Abstract
Clinch Dace (Chrosomus sp. cf. saylori) is a newly recognized and yet-undescribed species of minnow with a restricted and fragmented distribution in the upper Tennessee River basin in southwestern Virginia, USA. We collected Clinch Dace from seven streams and observed variations at nine selectively neutral microsatellite DNA loci to infer population genetic processes and identify units for conservation management. Bayesian cluster analysis showed that three of the seven surveyed populations were genetically distinct, while the other four populations showed signs of recent admixture. Estimated effective population sizes and m-ratios were low within most populations, suggesting loss of alleles due to recent genetic drift. Positive FIS values, high average individual inbreeding coefficients, and high degrees of inferred relatedness among individuals suggested that inbreeding is taking place in some populations. FST values were high, and analysis of molecular variance indicated genetic divergence among populations. These indicators suggest that Clinch Dace populations are subject to the genetic processes that are characteristic of small and isolated populations.
Key Contribution:
The findings inform conservation planning by identifying units of management and suggesting sources of individuals for translocations among wild or captive breeding populations, as well as demographic augmentation of targeted populations, to address genetic isolation and inbreeding.
1. Introduction
Clinch Dace (Chrosomus sp. cf. saylori) (Figure 1) is a currently undescribed species of minnow (Family Leuciscidae) that was first discovered in Tazewell County, Virginia, USA, in 1999 [1]. Clinch Dace was first thought to be a disjunct population of the closely related Laurel Dace (Chrosomus saylori). However, meristic and morphological differences between these congeners, such as a longer anal fin base and shallower head in the Clinch Dace, suggest otherwise [2]. Field identification of the Clinch Dace relies on differences in breeding colors between it and the Laurel Dace; the Clinch Dace has an upper black lateral band that extends all of the way to the caudal fin and two yellow spots at the caudal fin base [2]. Clinch Dace is currently being described formally as a new species (Dave Neeley, Tennessee Aquarium and Conservation Institute, personal communication).

Figure 1.
Clinch Dace (photo by R. Bourquin).
Surveys conducted since 1999 [3,4] have found that Clinch Dace occupy 16 streams in eight drainages of the upper Clinch River in Russell and Tazewell counties in southwestern Virginia, USA. Clinch Dace populations are isolated and have low densities [5]. White and Orth [3] showed negative correlations between Clinch Dace presence and substrate particle size, stream width, and conductivity. Clinch Dace occupancy was positively correlated with the proportion of forested land in the respective watersheds [5]. Clinch Dace occupies restricted habitats in isolated patches, especially in areas affected by coal mining and logging; hence, it is vulnerable to local extirpation. Virginia’s Wildlife Action Plan [6] classifies Clinch Dace as Tier II—Very High Conservation Need. Clinch Dace is under review for potential listing under the U.S. Endangered Species Act [7].
To promote species viability, it may be necessary to facilitate natural migration via removal of barriers to migration or initiate population augmentations through translocation, captive breeding, and stocking. Assessment of genetic variation above and below six road crossings found none to be barriers to genetically effective migration [8]. Before augmentation actions can be purposefully planned and implemented, managers need detailed information on population genetics of Clinch Dace. Individual populations might or might not show the signatures of small-population genetic processes, i.e., random genetic drift and inbreeding. As Clinch Dace are headwater specialists [3,5], populations are unlikely to disperse through larger unsuitable downstream habitats and, ultimately, the Clinch River itself. It is reasonable to hypothesize that some, if not all, of these populations should be regarded as demographically independent and distinct management units; therefore, we tested against the null hypothesis the notion that Clinch Dace populations are genetically homogenous. To characterize within-population genetic processes and population genetic divergence, and thereby inform management planning, we analyzed variations in microsatellite loci using DNA isolated from fin-clips of Clinch Dace collected across their distribution to assess populations’ genetic processes and structure.
2. Materials and Methods
2.1. Fish Sampling
In the summer of 2017, we sampled fish located in seven streams (Figure 2) known to be inhabited by Clinch Dace [9] using three-pass pulsed-DC electrofishing. Sample sites and sizes were Big Creek (n = 106), Greasy Creek (n = 6), Hart Creek, (n = 63), Hurricane Creek (n = 40), Lewis Creek (n = 3), Middle Creek (n = 11), and Pine Creek (n = 32). A small fin-clip was cut from the upper caudal fin of Clinch Dace and stored in 95% ethanol. Fin-clips were given a unique identifier that corresponded to stream, reach, and fish length, and, in some cases, a photograph was taken. Any Clinch Dace that died during sampling were preserved in 10% formalin. All fish sampling was performed in accordance with Virginia Tech IACUC protocol FWC 16-188 and approved on 1 November 2016.
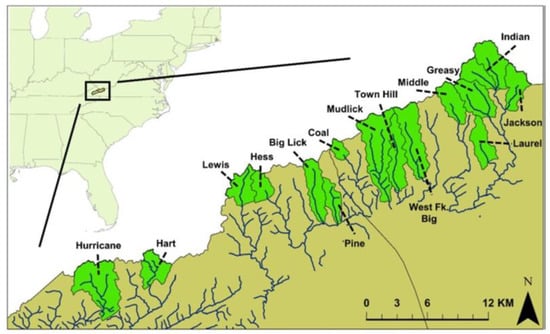
Figure 2.
Map of study site showing areas occupied by Clinch Dace based on prior surveys [9].
2.2. Genetic Markers
We extracted DNA from fin-clips using the DNeasy Blood and Tissue kit (Qiagen, Germantown, MD, USA). Concentration and purity of extracted DNA were quantified using a µLite spectrophotometer (BioDrop, Cambridge, UK). We screened the following 17 microsatellite loci using primer pairs that were developed to amplify the microsatellite DNA of European Minnow Phoxinus phoxinus [10]: Cto-A-247, LleB-072, LleC-090, Rru4, BLi-84, BLi-98, BLi-153 [11,12,13], CypG9 [14], LceC1 [15], Lco3 [16], Ppro132 [17], Rru4 [18], Lsou5, Lsou8 [19], Rhea20 [20], NLi-153 [21], CypG30 [22], and MFW1 [23]. Polymerase chain reaction amplification protocols, including reaction mixtures, were modified from those outlined by Grenier et al. [10] as necessary to promote amplification in this species. The PCR protocol was as follows: initial denaturation 94 °C for 3 min; 35 cycles of denaturation at 94 °C for one minute, annealing at 56 °C for 45 s, and extension at 72 °C for 1 min; and final extension at 72 °C for 5 min. Fragment-size analysis was conducted using GeneMarker (SoftGenetics, State College, PA, USA). A small subset of amplicons was sent to the Fralin Life Sciences Institute (Blacksburg, VA, USA) for Sanger sequencing to confirm successful amplification of the targeted microsatellite locus.
2.3. Data Analysis
We used Microchecker [24] to test for segregation of null alleles and PCR artifacts, such as stuttering or large-allele dropout. Departures from the Hardy–Weinberg equilibrium and the linkage disequilibrium were tested in Arlequin 3.5 [25], with exact tests using Markov chain with a forecasted chain length of 1,000,000 and 100,000 dememorization steps for all loci in all stream reaches.
To visualize and define population structures, the Bayesian cluster analysis implemented within STRUCTURE 2.3.4 [26] was applied to assess support for different numbers of population clusters (K) and assign individuals’ multilocus genotypes to those clusters. We used the admixture model to infer ancestry, with a burn-in length of 10,000 and 100,000 Monte Carlo Markov Chain iterations after the burn-in period. We assessed population divergence at several levels. Firstly, we ran STRUCTURE’s admixture model, with all samples from all collections with the set number of clusters (K) running from one to ten, as well as having five replications. On the basis of the results of this run, we then applied the algorithm to data from the four streams where admixture seemed most likely using K values from 1 to 5 and five replicate runs. Finally, we ran the admixture model for each stream individually, with each having K from one to five and five replicate runs.
Genetic diversity within inferred populations was quantified in terms of number of alleles per locus (A), allele frequencies, expected and observed heterozygosities (HE and HO), and allelic size ranges (number of repeats, R) for each locus and averaged across all loci for each population. M-ratios [27] were calculated as the number of alleles observed divided by the number possible within the observed allele size range; m-ratios lower than about 0.7 indicated the occurrence of prior genetic bottlenecks. The inbreeding coefficient (FIS) in each population was calculated using Fstat [28]. We employed the Bayesian analysis available in Inest 2.2 [29,30] using default settings to differentiate the effects of inbreeding from those of null alleles and genotyping errors. Effective population sizes (Ne) were estimated in NeEstimator v2 [31] using the linkage disequilibrium method. Rare microsatellite alleles were removed from the analysis to avoid upward bias of estimates. We used the program MLrelate [32] to estimate genetic relatedness among individual Clinch Dace within each stream.
Population-level genetic divergence was quantified using FST [33] and analysis of molecular variance (AMOVA [34]). FST was calculated in GENEALEX 6.5 [35]. AMOVA partitioning genetic variation within and among individuals and populations was executed in Arlequin 3.5 [25]. We measured the along-stream distances between the most-downstream Clinch Dace-occupied sites between each pair of streams in ArcGIS 10.2.2 [36]. We then plotted pairwise distances between the most-downstream Clinch Dace-occupied site on each stream against FST in a scatterplot and calculated r2 in order to assess the effects of isolation-by-distance.
3. Results
3.1. Polymorphism and Utility of Marker Loci
Of the 17 primer pairs tested, nine (Cto-A-247, LleC-090, BLi-84, BLi-153, Lco3, Lsou8, Rhea20, CypG30, and MFW1) consistently produced amplification products that were sufficiently clear to be analyzed for variation in fragment size (Table 1). Sequencing of amplicons for loci CtoA-247, BLI-84, BLI-153, Lco-3, Lsou-8, and MFW-1 all showed one tract of the repeated core motif, as reported for Phoxinus phoxinus and Gobio gobio by Grenier et al. [10]. Locus LleC-090 exhibited two tracts of the core motif, as reported for Leuciscus leuciscus by Dubut et al. [12].

Table 1.
Clinch Dace microsatellite loci amplified and analyzed to determine fragment size.
All microsatellite loci were proven to be polymorphic (Table 2), with populations exhibiting as many as eight (Hurricane Fork, CypG30) alleles per locus. Microchecker detected four instances of null alleles segregating within particular populations. No one locus consistently yielded null alleles, and data for all loci were included in subsequent analyses. Data for two loci in two populations (Hart Creek and Big Lick Creek) showed apparent linkage disequilibrium; since this outcome was observed in only these 2 cases among 72 tests for each of the 7 populations, we attributed this outcome to chance, and data from these loci were retained for further analyses.

Table 2.
Genetic diversity in Clinch Dace streams. Data for monomorphic loci are not shown. N = number of samples; HO = observed heterozygosity; HE = expected heterozygosity; NA = mean number of alleles at polymorphic loci; Range = mean range of allele sizes in base pairs for polymorphic loci; M-ratio = ratio of A to Range; Sig H-W = ratio of loci with significant departures of genotype frequencies from Hardy–Weinberg expectations to total number of polymorphic loci; Bonferroni α = Bonferroni-corrected critical p-values for assessing significance of a departure of genotype frequencies from Hardy–Weinberg expectations.
3.2. Recognition of Differentiated Genetic Units
STRUCTURE analysis of all samples revealed the strongest log-likelihood support for there being five genetic clusters (K = 5) among the seven stream populations surveyed (Figure 3). Populations in Hurricane Fork (population 1) and Hart Creek (population 2) clustered separately, while those in the remaining five streams (Pine Creek, Big Lick Creek, Middle Creek and Greasy Creek) displayed apparent admixture. Cluster analysis of populations in particular streams that run independently of one another showed no structure among sites or reaches.
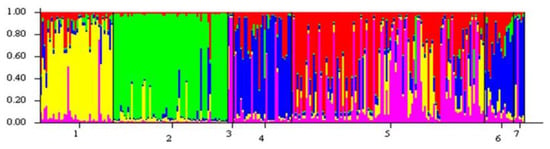
Figure 3.
Bar plot of results of Bayesian cluster and individual assignment analysis using STRUCTURE for the seven stream populations at K = 5; 1 = Hurricane Fork, 2 = Hart Creek, 3 = Lewis Creek, 4 = Pine Creek, 5 = Big Lick Creek, 6 = Middle Creek, 7 = Greasy Creek.
3.3. Within-Population Genetic Variation
Expected (HE) and observed (HO) heterozygosities (Table 2) were similar in most cases. Bonferroni-corrected alpha values varied because the number of polymorphic loci observed in each population varied; genotype frequencies at four loci distributed across four populations departed from Hardy–Weinberg equilibrium (Suppl. Table 1), and three of those departures involved locus CypG30. We retained data for all nine loci that consistently amplified Clinch Dace DNA for subsequent analyses.
At a locus-by-locus level, 25 of 30 m-ratios (Table S1), and at a multi-locus level, m-ratios for all seven populations (Table 2) were lower than the criterion level of 0.70 suggested by Garza and Williamson [27] (2001), suggesting that these populations underwent genetic bottlenecks in the recent past. Estimated effective population sizes (Ne) for Clinch Dace populations in each stream (Table 3) ranged from the low tens (five streams) to approximately 500 (Hart Creek). Small sample sizes for Lewis Creek and Middle Creek precluded the estimation of Ne for these populations using the linkage disequilibrium method. Indeed, only for Big Lick Creek, which had the largest sample size, was an upper limit for Ne estimated. In this population, the ratio of effective population size to our estimated population size was 0.26. The species does not exhibit distinguishable sexual dimorphism based on size or color [2], and as it is an imperiled species, we did not sacrifice individuals to observe gonadal development; hence, we cannot assess the impact of sex ratio upon Ne.

Table 3.
Estimated effective population sizes. N = total number of samples analyzed, and Ne = effective population size.
Some populations showed indication of ongoing inbreeding. In total, 17 of 30 locus-by-locus FIS values were positive and significantly greater than 0 (Table S1), but its multi-locus value was significantly above random expectation for only the Hart Creek population (Table 4). Average individual inbreeding coefficients Fi were greater than 0.1 in four populations—Big Lick Creek, Hart Creek, Lewis Creek, and Middle Creek. Results of maximum likelihood-based estimation showed that individuals within steams often showed relatedness, i.e., they were inferred to have full-sibling, half-sibling, or parent–offspring relationships (Figure 4). Not surprisingly, small population size and high frequency of relatedness seemed to be linked. In Big Lick Creek, which had an estimated Ne of 106, 19% of individual pairs were apparent parent/offspring, 10% were full-siblings, and 7% were half-siblings. In Greasy Creek, with an Ne of 58, 13% of individual pairs were half-siblings. In Hart Creek, where Ne was 492, 14% of individual pairs were full-siblings, 10% were half-siblings, and 11% had parent/offspring relationships. In Hurricane Fork, where Ne was 23.5, 1% of individual pairs were half-siblings, 22% had parent/offspring relationships, and 10% were full-siblings. In Lewis Creek, with an N of 3 and where Ne could not be estimated, two individuals were full-siblings. At Middle Creek, with an N of 16 and where Ne could not be estimated, 23% of individual pairs had parent/offspring relationships, 6% were half-siblings, and 3% were full-siblings. In Pine Creek, which had an Ne of 61, 23% of individual pairs were parent/offspring, 6% were half-siblings, and 3% were full-siblings.

Table 4.
Metrics of inbreeding within Clinch Dace populations. Multi-locus FIS values, with associated probability of a random FIS value being greater or equal to the observed FIS; significant p values are shown in bold font. Average individual inbreeding coefficient Fi with associated 95% confidence interval after accounting for segregation of null alleles and genotyping errors.
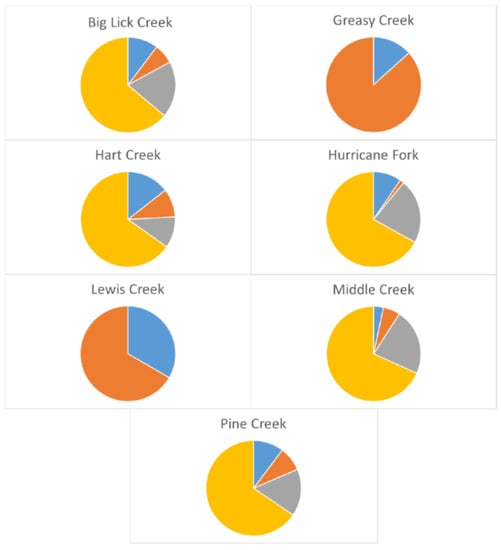
Figure 4.
Frequencies of inferred relatedness among individual Clinch Dace in seven streams. Yellow sections represent unrelated individuals, blue sections represent full-siblings, orange sections represent half-siblings, and grey sections represent parent/offspring relationships.
3.4. Between-Population Genetic Divergence
Fixation indices (FST) among populations in the respective streams ranged from a low of 0.037 between Pine Creek and Middle Creek to a high of 0.527 between Middle Creek and Lewis Creek (Table 5). The mean FST among populations in streams that are rather close together, such as Pine Creek and Big Lick Creek, which are separated by only two stream kilometers, was 0.2 (+SE = 0.02). The mean FST value among populations in streams that are most distant from one another, such as Greasy Creek and Hurricane Fork, which are separated from each other by 117 km, was 0.3 (+0.03). There was a weak and non-significant relationship between the distance between sites and pairwise FST (y = 0.0012x + 0.1983, r2 = 0.08). This non-significant relationship indicates that isolation-by-distance [37] was not an important factor driving genetic divergence among Clinch Dace populations.

Table 5.
Pairwise fixation index (FST) values among Clinch Dace populations between streams.
Basing our definition of populations on the results of the application of STRUCTURE, analysis of molecular variance (AMOVA) was conducted in two different ways: (1) for all streams as wholes independently, and (2) with Hart Creek, Hurricane Creek, and Lewis Creek as distinctive streams, while all other streams were combined into a single population cluster. For all streams analyzed separately (Table 6), AMOVA results showed that a considerable amount of genetic variance—26.0%—was among populations. Most of the remaining genetic variance—72.5%—was within individuals (72.5%), as is typical of populations of vertebrates. Only a small proportion of variance—1.5%—was among individuals within populations (1.5%). Results from both AMOVA analyses were largely convergent; there was more variation within populations than among populations, although there was considerable inter-population divergence.

Table 6.
AMOVA results for collection from all streams analyzed separately. Average FST over all loci = 0.259. Significance test (1023 permutations) for FST: p-value = 0.000.
4. Discussion
4.1. Population Genetic Processes and Structure
To inform conservation planning, we conducted analyses of variation at nine microsatellite DNA loci to assess population genetic processes within and divergence between seven Clinch Dace populations. Results of Bayesian cluster analysis showed that Clinch Dace populations in Hurricane Fork and Hart Creek were the most differentiated from those in other streams, while those in the other five streams (Lewis Creek, Big Lick Creek, Pine Creek, Greasy Creek, and Middle Creek) showed some degree of admixture. The isolation may occur because Hurricane Fork and Hart Creek are the western-most sites and less likely to have exchanged migrants with other sites. Apparent admixture among some populations (Figure 2) suggests that Clinch Dace can migrate among streams or could during episodes in the past. Our findings showed that the classical effects of small population size have led to genetic drift and apparent inbreeding within some of these populations, with co-occurring processes conforming to the small population paradigm [38]. Low estimated Ne values and m-ratios suggest that genetic drift has operated upon the respective gene pools in these streams, contributing to the divergence among them. Non-zero FIS values, high average Fi, and high degrees of relatedness in three of these populations suggest that inbreeding is taking place in some populations. AMOVA results show that over 25% of genetic variation occurs among populations and an overall FST of 0.26 indicates considerable genetic divergence.
Population viability is dependent on population size and genetic variation. A minimum effective population size would be at least 50 for short-term population viability and 500 for retaining long-term adaptive potential [39,40]. As all of the Clinch Dace populations that we sampled had estimated effective sizes under either 50 or 500, it would seem that all populations are vulnerable to extirpation over the short or long term. This problem is compounded by Clinch Dace populations being fragmented across the landscape and contemporary gene flow among populations being restricted or absent, exacerbating the likelihood of loss of genetic diversity within populations. Furthermore, the 3:1 imbalance in sex ratio observed by White and Orth [41] could decrease effective population sizes by 25% [42]. Competition for access to spawning opportunities at nests could make reproductive output unequal among individuals [43].
4.2. Management Implications
A first consideration for management of populations is defining biological units for conservation. Moritz [44] defined management units (MUs) as demographically independent populations, which might be diagnosed as populations that show divergence in allele frequencies at nuclear loci. Palsboll et al. [45] defined MUs as units within which population fluctuations are more dependent on births and deaths within the population than on immigration from other populations; they defined management units as populations of conspecific individuals among which the degree of connectivity is sufficiently low that each population should be monitored and managed separately. Against this background, MUs among Clinch Dace populations might be regarded, respectively, as Hurricane Fork, Hart Creek, and the remaining five streams where mixing has apparently taken place in the recent past.
Inference that individual populations have been subject to isolation, considerable random genetic drift, and, in some cases, apparent inbreeding informs choices of management options used to promote conservation and demographic recovery. Strategies might include actions to address inbreeding, increase effective population sizes, and promote genetically effective migration among populations. Hence, conservation actions might include translocations of individuals among populations, captive propagation and stocking, and habitat improvements to increase ecological carrying capacity and migration likelihood. We recommend adaptive management strategies that involve monitoring any translocations, population augmentations, reintroductions, or habitat improvements to assess the efficacy of any actions taken.
Translocations from large populations to small and at-risk populations might promote genetic rescue [46], addressing inbreeding and demographically boosting receiving populations. However, translocations might also pose the genetic hazard of outbreeding depression. Divergence at neutral genetic markers, such as microsatellites, does not address the possibility of adaptive variation among populations; if neutral variation is considerable, but adaptive variation is absent, then translocations may be safe. As it seems likely that these populations were in genetic contact in the recent past, the likelihood of outbreeding depression seems to be small [47]. Captive breeding and stocking of cultured individuals back into the same source populations avoids the risk of outbreeding depression, although there is a risk that artificial propagation of such a sample of the population and subsequent stocking could reduce the Ne content of the receiving population [48]. Tradeoffs related to genetic risks must be carefully balanced, and there are guidelines for genetically cognizant population augmentation practices [49,50].
This study identifies three possible donor populations (Hurricane Fork, Hart Creek, and Big Lick Creek) and four possible recipient populations (Lewis Creek, Middle Creek, Pine Creek, and Greasy Creek) for translocations. Despite relatively large Clinch Dace populations being present in Hart Creek and Hurricane Fork, these populations may not be suitable as donor populations because of their being genetically differentiated from other populations—that is, there may be risk of outbreeding depression if individuals from these streams were added to other Clinch Dace populations. However, this issue does not rule them out as donor populations from these streams for introductions into streams where Clinch Dace are currently not found. One low-risk translocation method could be to stock Hart Creek individuals into Alvy Creek within that same drainage, a potentially suitable stream with a downstream barrier, or an old mill dam.
Current Clinch Dace distribution may be more dependent on the legacy effects of previous mining activities and large-scale fish kills than the lack of instream habitat, suggesting that Clinch Dace might be introduced into streams with suitable water quality and habitat conditions, establishing new populations or re-establishing extirpated populations. Any such actions would have to stock collections of individuals with sufficient genetic variation to minimize risk of subsequent inbreeding and allow adaptation to local conditions. Fifteen conservation areas for protection of Clinch Dace were proposed by Moore et al. [9] (2018); within these areas, improvement in habitat would include measures to improve water quality and riparian zone integrity. Effective implementation would be complicated, however, by ongoing mining activities in the upper watersheds and the multiplicity of landowners in the lower watersheds, whose cooperation would be needed for habitat improvement on a meaningful geographic scale.
5. Conclusions
Genotype frequencies at nine microsatellite DNA loci within and among seven populations of Clinch Dace, which is an imperiled species endemic to the Clinch River drainage of southwestern Virginia, were indicative of small-population genetic processes. Small effective population size, random genetic drift, and inbreeding were apparent within populations. Genetic differentiation was observed among populations. Inference of these processes will inform conservation management, which may include habitat improvements, translocations of individuals among populations, and, perhaps, captive propagation and stocking to augment existing populations or establish new populations.
Supplementary Materials
The following supporting information can be downloaded via the following link: https://www.mdpi.com/article/10.3390/fishes8070365/s1, Table S1: Locus-by-locus genetic diversity metrics for Clinch Dace populations.
Author Contributions
Conceptualization, R.B., M.J.M., D.J.O. and E.M.H.; funding acquisition, D.J.O. and E.M.H.; project administration, D.J.O. and E.M.H.; methodology, R.B., M.J.M., D.J.O. and E.M.H.; field work, R.B.; laboratory work, R.B. and E.M.H.; formal analysis, R.B. and E.M.H.; writing, original draft, R.B.; writing—review and editing, R.B., M.J.M., D.J.O. and E.M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded through a U.S. Fish and Wildlife Service State Wildlife Grant managed by the Virginia Department of Wildlife Resources.
Institutional Review Board Statement
All work was performed in accordance with collection permits issued by the Virginia Department of Wildlife Resources and Protocol 16-188FIW, which was approved by the Virginia Tech Institutional Animal Care and Use Committee on 1 November 2016.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
Special thanks are offered to Miluska Hyde, Sheila Harris, Corbin Hilling, Kaitlin Hanak, and Chanz Hopkins for their assistance in the laboratory and the field. The participation of D.J. Orth and E.M. Hallerman was supported in part by the Virginia Agricultural Experiment Station under the U.S. Department of Agriculture’s National Institute of Food and Agriculture.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Skelton, C.E. Distribution and Status of Blackside Dace (Phoxinus cumberlandensis) and Clinch Dace (Phoxinus sp. cf. saylori) in the upper Clinch River System, Virginia; Final Report; Virginia Department of Game and Inland Fisheries: Blacksburg, VA, USA, 2007. [Google Scholar]
- White, S.L.; Orth, D.J. Ontogenetic and comparative morphology of Clinch Dace (Chrosomus sp. cf. saylori). Copeia 2013, 2013, 750–756. [Google Scholar] [CrossRef]
- White, S.L.; Orth, D.J. Distribution and habitat correlates of Clinch Dace (Chrosomus sp. cf. saylori) in the upper Clinch River watershed. Am. Midl. Nat. 2014, 171, 311–320. [Google Scholar] [CrossRef]
- Moore, M.J.; Orth, D.J.; Frimpong, E.A. Occupancy and detection of Clinch Dace using two gear types. J. Fish Wildl. Manag. 2017, 8, 530–543. [Google Scholar] [CrossRef]
- Moore, M.J.; Hallerman, E.M.; Orth, D.J. Densities and population sizes of Clinch Dace Chrosomus sp. cf. saylori in the upper Clinch River basin in Virginia. Copeia 2017, 105, 92–99. [Google Scholar]
- Virginia Department of Game and Inland Fishes. Virginia’s 2015 Wildlife Action Plan; VDGIF: Henrico, VA, USA, 2015. Available online: http://bewildvirginia.org/wildlife-action-plan/ (accessed on 30 December 2020).
- U.S. Fish and Wildlife Service. Clinch dace (Chrosomus Sp. Cf. saylori). Environmental Conservation Online System. 2023. Available online: https://ecos.fws.gov/ecp/species/10765 (accessed on 1 June 2023).
- Bourquin, R.; Orth, D.J.; Hallerman, E.M.; Stauffer, D.F. Are road crossings fragmenting populations of Clinch Dace? Northeast. Nat. 2020, 24, 709–722. [Google Scholar] [CrossRef]
- Moore, M.J.; Hallerman, E.M.; Orth, D.J. Multi-metric conservation assessment for the imperiled Clinch Dace. Southeast Fishes Counc. Proc. 2018, 58, 31–56. [Google Scholar]
- Grenier, R.; Costedoat, C.; Chappaz, R.; Dubut, V. Two multiplexed sets of 21 and 18 microsatellites for Phoxinus phoxinus (L.) and Gobio gobio (L.) developed by cross-species amplification. Eur. J. Wildl. Res. 2013, 59, 291–297. [Google Scholar] [CrossRef]
- Dubut, V.; Martin, J.F.; Costedoat, C.; Chappez, R.; Giles, A. Isolation and characterization of polymorphic microsatellite loci in the freshwater fishes Telestes souffia and Telestes muticellus (Teleostei: Cyprinidae). Mol. Ecol. Res. 2009, 9, 1001–1005. [Google Scholar] [CrossRef]
- Dubut, V.; Martin, J.F.; Giles, A.; van Houdt, J.; Chappez, R.; Costedoat, C. Isolation and characterization of polymorphic loci for the dace complex: Leuciscus leuciscus (Teleostei: Cyprinidae). Mol. Ecol. Res. 2009, 9, 1179–1183. [Google Scholar] [CrossRef]
- Dubut, V.; Sinama, M.; Martin, J.F.; Meglecz, E.; Fernandez, J.; Chappez, R.; Giles, A.; Costedoat, C. Cross-species amplification of 41 microsatellites in European cyprinids: A tool for evolutionary population genetics and hybridization studies. BMC Res. Notes 2010, 3, 135. [Google Scholar] [CrossRef]
- Baerwald, M.R.; May, B. Characterization of microsatellite loci for five members of the minnow family Cyprinidae found in the Sacramento-San Joaquin Delta and its tributaries. Mol. Ecol. Res. Notes 2004, 4, 385–390. [Google Scholar] [CrossRef]
- Larno, V.; Launey, S.; Devaux, A.; Loroche, J. Isolation and characterization of microsatellite loci from Chub Leusiscus cephalus (Pisces: Cyprinidae). Mol. Ecol. Res. Notes 2005, 5, 752–754. [Google Scholar] [CrossRef]
- Turner, T.F.; Dowling, T.E.; Broughton, R.E.; Gold, J.R. Variable microsatellite markers amplify across divergent lineages of cyprinid fishes (subfamily Leusiscinae). Conserv. Genet. 2004, 5, 279–281. [Google Scholar] [CrossRef]
- Bessert, M.L.; Orti, G. Microsatellite loci paternity analysis in the Fathead Minnow Pimephales promelas (Teleostei: Cyprinidae). Mol. Ecol. Notes 2003, 3, 532–534. [Google Scholar] [CrossRef]
- Barinova, M.R.; Yadrenkina, E.; Nakajima, M.; Taniguchi, N. Identification and characterization of microsatellite DNA markers developed in Ide Leuciscus idus and Siberian Roach Rutilus rutilus. Mol. Ecol. Notes 2004, 4, 86–88. [Google Scholar] [CrossRef]
- Muenzel, F.M.; Sanetra, M.; Salzburger, W.; Meyer, A. Microsatellites from the Vairone Leuciscus souffia (Pisces: Cyprinidae) and their application to closely related species. Mol. Ecol. Notes 2007, 7, 1048–1050. [Google Scholar] [CrossRef]
- Girard, P.; Angers, B. Characterization of microsatellite loci in Longnose Dace (Rhinichthys cataractae) and interspecific amplification in five other Leusiscinae species. Mol. Ecol. Notes 2006, 6, 69–71. [Google Scholar] [CrossRef]
- Dimsoski, P.; Toth, G.P.; Bagley, M.J. Microsatellite characterization in Central Stoneroller Campostoma anomalum (Pisces: Cyprinidae). Mol. Ecol. 2000, 9, 2187–2189. [Google Scholar] [CrossRef]
- Vyskoclova, M.; Simkova, A.; Martin, J.F. Isolation and characterization of microsatellites in Leuciscus cephalus (Cypriniformes, Cyprinidae) and cross-species amplification within the Family Cyprinidae. Mol. Ecol. Notes 2007, 55, 627–629. [Google Scholar]
- Crooijmans, R.P.M.A.; Bierbooms, V.A.F.; Komen, J.; Van der Poel, J.J.; Groenen, M.A.M. Microsatellite markers in Common Carp (Cyprinus carpio L.). Anim. Genet. 1997, 28, 129–134. [Google Scholar] [CrossRef]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. MICRO-CHECKER: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evol. Bioinformat. Online 2005, 1, 47–50. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.C.; Williamson, E.G. Detection of reduction in population size using data from microsatellite loci. Mol. Ecol. 2001, 10, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Goudet, J. Fstat, Version 1.2., a program for IBM PC compatibles to calculate Weir and Cockerham’s estimators of F-statistics. J. Hered. 1995, 86, 485–486. [Google Scholar] [CrossRef]
- Chybicki, I.J. Inest 2.2. 2020. Available online: https://www.ukw.edu.pl/pracownicy/strona/igor_chybicki/software_ukw/ (accessed on 2 June 2023).
- Campagne, P.; Smouse, P.E.; Varouchas, G.; Silvain, L.F.; Leru, B. Comparing the van Oosterhout and Chybicki-Burczyk methods of estimating null allele frequencies for inbred populations. Mol. Ecol. Resour. 2012, 12, 975–982. [Google Scholar] [CrossRef]
- Do, C.; Waples, R.S.; Peel, D.; Macbeth, G.M.; Tillett, B.J.; Ovenden, J.R. NeEstimator v2: Re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol. Ecol. Resour. 2014, 14, 209–214. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Wagner, A.P.; Taper, M.L. ML-Relate: A computer program for maximum likelihood estimation of relatedness and relationship. Mol. Ecol. Notes 2006, 6, 576–579. [Google Scholar] [CrossRef]
- Wright, S. The interpretation of population structure by F-statistics with special regard to mating. Evolution 1965, 19, 395–420. [Google Scholar] [CrossRef]
- Excoffier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- ESRI. ArcGIS for Desktop; Version 10.2.2; Environmental Systems Research Institute: Redlands, CA, USA, 2014. [Google Scholar]
- Wright, S. Isolation by distance. Genetics 1943, 28, 114–138. [Google Scholar] [CrossRef]
- Caughley, G. Directions in conservation biology. J. Anim. Ecol. 1994, 63, 215–244. [Google Scholar] [CrossRef]
- Newmark, W.D. Extinction of mammal populations in western North American national parks. Conserv. Biol. 1995, 9, 512–526. [Google Scholar] [CrossRef]
- O’Grady, J.J.; Reed, D.H.; Brook, B.W.; Frankham, R. What are the best correlates of predicted extinction risk? Biol. Cons. 2004, 118, 513–520. [Google Scholar] [CrossRef]
- White, S.L.; Orth, D.J. Reproductive biology of Clinch Dace, Chrosomus sp. cf. saylori. Southeast Natur. 2014, 13, 735–743. [Google Scholar] [CrossRef]
- Högland, J. Evolutionary Conservation Genetics; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Hatcher, H.R.; Moore, M.J.; Orth, D.J. Spawning observations of Clinch Dace: Comparison of Chrosomus spawning behavior. Am. Midl. Nat. 2017, 177, 318–326. [Google Scholar] [CrossRef]
- Moritz, C. Conservation units and translocations: Strategies for conserving evolutionary processes. Hereditas 1999, 130, 217–228. [Google Scholar] [CrossRef]
- Palsboll, P.J.; Berube, M.; Allendorf, F.W. Identification of management units using population genetic data. Trends Ecol. Evol. 2007, 22, 11–16. [Google Scholar] [CrossRef]
- Whiteley, A.R.; Fitzpatrick, S.W.; Funk, W.C.; Tallmon, D.A. Genetic rescue to the rescue. Trends Ecol. Evol. 2015, 30, 42–49. [Google Scholar] [CrossRef]
- Frankham, R.; Ballou, J.D.; Eldridge, M.D.; Lacy, R.C.; Ralls, K.; Dudash, M.R.; Fenster, C.B. Predicting the probability of outbreeding depression. Cons. Biol. 2011, 25, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Ryman, N.; Laikre, L. Effects of supportive breeding on the genetically effective population size. Cons. Biol. 1991, 5, 325–329. [Google Scholar] [CrossRef]
- Miller, L.M.; Kapuscinski, A.R. Genetic guidelines for hatchery supplementation programs. In Population Genetics: Principles and Applications for Fisheries Scientists; Hallerman, E., Ed.; American Fisheries Society: Bethesda, MD, USA, 2003; pp. 329–355. [Google Scholar]
- George, A.L.; Kuhajda, B.R.; Williams, J.D.; Cantrell, M.A.; Rakes, P.L.; Shute, J.R. Guidelines for propagation and translocation for freshwater fish conservation. Fisheries 2009, 34, 529–545. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).