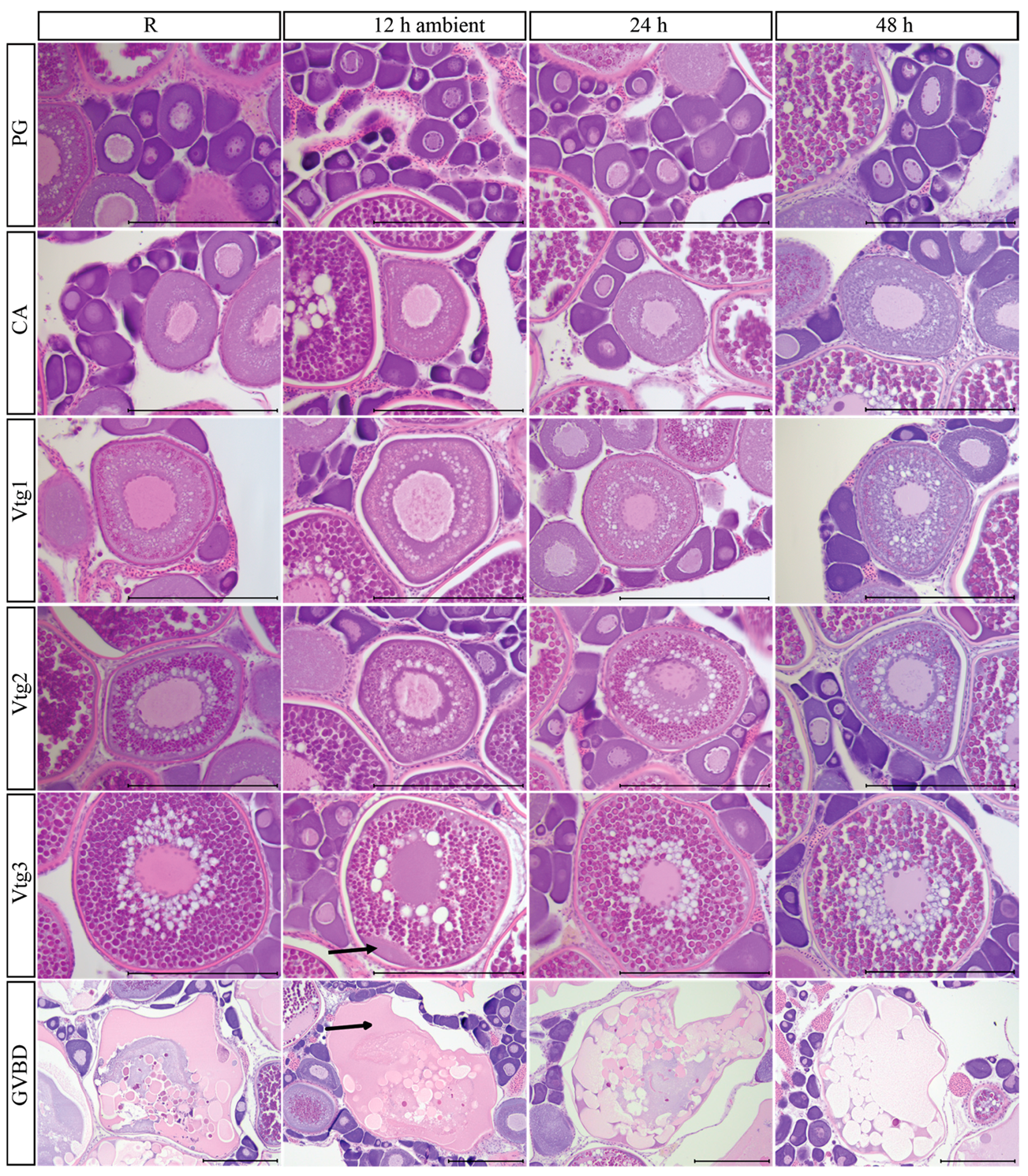
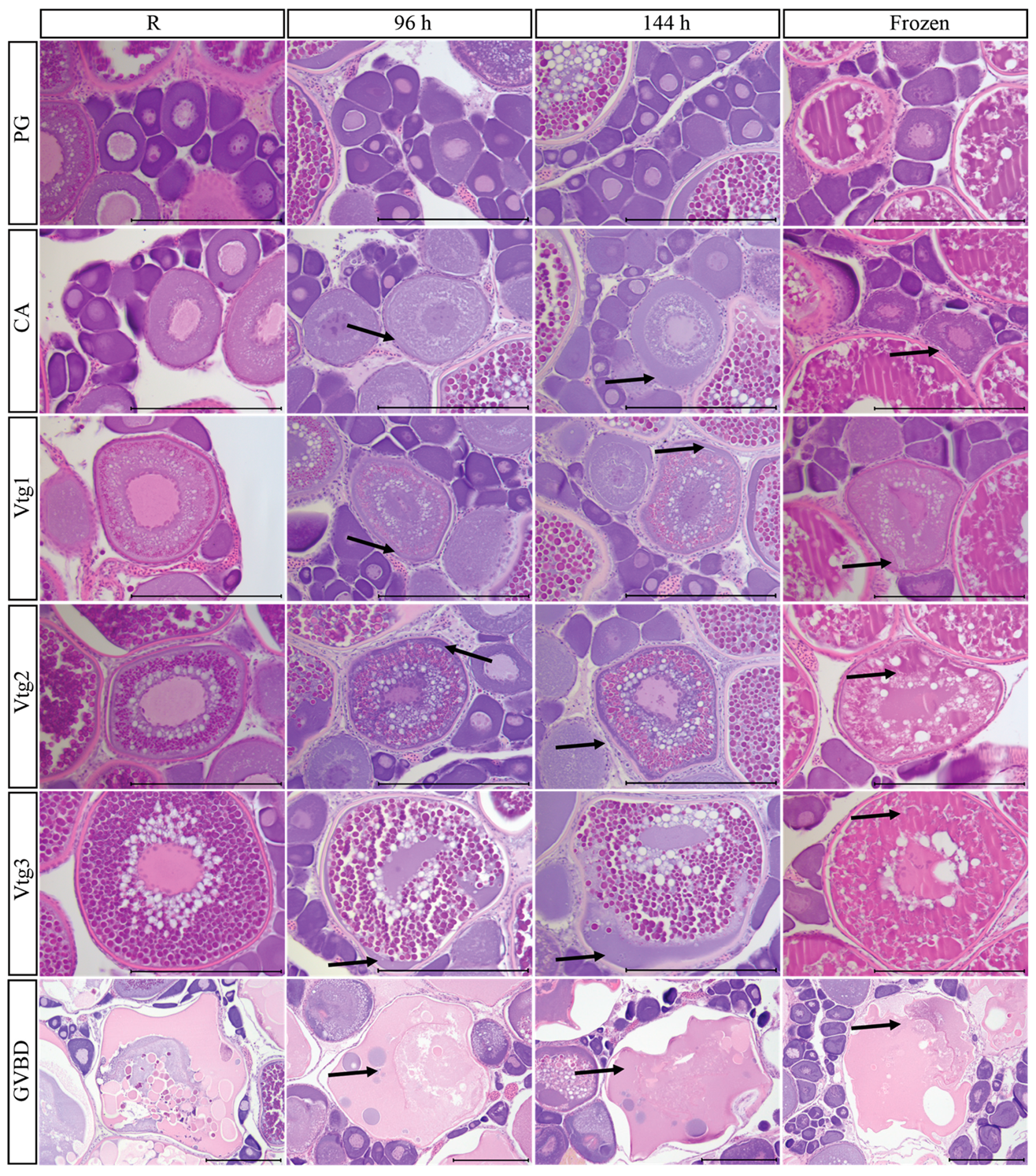
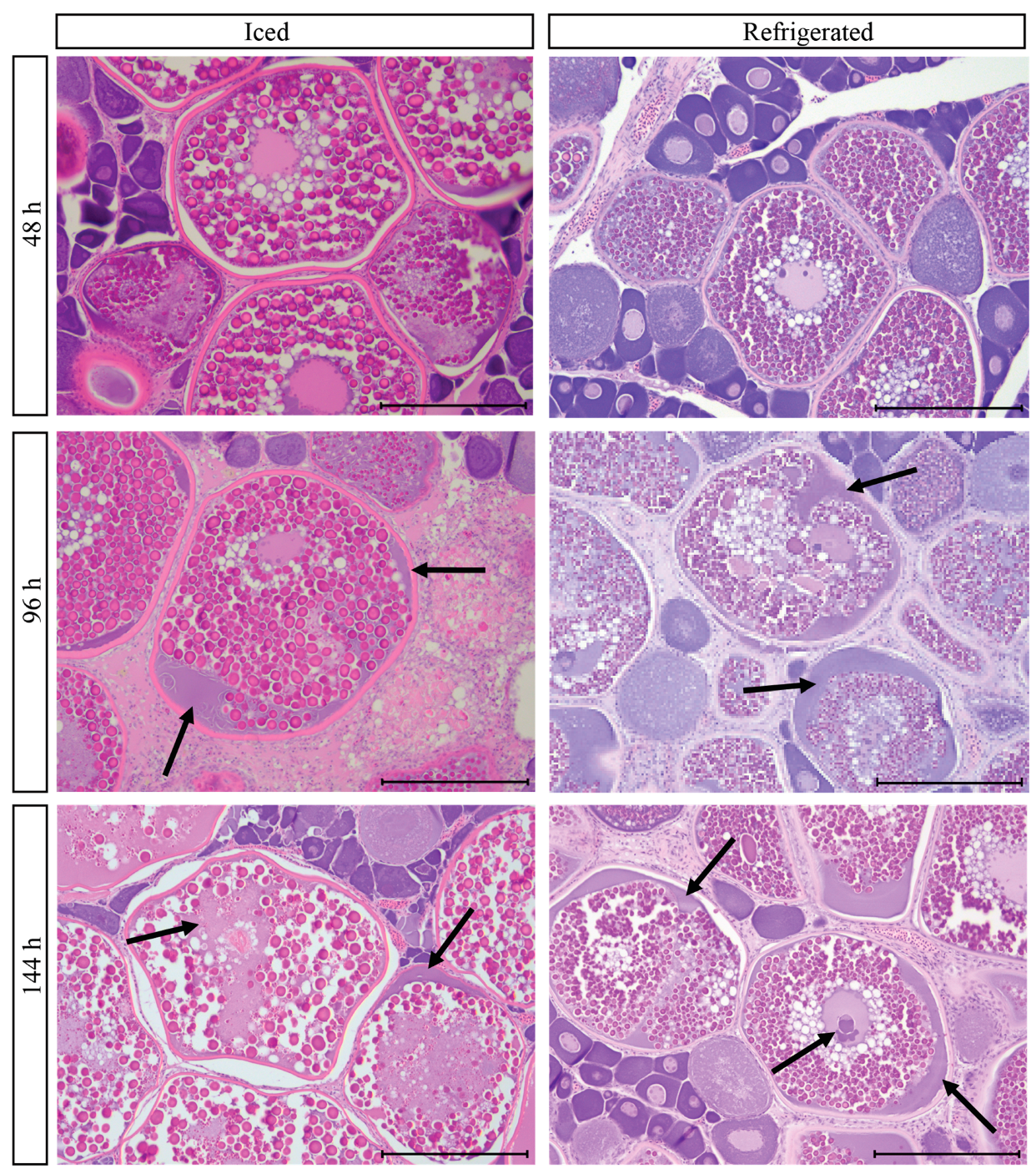
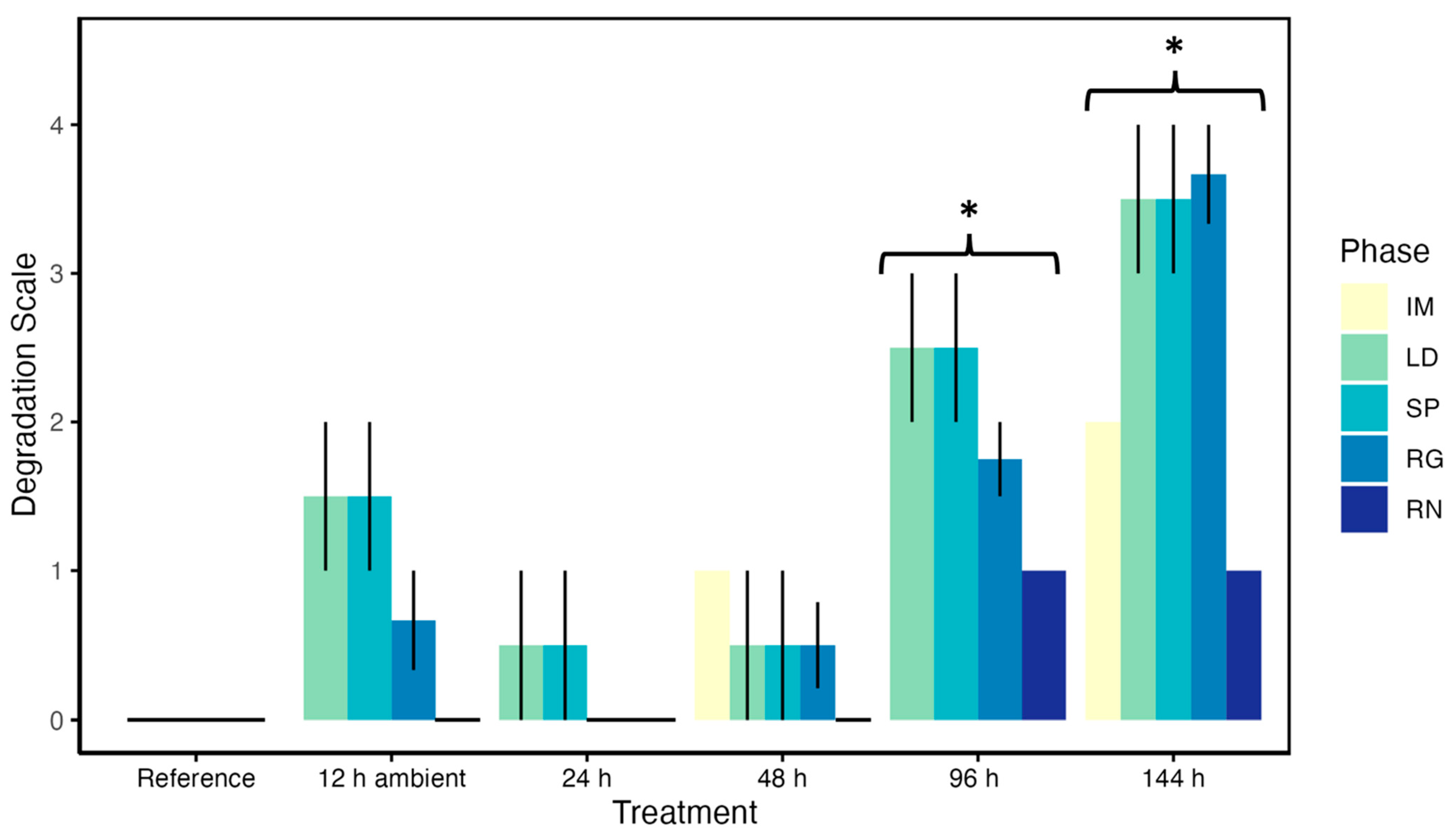
3.9. Post-Mortem Degradation in In Situ Ovaries
3.10. Impacts of Post-Mortem Degradation on Reproductive Classification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown-Peterson, N.J.; Wyanski, D.M.; Saborido-Rey, F.; Macewicz, B.J.; Lowerre-Barbieri, S.K. A standardized terminology for describing reproductive development in fishes. Mar. Coast. Fish. 2011, 3, 52–70. [Google Scholar] [CrossRef]
- Wuenschel, M.J.; Deroba, J.J. The reproductive biology of female Atlantic herring in US waters: Validating classification schemes for assessing the importance of spring and skipped spawning. Mar. Coast. Fish. 2019, 11, 487–505. [Google Scholar] [CrossRef] [Green Version]
- Young, J.; Drake, A.; Brickhill, M.; Farley, J.; Carter, T. Reproductive dynamics of broadbill swordfish, Xiphias gladius, in the domestic longline fishery off eastern Australia. Mar. Freshw. Res. 2003, 54, 315–332. [Google Scholar] [CrossRef]
- Corriero, A.; Zupa, R.; Mylonas, C.C.; Passantino, L. Atresia of ovarian follicles in fishes, and implications and uses in aquaculture and fisheries. J. Fish. Dis. 2021, 44, 1271–1291. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Bazzoli, N.; Rizzo, E.; Sato, Y. Ovarian follicular atresia in two teleost species: A histological and ultrastructural study. Tissue Cell 1999, 31, 480–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassel, M.; de Paiva Camargo, M.; Oliveira de Jesus, L.W.; Borella, M.I. Involution processes of follicular atresia and post-ovulatory complex in a characid fish ovary: A study of apoptosis and autophagy pathways. J. Mol. Histol. 2017, 48, 243–257. [Google Scholar] [CrossRef]
- George, J.; Van Wettere, A.J.; Michaels, B.B.; Crain, D.; Lewbart, G.A. Histopathologic evaluation of post-mortem autolytic changes in bluegill (Lepomis macrohirus) and crappie (Pomoxis anularis) at varied time intervals and storage temperatures. PeerJ 2016, 4, e1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowerre-Barbieri, S.; Fries, C.; Brown-Peterson, N.; Moncrief-Cox, H.; Barnett, B. Best Practices for Standardized Reproductive Data and Methodology to Estimate Reproductive Parameters for Red Snapper in the Gulf of Mexico. 2022. 43p. Available online: https://sedarweb.org/documents/sedar-74-dw-36-best-practices-for-standardized-reproductive-data-and-methodology-to-estimate-reproductive-parameters-for-red-snapper-in-the-gulf-of-mexico/ (accessed on 1 July 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 28 June 2023).
- Ogle, D.H.; Doll, J.C.; Wheeler, A.P.; Dinno, A. FSA: Simple Fisheries Stock Assessment Methods. R Package Version 0.9.4. 2023. Available online: https://CRAN.R-project.org/package=FSA (accessed on 28 June 2023).
- Yang, Y.; Wang, G.; Li, Y.; Hu, J.; Wang, Y.; Tao, Z. Oocytes skipped spawning through atresia is regulated by somatic cells revealed by transcriptome analysis in Pampus argenteus. Front. Mar. Sci. 2022, 9, 927548. [Google Scholar] [CrossRef]
- Zelazowska, M.; Kilarski, W.; Bilinski, S.M.; Podder, D.D.; Kloc, M. Balbiani cytoplasm in oocytes of a primitive fish, the sturgeon Acipenser gueldenstaedtii, and its potential homology to the Balbiani body (mitochondrial cloud) of Xenopus laevis oocytes. Cell Tissue Res. 2007, 329, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Pacicco, A.E.; Brown-Peterson, N.J.; Murie, D.J.; Allman, R.J.; Snodgrass, D.; Franks, J.S. Reproductive biology of yellowfin tuna (Thunnus albacares) in the northcentral US Gulf of Mexico. Fish. Res. 2023, 261, 106620. [Google Scholar] [CrossRef]
- Lemasters, J.J.; Nieminen, A.-L.; Qian, T.; Trost, L.C.; Elmore, S.P.; Nishimura, Y.; Crowe, R.A.; Cascio, W.E.; Bradham, C.A.; Brenner, D.A.; et al. The mitochondrial permeability transition in cell death: A common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta (BBA)—Bioenerg. 1998, 1366, 177–196. [Google Scholar] [CrossRef] [Green Version]
- Von Knebel Doeberitz, M.; Wentzensen, N. CHAPTER 1—The Cell: Basic Structure and Function. In Comprehensive Cytopathology, 3rd ed.; Bibbo, M., Wilbur, D., Eds.; W.B. Saunders: Edinburgh, UK, 2008; pp. 3–22. [Google Scholar]
- Lowerre-Barbieri, S.K.; Ganias, K.; Saborido-Rey, F.; Murua, H.; Hunter, J.R. Reproductive timing in marine fishes: Variability, temporal scales, and methods. Mar. Coast. Fish. 2011, 3, 71–91. [Google Scholar] [CrossRef] [Green Version]
- Hunter, J.R.; Macewicz, B. Improving the accuracy and precision of reproductive information used in fisheries. In Modern Approaches to Assess Maturity and Fecundity of Warm and Cold Water Fish and Squids; Havforskningsinstituttet: Bergen, Norway, 2003; pp. 57–68. [Google Scholar]
- Mackie, M.; Lewis, P. Assessment of Gonad Staging Systems and Other Methods Used in the Study of the Reproductive Biology of Narrow-Barred Spanish Mackerel, Scomberomorus commerson, in Western Australia; Fisheries Research Report; Department of Fisheries: Perth, Australia, 2001; No. 136. [Google Scholar]
- Ferreri, R.; Basilone, G.; D’Elia, M.; Traina, A.; Saborido-Rey, F.; Mazzola, S. Validation of macroscopic maturity stages according to microscopic histological examination for European anchovy. Mar. Ecol. 2009, 30, 181–187. [Google Scholar] [CrossRef]
- Kopf, R.; Davie, P.; Bromhead, D.; Young, J. Reproductive biology and spatiotemporal patterns of spawning in striped marlin Kajikia audax. J. Fish. Biol. 2012, 81, 1834–1858. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P.; Cole, K.W. Roles of unfrozen fraction, salt concentration, and changes in cell volume in the survival of frozen human erythrocytes. Cryobiology 1989, 26, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.F.; Gadella, B.M. In situ detection and localization of lipid peroxidation in individual bovine sperm cells. Free Radic. Bio Med. 2003, 35, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Kopf, R.K. Age, Growth, and Reproductive Dynamics of Striped Marlin, Kajikia audax in the Southwest Pacific Ocean; Charles Sturt University: Bathurst, Australia, 2010. [Google Scholar]





| Reproductive Phase | Most Advanced Oocyte Stage | Physiologically Mature | Characteristics |
|---|---|---|---|
| Immature | Oogonia, chromatin nucleolar (CN), or perinucleolar (PN) | No | Small ovary with CN and PN primary growth (PG) oocytes. Thin ovary wall, no muscle bundles or large blood vessels. |
| Early Developing | Cortical alveolar (CA) | Yes | Only PG and CA oocytes, no evidence of POFs, some atresia may be present, muscle bundles, and large blood vessels can be present in non-virgin females. |
| Late Developing | Vitellogenic oocytes (Vtg1, Vtg2, Vtg3) | Yes | Can have vitellogenic oocytes at any stage. No post-ovulatory follicle complexes (POFs) present. Some atresia may be present. |
| Spawning | Germinal vesicle migration (GVM), germinal vesicle breakdown (GVBD), hydrated oocytes (H), ovulated eggs, POFs | Yes | Undergoing oocyte maturation (lipid coalescence, GVM, GVBD, hydrated oocytes), ovulated eggs, or POF of any age. Can contain atretic oocytes. |
| Regressing | PN, CA, and/or vitellogenic | Yes | Ovaries dominated by oocytes in any stage of atresia (50% or more). May contain some CA or Vtg oocytes and POF > 24 h. |
| Regenerating | Primary growth oocytes (PG) | Yes | Only PG present (CN and PN). May contain unabsorbed material from past spawning events, gamma or delta atresia, large muscle bundles, large blood vessels, and thick ovarian wall. |
| Sample ID | Species | FL (cm) | Weight (kg) | Gonad Weight (g) | Reproductive Phase | Most Advanced Oocyte Present | Atresia Present (Alpha, Beta, Gamma, Delta) |
|---|---|---|---|---|---|---|---|
| SE-22-02-003 | PRFI | 30.2 | 0.5 | <0.1 | Immature | PG | None |
| SE-22-02-004 | PRFI | 24.2 | 0.3 | <0.1 | Immature | PG | None |
| SE-22-02-005 | PRFI | 26.2 | 0.3 | <0.1 | Immature | PG | None |
| SE-22-02-006 | PRFI | 24.5 | 0.3 | <0.1 | Immature | PG | None |
| SE-22-02-008 | PRFI | 35.0 | 0.5 | 3.4 | Regenerating | PG | delta |
| SE-22-02-009 | PRFI | 64.9 | 3.8 | 56.0 | Late Developing | Vtg3 | alpha, beta, and delta |
| SE-22-02-015 | PRFI | 37.5 | 0.7 | 9.4 | Regressing | Vtg2 | alpha, beta, gamma, and delta |
| SE-22-02-017 | PRFI | 38.0 | 0.7 | 6.4 | Regenerating | PG | delta |
| RA-22-01-001 | ETCA | 39.0 | 1.1 | 35.0 | Late Developing | Vtg3 | delta |
| RA-22-01-002 | ETCA | 32.2 | 0.6 | 15.0 | Spawning | Vtg3, POFs | gamma and delta |
| RA-22-01-003 | ETCA | 29.0 | 0.4 | 6.5 | Regressing | Vtg1 | alpha and beta-gamma |
| RA-22-01-006 | ETCA | 24.1 | 0.2 | 3.0 | Regressing | Vtg2 | alpha, beta, and gamma |
| RA-22-01-010 | PRAU | 28.5 | 0.5 | 8.8 | Spawning | GVBD | delta |
| RA-22-01-032 | ETCA | 35.2 | 0.9 | 16.0 | Late Developing | Vtg3 | gamma |
| RA-22-01-034 | ETCA | 40.3 | 1.1 | 17.0 | Late Developing | Vtg3 | beta and gamma |
| RA-22-01-035 | ETCA | 34.4 | 0.6 | 7.6 | Regressing | Vtg2 | alpha, beta, and gamma |
| RA-22-01-037 | ETCA | 26.6 | 0.3 | 6.0 | Spawning | GVM | alpha, beta, and gamma |
| RA-22-01-038 | ETCA | 27.3 | 0.4 | 2.9 | Regressing | CA | alpha, beta, and gamma |
| RA-22-01-039 | ETCA | 28.5 | 0.4 | 2.6 | Regenerating | PG | gamma, delta |
| RA-22-01-040 | ETCA | 28.0 | 0.4 | 2.6 | Regressing | Vtg1 | alpha, beta, and gamma |
| RA-22-01-043 | ETCA | 29.4 | 0.5 | 1.8 | Regressing | CA | alpha, beta, and gamma |
| RA-22-01-047 | ETCA | 24.9 | 0.3 | 1.3 | Regressing | CA | alpha, beta, and gamma |
| Phase | 0 (None) | 1 (Slight) | 2 (Moderate) | 3 (Substantial) | 4 (Severe) |
|---|---|---|---|---|---|
| Immature (IM) | No apparent changes from reference | PG cytoplasm slightly cloudy | PG cytoplasm cloudy and nucleolus no longer visible, PG shape changes | Not observed | Not observed |
| Late Developing (LD) | No apparent changes from reference | Minor dissolution of cytoplasm in oocyte periphery of Vtg | Increased dissolution of cytoplasm in Vtg, ~1/4 of Vtg oocyte periphery undergoing dissolution | 1/4 or more of cytoplasm of Vtg oocyte dissolution, nucleus of Vtg oocytes begin to rupture and breakdown | 1/2 or more of cytoplasm of Vtg oocyte dissolution, nucleus of Vtg oocytes broken down |
| Spawning (SP) | No apparent changes from reference | OM and POFs identifiable, minor dissolution of cytoplasm in oocyte periphery of Vtg | ~1/4 of Vtg and OM oocyte periphery undergoing dissolution, GVBD oocyte becoming cloudy, POFs identifiable | 1/4 or more of cytoplasm of Vtg oocyte dissolution, nucleus of Vtg oocytes begin to rupture and breakdown, GVBD oocyte becoming cloudy and shape changes, POFs small and hard to ID | 1/2 or more of cytoplasm of Vtg oocyte dissolution, nucleus of Vtg oocytes broken down, GVBD oocyte cloudy with increased space between the chorion and oocyte, POFs cannot be differentiated from atresia |
| Regressing (RG) | No apparent changes from reference | Slight reduced ID of atresia | Differentiation between late-stage atresia difficult | Atresia stages hard to determine | Atresia stages cannot be identified |
| Regenerating (RN) | No apparent changes from reference | PG cytoplasm slightly cloudy | Not observed | Not observed | Not observed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schemmel, E.; Brown-Peterson, N.J. Handling Effects on Histological Identification of Female Reproductive Status: Examples from Tropical Deepwater Snappers. Fishes 2023, 8, 406. https://doi.org/10.3390/fishes8080406
Schemmel E, Brown-Peterson NJ. Handling Effects on Histological Identification of Female Reproductive Status: Examples from Tropical Deepwater Snappers. Fishes. 2023; 8(8):406. https://doi.org/10.3390/fishes8080406
Chicago/Turabian StyleSchemmel, Eva, and Nancy J. Brown-Peterson. 2023. "Handling Effects on Histological Identification of Female Reproductive Status: Examples from Tropical Deepwater Snappers" Fishes 8, no. 8: 406. https://doi.org/10.3390/fishes8080406
APA StyleSchemmel, E., & Brown-Peterson, N. J. (2023). Handling Effects on Histological Identification of Female Reproductive Status: Examples from Tropical Deepwater Snappers. Fishes, 8(8), 406. https://doi.org/10.3390/fishes8080406