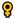Effect of Bacillus Probiotics on the Immunological Responses of Nile Tilapia (Oreochromis niloticus): A Review
Abstract
:1. Introduction
2. Common Bacillus Probiotics Used in Nile Tilapia Rearing and Farming
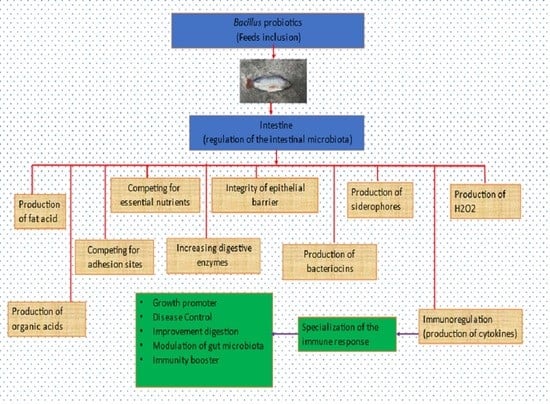
3. Bacillus Probiotics and Gut Microbiota
4. Mode of Action of Bacillus Probiotics in Nile Tilapia
5. Bacillus Probiotics and Fish Immunological Response
5.1. Bacillus Probiotics Improve Immune Response
5.2. Bacillus Probiotics Modulate Phagocytic Activity
5.3. Bacillus Probiotics Regulate Lysozyme Activity
5.4. Bacillus Probiotics Modulate the Respiratory Burst Process
5.5. Bacillus Probiotics Improve Complement Activity
5.6. Bacillus Probiotics Stimulate Immune-Related Gene Expression
6. Bacillus Probiotics Regulate Antioxidant Enzyme Activity
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohapatra, S.; Chakraborty, T.; Kumar, V.; DeBoeck, G.; Mohanta, K.N. Aquaculture and stress management: A review of probiotic intervention. J. Anim. Physiol. Anim. Nutr. 2013, 97, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.E.; McNevin, A.A.; Davis, R.P. The contribution of fisheries and aquaculture to the global protein supply. Food Secur. 2022, 14, 805–827. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, M.M.; Goedknegt, M.A.; Poulin, R.; Thieltges, D.W. Collateral diseases: Aquaculture impacts on wildlife infections. J. Appl. Ecol. 2021, 58, 453–464. [Google Scholar] [CrossRef]
- Akanmu, O.A. Probiotics, an Alternative Measure to Chemotherapy in Fish Production. In Probiotics-Current Knowledge and Future Prospects; IntechOpen: London, UK, 2018; Volume 1, pp. 9–12. [Google Scholar]
- Hoseinifar, S.H.; Sun, Y.-Z.; Wang, A.; Zhou, Z. Probiotics as Means of Diseases Control in Aquaculture, a Review of Current Knowledge and Future Perspectives. Front. Microbiol. 2018, 9, 2429. [Google Scholar] [CrossRef] [PubMed]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef] [PubMed]
- Zorriehzahra, M.J.; Delshad, S.T.; Adel, M.; Tiwari, R.; Karthik, K.; Dhama, K.; Lazado, C.C. Probiotics as beneficial microbes in aquaculture: An update on their multiple modes of action: A review. Vet. Q. 2016, 36, 228–241. [Google Scholar] [CrossRef]
- Goh, J.X.H.; Tan, L.T.H.; Law, J.W.F.; Ser, H.L.; Khaw, K.Y.; Letchumanan, V.; Lee, L.H.; Goh, B.H. Harnessing the potentialities of probiotics, prebiotics, synbiotics, paraprobiotics, and postbiotics for shrimp farming. Rev. Aquac. 2022, 14, 1478–1557. [Google Scholar] [CrossRef]
- Merrifield, D.L.; Dimitroglou, A.; Foey, A.; Davies, S.J.; Baker, R.T.; Bøgwald, J.; Castex, M.; Ringø, E. The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 2010, 302, 1–18. [Google Scholar] [CrossRef]
- Nayak, S. Probiotics and immunity: A fish perspective. Fish Shellfish Immunol. 2010, 29, 2–14. [Google Scholar] [CrossRef]
- Patel, S.; Shukla, R.; Goyal, A. Probiotics in valorization of innate immunity across various animal models. J. Funct. Foods 2015, 14, 549–561. [Google Scholar] [CrossRef]
- Marco, M.L.; Pavan, S.; Kleerebezem, M. Towards understanding molecular modes of probiotic action. Curr. Opin. Biotechnol. 2006, 17, 204–210. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Abarike, E.D.; Sakyi, M.E.; Lu, Y.; Wang, Z. Modulation of nutrient utilization, growth, and immunity of Nile tilapia, Oreochromis niloticus: The role of probiotics. Aquac. Int. 2020, 28, 277–291. [Google Scholar] [CrossRef]
- Olmos Soto, J. Bacillus Probiotic Enzymes: External Auxiliary Apparatus to Avoid Digestive Deficiencies, Water Pollution, Diseases, and Economic Problems in Marine Cultivated Animals, 1st ed.; Advances in Food and Nutrition Research; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 80, pp. 15–35. [Google Scholar]
- Amoah, K.; Huang, Q.-C.; Tan, B.-P.; Zhang, S.; Chi, S.-Y.; Yang, Q.-H.; Liu, H.-Y.; Dong, X.-H. Dietary supplementation of probiotic Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microflora, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 87, 796–808. [Google Scholar] [CrossRef]
- Lee, S.; Katya, K.; Hamidoghli, A.; Hong, J.; Kim, D.-J.; Bai, S.C. Synergistic effects of dietary supplementation of Bacillus subtilis WB60 and mannanoligosaccharide (MOS) on growth performance, immunity and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol. 2018, 83, 283–291. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, M.; Gao, F.; Lu, M.; Chen, G. Effects of dietary probiotic supplementation on the growth, gut health and disease resistance of juvenile Nile tilapia (Oreochromis niloticus). Anim. Nutr. 2020, 6, 69–79. [Google Scholar] [CrossRef]
- Addo, S.; Carrias, A.A.; Williams, M.A.; Liles, M.R.; Terhune, J.S.; Davis, D.A. Effects of Bacillus subtilis strains and the prebiotic Previda® on growth, immune parameters and susceptibility to Aeromonas hydrophila infection in Nile tilapia, Oreochromis niloticus. Aquac. Res. 2017, 48, 4798–4810. [Google Scholar] [CrossRef]
- Arora, M.; Baldi, A. Selective Identification and Characterization of Potential Probiotic Strains: A Review on Comprehensive Polyphasic Approach. Appl. Clin. Res. Clin. Trials. Regul. Aff. 2017, 4, 60–76. [Google Scholar] [CrossRef]
- Han, B.; Long, W.-Q.; He, J.-Y.; Liu, Y.-J.; Si, Y.-Q.; Tian, L.-X. Effects of dietary Bacillus licheniformis on growth performance, immunological parameters, intestinal morphology and resistance of juvenile Nile tilapia (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol. 2015, 46, 225–231. [Google Scholar] [CrossRef]
- Richardson, K.; Wilcox, C.; Vince, J.; Hardesty, B.D. Challenges and misperceptions around global fishing gear loss estimates. Mar. Policy 2021, 129, 104522. [Google Scholar] [CrossRef]
- Pilling, D.; Bélanger, J.; Hoffmann, I. Declining biodiversity for food and agriculture needs urgent global action. Nat. Food 2020, 1, 144–147. [Google Scholar] [CrossRef]
- Zhang, L. Global Fisheries Management and Community Interest. Sustainability 2021, 13, 8586. [Google Scholar] [CrossRef]
- Thanh, H.; Viet, V.; Dinh, H.; Sangsuriya, P.; Jitrakorn, S. Naturally concurrent infections of bacterial and viral pathogens in disease outbreaks in cultured Nile tilapia (Oreochromis niloticus) farms. Aquaculture 2015, 448, 427–435. [Google Scholar]
- Fitzsimmons, K. From Africa to the world—The journey of Nile tilapia. Rev. Aquac. 2023, 15, 6–21. [Google Scholar]
- Wang, M.; Liu, G.; Lu, M.; Ke, X.; Liu, Z.; Gao, F.; Cao, J.; Zhu, H.; Yi, M.; Yu, D. Effect of Bacillus cereus as a water or feed additive on the gut microbiota and immunological parameters of Nile tilapia. Aquac. Res. 2017, 48, 3163–3173. [Google Scholar] [CrossRef]
- Van Hai, N. Research findings from the use of probiotics in tilapia aquaculture: A review. Fish Shellfish Immunol. 2015, 45, 592–597. [Google Scholar]
- Elkamel, A. Immunomodulation of Nile Tilapia, Oreochromis niloticus, by Nigella sativa and Bacillus subtilis. J. Aquac. Res. Dev. 2018, 19, 3–6. [Google Scholar]
- Van Doan, H.; Hossein, S.; Tapingkae, W. Combined administration of low molecular weight sodium alginate boosted immunomodulatory, disease resistance and growth-enhancing effects of Lactobacillus plantarum in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2016, 58, 678–685. [Google Scholar] [CrossRef]
- Meena, D.K.; Behera, B.K.; Das, P. Probiotics in fish and shellfish culture: Immunomodulatory and ecophysiological responses Probiotics in fish and shellfish culture: Immunomodulatory and ecophysiological responses. Fish Physiol. Biochem. 2014, 1, 4–38. [Google Scholar]
- Nathanailides, C.; Kolygas, M.; Choremi, K.; Mavraganis, T.; Gouva, E.; Vidalis, K.; Athanassopoulou, F. Probiotics Have the Potential to Significantly Mitigate the Environmental Impact of Freshwater Fish Farms. Fishes 2021, 6, 76. [Google Scholar] [CrossRef]
- Buruiană, C.T.; Profir, A.G.; Vizireanu, C. Effects of probiotic Bacillus species in aquaculture—An overview. Ann. Univ. Dunarea Jos Galati. Fascicle VI-Food Technol. 2014, 38, 9–17. [Google Scholar]
- Emam, A.M.; Dunlap, C.A. Genomic and phenotypic characterization of Bacillus velezensis AMB-y1; a potential probiotic to control pathogens in aquaculture. Antonie Leeuwenhoek 2020, 113, 2041–2052. [Google Scholar] [CrossRef]
- Ya, X.L.; Li, L.J. Isolation and characterization of Bacillus spp. antagonistic to Vibrio parahaemolyticus for use as probiotics in aquaculture. J. Microbiol. Biotechnol. 2015, 2, 2–7. [Google Scholar]
- Soto, J.O. Feed intake improvement, gut microbiota modulation and pathogens control by using Bacillus species in shrimp aquaculture. World J. Microbiol. Biotechnol. 2021, 37, 28. [Google Scholar] [CrossRef]
- Aly, S.M.; Ahmed, Y.A.-G.; Ghareeb, A.A.-A.; Mohamed, M.F. Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol. 2008, 25, 128–136. [Google Scholar] [CrossRef]
- Galagarza, O.A.; Smith, S.A.; Drahos, D.J.; Eifert, J.D.; Williams, R.C.; Kuhn, D.D. Modulation of innate immunity in Nile tilapia (Oreochromis niloticus) by dietary supplementation of Bacillus subtilis endospores. Fish Shellfish Immunol. 2018, 83, 171–179. [Google Scholar] [CrossRef]
- Opiyo, M.A.; Jumbe, J.; Ngugi, C.C.; Charo-Karisa, H. Dietary administration of probiotics modulates non-specific immunity and gut microbiota of Nile tilapia (Oreochromis niloticus) cultured in low input ponds. Int. J. Vet. Sci. Med. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Cavalcante, R.B.; Telli, G.S.; Tachibana, L.; Dias, D.D.C.; Oshiro, E.; Natori, M.M.; da Silva, W.F.; Ranzani-Paiva, M.J. Probiotics, Prebiotics and Synbiotics for Nile tilapia: Growth performance and protection against Aeromonas hydrophila infection. Aquac. Rep. 2020, 17, 100343. [Google Scholar] [CrossRef]
- Ramos, E.A.; Relucio, J.L.V.; Torres-villanueva, C.A.T. Orginial Articles Gene Expression in Tilapia Following Oral Delivery of Chitosan-Encapsulated Plasmid DNA Incorporated into Fish Feeds. Mar Biotechnol. 2005, 7, 89–94. [Google Scholar] [CrossRef]
- Bakhrouf, A. Inhibitory activity and adhesive ability of potential probiotic Bacillus species to confer protection for Artemia gnotobiotic culture against pathogenic Vibrio spp. Inhibitory activity and adhesive ability of potential probiotic Bacillus species to confer. Turk. J. Vet. Sci. 2011, 35, 227–233. [Google Scholar]
- Zhang, C.N.; Li, X.F.; Xu, W.N.; Jiang, G.Z.; Lu, K.L.; Wang, L.N.; Liu, W.B. Combined effects of dietary fructooligosaccharide and Bacillus licheniformis on innate immunity, antioxidant capability and disease resistance of triangular bream (Megalobrama terminals). Fish Shellfish Immunol. 2013, 2, 1380–1386. [Google Scholar] [CrossRef]
- Liang, W.; Li, H.; Zhou, H.; Wang, M.; Zhao, X.; Sun, X.; Li, C.; Zhang, X. Effects of Taraxacum and Astragalus extracts combined with probiotic Bacillus subtilis and Lactobacillus on Escherichia coli—Infected broiler chickens. Poult. Sci. 2021, 100, 101007. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; El-Wahab, A.A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, Prebiotics, and Phytogenic Substances for Optimizing Gut Health in Poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Ogueke, C.; Owuamanam, C.; Ihediohanm, N.; Iwouno, J. Probiotics and Prebiotics: Unfolding Prospects for Better Human Health. Pak. J. Nutr. 2010, 9, 833–843. [Google Scholar] [CrossRef]
- Nayak, S.; Swain, P.; Mukherjee, S. Effect of dietary supplementation of probiotic and vitamin C on the immune response of Indian major carp, Labeo rohita (Ham.). Fish Shellfish Immunol. 2007, 23, 892–896. [Google Scholar] [CrossRef]
- Telli, G.S.; Ranzani-Paiva, M.J.T.; Dias, D.D.C.; Sussel, F.R.; Ishikawa, C.M.; Tachibana, L. Dietary administration of Bacillus subtilis on hematology and non-specific immunity of Nile tilapia Oreochromis niloticus raised at different stocking densities. Fish Shellfish. Immunol. 2014, 39, 305–311. [Google Scholar] [CrossRef]
- Wu, P.S.; Liu, C.H.; Hu, S.Y. Probiotic Bacillus safensis NPUST1 administration improves growth performance, gut microbiota, and innate immunity against Streptococcus iniae in nile tilapia (Oreochromis niloticus). Microorganisms 2021, 9, 2494. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.; Tang, J.; Cai, J.; Yu, H.; Wang, Z.; Abarike, E.D.; Lu, Y.; Li, Y.; Afriyie, G. In vivo assessment of the probiotic potentials of three host-associated Bacillus species on growth performance, health status and disease resistance of Oreochromis niloticus against Streptococcus agalactiae. Aquaculture 2020, 527, 735440. [Google Scholar] [CrossRef]
- Kuebutornye, F.K.A.; Wang, Z.W.; Lu, Y.S.; Abarike, E.D.; Sakyi, M.E.; Li, Y.; Xie, C.X.; Hlordzi, V. Effects of three host-associated Bacillus species on mucosal immunity and gut health of Nile tilapia, Oreochromis niloticus and its resistance against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2020, 97, 83–95. [Google Scholar] [CrossRef]
- Sciences, M. Dietary administration of a commercial mixed-species probiotic improves growth performance and modulates the intestinal immunity of tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2016, 1, 5–24. [Google Scholar]
- Saputra, F.; Shiu, Y.L.; Chen, Y.C.; Puspitasari, A.W.; Danata, R.H.; Liu, C.H.; Hu, S.Y. Dietary supplementation with xylanase-expressing B. amyloliquefaciens R8 improves growth performance and enhances immunity against Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2016, 58, 397–405. [Google Scholar] [CrossRef]
- Srisapoome, P.; Areechon, N. Efficacy of viable Bacillus pumilus isolated from farmed fish on immune responses and increased disease resistance in Nile tilapia (Oreochromis niloticus): Laboratory and on-farm trials. Fish Shellfish Immunol. 2017, 67, 199–210. [Google Scholar] [CrossRef]
- Ghanbari, M.; Kneifel, W.; Domig, K.J. A new view of the fish gut microbiome: Advances from next-generation sequencing. Aquaculture 2015, 448, 464–475. [Google Scholar] [CrossRef]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P. The Gut Microbiota of Marine Fish. Front. Microbiol. 2018, 9, 873. [Google Scholar] [CrossRef]
- Yu, L.; Qiao, N.; Li, T.; Yu, R.; Zhai, Q.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Dietary supplementation with probiotics regulates gut microbiota structure and function in Nile tilapia exposed to aluminum. PeerJ 2019, 7, e6963. [Google Scholar] [CrossRef]
- Li, X.; Ringø, E.; Hoseinifar, S.H.; Lauzon, H.L.; Birkbeck, H.; Yang, D. The adherence and colonization of microorganisms in the fish gastrointestinal tract. Rev. Aquac. 2019, 11, 603–618. [Google Scholar] [CrossRef]
- Tarnecki, A.; Burgos, F.; Ray, C.; Arias, C. Fish intestinal microbiome: Diversity and symbiosis unravelled by metagenomics. J. Appl. Microbiol. 2017, 123, 2–17. [Google Scholar] [CrossRef]
- Butt, R.L.; Volkoff, H. Gut Microbiota and Energy Homeostasis in Fish. Front. Endocrinol. 2019, 10, 9. [Google Scholar] [CrossRef]
- Adeoye, A.A.; Yomla, R.; Jaramillo-Torres, A.; Rodiles, A.; Merrifield, D.L.; Davies, S.J. Combined effects of exogenous enzymes and probiotics on Nile tilapia (Oreochromis niloticus) growth, intestinal morphology, and microbiome. Aquaculture 2016, 463, 61–70. [Google Scholar] [CrossRef]
- El-Ezabi, M.; Serafy, S.E.; Essa, M.; Daboor, S.; Esmael, N. The viability of probiotics as a factor influencing the immune response in the Nile tilapia, Oreochromis niloticus. Egypt. J. Aquat. Biol. Fish. 2011, 15, 105–124. [Google Scholar] [CrossRef]
- Jahangiri, L.; Esteban, M. Administration of Probiotics in the Water in Finfish Aquaculture Systems: A Review. Fishes 2018, 3, 33. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.-Q.; Tian, L.-X.; Liu, Y.-J.; Niu, J. Improvement of growth, intestinal short-chain fatty acids, non-specific immunity and ammonia resistance in Pacific white shrimp (Litopenaeus vannamei) fed dietary water-soluble chitosan and mixed probiotics. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2020, 236, 108791. [Google Scholar] [CrossRef] [PubMed]
- Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; Elashry, M.A.; Moustafa, M.M.; Wassel, M.A.; El-Garhy, H.A.; El-Haroun, E.R.; Elsaied, H.E. Synergistic effects of Bacillus pumilus and exogenous protease on Nile tilapia (Oreochromis niloticus) growth, gut microbes, immune response and gene expression fed plant protein diet. Anim. Feed. Sci. Technol. 2021, 275, 114892. [Google Scholar] [CrossRef]
- Yaqub, A.; Awan, M.N.; Kamran, M.; Majeed, I. Evaluation of potential applications of dietary probiotic (Bacillus licheniformis SB3086): Effect on growth, digestive enzyme activity, hematological, biochemical, and immune response of Tilapia (Oreochromis mossambicus). Turk. J. Fish. Aquat. Sci. 2022, 223, 5. [Google Scholar] [CrossRef]
- Giatsis, C.; Sipkema, D.; Smidt, H.; Verreth, J.; Verdegem, M. The Colonization Dynamics of the Gut Microbiota in Tilapia Larvae. PLoS ONE 2014, 9, e103641. [Google Scholar]
- Deng, Y.; Verdegem, M.C.; Eding, E.; Kokou, F. Effect of rearing systems and dietary probiotic supplementation on the growth and gut microbiota of Nile tilapia (Oreochromis niloticus) larvae. Aquaculture 2022, 546, 737297. [Google Scholar] [CrossRef]
- Bereded, N.K.; Curto, M.; Domig, K.J.; Abebe, G.B.; Fanta, S.W.; Waidbacher, H.; Meimberg, H. Metabarcoding Analyses of Gut Microbiota of Nile Tilapia (Oreochromis niloticus) from Lake Awassa and Lake Chamo, Ethiopia. Microorganisms 2020, 8, 1040. [Google Scholar] [CrossRef]
- Payne, C.J.; Turnbull, J.F.; MacKenzie, S.; Crumlish, M. Investigating the Effect of an Oxytetracycline Treatment on the Gut Microbiome and Antimicrobial Resistance Gene Dynamics in Nile Tilapia (Oreochromis niloticus). Antibiotics 2021, 10, 1213. [Google Scholar] [CrossRef]
- Zhai, Q.; Yu, L.; Li, T.; Zhu, J.; Zhang, C.; Zhao, J.; Zhang, H.; Chen, W. Effect of dietary probiotic supplementation on intestinal microbiota and physiological conditions of Nile tilapia (Oreochromis niloticus) under waterborne cadmium exposure. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2017, 110, 501–513. [Google Scholar] [CrossRef]
- Mannan, M.; Islam, S.R.; Osman, M.H.; Rahman, M.K.; Uddin, M.N.; Kamal, M.; Reza, S. Antibacterial activity of oxytetracycline on the microbial ecology of Nile tilapia (Oreochromis niloticus) gastrointestinal tract under laboratory conditions. Aquac. Res. 2020, 51, 2125–2133. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Naraballobh, W.; Jaturasitha, S.; Tongsiri, S.; Chitmanat, C.; Ringø, E. Dietary inclusion of Orange peels derived pectin and Lactobacillus plantarum for Nile tilapia (Oreochromis niloticus) cultured under indoor biofloc systems. Aquaculture 2019, 508, 98–105. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2015, 7, 17. [Google Scholar] [CrossRef]
- Wang, A.R.; Ran, C.; Ringø, E.; Zhou, Z.G. Progress in fish gastrointestinal microbiota research. Rev. Aquac. 2018, 10, 626–640. [Google Scholar] [CrossRef]
- Li, J.; Liu, G.; Li, C.; Deng, Y.; Tadda, M.A.; Lan, L.; Zhu, S.; Liu, D. Effects of different solid carbon sources on water quality, biofloc quality and gut microbiota of Nile tilapia (Oreochromis niloticus) larvae. Aquaculture 2018, 495, 919–931. [Google Scholar] [CrossRef]
- Thirabunyanon, M.; Thongwittaya, N. Protection activity of a novel probiotic strain of Bacillus subtilis against Salmonella Enteritidis infection. Res. Vet. Sci. 2012, 93, 74–81. [Google Scholar] [CrossRef]
- Kumar, V.; Roy, S.; Meena, D.K.; Sarkar, U.K. Application of Probiotics in Shrimp Aquaculture: Importance, Mechanisms of Action, and Methods of Administration. Rev. Fish. Sci. Aquac. 2016, 24, 342–368. [Google Scholar] [CrossRef]
- Maas, R.M.; Deng, Y.; Dersjant-Li, Y.; Petit, J.; Verdegem, M.C.J.; Schrama, J.W.; Kokou, F. Exogenous enzymes and probiotics alter digestion kinetics, volatile fatty acid content and microbial interactions in the gut of Nile tilapia. Sci. Rep. 2021, 11, 8221. [Google Scholar] [CrossRef]
- Cai, G.; Wu, D.; Li, X.; Lu, J. Levan from Bacillus amyloliquefaciens JN4 acts as a prebiotic for enhancing the intestinal adhesion capacity of Lactobacillus reuteri JN101. Int. J. Biol. Macromol. 2020, 146, 482–487. [Google Scholar] [CrossRef]
- Yang, J.; Huang, K.; Wang, J.; Wu, D.; Liu, Z.; Yu, P.; Wei, Z.; Chen, F. Combined Use of Bacillus subtilis yb-114,246 and Bacillus licheniformis yb-214,245 Improves Body Growth Performance of Chinese Huainan Partridge Shank Chickens by Enhancing Intestinal Digestive Profiles. Probiotics Antimicrob. Proteins 2020, 13, 327–342. [Google Scholar] [CrossRef]
- He, S.; Zhang, Y.; Xu, L.; Yang, Y.; Marubashi, T.; Zhou, Z.; Yao, B. Effects of dietary Bacillus subtilis C-3102 on the production, intestinal cytokine expression, and autochthonous bacteria of hybrid tilapia Oreochromis niloticus
× Oreochromis aureus
. Aquaculture 2013, 412–413, 125–130. [Google Scholar] [CrossRef]
- Sookchaiyaporn, N.; Srisapoome, P.; Unajak, S.; Areechon, N. Efficacy of Bacillus spp. isolated from Nile tilapia Oreochromis niloticus Linn. on its growth and immunity, and control of pathogenic bacteria. Fish. Sci. 2020, 86, 353–365. [Google Scholar] [CrossRef]
- Eladawy, M.M. Characterization of probiotic Bacillus subtilis isolated from Nile tilapia (Oreochromis niloticus) digestive tract and evaluation its positive impact on health and immunity. Egypt. J. Aquac. 2019, 9, 1–26. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, Y.; Ke, X.; Yi, M.; Liu, Z.; Han, X.; Shi, C.; Lu, M. Bacillus velezensis LF01: In vitro antimicrobial activity against fish pathogens, growth performance enhancement, and disease resistance against streptococcosis in Nile tilapia (Oreochromis niloticus). Appl. Microbiol. Biotechnol. 2019, 103, 9023–9035. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, T.; Sofi Uddin Mahamud, A.G.M.; Acharjee, T.K.; Hassan, R.; Akter Snigdha, T.; Islam, T.; Alam, R.; Khoiam, U.; Akter, F.; Azad, R.; et al. Probiotic supplementations improve the growth, water quality, hematology, gut microbiota, and intestinal morphology of Nile tilapia. Aquac Rep. 2021, 21, 100972. [Google Scholar] [CrossRef]
- Das, S.; Ward, L.R.; Burke, C. Screening of marine Streptomyces spp. for potential use as probiotics in aquaculture. Aquaculture 2010, 305, 32–41. [Google Scholar] [CrossRef]
- Arun, K.; Madhavan, A.; Sindhu, R.; Emmanual, S.; Binod, P.; Pugazhendhi, A.; Sirohi, R.; Reshmy, R.; Awasthi, M.K.; Gnansounou, E.; et al. Probiotics and gut microbiome—Prospects and challenges in remediating heavy metal toxicity. J. Hazard. Mater. 2021, 420, 126676. [Google Scholar] [CrossRef]
- Nayak, S.K. Multifaceted applications of probiotic Bacillus species in aquaculture with special reference to Bacillus subtilis. Rev. Aquac. 2021, 13, 862–906. [Google Scholar] [CrossRef]
- Al Masri, S.; Hünigen, H.; Al Aiyan, A.; Rieger, J.; Zentek, J.; Richardson, K.; Plendl, J. Influence of age at weaning and feeding regimes on the postnatal morphology of the porcine small intestine. J. Swine. Health Prod. 2015, 23, 186–203. [Google Scholar]
- Shim, K.-Y.; Lee, D.; Han, J.; Nguyen, N.-T.; Park, S.; Sung, J.H. Microfluidic gut-on-a-chip with three-dimensional villi structure. Biomed. Microdevices 2017, 19, 37. [Google Scholar] [CrossRef]
- Vallejos-Vidal, E.; Reyes-López, F.; Teles, M.; MacKenzie, S. The response of fish to immunostimulant diets. Fish Shellfish Immunol. 2016, 56, 34–69. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Cai, Y.; Guo, X.; Cao, Z.; Zhang, Y.; Liu, S.; Yuan, W.; Zhu, W.; Zheng, Y.; et al. Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2017, 60, 326–333. [Google Scholar] [CrossRef]
- Panase, A.; Thirabunyanon, M.; Promya, J.; Chitmanat, C. Influences of Bacillus subtilis and fructooligosaccharide on growth performances, immune responses, and disease resistance of Nile tilapia, Oreochromis niloticus. Front. Vet. Sci. 2023, 9, 1094681. [Google Scholar] [CrossRef]
- Ismail, T.; Hegazi, E.; Nassef, E.; Habotta, O.A.; Gewaily, M.S. The optimized inclusion level of Bacillus subtilis fermented Azolla pinnata in Nile tilapia (Oreochromis niloticus) diets: Immunity, antioxidative status, intestinal digestive enzymes and histomorphometry, and disease resistance. Fish Physiol. Biochem. 2022, 48, 767–783. [Google Scholar] [CrossRef]
- Al-deriny, S.H.; Dawood, M.A.O.; Abou, A.A.; El-tras, W.F.; Ahamad, B.; Van Doan, H.; Mohamed, R.A. The synergistic effects of Spirulina platensis and Bacillus amyloliquefaciens on the growth performance, intestinal histomorphology, and immune response of Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2020, 17, 100390. [Google Scholar] [CrossRef]
- Midhun, S.J.; Neethu, S.; Arun, D.; Vysakh, A.; Divya, L.; Radhakrishnan, E.; Jyothis, M. Dietary supplementation of Bacillus licheniformis HGA8B improves growth parameters, enzymatic profile and gene expression of Oreochromis niloticus. Aquaculture 2019, 505, 289–296. [Google Scholar] [CrossRef]
- Sutthi, N.; Van Doan, H. Saccharomyces crevices and Bacillus spp. effectively enhance the health tolerance of Nile tilapia under transportation stress. Aquaculture 2020, 528, 735527. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Khanongnuch, C.; Kanpiengjai, A.; Unban, K.; Van Kim, V.; Srichaiyo, S. Host-associated probiotics boosted mucosal and serum immunity, disease resistance, and growth performance of Nile tilapia (Oreochromis niloticus). Aquaculture 2018, 491, 94–100. [Google Scholar] [CrossRef]
- Wang, M.; Yi, M.M.; Lu, M.X.; Gao, F.Y.; Liu, Z.G.; Huang, Q.B.; Li, Q.Y.; Zhu, D.X. Effects of probiotics Bacillus cereus NY5 and Alcaligenes faecalis Y311 used as water additives on the microbiota and immune enzyme activities in three mucosal tissues in Nile tilapia Oreochromis niloticus reared in outdoor tanks. Aquac. Rep. 2020, 17, 100309. [Google Scholar] [CrossRef]
- Won, S.; Hamidoghli, A.; Choi, W.; Park, Y.; Jang, W.J.; Kong, I.S.; Bai, S.C. Effects of Bacillus subtilis wb60 and Lactococcus lactis on growth, immune responses, histology and gene expression in nile tilapia, Oreochromis niloticus. Microorganisms 2020, 8, 68. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defense molecule of the fish’s innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Gómez, G.D.; Balcázar, J.L. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunol. Med. Microbiol. 2008, 52, 145–154. [Google Scholar] [CrossRef]
- Mohammadi, M.; Imani, A.; Farhangi, M.; Gharaei, A.; Hafezieh, M. Replacement of fishmeal with processed canola meal in diets for juvenile Nile tilapia (Oreochromis niloticus): Growth performance, mucosal innate immunity, hepatic oxidative status, liver and intestine histology. Aquaculture 2020, 518, 734824. [Google Scholar] [CrossRef]
- Pirarat, N.; Pinpimai, K.; Endo, M.; Katagiri, T.; Ponpornpisit, A.; Chansue, N.; Maita, M. Modulation of intestinal morphology and immunity in nile tilapia (Oreochromis niloticus) by Lactobacillus rhamnosus GG. Res. Vet. Sci. 2011, 91, e92–e97. [Google Scholar] [CrossRef] [PubMed]
- Van Doan, H.; Hoseinifar, S.H.; Sringarm, K.; Jaturasitha, S.; Yuangsoi, B.; Dawood, M.A.; Esteban, M.; Ringø, E.; Faggio, C. Effects of Assam tea extract on growth, skin mucus, serum immunity and disease resistance of Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae. Fish Shellfish. Immunol. 2019, 93, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K.A. Main Components of Fish Immunity: An Overview of the Fish Immune System. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Monteiro, S.M.; Fontainhas-Fernandes, A.; Sousa, M. An immunohistochemical study of gill epithelium cells in the Nile tilapia, Oreochromis niloticus. Folia Histochem. Cytobiol. 2010, 48, 112–121. [Google Scholar] [CrossRef]
- Lazado, C.C.; Marlowe, C.; Caipang, A. Mucosal immunity and probiotics in fish. Fish Shellfish Immunol. 2014, 39, 78–89. [Google Scholar] [CrossRef]
- Rinaldi, E.; Consonni, A.; Guidesi, E.; Elli, M.; Mantegazza, R.; Baggi, F. Gut microbiota and probiotics: Novel immune system modulators in myasthenia gravis? Ann. N. Y. Acad. Sci. 2018, 1413, 49–58. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhang, R.; Zhang, J. The diversity of pattern recognition receptors (PRRs) involved with insect defense against pathogens. Curr. Opin. Insect Sci. 2019, 33, 105–110. [Google Scholar] [CrossRef]
- Iwashita, M.K.P.; Nakandakare, I.B.; Terhune, J.S.; Wood, T.; Ranzani-Paiva, M.J.T. Dietary supplementation with Bacillus subtilis, Saccharomyces cerevisiae and Aspergillus oryzae enhance immunity and disease resistance against Aeromonas hydrophila and Streptococcus iniae infection in juvenile tilapia Oreochromis niloticus. Fish Shellfish Immunol. 2015, 43, 60–66. [Google Scholar] [CrossRef]
- Akhter, N.; Wu, B.; Memon, A.M.; Mohsin, M. Probiotics and prebiotics associated with aquaculture: A review. Fish Shellfish Immunol. 2015, 45, 733–741. [Google Scholar] [CrossRef]
- Chu, H.; Mazmanian, S.K. Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nat. Immunol. 2013, 14, 668–675. [Google Scholar] [CrossRef]
- Wolf, A.J.; Underhill, D.M. Peptidoglycan recognition by the innate immune system. Nat. Rev. Immunol. 2018, 18, 243–254. [Google Scholar] [CrossRef]
- Irazoki, O.; Hernandez, S.B.; Cava, F. Peptidoglycan Muropeptides: Release, Perception, and Functions as Signaling Molecules. Front. Microbiol. 2019, 10, 500. [Google Scholar] [CrossRef]
- Ashraf, R.; Shah, N.P. Immune System Stimulation by Probiotic Microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef]
- Roayaei, M.; Mansouri-Tehrani, H.-A.; Rabbani-Khorasgani, M.; Hosseini, S.M.; Mokarian, F.; Mahdavi, H. Effect of supplements: Probiotics and probiotic plus honey on blood cell counts and serum IgA in patients receiving pelvic radiotherapy. J. Res. Med Sci. 2015, 20, 679–683. [Google Scholar] [CrossRef]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A Comparison of the Innate and Adaptive Immune Systems in Cartilaginous Fish, Ray-Finned Fish, and Lobe-Finned Fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef] [PubMed]
- Opiyo, M.A.; Jumbe, J.; Ngugi, C.C.; Charo-Karisa, H. Different levels of probiotics affect growth, survival and body composition of Nile tilapia (Oreochromis niloticus) cultured in low input ponds. Sci. Afr. 2019, 4, e00103. [Google Scholar] [CrossRef]
- Tachibana, L.; Telli, G.S.; de Dias, D.C.; Gonçalves, G.S.; Guimarães, M.C.; Ishikawa, C.M.; Cavalcante, R.B.; Natori, M.M.; Alarcon, M.F.F.; Tapia-Paniagua, S.; et al. Bacillus subtilis and Bacillus licheniformis in diets for Nile tilapia (Oreochromis niloticus): Effects on growth performance, gut microbiota modulation, and innate immunology. Aquac Res. 2021, 52, 1630–1642. [Google Scholar] [CrossRef]
- Reda, R.M.; Selim, K.M. Evaluation of Bacillus amyloliquefaciens on the growth performance, intestinal morphology, hematology and body composition of Nile tilapia, Oreochromis niloticus. Aquac. Int. 2015, 23, 203–217. [Google Scholar] [CrossRef]
- Ridha, M.T.; Azad, I.S. Preliminary evaluation of growth performance and immune response of Nile tilapia Oreochromis niloticus supplemented with two putative probiotic bacteria. Aquac. Res. 2012, 43, 843–852. [Google Scholar] [CrossRef]
- Cruvinel, W.M.; Júnior, D.M.; Araújo, J.A.P.; Catelan, T.T.T.; de Souza, A.W.S.; da Silva, N.P.; da Silva, N.P.; Andrade, L.E.C. Immune system—Part I fundamentals of innate immunity with emphasis on molecular and cellular mechanisms of inflammatory response. Rev. Bras. Reumatol. 2010, 50, 443–461. [Google Scholar]
- Ghalwash, H.R.; Salah, A.S.; El-Nokrashy, A.M.; Abozeid, A.M.; Zaki, V.H.; Mohamed, R.A. Dietary supplementation with Bacillus species improves growth, intestinal histomorphology, innate immunity, antioxidative status, and expression of growth and appetite-regulating genes of Nile tilapia fingerlings. Aquac Res. 2022, 53, 1378–1394. [Google Scholar] [CrossRef]
- Fath El-Bab, A.F.; Majrashi, K.A.; Sheikh, H.M.; Shafi, M.E.; El-Ratel, I.T.; Neamat-Allah, A.N.F.; El-Raghi, A.A.; Elazem, A.Y.A.; Abd-Elghany, M.F.; Abdelnour, S.A.; et al. Dietary supplementation of Nile tilapia (Oreochromis niloticus) with β-glucan and/or Bacillus coagulans: Synergistic impacts on performance, immune responses, redox status and expression of some related genes. Front Vet. Sci. 2022, 9, 4–9. [Google Scholar] [CrossRef]
- Tachibana, L.; Telli, G.S.; Dias, D.D.C.; Gonçalves, G.S.; Ishikawa, C.M.; Cavalcante, R.B.; Natori, M.M.; Ben Hamed, S.; Ranzani-Paiva, M.J.T. Effect of feeding strategy of probiotic Enterococcus faecium on growth performance, hematologic, biochemical parameters and non-specific immune response of Nile tilapia. Aquac. Rep. 2020, 16, 100277. [Google Scholar] [CrossRef]
- Mahboob, S. Environmental pollution of heavy metals as a cause of oxidative stress in fish: A review. Life Sci. J. 2013, 10, 336–347. [Google Scholar]
- Parkin, J.; Cohen, B. An overview of the immune system. Immunology 2001, 357, 1777–1787. [Google Scholar] [CrossRef]
- Giang, J.; Seelen, M.A.J.; Van Doorn, M.B.A.; Rissmann, R.; Prens, E.P.; Damman, J. Complement Activation in Inflammatory Skin Diseases. Front. Immunol. 2018, 9, 639. [Google Scholar] [CrossRef]
- Mohammadi, G.; Adorian, T.J.; Federal, U.; Maria, D.S.; Rafiee, G. Beneficial effects of Bacillus subtilis on water quality, growth, immune responses, endotoxemia and protection against lipopolysaccharide-induced damages in Oreochromis niloticus under biofloc technology system. Aquac. Nutr. 2020, 26, 1476–1492. [Google Scholar] [CrossRef]
- Ridha, M.T.; Azad, I.S. Effect of autochthonous and commercial probiotic bacteria on growth, persistence, immunity and disease resistance in juvenile and adult Nile tilapia Oreochromis niloticus. Aquac Res. 2015, 47, 2757–2767. [Google Scholar] [CrossRef]
- Ismail, T.; Hegazi, E.; Nassef, E.; El-Din, M.T.S.; Dawood, M.A.; Abdo, S.E.; Gewaily, M.S. Gut immune-related gene expression, histomorphometry and hematoimmunological assays in Nile tilapia (Oreochromis niloticus) fed Aspergillus oryzae fermented olive cake. Fish Shellfish Immunol. 2021, 117, 299–310. [Google Scholar] [CrossRef]
- Di, J.; Chu, Z.; Zhang, S.; Huang, J.; Du, H.; Wei, Q. Evaluation of the potential probiotic Bacillus subtilis isolated from two ancient sturgeons on growth performance, serum immunity and disease resistance of Acipenser dabryanus. Fish Shellfish. Immunol. 2019, 93, 711–719. [Google Scholar] [CrossRef]
- Tan, H.Y.; Chen, S.-W.; Hu, S.-Y. Improvements in the growth performance, immunity, disease resistance, and gut microbiota by the probiotic Rummelii Bacillus stabekisii in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 92, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Foysal, J.; Alam, M.; Kawser, A.Q.M.R.; Hasan, F.; Rahman, M.; Tay, C.; Prodhan, M.S.H.; Gupta, S.K. Meta-omics technologies reveals beneficiary effects of Lactobacillus plantarum as dietary supplements on gut microbiota, immune response and disease resistance of Nile tilapia (Oreochromis niloticus) J. Aquac. 2020, 734974. [Google Scholar] [CrossRef]
- Pérez-sánchez, T.; Luis, J.; Merri, D.L.; Carnevali, O.; Gioacchini, G.; De Blas, I.; Ruiz-Zarzuela, I. Expression of immune-related genes in rainbow trout (Oncorhynchus mykiss) induced by probiotic bacteria during Lactococcus garvieae infection. Fish Shellfish Immunol. 2011, 31, 196–201. [Google Scholar] [CrossRef]
- Huang, L.; Ran, C.; He, S.; Ren, P.; Hu, J.; Zhao, X.; Zhou, Z. Effects of dietary Saccharomyces cerevisiae culture or live cells with Bacillus amyloliquefaciens spores on growth performance, gut mucosal morphology, hsp70 gene expression, and disease resistance of juvenile common carp (Cyprinus carpio). Aquaculture 2015, 438, 33–38. [Google Scholar] [CrossRef]
- Ran, C.; Huang, L.; Hu, J.; Tacon, P.; He, S.; Li, Z.; Xu, L.; Yang, Y.; Zhou, Z. Effects of dietary live and heat-inactive baker’s yeast on growth, gut health, and disease resistance of Nile tilapia under high rearing density. Fish Shellfish Immunol. 2016, 56, 263–271. [Google Scholar] [CrossRef]
- Sung, Y.Y.; MacRae, T.H. Heat Shock Proteins and Disease Control in Aquatic Organisms Heat Shock Proteins and Disease Control in Aquatic Organisms. Aquac. Res. Dev. 2015, s2, 2–5. [Google Scholar]
- Avella, M.A.; Olivotto, I.; Silvi, S.; Place, A.R.; Carnevali, O. Effect of dietary probiotics on clownfish: A molecular approach to define how lactic acid bacteria modulate development in a marine fish. Am. J. Physiol. Soc. 2010, 298, R359–R371. [Google Scholar] [CrossRef]
- Tang, S.; Liu, S.; Zhang, J.; Zhou, L.; Wang, X.; Zhao, Q.; Weng, W.; Qin, J.G.; Chen, L.; Li, E. Relief of hypersaline stress in Nile tilapia Oreochromis niloticus by dietary supplementation of a host-derived Bacillus subtilis strain. Aquaculture 2020, 528, 735542. [Google Scholar] [CrossRef]
- Bakhrouf, A.; Mahdhi, A. Effect of dietary supplementation of probiotics and palm fruits extracts on the antioxidant enzyme gene expression in the mucosae of gilthead seabream (Sparus aurata L). Fish Shellfish Immunol. 2014, 39, 532–540. [Google Scholar]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2009, 5, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Article, R.; Bratovcic, A. Antioxidant Enzymes and their Role in Preventing Cell Damage. Acta Sci. Nutr. Health 2020, 4, 1–7. [Google Scholar]
- Dawood, M.A.O.; Eweedah, N.M.; Moustafa, E.; Farahat, E.M. Probiotic effects of Aspergillus oryzae on the oxidative status, heat shock protein, and immune-related gene expression of Nile tilapia (Oreochromis niloticus) under hypoxia challenge. Aquaculture 2019, 520, 734669. [Google Scholar] [CrossRef]
- Zhou, X.; Tian, Z.; Wang, Y. Effect of treatment with probiotics as water additive on tilapia (Oreochromis niloticus) growth performance and immune response Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune res. Fish Physiol. Biochem. 2009, 36, 501–509. [Google Scholar] [CrossRef]
- Makled, S.O.; Hamdan, A.M.; Fattah, A.; El Sayed, M. Effects of dietary supplementation of a marine thermotolerant bacterium, SO - 1, on growth performance and immune responses of Nile tilapia, Oreochromis niloticus. Aquac. Nutr. 2019, 25, 817–827. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X. Effects of Bacillus preparations on immunity and antioxidant activities in grass carp (Ctenopharyngodon idellus). Fish Physiol. Biochem. 2015, 38, 1585–1592. [Google Scholar]

| Probiotic Name | Probiotic Source | Dose (CFU/g) | Feeding Duration (Days) | Immunological Effects | Country of Investigation | Reference |
|---|---|---|---|---|---|---|
| B. subtilis HAINUP40 | Natural pond water | 108 | 56 | LYZ (↑ after 2 weeks), RB (↑ after 2 weeks), SOD (↑ after 14 weeks), T-AOC (↑ after 14 weeks) | Hainan, China | [92] |
| B. subtilis NZ86 | Commercial | Individual 107 | 51 | LYZ (↓), PG (↔), RB (↔), IL-1β (↔), TNF-α (↔), AH50 (↑ NZ86), LYZ (↑ NZ86) | Miami, IL, USA | [37] |
| Bacillus spp. | O. niloticus gastrointestinal tract | 108 | 28 | NO (↑), IgM (↑), LYZ (↑), AKP (↑), CAT (↑), SOD (↑) | Gaozhou, China | [49,50] |
| B. velezensis TPS3N, B. subtilis TPS4, B. amyloliquefaciens TPS17 | O. niloticus gastrointestinal tract | 108 individual, 108 + 108 + 108 combined | 28 | NO (↑), IgM (↑), LYZ (↑), AKP (↑), ACP (↑), SOD (↑), CAT (↑), MDA (↓) | Gaozhou, China | [49,50] |
| B. subtilis | Commercial | 1 × 108 and 1 × 1010 CFU/kg | 56 | IL-1β, TNF-α, IFN-γ, and hsp-70 in the live (↑) | Greentech Aquaculture Co. Ltd., Nonthaburi, Thailand | [93] |
| B. subtilis E20 | Culture collection strain | 0.3 mL of 108 cells/mL | 95 | Non-specific immunity parameters including LYZ, PG (↑) | Cairo, Egypt | [94] |
| B. subtilis | Commercial | 1010 individual | 180 | Serum TP (↑), WBCs (↑), LYZ (↑) | Sagana, Kenya | [38] |
| B. amyloliquefaciens | Culture collection strain | 2.1×109 | 60 | LYZ (↑), RB (↑), PG (↑), SOD (↔) | Pingtung, Taiwan | [52] |
| B. pumilus | O. niloticus | 107–109 | 120 | Peripheral blood leukocyte (↑), RB (↑), PG (↑) | Bangkok, Thailand | [53] |
| B. subtilis | Commercial | 3 g kg−1 | 42 | TLR-2 (↑), TNF-α (↑), IL-1β (↑), IL-10 (↑), TGFβ (↑), HSP70 (↑) | Plymouth, UK | [51] |
| B. amyloliquefaciens | Commercial | 109 | 60 | Blood TP (↑), IgM (↑), TNF-α (↑), HSP70 (↓), SOD (↔) | Kafr El-Sheikh farm, Kafr al-Shaykh, Egypt | [95] |
| B. licheniformis HGA8B | Anabas testudineus gastrointestinal tract | 106, 108 | 60 | MUC 2 (↑), SOD (↑), TLR-2 (↑), IL-10 (↑), TNF-α (↔), IL-8 (↔) GSH-Px (↑), CAT (↑), MPO (↑) | Panagad, Kerala, India | [96] |
| B. cereus | O. niloticus intestinal tract | 107, 108 via water | 42 | LYZ (↑), peroxidase activity (↑), ACP (↑), SOD (↑) | Guangzhou, China | [26] |
| B. subtilis, B. megaterium, and B. licheniformis | Culture collection strain | 106 | 120 | TNF-α (↑), HSP70 (↑), LYZ (↔) | Khon Kaen, Thailand | [97] |
| B. velezensis H3.1 | O. niloticus intestinal tract | 108 | 15 | LYZ (↑ mucus) (↔ serum) | Chiang Mai, Thailand | [98] |
| B. cereus NY5 | O. niloticus intestinal tract | 104 CFU mL−1 individual via water | 90 | SOD (↑ skin) | Guangzhou, China | [99] |
| B. subtilis WB60 | Anguilla japonica intestine | 108 individual | 56 | LYZ (↑), SOD (↑), HSP70 (↑), MPO (↑), IL-1β (↑), IFN-γ (↑), TNF-α (↑) | Busan, Republic of Korea | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shija, V.M.; Amoah, K.; Cai, J. Effect of Bacillus Probiotics on the Immunological Responses of Nile Tilapia (Oreochromis niloticus): A Review. Fishes 2023, 8, 366. https://doi.org/10.3390/fishes8070366
Shija VM, Amoah K, Cai J. Effect of Bacillus Probiotics on the Immunological Responses of Nile Tilapia (Oreochromis niloticus): A Review. Fishes. 2023; 8(7):366. https://doi.org/10.3390/fishes8070366
Chicago/Turabian StyleShija, Vicent Michael, Kwaku Amoah, and Jia Cai. 2023. "Effect of Bacillus Probiotics on the Immunological Responses of Nile Tilapia (Oreochromis niloticus): A Review" Fishes 8, no. 7: 366. https://doi.org/10.3390/fishes8070366
APA StyleShija, V. M., Amoah, K., & Cai, J. (2023). Effect of Bacillus Probiotics on the Immunological Responses of Nile Tilapia (Oreochromis niloticus): A Review. Fishes, 8(7), 366. https://doi.org/10.3390/fishes8070366