Abstract
This study aimed to evaluate the supplementation of peppermint essential oil (Mentha piperita; PEO) in diets for juvenile Nile tilapia (Oreochromis niloticus). A feeding experiment with diets containing graded levels (0.0, 0.2, 0.4, 0.6, 0.8, and 1.0 g kg−1) of PEO was carried out with fish of 0.58 ± 0.08 g. The inclusion of graded levels of PEO in the diet improved the weight gain (0.52 g kg−1), feed intake (0.51 g kg−1), and feed conversion (0.51 g kg−1) of juvenile Nile tilapia in a quadratic pattern. Increasing levels of PEO also led to a linear decrease in body lipid content. The gut activity of the digestive enzymes amylase (0.54 g kg−1) and protease (0.39 g kg−1) increased quadratically, whereas lipase activity increased linearly. PEO increased the activity of the antioxidant enzymes catalase (CAT; 0.52 g kg−1) and superoxide dismutase (SOD; 0.58 g kg−1) while reducing the production of malonaldehyde (MDA; 0.40 g kg−1) and nitric oxide (NO; 0.63 g kg−1) in the liver. The results of this study provide evidence of the beneficial effects of PEO on the growth and health of Nile tilapia and recommend a dose of 0.6 g kg−1 as the optimal level of supplementation.
Key Contribution:
Peppermint essential oil (PEO) at 0.6 g kg−1 of inclusion in the diet improves feed efficiency and growth performance of fish.
1. Introduction
Intensive aquaculture plays a critical role in meeting the global demand for fish production, with Nile tilapia (Oreochromis niloticus) being one of the most cultivated species due to its rapid growth rate and adaptability to intensive farming systems [1,2]. However, challenges related to fish health, such as stress and digestive issues, can have negative impacts on productivity in these systems. To address these challenges, the use of feed additives has gained significant attention as a strategy to improve fish growth, health, and overall productivity [3,4].
Among the wide variety of feed additives, phytoadditives are promising options in the aquafeed industry [5], as they are plant-derived products with recognized therapeutic effects [6] and biological safety [7]. Within this category, essential oils have attracted particular interest due to their complex composition of bioactive compounds, which offer several benefits for fish health and performance [8,9]. One key aspect of essential oils is their antimicrobial actions, which can help maintain healthy gut microbiota and reduce the risk of infections in fish [10,11]. Additionally, their antioxidant activities contribute to attenuating oxidative stress, often associated with intensive cultivation. Moreover, certain essential oils improve feed palatability and stimulate feed intake, thereby increasing nutrient utilization and promoting growth performance in fish [12].
Peppermint (Mentha Piperita) is one such plant that has been extensively studied for its potential as a phytoadditive in fish nutrition. Widely produced worldwide and commonly used to treat issues related to poor digestion, peppermint contains active compounds, including menthol, which promote relaxation of the gastrointestinal mucosa [13]. This relaxation effect can lead to beneficial changes in intestinal motility and increased secretion of digestive enzymes [13,14,15]. Peppermint plant extracts can be found in various forms, such as powder, aqueous extracts, or essential oil. However, peppermint essential oil (PEO) is a more concentrated extract, consisting of high proportions of menthol (33–54%), menthone (7–18%), and menthol acetate (4–13%) [16,17,18,19]. Therefore, in addition to improving digestion, peppermint exhibits potent antioxidant [16,17], antifungal [7,18,19], and antibacterial [7,19,20,21] properties, which may contribute to enhancing intestinal health through modulation of microbial activity and reduction in inflammatory processes [22], thus alleviating stress.
Several studies have demonstrated the potential of peppermint extracts to improve the digestibility of commercial feeds and promote growth and health in captive-raised fish. Among these studies, dietary supplementation with peppermint powder or aqueous extracts improved the growth performance and health of Oreochromis niloticus [15,22], Lates calcarifer [23], Rutilus frisii kutum [24], Salmo trutta caspius [25], Labeo rohita [26], and Rutilus caspicus [27]. However, few studies have investigated the use of PEO as a dietary supplement, leaving optimal levels of PEO supplementation for fish yet to be established in the literature.
Therefore, this study aims to evaluate the impact of dietary supplementation with PEO on growth performance, body composition, digestive enzyme activity, biochemical parameters, and markers of oxidative stress in Nile tilapia to assess its benefits for fish reared in intensive systems.
2. Materials and Methods
2.1. Ethics Statement
This study was approved by the Ethics Committee on the Use of Production Animals at the Universidade Federal de Viçosa (CEUAP/UFV—number 38/2016). The experimental phase was conducted in LaNup (Laboratory of Nutrition and Fish Production) from the UEPE (Teaching, Research, and Extension in Fish Farming) of the Federal University of Viçosa, Minas Gerais, Brazil.
2.2. Experimental Design and Diets
A quadruplicate feed trial in a completely randomized design was conducted to evaluate six diets (315.90 g kg−1 of crude protein and 19.09 MJ kg−1 of energy) containing different levels (0.0, 0.2, 0.4, 0.6, 0.8, and 1.0 g kg−1) of PEO (Table 1).

Table 1.
Formulation and composition of the basal diet.
The macro ingredients were ground with a knife mill (Nogueira®, DPM-Junior M.F., São João da Boa Vista, SP, Brazil) and manually mixed into the micro-ingredients. Then, the PEO was previously mixed with soy oil and added to the mixture. Increasing levels of PEO were added to the diets in replacement of the inert ingredient kaolin. After mixed, the diets were pelleted in an electric meat grinder (Filizola®, P-22, São Paulo, SP, Brazil) and dried in a forced air circulation oven (Marconi®, MA 035, Piracicaba, SP, Brazil) at 35 °C for 24 h. The obtained pellets were crushed in a grain mill (Botini®, Botini-1079, Bilac, SP, Brazil) and sieved (A Bronzinox®, Santo Amaro, SP, Brazil) to obtain smaller pellets with 4 mm.
Diets were analyzed for dry matter (official method 934.01), crude protein Kjeldahl N × 6.25 (official method 984.13), crude lipid with Soxhlet extractor (official method 920.39), ash (official method 942.05), and crude fiber (official method 962.09) [28]. The crude energy was determined with a calorimetric pump (IKA®-Werke, C5003 control, Satufen, BW, Germany). PEO chemical composition was provided by the manufacturer LASZLO® (Belo Horizonte, MG, Brazil). The chemical composition of the diets is shown in Table 2.

Table 2.
Composition of PEO (Mentha piperita) *.
2.3. Fish and Experimental Conditions
Nile tilapia juveniles (0.58 ± 0.08 g) were distributed in 24 tanks (70 L) at a stocking density of 20 fish per tank, a total of 480 fish used. The tanks were maintained in a recirculation system (1.7 L min−1) and equipped with continuous aeration, controlled temperature, and ultraviolet, mechanical, and biological filters. The laboratory photoperiod was adjusted to 12 h and controlled with an analogical timer.
The water temperature (26.81 ± 0.48 °C) and dissolved oxygen levels (6.07 ± 0.84 mg L−1) were measured daily (multiparameter YSI-550A, Yellow Springs, OH, USA). The water pH (6.85 ± 0.30) and total ammonia (0.19 ± 0.17 mg L−1) were measured weekly with colorimetric kits (LabconTest Alcon®, Camboriú, SC, Brazil). The non-ionized ammonia (0.001 ± 0.001 mg L−1) was calculated according to [29]. The tanks were cleaned weekly by siphoning with a 10% water change. The fish were fed daily for eight weeks at 8, 11, 14, and 17 h.
2.4. Sampling Collection
At the end experimental period, all fish were counted to calculate survival. Then, three fish per tank (n = 12 per treatment) were captured two hours after feeding and euthanized via immersion in clove oil (400 mg L−1) for gut-collection-containing digesta. Intestine samples were stored at −80 °C for later determination of the activities of digestive enzymes.
The remaining fish were fasted for 24 h, euthanized in clove oil immersion (400 mg L−1), and weighed to calculate the body weight gain, relative feed intake, feed conversion rate, and protein efficiency rate (n = 68 per treatment). Blood samples from four fish per tank (n = 16 per treatment) were collected in the caudal vein using a heparinized syringe to determine plasmatic levels of glucose, cholesterol, triacylglycerol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL). Subsequently, the liver and viscera of the fish were removed and separately weighed to calculate the viscerosomatic and hepatosomatic indexes, respectively. Furthermore, liver samples from two fish per tank (n = 8 per treatment) were collected to evaluate oxidative stress biomarkers and glycogen content. Samples for oxidative stress were preserved at −80 °C until analysis. Samples for glycogen determination were immediately placed in test tubes with potassium hydroxide solution (30% KOH). The remaining carcasses of thirteen fish per tank were separated to calculate the carcass yield (n = 52 per treatment) and polled (n = 1 per treatment) to determine the bodies’ chemical compositions. Figure 1 shows the experimental setup of the experiment.
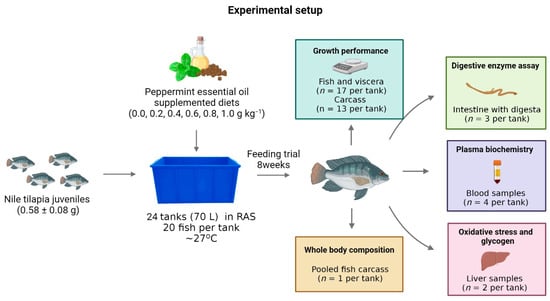
Figure 1.
Experimental setup and sampling scheme.
2.5. Growth Performance
The growth variables, somatic indices, and survival were calculated using the following equations:
- Body weight gain (g) = final body weight (g) − initial body weight (g);
- Relative feed intake (% body weight per day−1) = [dry feed intake (g)/average fish weigh (g)/days fed] × 100;
- Feed conversion ratio = body weight gain (g)/dry feed consumed (g);
- Protein efficiency ratio (%) = [protein gain (g)/protein intake (g)] × 100;
- Hepatosomatic index (%) = [liver weight (g)/final body weight (g)] × 100;
- Viscerosomatic index (%) = [viscera weight (g)/final body weight (g)] × 100;
- Survival (%) = [final number of fish/initial number of fish] × 100.
2.6. Whole-Body Composition
The carcasses were analyzed for dry matter (official method 934.01), crude protein Kjeldahl N × 6.25 (official method 984.13), crude lipid by Soxhlet extractor (official method 920.39), and ash (official method 942.05) according to [28]. The crude energy was determined with a calorimetric pump (IKA®-Werke, C5003 control, Satufen, BW, Germany).
2.7. Digestive Enzymes
The enzymatic extract was obtained by maceration of the intestine using a porcelain mortar and pistil in baths of liquid nitrogen. Subsequently, 5 mL of water (pH 3.0) was added to the macerated and the mixture was centrifuged at 10,000× g for 20 min (4 °C). The collected supernatant was used to determine the total protein concentration and digestive enzyme activities. Total protein content was measured according to [30], whose protocol used bovine serum albumin (BSA) as a standard, and the readings were performed in a spectrophotometer at 595 nm. Total protease activity was measured according to [31] in a spectrophotometer at 440 nm. The determination of lipase and amylase activities was performed using enzymatic kits (Bioclin® Bioclin Quibasa—Basic Chemistry, Belo Horizonte, MG, Brazil) following the manufacturer recommendations and based on [32,33] methods, respectively.
2.8. Biochemical Parameters
The blood samples were centrifuged for 15 min at 7000× g (Nova Técnica®, NT 805, Piracicaba, SP, Brazil), and the concentrations of glucose, cholesterol, triacylglycerol, HDL, and LDL were measured in the plasma. The plasma analyses were performed with Bioclin kits (Quibasa®—Quimica básica, Belo Horizonte, MG, Brazil) and the readings with BS 200 equipment (Mindray®, Clinical Chemistry Analyzer, Shenzhen, China) at the wavelengths indicated by the manufacturer.
The extraction and quantification of the hepatic glycogen content were measured according to [34]. After dissolving the samples in 30% KOH, the glycogen extraction phase was carried out using Na2SO4 and absolute alcohol. Then, the extracts were centrifuged (206-R, Fanem®, São Paulo, SP, Brazil) at 500× g for 10 min, and the precipitate was resuspended in distilled water. The anthrone reagent was added to the solution, and the samples were read in a spectrophotometer at 620 nm to quantify the glycogen content.
2.9. Oxidative Stress Indicators
Liver samples were homogenized in 1.0 M ethylenediamine tetra-acetic acid (EDTA) and 0.2 M phosphate-buffered potassium (PBS) solution. The pHs of the solutions were adjusted to 7.4. The homogenate was centrifuged at 15,000× g (4 °C) for 10 min, and the supernatant was used for characterizing the activities of antioxidant enzymes (superoxide dismutase, catalase, and glutathione-S-transferase) and oxidative stress biomarkers (malondialdehyde, carbonyl protein, nitric oxide, and hydrogen peroxide). The superoxide dismutase (SOD) activity was determined by the pyrogallol method, based on the ability of this enzyme to catalyze the reaction of superoxide anion (O−2) in hydrogen peroxide (H2O2) at 570 nm [35]. Catalase (CAT) activity was assessed by measuring the rate of decomposition of hydrogen peroxide (H2O2) for 60 s at 240 nm [36]. For those two enzymes, the results were expressed in U of enzyme mg of protein−1. Glutathione S-transferase activity (GST) was determined through the formation of glutathione-conjugated 2,4-dinitrochlorobenzene (CDNB) at 340 nm for 60 s, as described by [37]. The results were expressed in µmol min−1g−1 of tissue. SOD activity was evaluated in a microplate reader (Multiskan GO, Thermo Scientific, Waltham, MA, USA) and CAT and GST using a spectrophotometer (Model Mini 1240 UV-Vis Shimadzu Corporation, Kyoto, Japan).
Malondialdehyde (MDA) levels were estimated according to [38]. The tissue’s supernatant was homogenized in a solution containing trichloroacetic acid (15%), thiobarbituric acid (0.375%), and hydrochloric acid (0.6%). Samples were kept in a boiling water bath for 40 min, butyl alcohol was added, and tubes were centrifuged for 10 min at 9000× g. The supernatant was used to measure the absorbance at 540 nm. The results were expressed in µmol L−1 mg protein−1. The carbonyl protein group (CP) was quantified according to the method described by [39], using 2,4-dinitrophenylhydrazine (DNPH) and the DNPH molar extinction coefficient ε370 = 22 mmol L−1 cm−1 for the calculation. The reading was performed on a microplate reader under absorbance at 370 nm, and the results were expressed as nmol mg protein−1. Nitric oxide (NO) production was measured indirectly through the nitrite/nitrate content by the standard Griess reaction. The supernatants were incubated with Griess reagent (1% sulfanilamide, 0.1% N-(1-Naphthyl) ethylenediamine, in 2.5% H3PO4) for 10 min at 37° C in the dark. The absorbance was measured at 570 nm, and the results were expressed in µmol mg−1 protein−1. The H2O2 concentration was analyzed according to [40], adapted for a microplate reader at 374 nm, with the results expressed as mmol L−1. All analyses were performed in triplicates.
2.10. Statistical Analyses
Data were submitted to the Shapiro–Wilk test to verify the normality of the errors and the Bartlett test to verify the homogeneity of the variances. Effects of PEO dietary supplementation on different parameters were evaluated by one-way analysis of variance (ANOVA). A Dunnett test was used to evaluate significant differences of means from fish fed with PEO compared to fish fed with basal diet (p < 0.05). Orthogonal polynomial contrasts were performed to verify the linear and quadratic effects of the PEO dietary supplementation on the different parameters. The best regression models were chosen based on p-value (p < 0.05). All statistical analyses were performed using SAS® Studio software (SAS Inst. Inc., Cary, NC, USA).
3. Results
3.1. Growth Performance
Table 3 shows the growth performance of juvenile Nile tilapia fed the experimental diets. Body weight gain (p < 0.001; R2 = 0.676; Ymax. = 0.52 g kg−1 diet), relative feed intake (p < 0.001; R2 = 0.423; Ymax. = 0.51 g kg−1 diet), and feed conversion ratio (p < 0.001; R2 = 0.425; Ymin. = 0.51 g kg−1 diet) increased in a quadratic pattern in fish fed graded levels of PEO. Based on Dunnet’s test, fish fed 0.2 to 0.8 g kg−1 PEO showed higher body weight gain and feed conversion ratio and lower feed intake than that fed control diet (p < 0.05) (Figure 2). Differently, only fish fed the 0.4 g kg−1 PEO showed higher (p = 0.027) protein retention efficiency than those fed the diet control, whereas fish fed the diet 1.0 g kg−1 PEO revealed higher (p = 0.025) hepatosomatic indexes than those fed the diet control. However, the viscerosomatic index and survival rate were not affected (p < 0.05) by dietary treatments.

Table 3.
Growth performance of juvenile Nile tilapia fed the experimental diets with graded PEO levels over eight weeks 1.
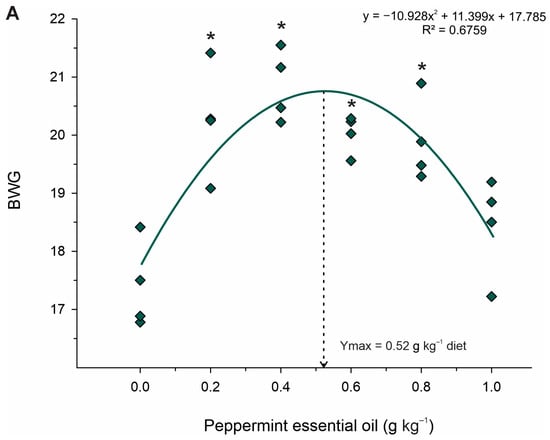
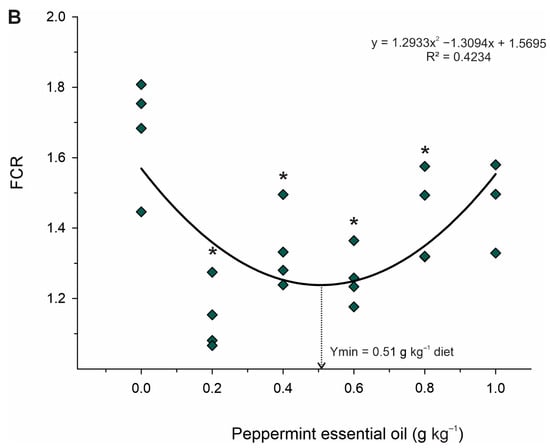
Figure 2.
Fitted orthogonal polynomial contrast for quadratic plots of body weight gain (BWG; (A)) and feed conversion ratio (FCR; (B)) as a function of the level of peppermint essential oil in diets for juvenile Nile tilapia. Each dot point represents the mean of each replicate, and superscripts (*) indicate significant differences from the control diet (peppermint essential oil = 0 g kg−1) by Dunnett’s test (p ≤ 0.05).
3.2. Whole-Body Composition
Whole-body compositions of juvenile Nile tilapia fed the experimental diets are shown in Table 4. Whole-body crude lipids (p < 0.001; R2 = 0.658) and gross energy decreased linearly according to increased PEO inclusion. Considering Dunnet’s test, fish fed 0.20 to 1.0 g kg−1 PEO showed lower (p < 0.001) whole-body crude lipids than those fed the diet control. However, dietary treatments unaffected whole-body moisture, crude protein, and mineral matter (p > 0.05).

Table 4.
Body compositions (g kg−1) of juvenile Nile tilapia fed the experimental diets with graded PEO levels over eight weeks 1.
3.3. Activity of Digestive Enzymes
Table 5 shows the activities of digestive enzymes of juvenile Nile tilapia fed diets with graded levels of PEO. The activities of the amylase (p < 0.001; R2 = 0.686; Ymax. = 0.54 g kg−1 diet) and protease (p = 0.003; R2 = 0.449; Ymax. = 0.39 g kg−1 diet) enzymes increased in a quadratic manner (Figure 3). However, lipase enzyme activity increased linearly (p < 0.001; R2 = 0.658), with graded levels of dietary PEO inclusion. Based on Dunnett’s test, fish fed diets with 0.2 to 0.8 g kg−1 diets of PEO showed higher (p < 0.001) activities of lipase, whereas only fish fed the diet with 0.4 g kg−1 of PEO revealed higher (p < 0.001) activities of protease than those fed the diet control. Differently, fish fed the 0.2 to 1.0 g kg−1 diet of PEO showed higher (p < 0.001) than those fed the diet control.

Table 5.
The gut activity of the digestive enzyme of juvenile Nile tilapia fed the experimental diets with graded PEO levels over eight weeks 1.
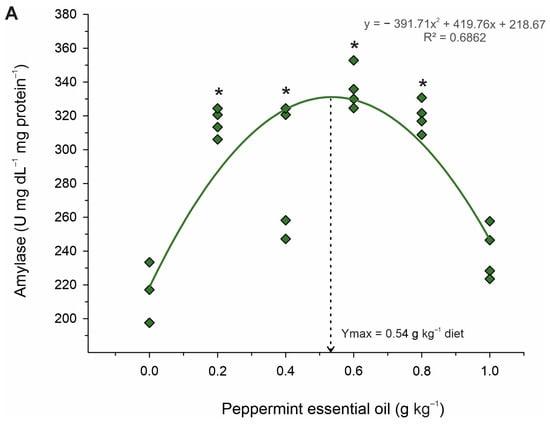
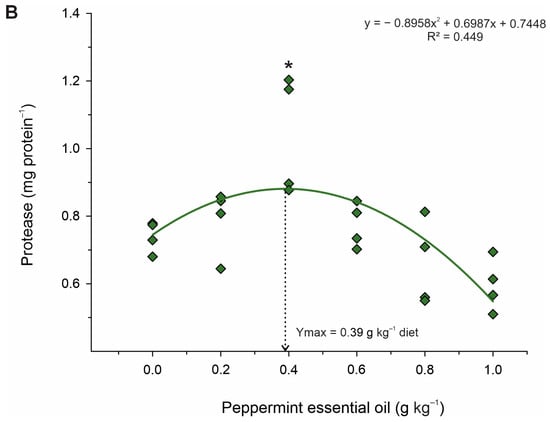
Figure 3.
Fitted orthogonal polynomial contrast for quadratic plots of enzymatic activity of amylase (A) and protease (B) in the gut as a function of the level of peppermint essential oil in diets of juvenile Nile tilapia. Each dot point represents the mean of each replicate, and superscripts (*) indicate significant differences from the control diet (peppermint essential oil = 0 g kg−1) by Dunnett’s test (p ≤ 0.05).
3.4. Biochemical Parameters
Plasma biochemical parameters of juvenile Nile tilapia fed the experimental diets are shown in Table 6. Plasma glucose (p = 0.013; Ymax. = 0.66 g kg−1 diet; R2 = 0.463), triacylglycerol (p < 0.001; Ymax. = 0.47 g kg−1 diet; R2 = 0.579), cholesterol (p = 0.013; Ymax. = 0.49 g kg−1 diet; R2 = 0.263), HDL (p = 0.002; Ymax. = 0.42 g kg−1 diet; R2 = 0.439), LDL (p = 0.021; Ymax. = 0.64 g kg−1 diet; R2 = 0.332), and hepatic glycogen (p = 0.028; Ymax. = 0.64 g kg−1 diet; R2 = 0.332) contents increased in a quadratic pattern according to graded dietary PEO levels. Based on Dunnett’s test, fish fed the diet with 0.6 g kg−1 PEO showed higher plasma triacylglycerides (p < 0.001), total cholesterol (p = 0.007), HDL (p < 0.001), LDL (p < 0.001), and higher hepatic glycogen (p = 0.010) than those fed diet control. Conversely, fish fed the 0.6 and 0.8 g kg−1 PEO plasmatic presented lower plasma glucose than those fed the diet control.

Table 6.
Plasma biochemical parameters (mg dL−1) and hepatic glycogen content (mg 100 mg−1 tissue) of juvenile Nile tilapia fed the experimental diets with graded PEO over eight weeks 1.
3.5. Oxidative Stress Indicators
Data on liver oxidative stress responses of juvenile Nile tilapia fed the experimental diets are shown in Table 7. The activity of the SOD (p = 0.004; Ymax. = 0.58 g kg−1 diet; R2 = 0.390) and CAT (p < 0.001; Ymax. = 0.52 g kg−1 diet; R2 = 0.499) enzymes increased, whereas MDA (p = 0.001; Ymax. = 0.40 g kg−1 diet; R2 = 0.535) and nitric oxide (p = 0.006; Ymax. = 0.63 g kg−1 diet; R2 = 0.607) contents decreased in a quadratic pattern (Figure 4). However, the activity of GST CP and H2O2 contents was unaffected (p > 0.05) by dietary treatments. Based on Dunnett’s test, fish fed 0.2 and 0.6 g kg−1 PEO showed higher (p < 0.001) activities of the CAT enzyme than those fed diet control. Additionally, fish fed the diets of 0.4 and 0.6 g kg−1 PEO showed higher activity of SOD (p = 0.011) and lower (p = 0.020) MDA and nitric oxide (p = 0.044) than those fed diet control. However, there were no effects of dietary treatments on the activities of the GST (p = 0.890) enzymes and the CP (p = 0.580) and hydrogen peroxide (p = 0.890) contents.

Table 7.
Antioxidant enzyme activity and MDA, CP, NO, and H2O2 contents in the liver (mg 100 mg−1 tissue) of juvenile Nile tilapia fed the experimental diets with graded PEO levels over eight weeks 1.
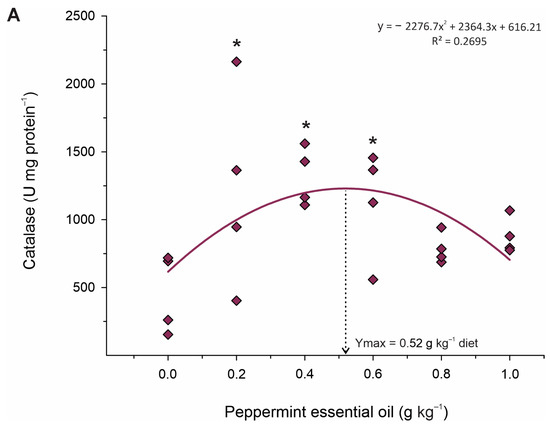
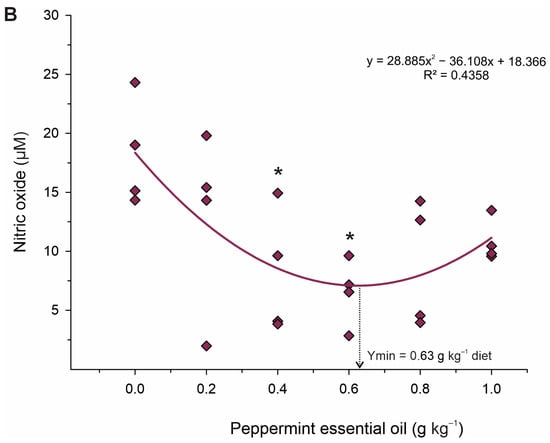
Figure 4.
Fitted orthogonal polynomial contrast for quadratic plots of activity of catalase (A) and nitric oxide content (B) in the liver as a function of the level of peppermint essential oil in diets of juvenile Nile tilapia. Each dot point represents the mean of each replicate, and superscripts (*) indicate significant differences from the control diet (peppermint essential oil = 0 g kg−1 diet) by Dunnett’s test (p ≤ 0.05).
4. Discussion
Dietary supplementation with PEO improved the condition of Nile tilapia juveniles, as evidenced by increased weight gain, feed conversion, specific growth rate, and protein efficiency rate. These findings may be related to PEO’s impact on intestinal motility and digestive enzyme secretion [14,15], as well as on the gut microbiota and morphology, potentially leading to an improvement in a fish’s digestive and absorptive capacity [25].
Previous studies, with products derived from M. piperita and with the active ingredient menthol, illustrated the beneficial effects of this plant on animal growth. Dietary supplementation with 30 g kg−1 ethanol extract of M. piperita improved body weight gain, specific growth rate, and feed conversion rate of R. frisii kutum [24] and S. trutta caspius [25]. Likewise, levels of 2.2 to 3.0 g kg−1 of menthol essential oil in the diets improved weight gain and feed conversion of Nile tilapia exposed to acute ammonia [15] and weight gain and specific growth rate when reared in intensive systems [3]. However, few studies have evaluated PEO as a feed additive. Ref. [21] evaluated dietary supplementation with levels of 0.75, 1.25, and 2.50 g kg−1 of PEO after challenging Streptococcus agalactiae, but no effect was observed on the growth performance of Nile tilapia. Therefore, the difference observed in our results may have been related to the optimal cultivation conditions. Furthermore, in our study, the active ingredients menthol and menthone were in higher concentrations (46.5% menthol and 26.2% menthone) compared to the aforementioned study (33.8% menthol and 15.2% menthone).
Although the inclusion of PEO in the diet altered the hepatosomatic index of juvenile Nile tilapia fed with 1.0 g kg−1 of PEO in this study, higher hepatic glycogen contents were observed in the organs of fish fed with 0.60 g kg−1. Concomitantly, fish fed this level of PEO showed reduced plasma glucose levels. This was probably due to the increase in carbohydrate digestion and glucose absorption by these animals, which promoted increased energy reserves stored in the liver. This result may have been related to improvements in glucose metabolism. Ref. [23] correlated the decrease in plasma glucose of L. calcarifer fed with 4.0 and 5.0 g kg−1 of powdered M. piperita leaves, with the increase in insulin activity induced by peppermint.
The higher plasmatic levels of cholesterol, triglycerides, LDL, and HDL found in juveniles fed diets containing intermediate levels of PEO (0.4 to 0.6 g kg−1) may have been related to increased digestion and absorption of nutrients, as there were increased activities of the total digestive enzymes protease, lipase, and amylase. Greater protease activity in the intestine of Nile tilapia [15] and greater amylase activity in the intestine of S. trutta caspius [25] have also been observed when fish were fed with menthol essential oil and an extract of M. piperita-supplemented diets, respectively. In the gastrointestinal tract, phytochemicals and other substances are recognized by specialized endocrine cells, which respond to the stimulus by activating Ca2+ channels operated by voltage-gated receptors [41]. In the case of PEO, menthol activates a cold-sensitive receptor called transient receptor potential channel (TRPA1) [42], which promotes muscle relaxation and triggers the release of CCK and digestive enzymes [13]. The muscle relaxation promoted by menthol decreases intestinal transit, which, when added to the increases in the activities of digestive enzymes, can improve nutrient digestibility and absorption by the fish’s gastrointestinal tract [43], which, consequently, could lead to improvements in fish growth performance. However, it should be taken into account that the effect of menthol on TRPA1 is bimodal, as higher concentrations lead to reversible blockades of this channel, causing hypersensitivity [42].
The improvement in the assimilation of nutrients, promoted by the greater secretion of digestive enzymes and the action of PEO on the metabolism of glucose and lipids, could have led to an improvement in the quality of the fish carcass, especially if we think of the effect of these nutrients as protein-sparing. However, the only change observed in the body composition of the fish was a reduction in lipid deposition in the body of the fish. Like other essential oils, PEO is known for its hypolipidemic effects [44]. Melissa officinalis essential oil, for example, has some active principles similar to PEO, which increase the expression of genes that encode lipoprotein lipases and fatty acid transport proteins [41]. Thus, PEO can act by promoting lipid catabolism, improving the growth and quality of fish carcasses.
The abundance of phenolic acids, flavones, and flavanones with antioxidant activities in PEO [45] gives this additive the ability to fight radicals and reduce cell damage caused by lipid peroxidation and inflammation [17,46]. Therefore, given the metabolic improvements observed through plasma biochemistry and analysis of energy reserves contained in the liver, in this study, we also observed that PEO also acted to protect the hepatic tissue. This was evidenced by the increased activity of antioxidant enzymes SOD and CAT in fish fed 0.4–0.6 g kg−1. SOD and CAT constitute a crucial part of the cellular antioxidant defense mechanism [47]. SOD catalyzes the conversion of O−2 into molecular oxygen and H2O2 and, subsequently, CAT decomposes H2O2 into oxygen [48]. Therefore, it is likely that these enzymes promoted the reduction in oxidative damage and thus reduced lipid peroxidation, which is corroborated by the lower production of MDA and NO in these groups. These results indicate that PEO can thereby improve the antioxidant status and contribute to the growth potential of fish exposed to stressful farming conditions.
This research found some limitations and needed attention in future studies. Firstly, understanding the response of environmentally challenged Nile tilapia to PEO supplementation is crucial for improving the precise utilization of essential oils in future aquafeed formulations. Furthermore, the dynamic nature of the aquatic environment and its inherent complexity, with various interacting variables and microbial communities, posed challenges in isolating the specific effects of PEO. Additionally, measuring and quantifying the precise concentrations of PEO in the diets and the resulting levels within the fish presented specific difficulties. Finally, diligent efforts to ensure accurate sampling, variations in feed intake, and digestion rates among individual fish could have influenced the results obtained in the current study. Despite these limitations, the study provided valuable insights into the beneficial effects of PEO on the growth and health of juvenile Nile tilapia.
5. Conclusions
In summary, this study identified a promising natural growth promoter in the form of PEO as an alternative to conventional synthetic growth promoters in aquaculture. Through its positive effects on digestion, nutrient utilization, and antioxidant status, PEO significantly improved the growth performance and body composition of Nile tilapia. These findings highlighted the potential of incorporating a 0.6 g kg−1 diet of PEO as a natural functional additive in Nile tilapia aquafeeds, thereby contributing to a more sustainable aquaculture industry.
Author Contributions
Conceptualization, G.A.C.C.d.A., C.L.d.S.C., D.A.V.C., B.B., J.A.S.Z., W.M.F. and A.L.S.; Methodology, G.A.C.C.d.A., C.L.d.S.C., R.C.T.R., J.F.R.M., A.V.d.O., M.G.d.A.O. and M.B.D.d.F.; Formal analysis, G.A.C.C.d.A., C.L.d.S.C., D.A.V.C., B.B., J.A.S.Z., W.M.F. and A.L.S.; Investigation, G.A.C.C.d.A., C.L.d.S.C., R.C.T.R., J.F.R.M. and A.L.S.; Resources, J.A.S.Z., A.V.d.O., M.G.d.A.O., M.B.D.d.F. and A.L.S.; Data curation, G.A.C.C.d.A., C.L.d.S.C., D.A.V.C., B.B., W.M.F. and A.L.S.; Writing—original draft, C.L.d.S.C., D.A.V.C., W.M.F. and A.L.S.; Writing—review and editing, G.A.C.C.d.A., R.C.T.R., J.F.R.M., B.B., J.A.S.Z., A.V.d.O., M.G.d.A.O. and M.B.D.d.F.; Visualization, G.A.C.C.d.A., C.L.d.S.C., D.A.V.C., W.M.F. and A.L.S.; Supervision, A.L.S.; Project administration, A.L.S.; Funding acquisition, D.A.V.C., J.A.S.Z. and A.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Research Support Foundation of the State of Minas Gerais (FAPEMIG) for funding this research (APQ430 01519-16) and for the undergraduate fellowship to R.C.T Rusth. and J.F.R. Maciel. We also would like to thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for the postgraduate scholarship (code 001) to G.A.C.C. Aguiar and the National Council for Scientific and Technological Development (CNPq) for the fellowships for A.L. Salaro (grant number 311402/2020-8), B. Baldiserotto (grant number 301816/2022-0), and W.M. Furuya (grant number 303366/2019-2).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee on the Use of Production Animals of the Federal University of Viçosa (Approval Code: 38/2016; Approval date: 2016).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We would like to thank BIOCLIN for supplying the kits used in the analyses.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- FAO. The State of World Fisheries and Aquaculture 2020: Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Santos, J.F.; Assis, C.R.D.; Soares, K.L.S.; Rafael, R.E.Q.; Oliveira, V.M.; De Vasconcelos Filho, J.E.; França, R.E.Q.; Lemos, D.; Bezerra, R.S. A comparative study on Nile tilapia under different culture systems: Effect on the growth parameters and proposition of new growth models. Aquaculture 2019, 503, 128–138. [Google Scholar] [CrossRef]
- Encarnação, P. Functional feed additives in aquaculture feeds. In Aquafeed Formulation; Nates, S.F., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 217–237. [Google Scholar] [CrossRef]
- Dawood, M.A.; Noreldin, A.E.; Ali, M.A.; Sewilam, H. Menthol essential oil is a practical choice for intensifying the production of Nile tilapia (Oreochromis niloticus): Effects on the growth and health performances. Aquaculture 2021, 543, 737027. [Google Scholar] [CrossRef]
- Kesbiç, O.S.; Acar, U.; Hassaan, M.S.; Yilmaz, S.; Guerrera, M.C.; Fazio, F. Effects of Tomato Paste By-Product Extract on Growth Performance and Blood Parameters in Common Carp (Cyprinus carpio). Animals 2022, 12, 3387. [Google Scholar] [CrossRef]
- Chakraborty, S.B.; Horn, P.; Hancz, C. Application of phytochemicals as growth-promoters and endocrine modulators in fish culture. Rev. Aquac. 2014, 6, 1–19. [Google Scholar] [CrossRef]
- Kalemba, D.A.A.K.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Sutili, F.J.; Gatlin III, D.M.; Heinzmann, B.M.; Baldisserotto, B. Plant essential oils as fish diet additives: Benefits on fish health and stability in feed. Rev. Aquac. 2018, 10, 716–726. [Google Scholar] [CrossRef]
- Carneiro, C.L.D.S.; De Assis, C.E.; Modesto, A.L.S.; Maciel, J.F.R.; Campelo, D.A.V.; Luz, R.K.; Salaro, A.L. Oregano essential oil (Origanum vulgare) dietary supplementation improved growth performance, body protein retention and muscle hyperplasia of the Neotropical catfish Lophiosilurus alexandri. Aquac. Nutr. 2021, 27, 1221–1231. [Google Scholar] [CrossRef]
- Ezzat Abd El-Hack, M.; Alagawany, M.; Ragab Farag, M.; Tiwari, R.; Karthik, K.; Dhama, K.; Adel, M. Beneficial impacts of thymol essential oil on health and production of animals, fish, and poultry: A review. J. Essent. Oil Res. 2016, 28, 365–382. [Google Scholar] [CrossRef]
- Zaminhan-Hassemer, M.; Zagolin, G.B.; Perazza, C.A.; Barbosa, D.A.; Menegidio, F.B.; Coutinho, L.L.; Hilsdorf, A.W.S. Adding an essential oil blend to the diet of juvenile Nile tilapia improves growth and alters the gut microbiota. Aquaculture 2022, 560, 738581. [Google Scholar] [CrossRef]
- Da Silva Cardoso, A.J.; Dos Santos, W.V.; Gomes, J.R.; Martins, M.T.S.; Coura, R.R.; De Almeida Oliveira, M.G.; Zuanon, J.A.S. Ginger oil, Zingiber officinale, improve palatability, growth and nutrient utilization efficiency in Nile tilapia fed with an excess of starch. Anim. Feed Sci. Technol. 2021, 272, 114756. [Google Scholar] [CrossRef]
- Valussi, M. Functional foods with digestion-enhancing properties. Int. J. Food Sci. Nutr. 2012, 63 (Suppl. S1), 82–89. [Google Scholar] [CrossRef] [PubMed]
- Goerg, K.J.; Spilker, T.H. Effect of peppermint oil and caraway oil on gastrointestinal motility in healthy volunteers: A pharmacodynamic study using simultaneous determination of gastric and gall-bladder emptying and orocaecal transit time. Aliment. Pharmacol. Ther. 2003, 17, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Magouz, F.I.; Mahmoud, S.A.; El-Morsy, R.A.; Paray, B.A.; Soliman, A.A.; Zaineldin, A.I.; Dawood, M.A. Dietary menthol essential oil enhanced the growth performance, digestive enzyme activity, immune-related genes, and resistance against acute ammonia exposure in Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 530, 735944. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; Malheiros, D.F.; Guilozki, I.C.; Majolo, C.; Chaves, F.C.M.; Chagas, E.C.; De Assis, H.C.S.; Tavares-Dias, M.; Yoshioka, E.T.O. Antioxidants effects and resistance against pathogens of Colossoma macropomum (Serassalmidae) fed Mentha piperita essential oil. Aquaculture 2018, 490, 29–34. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Ma, A.; Bao, Y.; Wang, M.; Sun, Z. In Vitro antiviral, anti-inflammatory, and antioxidant activities of the ethanol extract of Mentha piperita L. Food Sci. Biotechnol. 2017, 26, 1675–1683. [Google Scholar] [CrossRef]
- Freire, M.M.; Jham, G.N.; Dhingra, O.D.; Jardim, C.M.; Barcelos, R.C.; Valente, V.M.M. Composition, antifungal activity and main fungitoxic components of the essential oil of Mentha piperita L. J. Food Saf. 2012, 32, 29–36. [Google Scholar] [CrossRef]
- Desam, N.R.; Al-Rajab, A.J.; Sharma, M.; Mylabathula, M.M.; Gowkanapalli, R.R.; Albratty, M. Chemical constituents, in vitro antibacterial and antifungal activity of Mentha × Piperita L. (peppermint) essential oils. J. King Saud. Univ. Sci. 2019, 31, 528–533. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Nasiri, R.; Jafari Sales, A. In-Vitro antibacterial effects of methanolic extract of peppermint (Mentha piperita Lamiaceae) on standard Staphylococcus aureus, Bacillus cereus, Escherichia coli and Pseudomonas aeruginosa strain. Jorjani Biomed. J. 2019, 7, 4–10. [Google Scholar] [CrossRef]
- De Souza Silva, L.T.; De Pádua Pereira, U.; De Oliveira, H.M.; Brasil, E.M.; Pereira, S.A.; Chagas, E.C.; Jesus, G.F.A.; Cardoso, L.; Mouriño, J.L.P.; Martins, M.L. Hemato-immunological and zootechnical parameters of Nile tilapia fed essential oil of Mentha piperita after challenge with Streptococcus agalactiae. Aquaculture 2019, 506, 205–211. [Google Scholar] [CrossRef]
- Dawood, M.A.; Metwally, A.E.; Elkomy, A.H.; Gewaily, M.S.; Abdo, S.E.; Abdel-Razek, M.A.S.; Soliman, A.A.; Amer, A.A.; Abdel-Razik, N.I.; Abdel-Latif, H.M.R.; et al. The impact of menthol essential oil against inflammation, immunosuppression, and histopathological alterations induced by chlorpyrifos in Nile tilapia. Fish Shellfish Immunol. 2020, 102, 316–325. [Google Scholar] [CrossRef]
- Talpur, A.D. Mentha piperita (Peppermint) as feed additive enhanced growth performance, survival, immune response and disease resistance of Asian seabass, Lates calcarifer (Bloch) against Vibrio harveyi infection. Aquaculture 2014, 420, 71–78. [Google Scholar] [CrossRef]
- Adel, M.; Amiri, A.A.; Zorriehzahra, J.; Nematolahi, A.; Esteban, M.Á. Effects of dietary peppermint (Mentha piperita) on growth performance, chemical body composition and hematological and immune parameters of fry Caspian white fish (Rutilus frisii kutum). Fish Shellfish Immunol. 2015, 45, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Adel, M.; Safari, R.; Pourgholam, R.; Zorriehzahra, J.; Esteban, M.Á. Dietary peppermint (Mentha piperita) extracts promote growth performance and increase the main humoral immune parameters (both at the mucosal and systemic levels) of Caspian brown trout (Salmo trutta caspius Kessler, 1877). Fish Shellfish Immunol. 2015, 47, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Padala, D.; Ganapathi Naik, M.; Anjusha, K.V.; Ramesh, K.S.; Abhiman, P.B.; Rakesh, K. Growth promoter effect of peppermint (Mentha piperita) on rohu (Labeo rohita). Int. J. Fish. Aquat. 2018, 6, 537–539. [Google Scholar]
- Paknejad, H.; Shekarabi, S.P.H.; Mehrgan, M.S.; Hajimoradloo, A.; Khorshidi, Z.; Rastegari, S. Dietary peppermint (Mentha piperita) powder affects growth performance, hematological indices, skin mucosal immune parameters, and expression of growth and stress-related genes in Caspian roach (Rutilus caspicus). Fish Physiol. Biochem. 2020, 46, 1883–1895. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; AOAC: Arlington, VA, USA, 2016. [Google Scholar]
- Emerson, K.; Russo, R.C.; Lund, R.E.; Thurston, R.V. Aqueous ammonia equilibrium calculations: Effect of pH and temperature. J. Fish. Res. 1975, 32, 2379–2383. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tomarelli, R.M.; Charney, J.; Harding, M.L. The use of azoalbumin as a substrate in the colorimetric determination or peptic and tryptic activity. J. Lab. Clin. Med. 1949, 34, 428–433. [Google Scholar]
- Cherry, I.S.; Crandall, L.A., Jr. The specificity of pancreatic lipase: Its appearance in the blood after pancreatic injury. Am. J. Physiol. Leg. Content 1932, 100, 266–273. [Google Scholar] [CrossRef]
- Caraway, W.T. A stable starch substrate for the determination of amylase in serum and other body fluids. Am. J. Clin. Pathol. 1959, 32, 97–99. [Google Scholar] [CrossRef]
- Carroll, N.V.; Longley, R.W.; Roe, J.H. The determination of glycogen in liver and muscle by use of anthrone reagent. J. Biol. Chem. 1956, 220, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, S.; Bieligk, U.; Beulich, K.; Hasenfuss, G.; Prestle, J. Gene expression of antioxidative enzymes in the human heart: Increased expression of catalase in the end-stage failing heart. Circulation 2000, 101, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology: Oxygen Radicals in Biological Systems; Packer, L., Ed.; Academic Press: San Diego, CA, USA, 1984; pp. 121–126. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Fleischer, S., Packer, L., Eds.; Academic Press: San Diego, CA, USA, 1978; pp. 302–310. [Google Scholar] [CrossRef]
- Levine, R.L.; Williams, J.A.; Stadtman, E.P.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. In Methods in Enzymology; Shukla, A., Ed.; Academic Press: San Diego, CA, USA, 1994; Volume 233, pp. 346–357. [Google Scholar]
- Goth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta. 1991, 196, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Racke, K.; Reimann, A.; Schwörer, H.; Kilbinger, H. Regulation of 5-HT release from enterochromaffin cells. Behav. Brain Res. 1995, 73, 83–87. [Google Scholar] [CrossRef]
- Karashima, Y.; Damann, N.; Prenen, J.; Talavera, K.; Segal, A.; Voets, T.; Nilius, B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J. Neurosci. 2007, 27, 9874–9884. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Bhilave, M.P.; Nadaf, S.B. Formulation of fish feed using ingredients from plant sources. Res. J. Agric. Sci. 2010, 1, 284–287. [Google Scholar]
- Sosa, B.D.S.; Moro, E.B.; Gomes, R.L.M.; Cardoso, M.D.S.; Cardoso, L.M.; Boscolo, W.R.; Oliveira, J.D.S.D.; Signor, A.; Bittencourt, F. Essential oils in diets for Nile tilapia juveniles: Productive performance and plasmatic biochemistry. Aquac. Res. 2020, 51, 2758–2765. [Google Scholar] [CrossRef]
- Riachi, L.G.; Maria, C.A. Peppermint antioxidants revisited. Food Chem. 2015, 176, 72–81. [Google Scholar] [CrossRef]
- Tafrihi, M.; Imran, M.; Tufail, T.; Gondal, T.A.; Caruso, G.; Sharma, S.; Pezzani, R. The wonderful activities of the genus Mentha: Not only antioxidant properties. Molecules 2021, 26, 1118. [Google Scholar] [CrossRef]
- Zheng, Z.L.; Tan, J.Y.; Liu, H.Y.; Zhou, X.H.; Xiang, X.; Wang, K.Y. Evaluation of oregano essential oil (Origanum heracleoticum L.) on growth, antioxidant effect and resistance against Aeromonas hydrophila in channel catfish (Ictalurus punctatus). Aquaculture 2009, 292, 214–218. [Google Scholar] [CrossRef]
- Dröge, W. Aging-related changes in the thiol/disulfide redox state: Implications for the use of thiol antioxidants. Exp. Gerontol. 2002, 37, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).