Abstract
This study aimed to investigate the immunoprotective effect of Coptis chinensis (CC) on Streptococcus agalactiae (SA) infection in tilapia. Experimental fish were randomly divided into two groups feeding on a normal diet (ND) and a CC-supplemented diet (CCD) for 2 weeks and then injected with SA. After the inoculation experiment, the ND and CCD groups were named PI_ND and PI_CCD, respectively. CCD increased superoxide dismutase (SOD) activity and decreased malondialdehyde (MDA) activity significantly before and after infection. Immunological assays revealed that the serum interleukin-1β (IL-1β), complement 3 (C3), immunoglobulin M (IgM), Interferon-gamma (IFN-γ), and tumor necrosis factor-α (TNF-α) levels in the CCD group were significantly higher than in the ND group both before and after infection. In addition, proteomics analysis of liver tissue identified 62 differentially expressed proteins (DEPs) in CCD vs. ND, and 36 DEPs in the PI_CCD vs. the PI_ND groups. Furthermore, 80 specific upregulated proteins and 49 specific downregulated proteins were screened in the CCD group. The specific upregulated proteins included important antimicrobial enzymes such as lysozymes and cathepsin D, and antimicrobial peptides such as septins, granulin, and grancalcin, involving multiple KEGG brite categories such as enzymes, exosomes, membrane transport, and proteolipid proteins. Furthermore, specific downregulated proteins were enriched in glycolysis/gluconeogenesis and TCA cycle pathways. In conclusion, CC supplementation effectively enhances the ability of tilapia to resist SA infection by modulating various antioxidant enzymes, immune factors, antimicrobial enzymes, and antimicrobial peptides, and by moderately inhibiting central carbon metabolism. These findings provide a basis for replacing antibiotics with environmentally-friendly functional aquatic feeds to control bacterial diseases.
Keywords:
tilapia; Streptococcus agalactiae; Coptis chinensis; proteomic; antimicrobial peptide; immune factor; antioxidant enzyme Key Contribution:
Coptis chinensis supplementation effectively improved tilapia’s ability to resist SA infection. This study provides a basis for replacing antibiotics with environmentally-friendly functional aquafeeds to control bacterial diseases.
1. Introduction
Streptococcus agalactiae (SA) (family Streptococcaceae, genus Streptococcus), also known as group B Streptococcus (GBS), is a facultatively anaerobic, Gram-positive diplococcus and a common pathogenic bacteria in humans, animals, and fish [1]. Streptococcicosis in fish is characterized by wide distribution, high incidence, and severe outcomes. The number of reports of SA infection has increased gradually in recent years, and SA infection affects various marine and freshwater cultured fish worldwide. Among them, tilapia is the most sensitive to SA, and SA infection is the most common and serious infection reported in this species. Since 2009, several serious outbreaks of SA infection in tilapia have been reported in various regions of Asia, including China, which have caused serious damage to the tilapia culture industry owing to the high morbidity and mortality [2,3]. Therefore, research on the pathogenesis and resistance mechanism underlying SA infection in tilapia is essential for prevention and control.
The use of traditional herbal medicines can help reduce indiscriminate overuse of drugs for the suppression of Streptococcus resistance to multiple antibiotics and lower the levels of drug residues in aquatic products. Additionally, these medicines help address the issues associated with the arduous developmental process of vaccines. Coptis chinensis (CC) is the dried rhizome of the buttercup plants Huanglian, C. deltoidea, or C. teeta Wall. CC, and can help to reduce heat and purge pathogenic fire (excessive internal heat, which is a concept from traditional Chinese medicine), aid detoxification, and dispel dryness [4]. In addition, CC exerts various biological effects that aid in the treatment of arrhythmia, hyperglycemia, hyperlipidemia, platelet aggregation, and tumors [5]. The experimental results shown by Yu et al. [6] and Wang et al. [7] suggested that CC can improve the resistance of tilapia to various bacteria. Niu et al. [8] conducted a meta-analysis by collecting data on the antibacterial effect of herbs on SA from databases and concluded that CC was more effective in inhibiting SA than Rheum and Rhus chinensi.
At present, reports on the immune response of CC-treated tilapia to SA and the molecular mechanism of action of herbal medicine on fish immunity are limited. In this study, using iTRAQ-labeled proteomics, we analyzed the differences in protein expression in the liver tissues of SA-infected fish before and after CC treatment to determine the major biological processes and the signaling pathways that differentially expressed proteins (DEPs) are associated with. In addition, we explored the mechanism underlying the immune reaction of tilapia against SA by monitoring the biochemical indexes in blood and related tissues in CC-treated tilapia. Overall, our findings provide the theoretical basis for additional investigations on CC-based treatment for the ecological prevention and control of SA infection.
2. Materials and Methods
2.1. Experimental Fish and Pathogenic Bacteria
Tilapia and SA strains were provided by the Guangxi Research Institute of Aquatic Sciences and Pearl River Institute of Aquatic Sciences, respectively. The culture experiments were conducted in an indoor recirculating aquaculture system at the aquatic base at Guangxi University. The culture tank was disinfected with 0.5% potassium permanganate solution for 2 h before the SA infection test of tilapia (270 fish, with an average weight of 30 g). The tilapia were temporarily housed in the tank for 14 days and fed with bait twice a day (at 09:00 a.m. and 17:00 p.m.). During cultivation, the water temperature was 30.21 ± 0.75 °C and the dissolved oxygen was 5.37 ± 1.35 mg/L. The water was changed every 2 days (half of the pool at a time, normal aeration). Feeding was stopped at 24 h before sampling.
2.2. Experimental Feed
The 1% CC (20:1) added to the feed was provided by Ningxia Guosheng Biotech. The feed preparation process was as follows: (1) CC was ground into a powder, mixed with distilled water in a certain ratio in a round-bottom flask, and extracted in a water bath at 80 °C for 1 h. After centrifugation, the supernatant was extracted, concentrated to 5 mg/mL, and stored at 4 °C for subsequent use; (2) ordinary granular feeds were mechanically ground into a powder, dried, and stored for subsequent use; and (3) the basal feed was mixed with distilled water in equal volumes. In addition, 1 mL of the CC liquid was added per gram of the feed to alter the drug content of the feed to 0.5%. Subsequently, the mixture was dried at 60 °C, cooled, and stored at 4 °C. Feed formulation and proximate composition of experimental diets are shown in Table 1.

Table 1.
Formulation and proximate analysis of the tilapia diets.
2.3. Experimental Stage and Grouping
Prior to the infection experiment, a feeding period of 14 days was carried out. The tilapia were divided into two groups: the normal group (conventional feed + saline, ND group) and the experimental group (conventional feed + 1% CC, CCD group). A total of 3 replicate culture buckets were used for each group (30 fish per bucket, 180 tilapia in all). After the feeding stage, 10 fish were randomly selected from each parallel culture bucket of each group for the 24 h inoculation experiment (named PI_ND and PI_CCD groups, respectively).
2.4. Bacterial Culture and Inoculation Experiments
The preserved SA was activated, diluted, and coated on BHI agar plates. Subsequently, monoclones were selected for 16S rRNA gene sequencing (Table S1). The sequencing-cleared monoclones were cultured till the logarithmic growth stage, and the concentration of the bacterial solution was adjusted to 5 × 1011 CFUs/mL using saline (0.65%) and diluted in five concentration gradients (1×, 10×, 100×, 1000×, and 10,000×). Subsequently, 120 fish were randomly kept in six experimental pools, and the bacterial solution was injected intraperitoneally (at the base of the ventral fin) in each fish (0.2 mL/fish) in the five groups at the five concentrations (mentioned above). In addition, tilapia in the control group were injected with 0.2 mL of 0.65% saline. After the injection, the fish were placed in the original test tank (water temperature: 30 °C, with normal feeding, aeration, and water replacement). The dead fish were retrieved and recorded for a number every 3 h. After SA inoculation, we counted the number of dead fish and confirmed that the lethal concentration for 50% (LC50) of the SA strain was 1.5 × 108 CFU/mL [7] using the Reed–Muench method [9].
After feeding, the inoculation experiment was conducted. First, ten fish from each parallel group were randomly selected for the inoculation experiment by injecting 0.2 mL of 1.5 × 108 CFU/mL bacterial solution in the ventral cavity (base of ventral fin). Tilapia in the control group were injected with 0.2 mL of 0.65% saline. After the injection, the fish were placed in the original test bucket. After a duration of 24 h, the tilapia were anesthetized using 200 mg/L MS-222 for sampling.
2.5. Sample Collection
After feeding and inoculation, 10 fish were randomly anesthetized with 200 mg/L MS-222 from each of the 3 parallel culture buckets of the 2 feeding groups. Later, fresh blood was collected from the tail vein, placed in a freezing tube for 2 h, and centrifuged at 2000× g for 10 min to separate the serum. The extracted serum was then stored in a refrigerator at −20 °C. In addition, liver samples were collected, placed in centrifuge tubes, snap-frozen in liquid nitrogen, and stored in an ultra-low temperature refrigerator (−80 °C) for subsequent biochemical and proteomic assays.
2.6. Biochemical and Immunological Index Determination
For examination of the biochemical characteristics, including total protein (TP), alanine transaminase (ALT), aspartate aminotransferase (AST), malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD) of the liver, and immunological indexes, such as complement C3 (C3), immunoglobulin M (IgM), tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), and Interleukin 1β (IL-1β) of serum samples, the detection kits were used according to the manufacturer’s instructions (Nanjing Jiancheng Biology Engineering Institute, Nanjing, China).
2.7. iTRAQ Differential Proteomics Assay
The liver tissue samples were ground to a powder form, and the proteins were extracted using UED Lysis Buffer. After determining the protein concentrations using a Bradford kit, samples of 100 µg were reduced, alkylated, and digested into peptides with trypsin. Subsequently, the peptides were labeled by iTRAQ-8plex Amine-Modifying Labeling. The mixed labeled peptides were subjected to HPLC component separation, and the 12 resultant components were analyzed individually using LC–MS/MS. The experiments were performed in three biological replicates.
2.8. Database Search and Bioinformatics Analysis
A database search against the tilapia Uniprot proteome sequences was performed using the SEQUEST software of the Proteome Discoverer platform. The database search settings were as follows–trypsin: digestive enzyme, up to two missed cuts allowed; parent ion error: 20 ppm; secondary ion error: 0.05 Da; iTRAQ-8plex (N-term, R, K); Oxidation (M): variable modification; Carbamidomethyl (C): fixed modification; and peptide FDR: 0.01. The identified proteins were annotated using the Uniprot database for GO annotation and the KAAS online tool for KEGG pathway annotation.
2.9. Statistical Analysis
Data obtained were recorded and initially stratified using Excel 2010 (Microsoft, Redmond, WA, USA, 2010). One-way analysis of variance (ANOVA) was performed using SPSS version 21 (IBM, Armonk, New York, NY, USA, 2012) to determine the significance of differences (p < 0.05). Values were expressed as mean ± standard error (Mean ± SE). DEPs were screened using the criteria of fold change > 1.2 or < 0.83 and p value < 0.05 (t-test).
3. Results
3.1. Effect of CC on Biochemical Indexes in the Liver of Healthy and SA-Infected Tilapia
The results of the liver biochemical indexes suggested that the liver TP level, AST, and SOD activities were significantly increased, whereas the MDA content was significantly decreased in the CCD group compared to the ND group (Figure 1A,C,F). However, the difference in the liver ALT and CAT activities shown was not significant in the CCD group compared to the ND group (Figure 1B,D). Results showed that SA infection decreased the TP level, CAT, and SOD activities, and significantly increased the MDA level and AST activity in the ND group. After SA infection, the PI_CCD group showed a significantly lower ALT activity and MDA content and higher SOD activity than those from the PI_ND group (Figure 1B,E,F). In addition, the liver TP content and CAT activity were higher and the AST activity was lower in the PI_CCD group compared to the PI_ND group; however, the differences were not significant (Figure 1A,C,D).
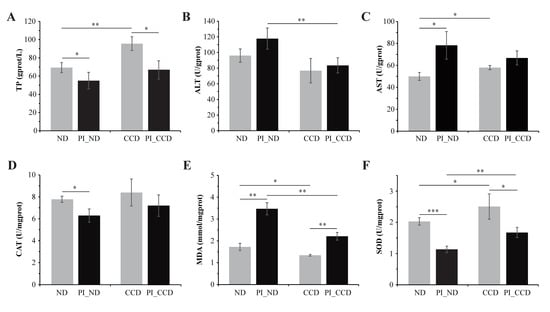
Figure 1.
Biochemical indexes in tilapia liver in the ND and CCD groups before and after SA infection. (A) TP content; (B) ALT level; (C) AST level; (D) CAT level; (E) MDA content; (F) SOD activity. * indicates significant difference (p < 0.05); ** indicates extremely significant difference (p < 0.01). *** indicates an extremely significant difference (p < 0.001).
3.2. Effect of CC on the Serum Immune Indexes of Healthy and SA-Infected Tilapia
The serum immune indexes, including C3, IgM, TNF-α, IFN-γ, and IL-1β levels, were higher in the serum of tilapia from the CCD group than in the ND group, especially after SA infection (Figure 2A–E). The increased rates of these immune indexes of the CCD vs. ND comparison were also higher in SA-infected tilapia than in healthy tilapia, especially C3 and IFN-γ (Figure 2F).
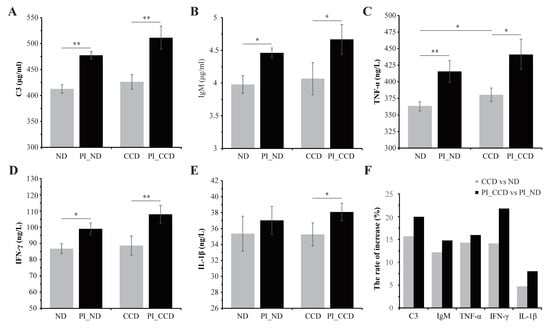
Figure 2.
Immunological indexes of tilapia liver in the ND and CCD groups before and after SA infection. (A) C3 content; (B) IgM content; (C) TNF-α content; (D) IFN-γ content; (E) IL-1β content; (F) increase rate for each index in the CCD vs. ND comparison before and after SA infection. * indicates significant difference (p < 0.05); ** indicates extremely significant difference (p < 0.01).
3.3. Protein Expression in SA-Infected Tilapia before and after CC Treatment
A high-throughput differential proteomics analysis based on iTRAQ label coupled with LC–MS/MS was performed for multiple groups in this study. Protein identification, quantification, and annotation details were listed in Table S2. The correlation heatmap of the overall protein expression levels in each group indicated a good positive correlation within the groups and low correlation between the groups (especially before and after infection) (Figure 3A). These results indicated that the samples were reproducible and that there were significant differences in the protein expression levels in the liver before and after infection. Expression heatmap clustering analysis of the quantified proteins in each sample suggested that the samples in various groups were clustered together, as were the pre-infection and post-infection samples (Figure 3B). These results further suggested that the samples had good reproducibility and that substantial differences were present in the liver protein expression levels before and after infection. DEP screening (FC > 2 or FC < 0.5, and p < 0.05) yielded 15 upregulated DEPs and 47 downregulated DEPs between the CCD and ND groups, 55 upregulated DEPs and 51 downregulated DEPs between the PI_ND and ND groups, 65 upregulated DEPs and 94 downregulated DEPs between the PI_CCD and CCD groups, and 22 upregulated DEPs and 14 downregulated DEPs between the PI_CCD and PI_ND groups (Figure 3C).
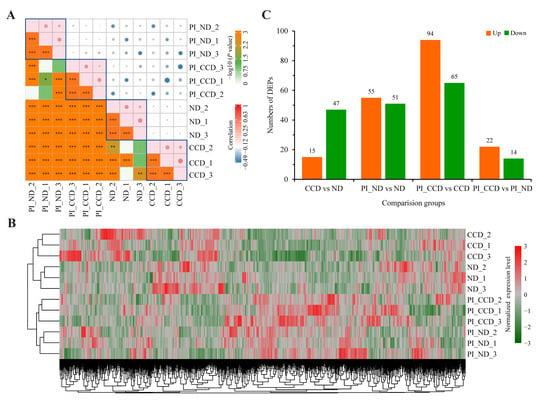
Figure 3.
Statistical analysis of the results of high-throughput differential proteomics analysis. (A) Correlation analysis among samples, asterisks represent significance of differences and dots represent correlation; (B) clustering heat map of protein expression for each sample; (C) differentially expressed proteins between various groups.
3.4. Changes in Protein Expression in CC-Treated Healthy Tilapia
A volcano plot of the differences in proteomic expression data between CCD and ND groups indicated the protein expression patterns in the two groups (Figure 4A). KOG functional classification of 47 downregulated DEPs yielded ten DEPs involved in C (energy production and conversion process) and some proteins involved in J (translation, ribosomal structure, and biogenesis), O (Posttranslational modification, protein turnover, chaperones), and other categories (Figure 4B). The KOG functional classification of 15 upregulated DEPs revealed that three DEPs were involved in C and O functional categories, respectively (Figure 4C).
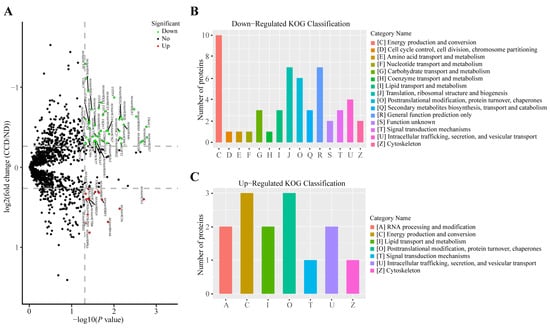
Figure 4.
Differential protein expression analysis of tilapia liver between the CCD and ND groups. (A) Volcano plot of differential protein expression between the CCD and ND groups; (B) KOG statistics of downregulated differentially expressed proteins (DEPs) between the CCD and ND groups; (C) KOG statistics of upregulated DEPs between the CCD and ND groups.
3.5. Changes in Protein Expression in CC-Treated SA-Infected Tilapia
The volcano plot of the differences in proteomic expression data between the PI_CCD and PI_ND groups indicates the protein expression patterns in the two groups (Figure 5A). KOG functional classification of the 14 downregulated DEPs showed three proteins each in the O (posttranslational modification, protein turnover, and chaperones) and R (general function prediction only) categories (Figure 5B). In addition, KOG functional classification of 22 upregulated DEPs revealed that four DEPs were involved in the O and R functional categories, and that three DEPs were involved in the Q (secondary metabolites biosynthesis, transport, and catabolism) category (Figure 5C).
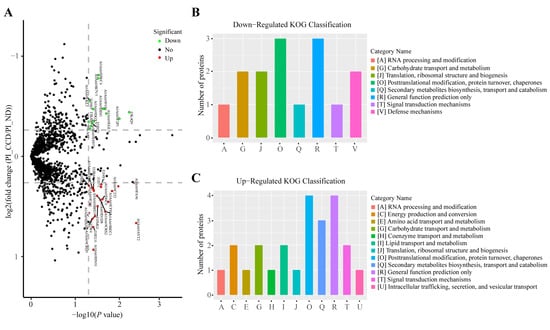
Figure 5.
Differential analysis of protein expression between PI_CCD group and PI_ND group of tilapia liver infected with Streptococcus agalactiae. (A) Volcano plot of differential protein expression between the PI_CCD and PI_ND groups; (B) KOG statistics of downregulated differentially expressed proteins (DEPs) between the PI_CCD and PI_ND groups; (C) KOG statistics of up-regulated DEPs between the PI_CCD and PI_ND groups.
3.6. Functional Analysis of CC-Specific Regulatory Proteins in Tilapia before and after SA Infection
To better understand the protein expression profiles of CC-treated SA-infected tilapia, we defined the DEPs that were upregulated between the PI_CCD and CCD groups but not between the PI_ND and ND groups as CCD-specific upregulated DEPs, and those that were downregulated between the PI_CCD and CCD groups but not between the PI_ND and ND groups as CCD-specific downregulated DEPs. In all, 80 CCD-specific upregulated proteins (Figure 6A) and 49 CCD-specific downregulated DEPs (Figure 6B) were screened from the Venn diagram. According to the results of the KEGG pathway analysis, upregulated DEPs were primarily associated with neurodegeneration, lysosome, lipid metabolism, mitochondrial autophagy, endocytosis, MAPK, and Toll-like receptor, whereas downregulated DEPs were primarily associated with glycolysis/gluconeogenesis, TCA cycle, cGMP-PKG, and pancreatic secretion (Figure 6C). In addition, upregulated DEPs were primarily involved in KEGG BRITE categories, such as exosomes, membrane transport, cytoskeleton, and proteolipid protein. In contrast, the number of downregulated DEPs involved in enzymatic activity-relayed KEGG BRITES was greater than the number of upregulated DEPs (Figure 6D).
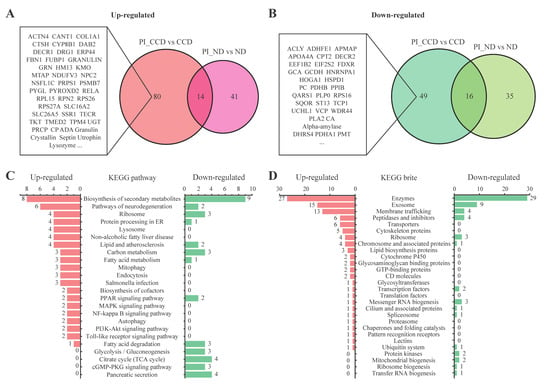
Figure 6.
Functional analysis of Streptococcus agalactiae-infected tilapia before and after Coptis chinensis (CC) treatment. (A) CCD-specific upregulated differentially expressed proteins (DEPs) in SA-infected tilapia before and after CC treatment and a representative Venn diagram; (B) CCD-specific downregulated DEPs in SA-infected tilapia before and after CC treatment and a representative Venn diagram; (C) KEGG pathway annotation of CCD-specific regulatory DEPs in SA-infected tilapia before and after CC treatment; (D) KEGG BRITE annotation of CCD-specific regulatory DEPs in SA-infected tilapia before and after CC treatment.
4. Discussion
CC, a traditional Chinese herb, has been recognized for its immune-enhancing effects in aquaculture. Choi et al. [10] reported that a modified detoxification decoction supplemented with 1% CC significantly enhanced the resistance of grey mullet (Mugil cephalus) to Lactococcus garvieae infection. However, studies on the mechanism of action of CC in enhancing antioxidant and immune effects in fish have been lacking. In this study, we determined the biochemical and immunological indexes and the differences in the protein expression of SA-infected tilapia before and after CC treatment to identify the potential proteins in CC that can improve the immune function of tilapia.
4.1. CC Supplementation in Feed Enhances the Antioxidant Function of Tilapia
The TP content is closely associated with protein synthesis in the liver, which can be impaired by liver damage [11]. When liver function is severely impaired, protein synthesis is significantly reduced. A study by Xu et al. [12] on Vibrio harveyi-infected Pseudosciaena crocea revealed that the TP and globulin levels were significantly lower in diseased P. crocea than in normal P. crocea. In the present study, the TP content in the liver of tilapia from the CCD group was significantly higher than that in those from the control group both before and after SA infection, indicating that CC can promote protein synthesis in the liver to an extent. AST/ALT is an essential aminotransferase involved in amino acid metabolism. When hepatocytes are damaged, hepatocyte membrane permeability increases, resulting in the entry of AST into the bloodstream and a decrease in enzyme activity in the liver [12]. In the present study, liver AST levels were significantly higher after SA infection than before infection. We hypothesized that SA infection affected hepatic amino acid metabolism and disrupted liver function.
SOD, CAT, and MDA are key antioxidant enzymes in living organisms and play a crucial role in the antioxidant system. SOD protects cells from peroxide-mediated damage by scavenging reactive oxygen species through disproportionation. CAT is commonly found in nearly all tissues of the animal body, and it is most abundant in the liver. It provides oxygen to cells while scavenging excess peroxyl radicals to reduce oxidative damage. CAT and SOD are strongly associated and act in conjunction to maintain the dynamic balance in the antioxidant system of organisms. MDA, one of the products of peroxidation by oxygen radicals, severely affects normal functions, such as cellular metabolism, by destroying the structure of the cell membrane by polymerizing with intracellular proteins. The alteration of these factors can be used as a biomarker for oxidative damage in the cells of the organism. Mu et al. [13] revealed that propolis supplementation in a basal diet increased the activity of antioxidant enzymes, such as SOD and CAT, and the expression of several cytokines in turbot (Scophthalmus maximus) infected with Edwardsiella piscicida. In the present study, the activities of SOD were increased in the CCD group compared to those in the ND group; in addition, the activity of SOD was significantly higher in the CCD group than in the ND group pre- and post-infection, indicating that CC enhanced SOD activity, reduced the oxidation of free radicals in vivo, and protected the body from oxidative damage. In an experiment to study the effects of different strains of SA on blood and related biochemical indexes in tilapia, Ao et al. [14] found that the SOD and CAT levels in the liver of tilapia were lower than normal at 24 h after SA infection. Consistently, results showed that the MDA contents were increased in ND and CCD groups post-SA infection. However, the MDA content was significantly lower in the CCD group than the ND group before and after infection. These results suggest that SA infection enhances lipid oxidation and produces harmful substances, such as MDA. However, CCD alleviates the damage to the antioxidant system by enhancing the activity of antioxidant enzymes, thus establishing dynamic balance.
4.2. CC Supplementation in Feed Enhances the Immune Function of Tilapia
Tilapia (a lower, poikilothermic, and bony type of fish) fight against pathogenic bacteria using nonspecific immune mechanisms. Three cytokines, IL-1β, IFN-γ, and TNF-α, are primarily involved in innate nonspecific immunity. IL-1β, an important member of the IL-1 family, is a pro-inflammatory factor secreted primarily by activated macrophages and monocytes, and it is involved in the physiological and pathological responses of the body. When the body is stimulated by cytokines, microorganisms, and their products, IL-1β bind to cell surface receptors to induce cellular inflammatory responses and modulate other factors to enhance immune regulation [15,16]. IFN-γ enhances the phagocytic potential of macrophages and the disruptive power of NK cells and promotes the differentiation of B cells to produce antibodies. In addition, it can influence B and T lymphocytes to regulate the immune response [17]. TNF-α, also a pro-inflammatory factor, kills invading pathogens by activating neutrophils, promotes the degradation of extracellular proteins in inflammation, and causes inflammatory cells to release more cytokines and inflammatory mediators [18]. Wang et al. [19] investigated the effect of Chinese yam (Dioscoreae Rhizoma) extract on the growth and nonspecific immunity of rainbow trout, and they found that the extract increased the levels of pro-inflammatory factors, such as TNF-α, in serum. The results of the present study indicated that CC supplementation in the feed promoted the secretion of cytokines, especially pro-inflammatory factors, to enhance non-specific immunity and improve the immunity of the organism. In addition, the increased ratios of various serum cytokine levels were significantly higher in the comparison of the CCD group vs. ND group after SA infection than before infection. After the SA infection of tilapia, inflammatory cells were stimulated to secrete inflammatory factors that enhanced the pro-inflammatory effect, which promoted the differentiation of B cells, thus boosting the production of antibodies and enhancing phagocytosis. The immunoglobulins of bony fish, including IgM, IgD, and IgT/Z, play a crucial role in humoral immunity [20,21]. IgM, the most abundant and earliest identified immunoglobulin, is primarily found in the serum and mucosa [22]. The level of immunoglobulins in serum can indicate the expression level of immunoglobulins. C3 has functions in bony fish similar to those in mammals and is primarily found in serum. C3 is not biologically active and functions only when cleaved into C3a and C3b by other factors. These factors play an important role in the defense mechanism and homeostasis of the organism and can assist phagocytes and antibodies to eliminate pathogenic microorganisms from the organism [19,23]. Kui and Liu [24] reported the elevation of C3 levels in Pelteobagrus fulvidraco injected with an inactivated E. ictaluri vaccine, indicating an elevated physiological immune response. In the present study, the C3 levels increased significantly in response to SA injection, indicating an improved immune response in tilapia, which was consistent with the findings of the abovementioned study. In addition, the serum levels of IgM and C3 differed significantly in the pairwise comparisons among the two groups, with the highest levels observed in the PI_CCD group. Therefore, the addition of 1% CC to the feed can significantly improve the humoral immune function of tilapia and enhance their immunity to an extent.
4.3. CC Regulates the Expression of Antimicrobial Proteins in the Liver of Tilapia
Lysozymes and cathepsins, two classes of small-molecule lyases, play a crucial role in host innate defense [25]. Lysozymes can provide defense against bacterial infection by cleaving peptidoglycan molecules in cell walls and act as antimicrobial peptides by disrupting the bacterial membrane structure and activating autolytic enzymes in the bacterial cell wall [26]. Extensive studies in the field of aquaculture have shown the essential role of fish lysozymes in their innate immune system. The expression and activities of lysozymes were significantly elevated in catfish (Ictalurus punctatus) to provide defense against pathogenic microbial infection [27,28]. The addition of Glycyrrhiza uralensis extracts to the feed significantly increased lysozyme activity in the serum and the expression of genes encoding lysozymes in the head kidney of yellow catfish (P. fulvidraco), thus enhancing their antimicrobial activity [29]. Moreover, the addition of fermented lemon peel to the diet effectively increased lysozyme activity and enhanced the resistance of orange-spotted grouper to Photobacterium [30]. Similarly, in the present study, we found that CC supplementation in the feed was effective in increasing the expression level of hepatic lysozymes, which could enhance the resistance of tilapia to SA to an extent. Lysosomal cathepsins are cysteine peptidases distributed ubiquitously during autophagic apoptosis that are involved in various biological processes, such as extracellular matrix remodeling, inflammation, pathogenic bacterial infection, and cancer [31]. Fish cathepsin B plays a crucial role in the defense of several species against pathogenic microbial infections, including olive flounder (Paralichthys olivaceus) against rhabdovirus [32], channel catfish (I. punctatus) against E. ictaluri and Flavobacterium [33], and tilapia (Oreochromis niloticus) against S. agalactiae [34]. Furthermore, the results of our proteomic analysis also indicated a significantly high expression of cathepsin protein in tilapia infected with SA.
Significant differences were observed in the expression between multiple antimicrobial peptides and antimicrobial proteins during SA infection. Septins, which are highly conserved cytoskeletal proteins, play a vital role in the innate immune and inflammatory response to bacterial infection in fish. According to Maria et al. [35], the depletion of Sept15 and Sept7b significantly increases susceptibility to bacteria in zebrafish. In catfish (I. punctatus) and rohu (Labeo rohita), the tissue expression levels of several septins are regulated by pathogenic bacterial infection and are closely related to their defense processes [36,37]. We observed that septin proteins were significantly upregulated in the CCD group, which may be attributed to the enhanced resistance to SA infection. In addition, a secreted glycosylated peptide, granulin a, and granulin domain-containing protein was also specifically regulated by CC. Granulin is a cysteine-rich regulatory growth factor that forms multiple pairs of disulfide bonds and regulates various processes, such as cell proliferation, tumor invasion, and immune response [38]. Potent peptide GRN-41 induces the expression of multiple immune factors, such as TNF-α, TNF-β, IL-8, IL-1β, IL-6, IL-26, IL-21, and IL-10 in transgenic zebrafish, thus effectively preventing V. vulnificus infection [39]. The above research team further identified the good antimicrobial activity of GRN-41 against Vibrio species in Mozambique tilapia [40]. Saleh et al. [41], in their proteomic study of Ichthyophthirius multifiliis-infected carp, found that granulin was significantly upregulated in the skin mucus, which was also consistent with the results of the present study. Moreover, a member of the penta-EF-hand protein family, Grancalcin, was reported to be translocated in macrophages in response to stimulation with bacterial LPS [42]. We also found that this protein was upregulated in the livers of tilapia fed with CC.
5. Conclusions
In conclusion, we applied a comprehensive iTRAQ-based liver proteomic analysis to evaluate the anti-SA effects on the molecular level of CCD. The results showed that CC supplementation can effectively improve the ability of tilapia to resist SA infection by modulating various antioxidant enzymes, immune factors, antimicrobial enzymes, and antimicrobial peptides, and can moderately inhibit central carbon metabolism. The results of the study provide a basis for replacing antibiotics with environmentally-friendly functional feed additives for the control of bacterial diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes8070370/s1, Table S1: The sequence of 16S rDNA of Streptococcus agalactiae strain. Table S2: Liver proteomic details in ND and CCD groups before and after SA infection of tilapia by LC-MS/MS.
Author Contributions
Conceptualization, K.H. and Q.L.; methodology, K.Y., R.G. and T.L.; funding acquisition, K.H.; proteomic analysis and bioinformatic analysis, K.Y.; writing—original draft preparation, R.G. and K.Y.; writing—review and editing, K.H. and K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Development Project of Key Laboratory on the Creation of Fishery Drugs of the Ministry of Chinese Agriculture and Rural Affairs (201807) and the National Natural Science Foundation of China (No. 32160865).
Institutional Review Board Statement
The animal experiments were approved and monitored by the Animal Experiments Ethical Review Committee of Guangxi University (approval code: GXU-2022-255).
Data Availability Statement
The original proteomic data (PXD00000, the accession No. will provide during review) can by fully accessed from the ProteomeXchange consortium via the PRIDE partner repository.
Acknowledgments
We acknowledge the State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources for the support of LC-MS/MS platform. We thank Nanning Current Science Biotechnology Co., Ltd. for optimizing the figures and editing this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Z. Research Advances on Tilapia Streptococcosis. Pathogens 2021, 10, 558. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhu, J.; Chen, K.; Gao, T.; Yao, H.; Liu, Y.; Zhang, W.; Lu, C. Development of Streptococcus agalactiae vaccines for tilapia. Dis. Aquat. Org. 2016, 122, 163–170. [Google Scholar] [CrossRef]
- Barkham, T.; Zadoks, R.N.; Azmai, M.N.A.; Baker, S.; Bich, V.T.N.; Chalker, V.; Chau, M.L.; Dance, D.; Deepak, R.N.; van Doorn, H.R.; et al. One hypervirulent clone, sequence type 283, accounts for a large proportion of invasive Streptococcus agalactiae isolated from humans and diseased tilapia in Southeast Asia. PLOS Negl. Trop. Dis. 2019, 13, e0007421. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Lou, G.; Zeng, H.-R.; Hu, J.; Huang, Q.; Peng, W.; Yang, X.-B. Coptidis Rhizoma: A comprehensive review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. Pharm. Biol. 2019, 57, 193–225. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.Q.; Liu, D.L. Progress in pharmacological research of Coptis chinensis. Gansu Agric. 2019, 10, 97–99. [Google Scholar]
- Yu, F.X.; Cao, M.X.; Liang, W.W.; Wang, C.H.; Wang, S.; Wei, Y.Y.; Hu, T.J. Clinical application of Sanhuanglian powder in prevention of Streptococcicosis in tilapia. Anim. Husb. Feed Sci. 2020, 41, 86–91. [Google Scholar]
- Wang, C.H.; Yu, F.X.; Zhao, Y.; Liang, W.W.; Wei, Y.Y.; Hu, T.J. Clinical application effect of Sanhuanglian mixture in preventing tilapia against Streptococcosis. Guangxi J. Anim. Husb. Vet. Med. 2020, 36, 147–150. [Google Scholar]
- Niu, Z.W.; Fan, H.L.; Long, S.; Huang, J.; Zhu, H.F.; Huang, G.C.; Liang, J.Z. A Meta-analysis: The effect of three kinds of Chinese herbalmedicines for tilapia Streptococcus agalactiae. J. Fish. Res. 2016, 38, 409–414. [Google Scholar]
- Matumoto, M. A note on some points of calculation method of LD50 by Reed and Muench. Jpn. J. Exp. Med. 1949, 20, 175–179. [Google Scholar]
- Choi, W.; Lam, C.; Mo, W.; Cheng, Z.; Mak, N.; Bian, Z.; Wong, M. Effects of the modified Huanglian Jiedu decoction on the disease resistance in grey mullet (Mugil cephalus) to Lactococcus garvieae. Mar. Pollut. Bull. 2014, 85, 816–823. [Google Scholar] [CrossRef]
- Ji, D.W.; Li, M.Y.; Wang, T.Z.; Zhang, C.N.; Xu, Z.; Xu, W.T. Effects of low temperature stress periods on serum biochemical indexes in large yellow croaker Pseudosciaena crocea. Fish. Sci. 2009, 28, 1–4. [Google Scholar]
- Xu, X.-J.; Xu, B.; Wang, J.; Su, Y.-Q.; Zhang, Z.-W.; Chen, X. Studies on blood chemistry indices and histopathology of Pseudosciaena crocea artificially challenged with Vibrio harveyi. J. Fish. China 2010, 34, 618–625. [Google Scholar] [CrossRef]
- Mu, D.; Jiang, Y.; He, J.; Zhang, Y.; Yang, D.; Liu, Q.; Wang, Z. Dietary supplementation of propolis enhanced the innate immune response against Edwardsiella piscicida challenge in turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2022, 124, 273–279. [Google Scholar] [CrossRef]
- Ao, Q.W.; Lu, Y.J.; Lu, M.; Zhu, J.J. Effects of Streptococcus agalactiae infection on blood and hepatopancreatic tissue biochemical indices in different species of tilapia. Prog. Fish. Sci. 2020, 41, 167–173. [Google Scholar]
- Bird, S.; Zou, J.; Wang, T.; Munday, B.; Cunningham, C.; Secombes, C.J. Evolution of interleukin-1β. Cytokine Growth Factor Rev. 2002, 13, 483–502. [Google Scholar] [CrossRef] [PubMed]
- Danilko, K.V.; Korytina, G.F.; Akhmidishina, L.Z.; Ianbaeva, D.G.; Zagidullin, S.Z.; Victorova, T.V. Association of cytokines genes (IL1B, IL1RN, TNFA, LTA, IL6, IL8, and IL10) polymorphic markers with chronic obstructive pulmonary disease. Mol. Biol. 2007, 41, 26–36. [Google Scholar] [CrossRef]
- Roberts-Thomson, I.C.; Fon, J.; Uylaki, W.; Cummins, A.G.; Barry, S. Cells, cytokines and inflammatory bowel disease: A clinical perspective. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Salomon, B.L. Insights into the biology and therapeutic implications of TNF and regulatory T cells. Nat. Rev. Rheumatol. 2021, 17, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, C.G.; Ma, Y.W.; Liu, F.; Meng, X.J. Effects of yam extract on growth performance and non-specific immune parameters of rainbow trout (Oncorhynchus mykiss). J. Henan Agric. Sci. 2020, 49, 167–172. [Google Scholar] [CrossRef]
- Castro, R.; Bromage, E.; Abós, B.; Pignatelli, J.; Granja, A.G.; Luque, A.; Tafalla, C. CCR7 Is Mainly Expressed in Teleost Gills, Where It Defines an IgD+IgM− B Lymphocyte Subset. J. Immunol. 2014, 192, 1257–1266. [Google Scholar] [CrossRef]
- Zhang, Y.-A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; La Patra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, M.R.; Notley, C.A. The importance of natural IgM: Scavenger, protector and regulator. Nat. Rev. Immunol. 2010, 10, 778–786. [Google Scholar] [CrossRef]
- Li, X.T.; Ke, X.L.; Lu, M.X.; Liu, Z.G.; Wang, M.; Pang, J.C. Cloning and tissues expression analysis of complement C3 of tilapia. Biotechnology 2016, 26, 205–212. [Google Scholar] [CrossRef]
- Kui, L.L.; Liu, Y. Effects of Edwardsiella ictaluri inactivated vaccine on immune gene wxpression and activities of CAT, SOD and complement C3 in yellow catfish Pelteobagrus fulvidraco. Fish. Sci. 2015, 34, 150–154. [Google Scholar] [CrossRef]
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef]
- Farnsworth, R.H.; Achen, M.G.; Stacker, S.A. Lymphatic endothelium: An important interactive surface for malignant cells. Pulm. Pharmacol. Ther. 2006, 19, 51–60. [Google Scholar] [CrossRef]
- Bilodeau-Bourgeois, L.; Bosworth, B.G.; Peterson, B.C. Differences in mortality, growth, lysozyme, and Toll-like receptor gene expression among genetic groups of catfish exposed to virulent Edwardsiella ictaluri. Fish Shellfish Immunol. 2008, 24, 82–89. [Google Scholar] [CrossRef]
- Small, B.C.; Bilodeau, A.L. Effects of cortisol and stress on channel catfish (Ictalurus punctatus) pathogen susceptibility and lysozyme activity following exposure to Edwardsiella ictaluri. Gen. Comp. Endocrinol. 2005, 142, 256–262. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, J.; Yan, Z.; Xiang, X.; Mu, R.; Zhu, P.; Yao, Y.; Zhu, F.; Chen, K.; Chi, S.; et al. Dietary Glycyrrhiza uralensis extracts supplementation elevated growth performance, immune responses and disease resistance against Flavobacterium columnare in yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2020, 97, 153–164. [Google Scholar] [CrossRef]
- Zhuo, L.-C.; Chen, C.-F.; Lin, Y.-H. Dietary supplementation of fermented lemon peel enhances lysozyme activity and susceptibility to Photobacterium damselae for orange-spotted grouper, Epinephelus coioides. Fish Shellfish Immunol. 2021, 117, 248–252. [Google Scholar] [CrossRef]
- Gobec, S. Inhibitors of Cathepsin B. Curr. Med. Chem. 2006, 13, 2309–2327. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Chen, Y.; Zhu, R.; Dong, C.; Li, Y.; Zhang, Q.; Gui, J. Expressional induction of Paralichthys olivaceus cathepsin B gene in response to virus, poly I:C and lipopolysaccharide. Fish Shellfish Immunol. 2008, 25, 542–549. [Google Scholar] [CrossRef]
- Li, C.; Song, L.; Tan, F.; Su, B.; Zhang, D.; Zhao, H.; Peatman, E. Identification and mucosal expression analysis of cathepsin B in channel catfish (Ictalurus punctatus) following bacterial challenge. Fish Shellfish Immunol. 2015, 47, 751–757. [Google Scholar] [CrossRef]
- Li, L.; Shi, Y.; Wang, R.; Huang, T.; Liang, W.; Luo, H.; Gan, X.; Huang, W.; Li, J.; Lei, A.; et al. Proteomic analysis of tilapia Oreochromis niloticus Streptococcus agalactiae strains with different genotypes and serotypes. J. Fish Biol. 2015, 86, 615–636. [Google Scholar] [CrossRef]
- Mazon-Moya, M.J.; Willis, A.R.; Torraca, V.; Boucontet, L.; Shenoy, A.R.; Colucci-Guyon, E.; Mostowy, S. Septins restrict inflammation and protect zebrafish larvae from Shigella infection. PLOS Pathog. 2017, 13, e1006467. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Li, Y.; Yang, Y.; Li, C.; Yao, J.; Zeng, Q.; Qin, Z.; Liu, S.; Li, D.; Liu, Z. Septin genes in channel catfish (Ictalurus punctatus) and their involvement in disease defense responses. Fish Shellfish Immunol. 2016, 49, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chhottaray, C.; Das Mahapatra, K.; Saha, J.N.; Baranski, M.; Robinson, N.; Sahoo, P.K. Analysis of immune-related ESTs and differential expression analysis of few important genes in lines of rohu (Labeo rohita) selected for resistance and susceptibility to Aeromonas hydrophila infection. Mol. Biol. Rep. 2014, 41, 7361–7371. [Google Scholar] [CrossRef] [PubMed]
- Klupp, F.; Kahlert, C.; Franz, C.; Halama, N.; Schleussner, N.; Wirsik, N.M.; Warth, A.; Schmidt, T.; Ulrich, A.B. Granulin: An Invasive and Survival-Determining Marker in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 6436. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Lin, H.-J.; Lin, W.-F.; Wu, J.-L.; Gong, H.-Y. A potent tilapia secreted granulin peptide enhances the survival of transgenic zebrafish infected by Vibrio vulnificus via modulation of innate immunity. Fish Shellfish Immunol. 2018, 75, 74–90. [Google Scholar] [CrossRef]
- Wu, S.-H.; Chou, H.-Y.; Liu, P.-C.; Wu, J.-L.; Gong, H.-Y. Granulin peptide GRN-41 of Mozambique tilapia is a novel antimicrobial peptide against Vibrio species. Biochem. Biophys. Res. Commun. 2019, 515, 706–711. [Google Scholar] [CrossRef]
- Saleh, M.; Kumar, G.; Abdel-Baki, A.-A.S.; Dkhil, M.A.; El-Matbouli, M.; Al-Quraishy, S. Quantitative proteomic profiling of immune responses to Ichthyophthirius multifiliis in common carp skin mucus. Fish Shellfish Immunol. 2019, 84, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Shinomiya, H.; Kirikae, T.; Hirata, H.; Asano, Y. Characterization of Murine Grancalcin Specifically Expressed in Leukocytes and Its Possible Role in Host Defense against Bacterial Infection. Biosci. Biotechnol. Biochem. 2004, 68, 894–902. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).