The Effects of Replacing Fish Meal with Enzymatic Soybean Meal on the Growth Performance, Whole-Body Composition, and Health of Juvenile Gibel Carp (Carassius auratus gibelio)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Fish and Experimental Rearing Conditions
2.3. Collection of Samples
2.4. Chemical Analysis
2.5. Hematoxylin and Eosin (HE) Staining
2.6. Statistics Analysis
3. Results
3.1. Growth Performance
3.2. Whole-Body Composition
3.3. Plasma Biochemical Indices
3.4. Results of Plasma Antioxidants Indices
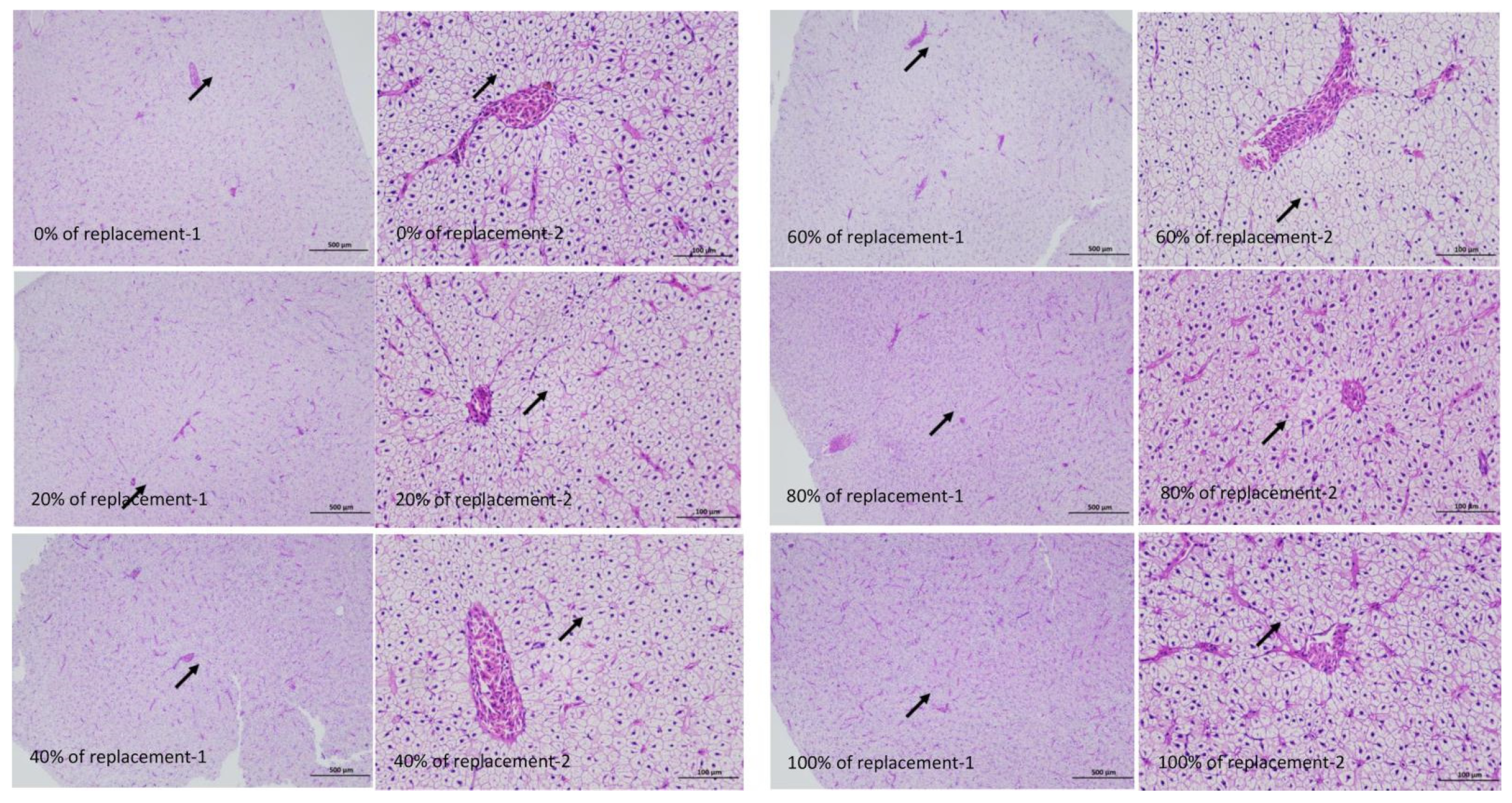
3.5. Results of Hepatic Tissue Structure
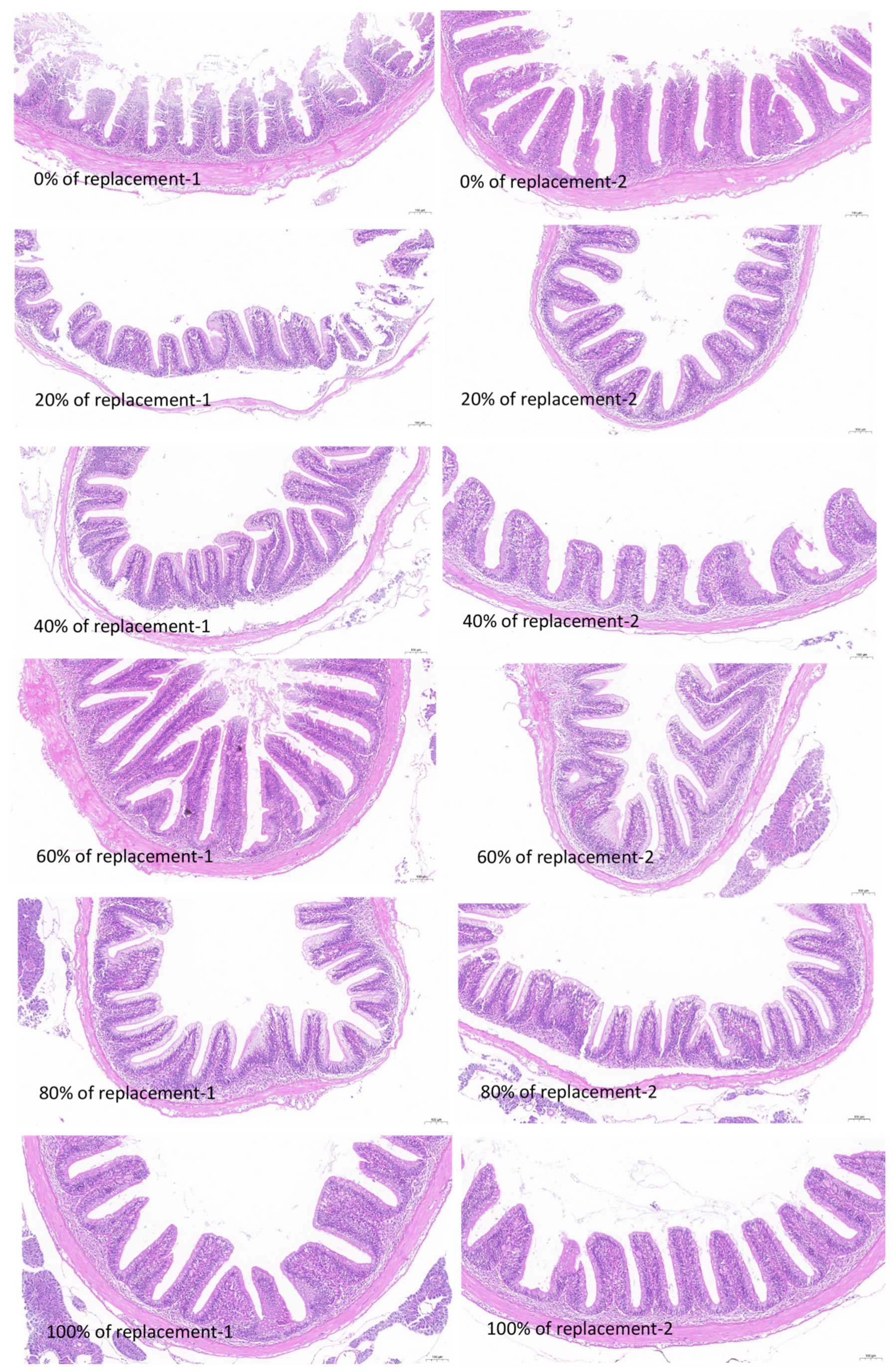
3.6. Results of Intestine Tissue Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyd, C.E.; McNevin, A.A.; Davis, R.P. The contribution of fisheries and aquaculture to the global protein supply. Food Secur. 2022, 14, 805–827. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Deng, P.; Dai, C.J.; Wu, M.K.; Liu, X.Q.; Li, L.J.; Pan, X.Y.; Yuan, J.F. Investigation of putative antimicrobial peptides in Carassius gibel, revealing a practical approach to screening antimicrobials. Fish Shellfish Immunol. 2022, 121, 254–264. [Google Scholar] [CrossRef]
- Zhou, Q.L.; Xie, J.; Ge, X.P.; Habte-Tsion, H.M.; Liu, B.; Ren, M.C. Growth performance and immune response of gibel carp, Carassius auratus gibelio, fed with graded level of rare earth-chitosan chelate. Aquacult. Int. 2016, 24, 453–463. [Google Scholar] [CrossRef]
- Thompson, K.R.; Muzinic, L.A.; Engler, L.S.; Morton, S.R.; Webster, C.D. Effects of feeding practical diets containing various protein levels on growth, survival, body composition, and processing traits of Australian red claw crayfish (Cherax quadricarinatus) and on pond water quality. Aquac. Res. 2004, 35, 659–668. [Google Scholar] [CrossRef]
- Ji, K.; He, J.Y.; Liang, H.L.; Ren, M.C.; Ge, X.P.; Masagounder, K. Response of gibel carp (Carassius auratus gibelio) to increasing levels of dietary lysine in zero fish meal diets. Aquacult. Nutr. 2021, 27, 49–62. [Google Scholar] [CrossRef]
- Ren, M.C.; Liang, H.L.; He, J.Y.; Masagounder, K.; Yue, Y.; Yang, H.; Ge, X.P.; Xi, B. Effects of DL-methionine supplementation on the success of fish meal replacement by plant proteins in practical diets for juvenile gibel carp (Carassius auratus gibelio). Aquacult. Nutr. 2017, 23, 934–941. [Google Scholar] [CrossRef]
- Liu, T.; Han, T.; Wang, J.T.; Liu, T.; Bian, P.; Wang, Y.B.; Cai, X.J. Effects of replacing fish meal with soybean meal on growth performance, feed utilization and physiological status of juvenile redlip mullet Liza haematocheila. Aquacul. Rep. 2021, 20, 100756. [Google Scholar] [CrossRef]
- Dersjant-Li, Y. The use of soy protein in aquafeeds. Oilseeds Focus 2021, 7, 29–31. Available online: https://hdl.handle.net/10520/ejc-vp_oilseeds_v7_n2_a10 (accessed on 26 June 2023).
- Porter, M.A.; Jones, A.M. Variability in soy flour composition. J. Am. Oil Chem. Soc. 2003, 80, 557–562. [Google Scholar] [CrossRef]
- Tan, C.; Zhou, H.H.; Wang, X.; Mai, K.S.; He, G. Resveratrol attenuates oxidative stress and inflammatory response in turbot fed with soybean meal based diet. Fish Shellfish Immunol. 2019, 91, 130–135. [Google Scholar] [CrossRef]
- Gatlin, D.M.; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W.; Wurtele, E. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Hernández, M.D.; Martínez, F.J.; Jover, M.; Garcia, B.G. Effects of partial replacement of fish meal by soybean meal in sharpsnout seabream (Diplodus puntazzo) diet. Aquaculture 2007, 263, 159–167. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, Q.C.; Feng, J.; He, J.J.; Lou, Y.D.; Zhu, J.Q. Effect of dietary fermented soybean meal on growth, intestinal morphology and microbiota in juvenile large yellow croaker, Larimichthys crocea. Aquac. Res. 2019, 50, 748–757. [Google Scholar] [CrossRef]
- NRC (National Research Council). Nutrient Requirements of Fish and Shrimp; National Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Sun, X.D. Enzymatic hydrolysis of soy proteins and the hydrolysates utilisation. Int. J. Food Sci. Technol. 2011, 46, 2447–2459. [Google Scholar] [CrossRef]
- Jacobsen, H.J.; Kousoulaki, K.; Sandberg, A.S.; Carlsson, N.G.; Ahlstrom, O.; Oterhals, A. Enzyme pre-treatment of soybean meal: Effects on non-starch carbohydrates, protein, phytic acid, and saponin biotransformation and digestibility in mink (Neovison vison). Anim. Feed Sci. Technol. 2018, 236, 1–13. [Google Scholar] [CrossRef]
- Tibaldi, E.; Hakim, Y.; Uni, Z.; Tulli, F.; Francesco, M.D.; Luzzana, U.; Harpaz, H. Effects of the partial substitution of dietary fish meal by differently processed soybean meals on growth performance, nutrient digestibility and activity of intestinal brush border enzymes in the European sea bass (Dicentrarchus labrax). Aquaculture 2006, 261, 182–193. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists, 15th ed.; Association of Official Analytical Chemists Inc.: Arlington, TX, USA, 2003. [Google Scholar]
- Liang, H.L.; Wu, L.H.; Chama, M.K.H.; Ge, X.P.; Ren, M.C.; Chen, X.R.; Pan, L.K.; Xia, D. Tributyrin Plays an Important Role in Regulating the Growth and Health Status of Juvenile Blunt Snout Bream (Megalobrama amblycephala), as Evidenced by Pathological Examination. Front. Immunol. 2021, 12, 652294. [Google Scholar] [CrossRef]
- Gui, D.; Liu, W.B.; Shao, X.P.; Xu, W.N. Effects of different dietary levels of cottonseed meal protein hydrolysate on growth, digestibility, body composition and serum biochemical indices in crucian carp (Carassius auratus gibelio). Anim. Feed Sci. Technol. 2010, 156, 112–120. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, L.K.; Li, C.; Bureau, D.P. Effect of replacing fish meal with soybean meal on growth, feed utilization and carcass composition of Cuneate drum (Nibea miichthioides). Aquaculture 2006, 261, 1307–1313. [Google Scholar] [CrossRef]
- Liu, X.; Chi, S.Y.; Li, S.; Cheng, X.L.; Gao, W.H.; Xu, Q.Q.; Zhang, W.B.; Zhou, X.Q. Substitution of fish meal with enzyme-treated soybean in diets for juvenile largemouth bass (Micropterus salmoides). Aquacult. Nutr. 2021, 27, 1569–1577. [Google Scholar] [CrossRef]
- Haghbayan, S.; Mehrgan, M.S. The Effect of Replacing Fish meal in the Diet with Enzyme-Treated Soybean Meal (HP310) on Growth and Body Composition of Rainbow Trout Fry. Molecules 2015, 20, 21058–21066. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Luo, K.; Guo, Y.L.; Gao, W.H.; Xu, Q.Y.; Zhang, W.B.; Mai, K.S. Replacement of fish meal by enzyme-treated soybean on the growth performance, intestine microflora, immune responses and disease resistance of Pacific white shrimp (Litopenaeus vannamei). Aquac. Res. 2021, 52, 4619–4628. [Google Scholar] [CrossRef]
- Lin, H.; Tan, B.; Ray, G.W.; Zeng, M.; Li, M.; Chi, S.; Yang, Q. A Challenge to Conventional Fish Meal: Effects of Soy Protein Peptides on Growth, Histomorphology, Lipid Metabolism and Intestinal Health for Juvenile Pompano Trachinotus ovatus. Front. Mar. Sci. 2022, 8, 815323. [Google Scholar] [CrossRef]
- Voorhees, J.M.; Barnes, M.; Chipps, S.; Brown, M. Direct substitution of fishmeal with bioprocessed soybean meal in brown trout diets. J. Fish. Aqua. Dev. 2018, 1, 100043. [Google Scholar] [CrossRef]
- Liang, H.; Xu, G.; Xu, P.; Zhu, J.; Li, S.; Ren, M. Dietary Histidine Supplementation Maintained Amino Acid Homeostasis and Reduced Hepatic Lipid Accumulation of Juvenile Largemouth Bass, Micropterus Salmoides. Aquac. Nutr. 2022, 2022, 4034922. [Google Scholar] [CrossRef]
- Li, S.L.; Ding, G.T.; Song, F.; Sang, C.Y.; Wang, A.; Chen, N.S. Comparison of dehulled, fermented and enzyme-treated soybean meal in diets for largemouth bass, Micropterus salmoides: Effects on growth performance, feed utilization, immune response and intestinal morphology. Anim. Feed Sci. Technol. 2020, 267, 114548. [Google Scholar] [CrossRef]
- Liu, X.R.; Han, B.; Xu, J.; Zhu, J.Y.; Hu, J.T.; Wan, W.L.; Miao, S.Y. Replacement of fish meal with soybean meal affects the growth performance, digestive enzymes, intestinal microbiota and immunity of Carassius auratus gibelio♀ × Cyprinus carpio♂. Aquacult. Rep. 2020, 18, 100472. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Lu, K.L.; Wang, L.; Song, K.; Mai, K.S.; Davis, D.A.; Zhang, C.X. Replacement of fish meal with Bacillus pumillus SE5 and Pseudozyma aphidis ZR1 fermented soybean meal in diets for Japanese seabass (Lateolabrax japonicus). Fish Shellfish Immunol. 2019, 84, 987–997. [Google Scholar] [CrossRef]
- Duan, Z.P.; Zhang, C.Y.; Huang, L.L.; Lan, Q.; Hu, J.; Li, X.Q.; Leng, X.J. An Evaluation of Replacing Fish Meal with Fermented Soybean Meal in Diet of Hybrid Snakehead (Channa argus × Channa maculata): Growth, Nutrient Utilization, Serum Biochemical Indices, Intestinal Histology, and Microbial Community. Aquac. Nutr. 2022, 2022, 2964779. [Google Scholar] [CrossRef]
- Roques, S.; Deborde, C.; Richard, N.; Skiba-Cassy, S.; Moing, A.; Fauconneau, B. Metabolomics and fish nutrition: A review in the context of sustainable feed development. Rev. Aquacult. 2020, 12, 261–282. [Google Scholar] [CrossRef]
- Sardar, P.; Abid, M.; Randhawa, H.S.; Prabhakar, S.K. Effect of dietary lysine and methionine supplementation on growth, nutrient utilization, carcass compositions and haemato-biochemical status in Indian Major Carp, Rohu (Labeo rohita H.) fed soy protein diet. Aquac. Nutr. 2009, 15, 339–346. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Liang, H.L.; Xu, P.; Xu, G.C.; Zhang, L.; Wang, Y.L.; Ren, M.C.; Chen, R. Effects of Enzymatic Cottonseed Protein Concentrate as a Feed Protein Source on the Growth, Plasma Parameters, Liver Antioxidant Capacity and Immune Status of Largemouth Bass (Micropterus salmoides). Metabolites 2022, 12, 1233. [Google Scholar] [CrossRef]
- Ajani, E.K.; Orisasona, O.; Omitoyin, B.O.; Osho, E.F. Total Replacement of Fish meal by Soybean Meal with or Without Methionine Fortification in the Diets of Nile Tilapia, Oreochromis niloticus. J. Fish. Aquat. Sci. 2016, 11, 238–243. [Google Scholar] [CrossRef]
- Martin, J.L.K.; Black, M.C. Biomarker assessment of the effects of coal-strip mine contamination on channel catfish. Ecotoxicol. Environ. Saf. 1998, 41, 307–320. [Google Scholar] [CrossRef]
- Ye, H.Q.; Xu, M.L.; Liu, Q.Y.; Sun, Z.Z.; Zou, C.Y.; Chen, L.L.; Su, N.N.; Ye, C.X. Effects of replacing fish meal with soybean meal on growth performance, feed utilization and physiological status of juvenile obscure puffer, Takifugu obscurus. Comp. Biochem. Phys. C 2019, 216, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Do, T.V.; Tran, H.D.; Nguyen, T.T. Replacement of fish meal with defatted and fermented soybean meals in pompano Trachinotus blochii (Lacepède, 1801) diets. Ann. Anim. Sci. 2021, 21, 575–587. [Google Scholar] [CrossRef]
- Setchell, K.D.; Cassidy, A. Dietary isoflavones: Biological effects and relevance to human health; 1999; Symposium on Phytochemicals: Biochemistry and Physiology. J. Nutr. 1999, 129, 758S–767S. [Google Scholar] [CrossRef]
- Moure, A.; Domínguez, H.; Parajo, J.C. Antioxidant properties of ultrafiltration-recovered soy protein fractions from industrial effluents and their hydrolysates. Process Biochem. 2006, 41, 447–456. [Google Scholar] [CrossRef]
- Del, R.D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Kong, F.K.; Liu, T.; Liu, Y.J.; Yu, Z.K.; Zhang, W.; Fan, D.F.; Fan, J.Q.; Kong, L.C.; Li, B.; Chen, S.J.; et al. Dietary effects of enzymolytic soybean meal inclusion on antioxidant capacity, intestinal morphology and caecal microbiota of Rex rabbits. Ital. J. Anim. Sci. 2022, 21, 1220–1231. [Google Scholar] [CrossRef]
- Zakaria, M.K.; Kari, Z.A.; Doan, H.A.; Kabir, M.A.; Harun, H.C.; Sukri, S.A.M.; Khang Wee, K.W.G.W.; Khoo, M.I.; Wei, L.S. Fermented Soybean Meal (FSBM) in African Catfish (Clarias gariepinus) Diets: Effects on Growth Performance, Fish Gut Microbiota Analysis, Blood Haematology, and Liver Morphology. Life 2022, 12, 1851. [Google Scholar] [CrossRef] [PubMed]
- Kokou, F.; Sarropoulou, E.; Cotou, E.; Rigos, G.; Henry, M.; Alexis, M.; Kentouri, M. Effects of Fish meal Replacement by a Soybean Protein on Growth, Histology, Selected Immune and Oxidative Status Markers of Gilthead Sea Bream, Sparus aurata. J. World Aquacult. Soc. 2015, 46, 115–128. [Google Scholar] [CrossRef]
- Jacobsen, H.J.; Samuelsen, T.A.; Girons, A.; Kousoulaki, K. Different enzyme incorporation strategies in atlantic salmon diet containing soybean meal: Effects on feed quality, fish performance, nutrient digestibility and distal intestinal morphology. Aquaculture 2018, 491, 302–309. [Google Scholar] [CrossRef]
| Raw Material (%) | Replacing FM with ESBM Levels | |||||
|---|---|---|---|---|---|---|
| 0 | 20% | 40% | 60% | 80% | 100% | |
| Fish meal 1 | 15 | 12 | 9 | 6 | 3 | 0 |
| Chicken meal 1 | 5 | 5 | 5 | 5 | 5 | 5 |
| Soybean meal 1 | 15 | 15 | 15 | 15 | 15 | 15 |
| Enzymatic soybean meal 2 | 0 | 4 | 8 | 12 | 16 | 20 |
| Cottonseed meal 1 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 | 5.5 |
| Rapeseed meal 1 | 24 | 24 | 24 | 24 | 24 | 24 |
| Wheat meal 1 | 14.33 | 14.33 | 14.33 | 14.33 | 14.33 | 14.33 |
| Rice bran 1 | 15.65 | 13.64 | 11.62 | 9.60 | 7.57 | 5.55 |
| Soybean oil | 0.67 | 1.29 | 1.92 | 2.55 | 3.18 | 3.81 |
| Monocalcium phosphate | 2.00 | 2.33 | 2.66 | 3.00 | 3.33 | 3.66 |
| Vitamin premix for omnivorous fish 3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Trace element premix for omnivorous fish 4 | 2 | 2 | 2 | 2 | 2 | 2 |
| Lysine (98.5%) | 0.30 | 0.33 | 0.36 | 0.39 | 0.43 | 0.46 |
| DL-methionine | 0.10 | 0.13 | 0.16 | 0.18 | 0.21 | 0.24 |
| Vitamin C phosphates | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Choline chloride | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Proximate Composition (dry basis) | ||||||
| Moisture (%) | 8.56 | 8.51 | 8.47 | 8.51 | 8.55 | 8.47 |
| Crude protein (%) | 35.31 | 35.26 | 35.32 | 35.31 | 35.32 | 35.33 |
| Gross energy (MJ/kg) | 15.31 | 15.29 | 15.28 | 15.26 | 15.22 | 15.21 |
| Crude lipid (%) | 7.83 | 7.79 | 7.81 | 7.83 | 7.84 | 7.83 |
| Crude fiber (%) | 7.62 | 7.35 | 7.08 | 6.81 | 6.53 | 6.26 |
| P (%) | 1.47 | 1.45 | 1.46 | 1.48 | 1.46 | 1.48 |
| Item | Methods and Testing Equipment |
|---|---|
| Moisture | Dried to constant weight in an oven at 105 °C. |
| Protein | Determined by Hanon K1100 auto Kjeldahl apparatus (Jinan Hanon Instruments Co., Ltd., Jinan, China). |
| Lipid | Determined by Hanon SOX606 auto fat analyzer (Jinan Hanon Instruments Co., Ltd., Jinan, China). |
| Ash | Determined by burning at 550 °C for 5 h in an XL-2A intelligent muffle furnace (Hangzhou Zhuochi Instruments Co., Ltd., Hangzhou, China). |
| Alanine transaminase (ALT) | All plasma parameters were determined by assay kits (Mindray Bio Medical Co., Ltd., Shenzhen, China) with a Mindray BS-400 automatic biochemical analyzer (Mindray Medical International Ltd., Shenzhen, China). |
| Total cholesterol (TC) | |
| Glucose (GLU) | |
| Alkaline phosphatase (ALP) | |
| Albumin (ALB) | |
| Triglyceride (TG) | |
| Aspartic transaminase (AST) | |
| Total protein (TP) | |
| Total antioxidant capacity (T-AOC) | All hepatic antioxidant parameters and MDA levels were tested according to instructions of assay kits purchased from Jian Cheng Bioengineering Institute (Nanjing, China). |
| Superoxide dismutase (SOD) | |
| Catalase (CAT) | |
| Glutathione (GSH) | |
| Glutathione peroxidase (GPx) | |
| Malondialdehyde (MDA) |
| Parameters | Replacement Levels | ||||||
|---|---|---|---|---|---|---|---|
| 0% | 20% | 40% | 60% | 80% | 100% | p Value | |
| 2 IW (g) | 45.00 ± 0.23 | 45.02 ± 0.13 | 44.98 ± 0.12 | 45.05 ± 0.13 | 45.05 ± 0.23 | 45.00 ± 0.15 | 0.99 |
| 3 FW (g) | 229.89 ± 4.62 | 231.44 ± 0.51 | 226.62 ± 3.57 | 231.95 ± 2.85 | 230.45 ± 1.17 | 229.03 ± 2.24 | 0.31 |
| 4 FCR | 1.27 ± 0.03 | 1.21 ± 0.02 | 1.22 ± 0.02 | 1.20 ± 0.08 | 1.26 ± 0.08 | 1.30 ± 0.05 | 0.21 |
| 5 SGR (%/d) | 1.03 ± 0.011 | 1.03 ± 0.003 | 1.02 ± 0.010 | 1.03 ± 0.008 | 1.02 ± 0.006 | 1.02 ± 0.008 | 0.61 |
| 6 WGR (%) | 410.86 ± 9.35 | 414.13 ± 2.54 | 403.80 ± 7.93 | 414.87 ± 6.86 | 411.56 ± 4.82 | 408.97 ± 6.18 | 0.41 |
| 7 VSI (%) | 5.77 ± 1.12 | 6.23 ± 1.04 | 6.89 ± 0.66 | 6.60 ± 0.92 | 6.64 ± 0.34 | 6.68 ± 0.81 | 0.27 |
| 8 SR (%) | 97.78 ± 3.85 | 100.00 | 97.78 ± 3.85 | 95.56 ± 7.70 | 97.78 ± 3.85 | 97.78 ± 3.85 | 0.90 |
| 9 HSI (%) | 4.19 ± 0.96 | 4.53 ± 0.71 | 5.36 ± 1.19 | 4.97 ± 0.91 | 5.28 ± 0.40 | 5.21 ± 0.88 | 0.16 |
| 10 CF (g/cm3) | 2.57 ± 0.31 | 2.62 ± 0.07 | 2.74 ± 0.20 | 2.64 ± 0.14 | 2.50 ± 0.11 | 2.47 ± 0.07 | 0.09 |
| Parameter (% Wet Matter) | Replacement Levels | ||||||
|---|---|---|---|---|---|---|---|
| 0% | 20% | 40% | 60% | 80% | 100% | p Value | |
| Moisture | 75.39 ± 0.40 | 76.12 ± 0.84 | 75.78 ± 0.59 | 75.59 ± 0.90 | 76.00 ± 0.83 | 75.72 ± 0.94 | 0.87 |
| Crude protein | 16.83 ± 0.78 | 16.61 ± 0.31 | 16.11 ± 0.59 | 17.10 ± 0.84 | 16.69 ± 0.61 | 16.72 ± 0.61 | 0.83 |
| Crude lipid | 1.84 ± 0.10 | 1.70 ± 0.04 | 1.66 ± 0.12 | 1.64 ± 0.16 | 1.61 ± 0.02 | 1.66 ± 0.10 | 0.16 |
| Crude ash | 4.77 ± 0.12 | 4.69 ± 0.24 | 4.65 ± 0.37 | 4.55 ± 0.13 | 4.32 ± 0.12 | 4.58 ± 0.35 | 0.37 |
| Parameter | Replacement Levels | ||||||
|---|---|---|---|---|---|---|---|
| 0% | 20% | 40% | 60% | 80% | 100% | p Value | |
| ALB (g/L) | 11.13 ± 0.82 | 11.11 ± 0.84 | 11.53 ± 1.46 | 10.75 ± 0.94 | 12.66 ± 2.26 | 11.74 ± 1.64 | 0.14 |
| ALT (U/L) | 4.60 ± 1.53 | 4.43 ± 0.69 | 5.15 ± 0.73 | 4.24 ± 0.79 | 4.69 ± 1.25 | 5.19 ± 1.19 | 0.41 |
| AST (U/L) | 100.28 ± 14.17 a | 98.94 ± 12.41 a | 108.85 ± 24.13 ab | 111.81 ± 10.83 ab | 114.61 ± 12.82 ab | 123.19 ± 11.79 b | 0.02 |
| TC (mmol/L) | 5.73 ± 0.63 | 5.50 ± 0.35 | 5.60 ± 0.50 | 5.40 ± 0.59 | 5.73 ± 0.23 | 5.46 ± 0.48 | 0.65 |
| TG (mmol/L) | 1.91 ± 0.23 c | 1.70 ± 0.11 ab | 1.76 ± 0.15 bc | 1.57 ± 0.13 ab | 1.63 ± 0.20 ab | 1.51 ± 0.18 a | 0.01 |
| GLU (g/L) | 6.11 ± 1.64 | 6.92 ± 1.21 | 5.62 ± 1.21 | 5.85 ± 1.04 | 6.64 ± 1.29 | 6.94 ± 0.78 | 0.15 |
| TP (g/L) | 36.74 ± 1.15 a | 36.85 ± 2.02 a | 37.74 ± 3.57 ab | 36.30 ± 2.35 a | 40.92 ± 4.43 b | 38.79 ± 4.63 ab | 0.04 |
| ALP (U/L) | 12.21 ± 4.71 | 9.58 ± 3.57 | 12.15 ± 4.99 | 10.14 ± 3.09 | 12.13 ± 4.38 | 11.88 ± 4.12 | 0.68 |
| Parameters | Replacement Levels | ||||||
|---|---|---|---|---|---|---|---|
| 0% | 20% | 40% | 60% | 80% | 100% | p Value | |
| T-AOC (U/mL) | 0.38 ± 0.02 | 0.40 ± 0.03 | 0.37 ± 0.05 | 0.38 ± 0.02 | 0.39 ± 0.02 | 0.38 ± 0.03 | 0.49 |
| SOD (U/mL) | 22.22 ± 1.07 | 22.80 ± 0.92 | 22.46 ± 0.96 | 22.71 ± 0.46 | 22.82 ± 0.95 | 22.07 ± 1.37 | 0.56 |
| CAT (U/mL) | 21.79 ± 7.49 abc | 13.79 ± 6.65 a | 30.78 ± 7.47 cd | 34.66 ± 9.76 d | 25.90 ± 11.50 bcd | 17.55 ± 10.05 ab | 0.01 |
| GSH (mg/L) | 30.56 ± 9.93 ab | 51.24 ± 20.15 c | 36.84 ± 23.21 abc | 47.92 ± 21.33 bc | 30.28 ± 8.71 ab | 26.30 ± 9.25 a | 0.04 |
| GPx (μmol/L) | 104.70 ± 42.39 a | 118.16 ± 36.44 a | 178.74 ± 10.42 c | 165.08 ± 41.42 bc | 119.09 ± 44.16 a | 128.95 ± 30.09 ab | 0.01 |
| MDA (nmol/mL) | 14.17 ± 5.50 | 12.52 ± 3.34 | 12.77 ± 4.24 | 13.80 ± 2.47 | 13.16 ± 4.17 | 9.88 ± 2.40 | 0.30 |
| Replacement Levels (%) | Thickness of Mucosal Layer (mm) | Length of the Villous Epithelium (mm) | Number of Goblet Cells | Number of Goblet Cells per Unit Length (Cell/mm) |
|---|---|---|---|---|
| 0 | 0.48 ± 0.14 | 0.54 ± 0.12 | 14.93 ± 7.19 | 27.66 ± 10.52 |
| 20 | 0.33 ± 0.05 | 0.46 ± 0.02 | 12.90 ± 6.65 | 27.81 ± 13.26 |
| 40 | 0.41 ± 0.03 | 0.55 ± 0.03 | 13.00 ± 5.30 | 23.74 ± 8.95 |
| 60 | 0.52 ± 0.22 | 0.69 ± 0.30 | 15.47 ± 5.67 | 22.98 ± 3.33 |
| 80 | 0.40 ± 0.05 | 0.55 ± 0.03 | 11.47 ± 3.65 | 20.70 ± 6.37 |
| 100 | 0.43 ± 0.07 | 0.61 ± 0.13 | 24.13 ± 7.71 | 39.70 ± 9.21 |
| p Value | 0.58 | 0.64 | 0.23 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uyisenga, A.; Liang, H.; Ren, M.; Huang, D.; Xue, C.; Yin, H.; Mi, H. The Effects of Replacing Fish Meal with Enzymatic Soybean Meal on the Growth Performance, Whole-Body Composition, and Health of Juvenile Gibel Carp (Carassius auratus gibelio). Fishes 2023, 8, 423. https://doi.org/10.3390/fishes8080423
Uyisenga A, Liang H, Ren M, Huang D, Xue C, Yin H, Mi H. The Effects of Replacing Fish Meal with Enzymatic Soybean Meal on the Growth Performance, Whole-Body Composition, and Health of Juvenile Gibel Carp (Carassius auratus gibelio). Fishes. 2023; 8(8):423. https://doi.org/10.3390/fishes8080423
Chicago/Turabian StyleUyisenga, Adolphe, Hualiang Liang, Mingchun Ren, Dongyu Huang, Chunyu Xue, Heng Yin, and Haifeng Mi. 2023. "The Effects of Replacing Fish Meal with Enzymatic Soybean Meal on the Growth Performance, Whole-Body Composition, and Health of Juvenile Gibel Carp (Carassius auratus gibelio)" Fishes 8, no. 8: 423. https://doi.org/10.3390/fishes8080423
APA StyleUyisenga, A., Liang, H., Ren, M., Huang, D., Xue, C., Yin, H., & Mi, H. (2023). The Effects of Replacing Fish Meal with Enzymatic Soybean Meal on the Growth Performance, Whole-Body Composition, and Health of Juvenile Gibel Carp (Carassius auratus gibelio). Fishes, 8(8), 423. https://doi.org/10.3390/fishes8080423








