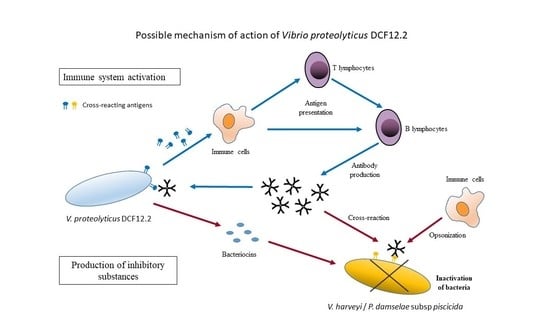
Effect of the Potential Probiotic Vibrio proteolyticus DCF12.2 on the Immune System of Solea senegalensis and Protection against Photobacterium damselae subsp. piscicida and Vibrio harveyi
Abstract
1. Introduction
2. Materials and Methods
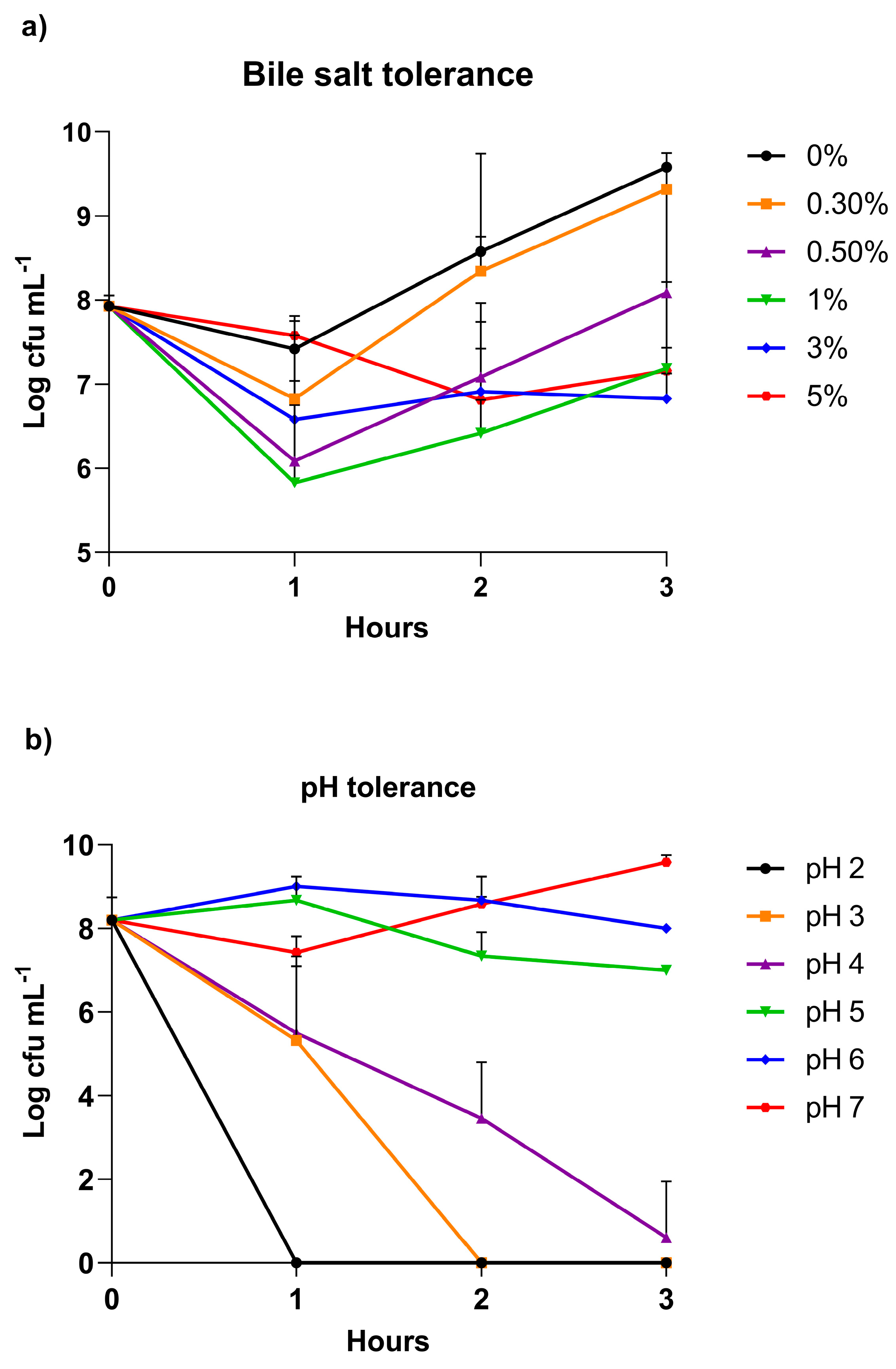
2.1. In Vitro Test to Determine DCF12.2 Survival in Intestinal Tract: pH and Bile Tolerance
2.2. Experimental Diets and Feeding Trial
- (i)
- Control diet (CTRL group): fish fed a commercial diet (Gemma, Skretting, Burgos, Spain).
- (ii)
- Fish intraperitoneally injected with V. proteolyticus DCF12.2 (IP group): V. proteolyticus DCF12.2 was grown on TSAs medium and resuspended in PBS at a concentration of 109 colony-forming units (cfu) mL−1. The fish were anesthetized with 100 ppm of isoeugenol (clove oil) and intraperitoneally injected using 0.1 mL of bacterial suspension [15].
- (iii)
- Fish immersed in a V. proteolyticus DCF12.2 suspension at 107 cfu mL−1 added in the tanks (BATH group), within the range used by other authors when probiotics were administrated though water [17]. One hour later, the water was changed to remove the probiotic cells. The treatment was repeated on the same fish 15 days later.
- (iv)
- Fish fed with a commercial diet (Gemma, Skretting, Burgos, Spain) supplemented with 109 cfu g−1 of V. proteolyticus DCF12.2 (DIET group). This experimental diet was prepared just before the administration by spraying the probiotic suspension on the feed surface [18].
2.3. Fish Sampling
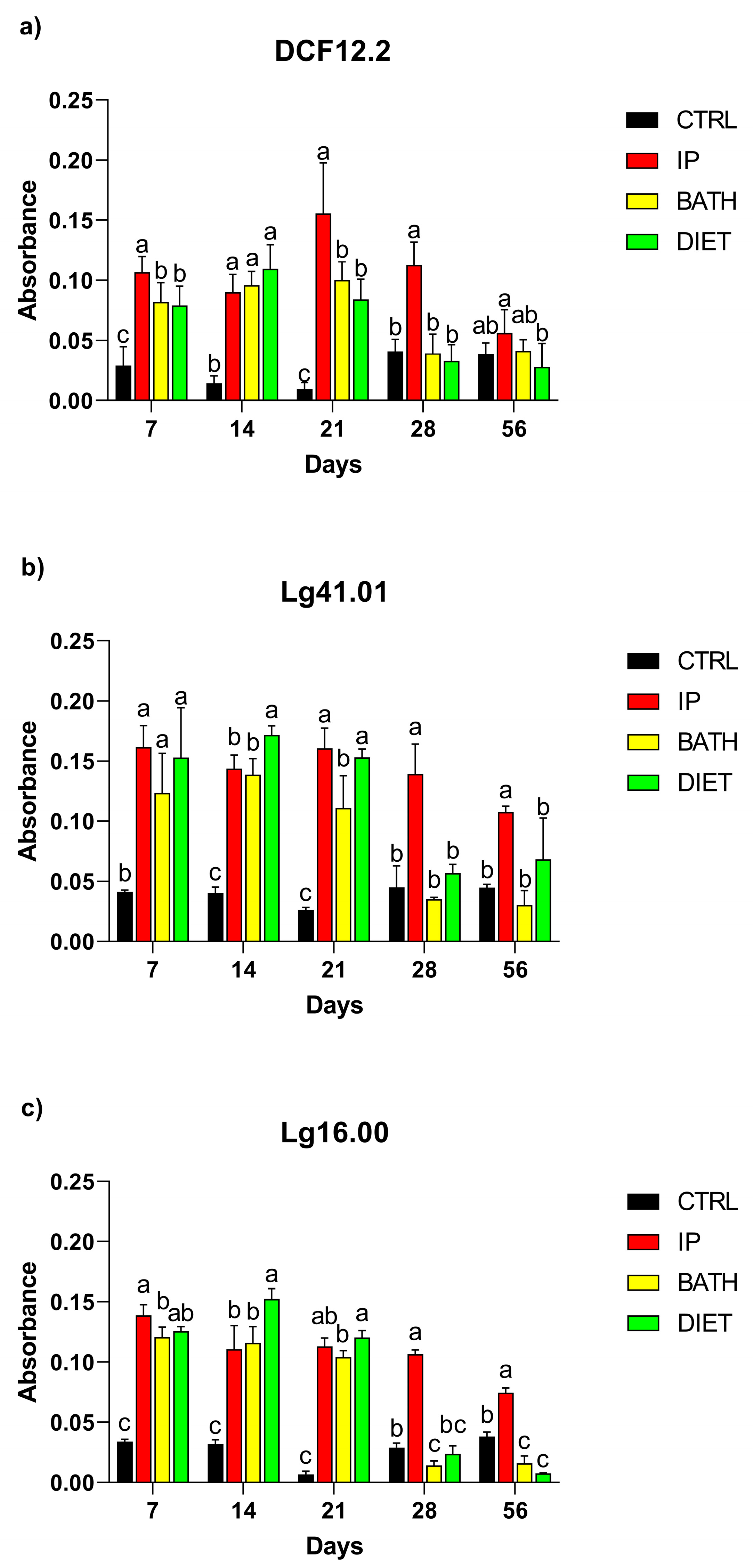
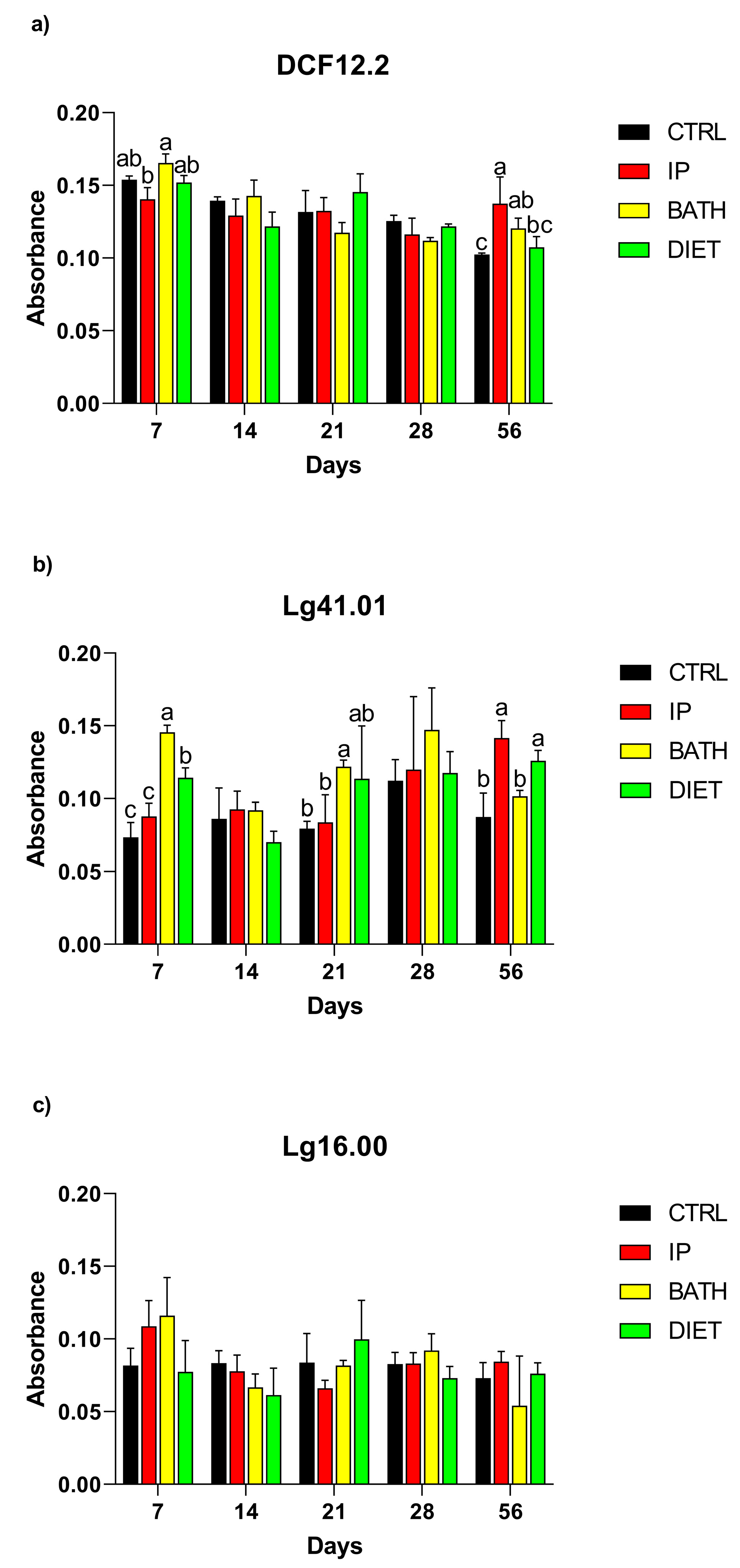
2.4. Detection of Antibodies in Serum, Intestinal Mucus, and Skin
2.5. Determination of Genes Involved in Innate Immune Response
2.6. Experimental Infection with P. damselae subsp. piscicida and V. harveyi
2.7. Statistical Analysis
3. Results
3.1. Growth of Vibrio Proteolyticus DCF12.2 in the Presence of Bile and Different pH
3.2. Detection of Antibodies in Serum, Intestinal Mucus, and Skin
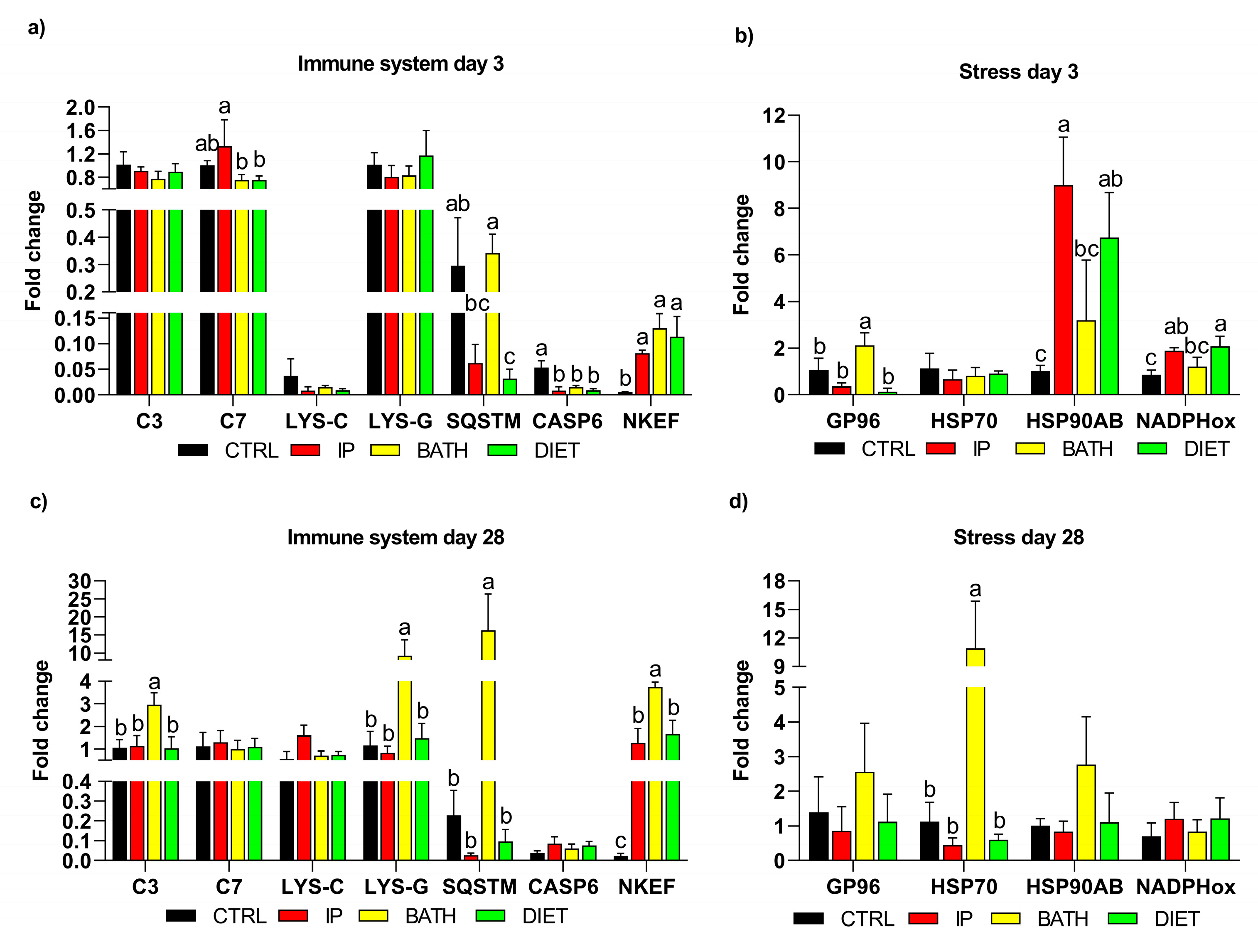
3.3. Determination of Genes Involved in Innate Immune Response
3.4. Determination of the Survival of Fish Administered with Probiotic and Infected with P. damselae subsp. piscicida and V. harveyi
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [PubMed]
- El-Hack, M.E.A.; El-Saadony, M.T.; Shafi, M.E.; Qattan, S.Y.A.; Batiha, G.E.; Khafaga, A.F.; Abdel-Moneim, A.-M.E.; Alagawany, M. Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1835–1850. [Google Scholar] [CrossRef] [PubMed]
- Ringø, E.; Doan, H.; Van Lee, S.H.; Soltani, M.; Hoseinifar, S.H.; Harikrishnan, R.; Song, S.K. Probiotics, lactic acid bacteria and bacilli: Interesting supplementation for aquaculture. J. Appl. Microbiol. 2020, 129, 116–136. [Google Scholar] [CrossRef]
- Patel, S.; Shukla, R.; Goyal, A. Probiotics in valorization of innate immunity across various animal models. J. Funct. Foods 2015, 14, 549–561. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Gao, C.; Ganesh, B.P.; Shi, Z.; Shah, R.R.; Fultz, R.; Major, A.; Venable, S.; Lugo, M.; Hoch, K.; Chen, X.; et al. Gut Microbe–Mediated Suppression of Inflammation-Associated Colon Carcinogenesis by Luminal Histamine Production. Am. J. Pathol. 2017, 187, 2323–2336. [Google Scholar] [CrossRef]
- Jobin, C. Precision medicine using microbiota. Science 2018, 359, 32–34. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Alagawany, M.; Patra, A.K.; Kar, I.; Tiwari, R.; Dawood, M.A.O.; Dhama, K.; Abdel-Latif, H.M.R. The functionality of probiotics in aquaculture: An overview. Fish Shellfish Immunol. 2021, 117, 36–52. [Google Scholar] [CrossRef]
- Sharifuzzaman, S.M.; Abbass, A.; Tinsley, J.W.; Austin, B. Subcellular components of probiotics Kocuria SM1 and Rhodococcus SM2 induce protective immunity in rainbow trout (Oncorhynchus mykiss, Walbaum) against Vibrio anguillarum. Fish Shellfish Immunol. 2011, 30, 347–353. [Google Scholar] [CrossRef]
- Palaksha, K.J.; Shin, G.W.; Kim, Y.R.; Jung, T.S. Evaluation of non-specific immune components from the skin mucus of olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2008, 24, 479–488. [Google Scholar] [CrossRef]
- Maassen, C.B.M.; Boersma, W.J.A.; Van Holten-Neelen, C.; Claassen, E.; Laman, J.D. Growth phase of orally administered Lactobacillus strains differentially affects IgG1/IgG2a ratio for soluble antigens: Implications for vaccine development. Vaccine 2003, 21, 2751–2757. [Google Scholar] [CrossRef]
- Beal, R.K.; Wigley, P.; Powers, C.; Barrow, P.A.; Smith, A.L. Cross-reactive cellular and humoral immune responses to Salmonella enterica serovars Typhimurium and Enteritidis are associated with protection to heterologous re-challenge. Vet. Immunol. Immunopathol. 2006, 114, 84–93. [Google Scholar] [CrossRef]
- Daniel, C.; Sebbane, F.; Poiret, S.; Goudercourt, D.; Dewulf, J.; Mullet, C.; Simonet, M.; Pot, B. Protection against Yersinia pseudotuberculosis infection conferred by a Lactococcus lactis mucosal delivery vector secreting LcrV. Vaccine 2009, 27, 1141–1144. [Google Scholar] [CrossRef]
- Medina, A.; Moriñigo, M.Á.; Arijo, S. Selection of putative probiotics based on antigen-antibody cross-reaction with Photobacterium damselae subsp. piscicida and Vibrio harveyi for use in Senegalese sole (Solea senegalensis). Aquac. Rep 2020, 17, 100366. [Google Scholar] [CrossRef]
- Thankappan, B.; Ramesh, D.; Ramkumar, S.; Natarajaseenivasan, K.; Anbarasu, K. Characterization of Bacillus spp. from the gastrointestinal tract of Labeo rohita—Towards to identify novel probiotics against fish pathogens. Appl. Biochem. Biotechnol. 2015, 175, 340–353. [Google Scholar] [CrossRef]
- Jahangiri, L.; Esteban, M.A. Administration of Probiotics in the Water in Finfish Aquaculture Systems: A Review. Fishes 2018, 3, 33. [Google Scholar] [CrossRef]
- Sultana, S.; Saifuddin, M.; Kaizer, M. Potential probiotic and health fostering effect of host gut-derived Enterococcus faecalis on freshwater prawn, Macrobrachium rosenbergii. Aquacul. Fish 2020, 7, 59–66. [Google Scholar] [CrossRef]
- García-Márquez, J.; Rico, R.M.; Sánchez-Saavedra, M.P.; Gómez-Pinchetti, J.L.; Acién, F.G.; Figueroa, F.L.; Alarcón, F.J.; Moriñigo, M.Á.; Abdala-Díaz, R.T. A short pulse of dietary algae boosts immune response and modulates fatty acid composition in juvenile Oreochromis niloticus. Aquac. Res. 2020, 51, 4397–4409. [Google Scholar] [CrossRef]
- Guardiola, F.A.; Cuesta, A.; Arizcun, M.; Meseguer, J.; Esteban, M.A. Comparative skin mucus and serum humoral defence mechanisms in the teleost gilthead seabream (Sparus aurata). Fish Shellfish Immunol. 2014, 36, 545–551. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Robertson, P.A.W.; Xu, H.S.; Austin, B. An enzyme-linked immunosorbent assay (ELISA) for the detection of Vibrio harvey in penaeid shrimp and water. J. Microbiol. Methods 1998, 34, 31–39. [Google Scholar] [CrossRef]
- Tapia-Paniagua, S.T.; Vidal, S.; Lobo, C.; Prieto-Álamo, M.J.; Jurado, J.; Cordero, H.; Cerezuela, R.; García de la Banda, I.; Esteban, M.A.; Balebona, M.C.; et al. The treatment with the probiotic Shewanella putrefaciens Pdp11 of specimens of Solea senegalensis exposed to high stocking densities to enhance their resistance to disease. Fish Shellfish Immunol. 2014, 41, 209–221. [Google Scholar] [CrossRef]
- Manchado, M.; Infante, C.; Asensio, E.; Cañavate, J.P. Differential gene expression and dependence on thyroid hormones of two glyceraldehyde-3-phosphate dehydrogenases in the flatfish Senegalese sole (Solea senegalensis Kaup). Gene 2007, 400, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Jiménez, I.; Williams, T.D.; Prieto-Álamo, M.J.; Abril, N.; Chipman, J.K.; Pueyo, C. Immune- and stress-related transcriptomic responses of Solea senegalensis stimulated with lipopolysaccharide and copper sulphate using heterologous cDNA microarrays. Fish Shellfish Immunol. 2009, 26, 699–706. [Google Scholar] [CrossRef]
- Prieto-Álamo, M.J.; Abril, N.; Osuna-Jiménez, I.; Pueyo, C. Solea senegalensis genes responding to lipopolysaccharide and copper sulphate challenges: Large-scale identification by suppression subtractive hybridization and absolute quantification of transcriptional profiles by real-time RT-PCR. Aquat. Toxicol. 2009, 91, 312–319. [Google Scholar] [CrossRef]
- Fernández-Trujillo, M.; Porta, J.; Manchado, M.; Borrego, J.J.; Alvarez, M.; Béjar, J. c-Lysozyme from Senegalese sole (Solea senegalensis): cDNA cloning and expression pattern. Fish Shellfish Immunol. 2008, 25, 697–700. [Google Scholar] [CrossRef]
- Salas-Leiton, E.; Anguis, V.; Martín-Antonio, B.; Crespo, D.; Planas, J.V.; Infante, C.; Cañavate, J.P.; Manchado, M. Effects of stocking density and feed ration on growth and gene expression in the Senegalese sole (Solea senegalensis): Potential effects on the immune response. Fish Shellfish Immunol. 2010, 28, 296–302. [Google Scholar] [CrossRef]
- Sarasquete, C.; Úbeda-Manzanaro, M.; Ortiz-Delgado, J.B. Effects of the isoflavone genistein in early life stages of the Senegalese sole, Solea senegalensis: Role of the Survivin and proliferation versus apoptosis pathways. BMC Vet. Res. 2018, 14, 16. [Google Scholar] [CrossRef]
- Teles, M.; Mackenzie, S.; Boltaña, S.; Callol, A.; Tort, L. Gene expression and TNF-alpha secretion profile in rainbow trout macrophages following exposures to copper and bacterial lipopolysaccharide. Fish Shellfish Immunol. 2011, 30, 340–346. [Google Scholar] [CrossRef]
- Manchado, M.; Salas-Leiton, E.; Infante, C.; Ponce, M.; Asensio, E.; Crespo, A.; Zuasti, E.; Cañavate, J.P. Molecular characterization, gene expression and transcriptional regulation of cytosolic HSP90 genes in the flatfish Senegalese sole (Solea senegalensis Kaup). Gene 2008, 416, 77–84. [Google Scholar] [CrossRef]
- Pfaffl, M. Development and Validation of an Externally Standardised Quantitative Insulin-like Growth Factor-1 RT-PCR Using LightCycler SYBR Green I Technology. In Rapid Cycle Real-Time PCR; Meuer, S., Wittwer, C., Nakagawara, K.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 281–291. [Google Scholar] [CrossRef]
- Arijo, S.; Chabrillón, M.; Díaz-Rosales, P.; Rico, R.; Martínez-Manzanares, E.; Balebona, M.C.; Toranzo, A.E.; Moriñigo, M.A. Bacteria isolated from outbreaks affecting cultured sole, Solea senegalensis (Kaup). Bull. Eur. Assoc. Fish Pathol. 2005, 25, 148–154. [Google Scholar]
- Arijo, S.; Rico, R.; Chabrillón, M.; Díaz-Rosales, P.; Martinez-Manzanares, E.; Balebona, M.C.; Magariños, B.; Toranzo, A.E.; Moriñigo, M.A. Effectiveness of a divalent vaccine for sole, Solea senegalensis (Kaup), against Vibrio harveyi and Photobacterium damselae subsp. piscicida. J. Fish Dis. 2005, 28, 33–38. [Google Scholar] [CrossRef]
- Amend, D.F. Potency testing of fish vaccines. In Fish Biologics: Serodiagnostics and Vaccines; Karger: Berlin, Germany, 1981; pp. 447–454. [Google Scholar]
- Pérez-Sánchez, T.; Ruiz-Zarzuela, I.; de Blas, I.; Balcázar, J.L. Probiotics in aquaculture: A current assessment. Rev. Aquac. 2014, 6, 133–146. [Google Scholar] [CrossRef]
- Gómez, G.D.; Balcázar, J.L. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Microbiol. Immunol. 2008, 52, 145–154. [Google Scholar] [CrossRef]
- Begley, M.; Gahan, C.G.M.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef] [PubMed]
- Balcázar, J.L.; Vendrell, D.; de Blas, I.; Ruiz-Zarzuela, I.; Muzquiz, J.L.; Girones, O. Characterization of probiotic properties of lactic acid bacteria isolated from intestinal microbiota of fish. Aquaculture 2008, 1–4, 188–191. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Xiao, P.; Li, G.Y.; Yue, S.; Huang, J.; Zhu, W.Y.; Mo, Z.L. Isolation and characterization of Bacillus spp. M001 for potential application in turbot (Scophthalmus maximus L.) against Vibrio anguillarum. Aquac. Nut. 2016, 22, 374–381. [Google Scholar] [CrossRef]
- Yúfera, M.; Darías, M.J. Changes in the gastrointestinal pH from larvae to adult in Senegal sole (Solea senegalensis). Aquaculture 2007, 267, 94–99. [Google Scholar] [CrossRef]
- Ringø, E.; Olsen, R.E.; Mayhew, T.M.; Myklebust, R. Electron microscopy of the intestinal microflora of fish. Aquaculture 2003, 227, 395–415. [Google Scholar] [CrossRef]
- Nikoskelainen, S.; Ouwehand, A.; Salminen, S.; Bylund, G. Protection of rainbow trout (Oncorhynchus mykiss) from furunculosis by Lactobacillus rhamnosus. Aquaculture 2001, 198, 229–236. [Google Scholar] [CrossRef]
- Salinas, I. The Mucosal Immune System of Teleost Fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef]
- Magor, B.G. Antibody Affinity Maturation in Fishes-Our Current Understanding. Biology 2015, 4, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; Lapatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Esteve-Gassent, M.D.; Fouz, B.; Barrera, R.; Amaro, C. Efficacy of oral reimmunisation after immersion vaccination against Vibrio vulnificus in farmed European eels. Aquaculture 2004, 231, 9–22. [Google Scholar] [CrossRef]
- Irie, T.; Watarai, S.; Iwasaki, T.; Kodama, H. Protection against experimental Aeromonas salmonicida infection in carp by oral immunisation with bacterial antigen entrapped liposomes. Fish Shellfish Immunol. 2005, 18, 235–242. [Google Scholar] [CrossRef]
- Núñez-Díaz, J.A.; Fumanal, M.; Mancera, J.M.; Moriñigo, M.A.; Balebona, M.C. Two routes of infection with Photobacterium damselae subsp. piscicida are effective in the modulation of the transcription of immune related genes in Solea senegalensis. Vet. Immunol. Immunopathol. 2016, 179, 8–17. [Google Scholar] [CrossRef]
- Zhang, X.H.; He, X.; Austin, B. Vibrio harveyi: A serious pathogen of fish and invertebrates in mariculture. Mar. Life Sci. Technol. 2020, 2, 231–245. [Google Scholar] [CrossRef]
- Tapia-Paniagua, S.T.; Fumanal, M.; Anguís, V.; Fernández-Díaz, C.; Alarcón, F.J.; Moriñigo, M.A.; Balebona, M.C. Modulation of Intestinal Microbiota in Solea senegalensis Fed Low Dietary Level of Ulva ohnoi. Front. Microbiol. 2019, 10, 171. [Google Scholar] [CrossRef]
- Lobb, C.J.; Clem, W.L. Phylogeny of immunoglobulin structure and function-XII. Secretory immunoglobulins in the bile of the marine teleost Archosargus probatocephalus. Mol. Immunol. 1981, 18, 615–619. [Google Scholar] [CrossRef]
- Vervarcke, S.; Ollevier, F.; Kinget, R.; Michoel, A. Oral vaccination of African catfish with Vibrio anguillarum O2: Effect on antigen uptake and immune response by absorption enhancers in lag time coated pellets. Fish Shellfish Immunol. 2004, 16, 407–414. [Google Scholar] [CrossRef]
- Hatten, F.; Fredriksen, Å.; Hordvik, I.; Endresen, C. Presence of IgM in cutaneous mucus, but not in gut mucus of Atlantic salmon, Salmo salar. Serum IgM is rapidly degraded when added to gut mucus. Fish Shellfish Immunol. 2001, 11, 257–268. [Google Scholar] [CrossRef]
- Klesius, P.H.; Shoemaker, C.A.; Evans, J.J. Efficacy of a killed Streptococcus iniae vaccine in tilapia (Oreochromis niloticus). Bull. Eur. Ass. Fish Pathol. 1999, 19, 39. [Google Scholar]
- Holland, M.C.H.; Lambris, J.D. The complement system in teleosts. Fish Shellfish Immunol. 2002, 12, 399–420. [Google Scholar] [CrossRef]
- Ichiki, S.; Kato-Unoki, Y.; Somamoto, T.; Nakao, M. The binding spectra of carp C3 isotypes against natural targets independent of the binding specificity of their thioester. Dev. Comp. Immunol. 2012, 38, 10–16. [Google Scholar] [CrossRef]
- Ye, X.; Zhang, L.; Tian, Y.; Tan, A.; Bai, J.; Li, S. Identification and expression analysis of the g-type and c-type lysozymes in grass carp Ctenopharyngodon idellus. Dev. Comp. Immunol. 2010, 34, 501–509. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Mauri, I.; Romero, A.; Acerete, L.; MacKenzie, S.; Roher, N.; Callol, A.; Cano, I.; Alvarez, M.C.; Tort, L. Changes in complement responses in Gilthead seabream (Sparus aurata) and European seabass (Dicentrarchus labrax) under crowding stress, plus viral and bacterial challenges. Fish Shellfish Immunol. 2011, 30, 182–188. [Google Scholar] [CrossRef]
- Moscat, J.; Diaz-Meco, M.T. p62: A versatile multitasker takes on cancer. Trends Biochem. Sci. 2012, 37, 230–236. [Google Scholar] [CrossRef]
- Miao, Z.; Miao, Z.; Wang, S.; Wu, H.; Xu, S. Exposure to imidacloprid induce oxidative stress, mitochondrial dysfunction, inflammation, apoptosis and mitophagy via NF-kappaB/JNK pathway in grass carp hepatocytes. Fish Shellfish Immunol. 2022, 120, 674–685. [Google Scholar] [CrossRef]
- Cui, T.; Liu, P.; Chen, X.; Liu, Z.; Wang, B.; Gao, C.; Wang, Z.; Li, C.; Yang, N. Identification and functional characterization of caspases in turbot (Scophthalmus maximus) in response to bacterial infection. Fish Shellfish Immunol. 2023, 137, 108757. [Google Scholar] [CrossRef] [PubMed]
- Bolívar, B.E.; Vogel, T.P.; Bouchier-Hayes, L. Inflammatory caspase regulation: Maintaining balance between inflammation and cell death in health and disease. FEBS J. 2019, 286, 2628–2644. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Chae, H.Z.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005, 38, 1543–1552. [Google Scholar] [CrossRef]
- Ishii, T.; Warabi, E.; Yanagawa, T. Novel roles of peroxiredoxins in inflammation, cancer and innate immunity. J. Clin. Biochem. Nutr. 2012, 50, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, H.Q.; Niu, H.; Shi, Y.H.; Li, M.Y. Increased liver protein and mRNA expression of natural killer cell-enhancing factor B (NKEF-B) in ayu (Plecoglossus altivelis) after Aeromonas hydrophila infection. Fish Shellfish Immunol. 2009, 26, 567–571. [Google Scholar] [CrossRef]
- Bethke, J.; Rojas, V.; Berendsen, J.; Cárdenas, C.; Guzmán, F.; Gallardo, J.A.; Mercado, L. Development of a new antibody for detecting natural killer enhancing factor (NKEF)-like protein in infected salmonids. J. Fish Dis. 2012, 35, 379–388. [Google Scholar] [CrossRef]
- Loo, G.H.; Sutton, D.L.; Schuller, K.A. Cloning and functional characterisation of a peroxiredoxin 1 (NKEF A) cDNA from Atlantic salmon (Salmo salar) and its expression in fish infected with Neoparamoeba perurans. Fish Shellfish Immunol. 2012, 32, 1074–1082. [Google Scholar] [CrossRef]
- Esteban, M.A.; Chaves-Pozo, E.; Arizcun, M.; Meseguer, J.; Cuesta, A. Regulation of natural killer enhancing factor (NKEF) genes in teleost fish, gilthead seabream and European sea bass. Mol. Immunol. 2013, 55, 275–282. [Google Scholar] [CrossRef]
- Strbo, N.; Podack, E.R. REVIEW ARTICLE: Secreted Heat Shock Protein gp96-Ig: An Innovative Vaccine Approach. Am. J. Reprod. Immunol. 2008, 59, 407–416. [Google Scholar] [CrossRef]
- Núñez-Díaz, J.A.; García de la Banda, I.; Lobo, C.; Moriñigo, M.A.; Balebona, M.C. Transcription of immune related genes in Solea senegalensis vaccinated against Photobacterium damselae subsp. piscicida. Identification of surrogates of protection. Fish Shellfish Immunol. 2017, 66, 455–465. [Google Scholar] [CrossRef]
- Huo, D.; Sun, L.; Zhang, L.; Yang, H.; Liu, S.; Sun, J.; Su, F. Time course analysis of immunity-related gene expression in the sea cucumber Apostichopus japonicus during exposure to thermal and hypoxic stress. Fish Shellfish Immunol. 2019, 95, 383–390. [Google Scholar] [CrossRef]
- Guo, S.; Wharton, W.; Moseley, P.; Shi, H. Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaperones 2007, 12, 245. [Google Scholar] [CrossRef]
- Zininga, T.; Ramatsui, L.; Shonhai, A. Heat Shock Proteins as Immunomodulants. Molecules 2018, 23, 2846. [Google Scholar] [CrossRef]
- Azad, P.; Ryu, J.; Haddad, G.G. Distinct role of Hsp70 in Drosophila hemocytes during severe hypoxia. Free Radic. Biol. Med. 2011, 51, 530–538. [Google Scholar] [CrossRef]
- Chen, Y.M.; Kuo, C.E.; Wang, T.Y.; Shie, P.S.; Wang, W.C.; Huang, S.L.; Tsai, T.J.; Chen, P.P.; Chen, J.C.; Chen, T.Y. Cloning of an orange-spotted grouper Epinephelus coioides heat shock protein 90AB (HSP90AB) and characterization of its expression in response to nodavirus. Fish Shellfish Immunol. 2010, 28, 895–904. [Google Scholar] [CrossRef]
- Segal, B.H.; Grimm, M.J.; Khan, A.N.H.; Han, W.; Blackwell, T.S. Regulation of innate immunity by NADPH oxidase. Free Radic. Biol. Med. 2012, 53, 72–80. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Yousefi, S.; Van Doan, H.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative Stress and Antioxidant Defense in Fish: The Implications of Probiotic, Prebiotic, and Synbiotics. Rev. Fish. Sci. Aquac. 2020, 29, 198–217. [Google Scholar] [CrossRef]
- Ben Mkaddem, S.; Pedruzzi, E.; Werts, C.; Coant, N.; Bens, M.; Cluzeaud, F.; Goujon, J.M.; Ogier-Denis, E.; Vandewalle, A. Heat shock protein gp96 and NAD(P)H oxidase 4 play key roles in Toll-like receptor 4-activated apoptosis during renal ischemia/reperfusion injury. Cell Death Differ. 2010, 17, 1474–1485. [Google Scholar] [CrossRef]
- Acosta, F.; Real, F.; Ellis, A.E.; Tabraue, C.; Padilla, D.; Ruiz De Galarreta, C.M. Influence of vaccination on the nitric oxide response of gilthead seabream following infection with Photobacterium damselae subsp. piscicida. Fish Shellfish Immunol. 2005, 18, 31–38. [Google Scholar] [CrossRef]
- Lin, J.H.Y.; Chen, T.Y.; Chen, M.S.; Chen, H.E.; Chou, R.L.; Chen, T.I.; Su, M.-S.; Yang, H.L. Vaccination with three inactivated pathogens of cobia (Rachycentron canadum) stimulates protective immunity. Aquaculture 2006, 255, 125–132. [Google Scholar] [CrossRef]
- García de la Banda, I.; Lobo, C.; Chabrillón, M.; León-Rubio, J.M.; Arijo, S.; Pazos, G.; María Lucas, L.; Moriñigo, M.Á. Influence of dietary administration of a probiotic strain Shewanella putrefaciens on senegalese sole (Solea senegalensis, Kaup 1858) growth, body composition and resistance to Photobacterium damselae subsp piscicida. Aquac. Res. 2012, 43, 662–669. [Google Scholar] [CrossRef]
- Brunt, J.; Newaj-Fyzul, A.; Austin, B. The development of probiotics for the control of multiple bacterial diseases of rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2007, 30, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Brunt, J.; Austin, B. Use of a probiotic to control lactococcosis and streptococcosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2005, 28, 693–701. [Google Scholar] [CrossRef]
- Abbass, A.; Sharifuzzaman, S.M.; Austin, B. Cellular components of probiotics control Yersinia ruckeri infection in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2010, 33, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Arijo, S.; Brunt, J.; Chabrillón, M.; Díaz-Rosales, P.; Austin, B. Subcellular components of Vibrio harveyi and probiotics induce immune responses in rainbow trout, Oncorhynchus mykiss (Walbaum), against V. harveyi. J. Fish Dis. 2008, 31, 579–590. [Google Scholar] [CrossRef]
- Plant, K.P.; LaPatra, S.E. Advances in fish vaccine delivery. Dev. Comp. Immunol. 2011, 35, 1256–1262. [Google Scholar] [CrossRef]
- Nakanishi, T.; Kiryu, I.; Ototake, M. Development of a new vaccine delivery method for fish: Percutaneous administration by immersion with application of a multiple puncture instrument. Vaccine 2002, 20, 3764–3769. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Kim, J.S.; Balasundaram, C.; Heo, M.S. Vaccination effect of liposomes entrapped whole cell bacterial vaccine on immune response and disease protection in Epinephelus bruneus against Vibrio harveyi. Aquaculture 2012, 342–343, 69–74. [Google Scholar] [CrossRef]
- Mao, Z.; He, C.; Qiu, Y.; Chen, J. Expression of Vibrio harveyi ompK in the yeast Pichia pastoris: The first step in developing an oral vaccine against vibriosis? Aquaculture 2011, 318, 268–272. [Google Scholar] [CrossRef]

| Protein | Gen | Reference |
|---|---|---|
| Immune response | ||
| Complement component 3 | C3 | Prieto-Álamo et al. [26] |
| Complement component 7 | C7 | Prieto-Álamo et al. [26] |
| Lysozyme C | C-LYS | Fernández-Trujillo et al. [27] |
| Lysozyme G | G-LYS | Salas-Leiton et al. [28] |
| Sequestosome 1 | SQSTM-1 | Prieto-Álamo et al. [26] |
| Caspase 6 | Casp6 | Sarasquete et al. [29] |
| Natural killer enhancing factor | NKEF | Prieto-Álamo et al. [26] |
| Stress response | ||
| NADPH oxidase | NADPHox | Teles et al. [30] |
| Heat shock protein 70 | HSP70 | Salas-Leiton et al. [28] |
| Heat shock protein 90 | HSP90AB | Manchado et al. [31] |
| Heat shock protein gp96 | GP96 | Osuna-Jiménez et al. [25] |
| Reference genes | ||
| Gliceraldehide-3-phosfatase 1 | GADPH1 | Osuna-Jiménez et al. [25] |
| Gliceraldehide-3-phosfatase 2 | GADPH2 | Manchado et al. [24] |
| Treatment | P. damselae subsp. piscicida | V. harveyi | ||
|---|---|---|---|---|
| Mortality | RPS (%) | Mortality | RPS (%) | |
| Control | 100 | - | 60 | - |
| IP | 70 | 30 | 20 | 67 |
| BATH | 60 | 40 | 70 | - |
| DIET | 70 | 30 | 70 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina, A.; García-Márquez, J.; Moriñigo, M.Á.; Arijo, S. Effect of the Potential Probiotic Vibrio proteolyticus DCF12.2 on the Immune System of Solea senegalensis and Protection against Photobacterium damselae subsp. piscicida and Vibrio harveyi. Fishes 2023, 8, 344. https://doi.org/10.3390/fishes8070344
Medina A, García-Márquez J, Moriñigo MÁ, Arijo S. Effect of the Potential Probiotic Vibrio proteolyticus DCF12.2 on the Immune System of Solea senegalensis and Protection against Photobacterium damselae subsp. piscicida and Vibrio harveyi. Fishes. 2023; 8(7):344. https://doi.org/10.3390/fishes8070344
Chicago/Turabian StyleMedina, Alberto, Jorge García-Márquez, Miguel Ángel Moriñigo, and Salvador Arijo. 2023. "Effect of the Potential Probiotic Vibrio proteolyticus DCF12.2 on the Immune System of Solea senegalensis and Protection against Photobacterium damselae subsp. piscicida and Vibrio harveyi" Fishes 8, no. 7: 344. https://doi.org/10.3390/fishes8070344
APA StyleMedina, A., García-Márquez, J., Moriñigo, M. Á., & Arijo, S. (2023). Effect of the Potential Probiotic Vibrio proteolyticus DCF12.2 on the Immune System of Solea senegalensis and Protection against Photobacterium damselae subsp. piscicida and Vibrio harveyi. Fishes, 8(7), 344. https://doi.org/10.3390/fishes8070344