Abstract
Ovarian follicle maturation (OFM), including the resumption of meiosis in the oocyte, is under hormonal regulation. Insulin-like growth factors (IGF) have been shown to participate in the regulation of OFM with species-specific actions in teleost fishes. In the present study, in vitro treatment of rainbow trout ovarian follicles with recombinant human insulin-like growth factor-1 (rhIGF1) or rhIGF2 did not induce germinal vesicle breakdown (GVBD), a marker for the resumption of meiosis, in the oocytes. Co-incubation of follicle-enclosed oocytes with rhIGF1 and the maturation-inducing steroid (MIS) in rainbow trout, 17α,20β-dihydroxy-4-pregnen-3-one (17,20βP), also did not induce GVBD in follicles from rainbow trout that were not able to respond to the MIS alone suggesting IGFs cannot induce oocyte maturational competence (OMC), which is the ability of the oocyte to respond to the MIS. Nevertheless, the addition of rhIGF1 with the MIS increased the proportion of oocytes completing GVBD compared with MIS alone, although this potentiation was small and varied greatly among clutches of follicles from fish with oocytes at different stages of germinal vesicle migration. Collectively, these observations suggest IGFs may have synergistic actions with the MIS but cannot induce resumption of meiosis directly at the oocyte and are not potent inducers of OMC in rainbow trout. Rainbow trout are the first teleost fish in which IGFs were found to induce neither OMC nor resumption of meiosis in vitro.
Key Contribution:
Insulin-like growth factors IGF1 and IGF2 did not induce oocyte maturation and IGF1 did not induce oocyte maturational competence in rainbow trout follicles in vitro. The rainbow trout is the first fish in which neither of these processes was found to be regulated by IGFs.
1. Introduction
The oocyte is arrested at prophase 1 of the first meiotic division during most of oocyte growth including vitellogenesis. Ovarian follicle maturation (OFM) takes place following oocyte growth and encompasses changes in the somatic follicle cells and the oocyte leading to the resumption of meiosis or oocyte maturation (OM). In most vertebrates, OFM is regulated by luteinizing hormone (LH) and in teleosts includes a switch from the production of estradiol-17β (E2) to the progestin maturation-inducing steroid (MIS), and the acquisition of oocyte maturation competence (OMC). Oocyte maturational competence is the ability of the oocyte to respond to the MIS and includes the formation of homologous and heterologous gap junctions among the somatic follicle cells and between the follicle cells and the oocyte and an increase in oocyte membrane progestin receptors (mPR). Competence is attained as the oocyte nucleus or germinal vesicle (GV) migrates from the center to the periphery of the oocyte. Germinal vesicle migration (GVM) is used to roughly predict maturational competence. The MIS then induces the formation and activation of maturation promoting factor (MPF) in the oocyte which is the final inducer of the resumption of meiosis, OM [1,2,3]. The MIS in many species of fish including rainbow trout (Oncorhynchus mykiss) is 17α,20β-dihydroxy-4-pregnen-3-one (17,20βP) [2,4]. Several hormonal factors other than gonadotropins (GtH) and sex steroids can meditate or modulate the actions of LH and MIS in OFM including insulin-like growth factors (IGF) [3,5,6]. The described actions of IGFs in regulating OFM are extensive and vary among fishes. Insulin-like growth factors have been shown to regulate the expression of LH receptors on the somatic follicle cells, affect the switch from E2 to progestin production, increase OMC, and act at the oocyte membrane to promote the resumption of meiosis [7,8,9].
Complex intraovarian IGF systems have been identified in fish species including the rainbow trout [7,10,11,12,13]. The ovarian IGF system in fish includes the ligands IGF1, IGF2a, IGF2B, and IGF3; the IGF1 receptors (IGF1r) IGF1ra and IGF1rb; and multiple IGF binding proteins (IGFBP). Despite salmonids being a primary model for the study of OFM and the IGF system, little is known about the actions of IGFs in OFM in this order. Intraovarian expression profiles of IGF system components are consistent with the system being involved in the regulation of OFM in salmonids. Expression of igf1 and igf2 transcripts increase in rainbow trout somatic follicle cells as follicles increase in OMC [11] and igfbp3 decreases whereas igfbp6 and IGFBP-related protein-1 (igfbp-rP1) increase as follicles progress through maturation [12]. The binding of IGF1 takes place in both the theca-interstitial cell layer and granulosa cell layer of the ovarian follicle in brown trout (Salmo trutta) and coho salmon (Oncorhynchus kisutch) with a slight increase from the preovulatory period compared with the end of vitellogenesis in brown trout [14,15]. Testosterone and 17α-hydroxyprogesterone are the steroid precursors to E2 and MIS, respectively, in the salmonid follicle [16,17]. In Coho salmon, IGF1 reduces basal and LH-induced production of testosterone and 17α-hydroxyprogesterone from the theca-interstitial layer of the follicle throughout the preovulatory period, but IGF1 switches from stimulating both E2 and 17,20βP from the granulosa cell layer early in maturation to only 17,20βP late in maturation [14,18]. These actions suggest a role for IGFs in the switch from E2 to MIS production during OFM, however, direct actions of IGFs on induction of OM or OMC have not been reported in a salmonid.
Fish exhibit a wide range of reproductive strategies, which can vary considerably among closely related species. Among this diversity are fish that exhibit synchronous egg development in which all the oocytes develop as a single clutch and ovulate at the same time; asynchronous egg development in which all stages of oocytes are found in the ovary at the same time without a dominant pool of a certain size and the fish tend to ovulate daily during the spawning season; and group-synchronous in which a single clutch or multiple clutches of oocytes from the oocyte pool develop in unison and either ovulate as a single clutch each spawning season or grow and mature as multiple clutches that ovulate more than once over the spawning season. Insulin-like growth factors have been shown to induce OM in striped bass (Morone saxatilis), a group-synchronous single-clutch ovulator whereas they induce OMC in the congeneric white bass (Morone crysops) and white perch (Morone americana) which are both group-synchronous multiple-clutch ovulators [19,20,21]. The possibility that divergence of reproductive strategies may derive in part from changes in IGF system regulation of OFM might be considered. Thus far, actions of IGFs in regulating OFM have been investigated in only a limited number of species but represent a high degree of phylogenetic diversity and reproductive strategies (Table 1). Knowledge of the actions of IGFs in regulating OM and OMC in salmonids would be valuable to understanding the control of OFM in this order as well as the evolution of IGF functions among teleost fishes. We, therefore, investigated whether IGFs can induce OM or OMC in rainbow trout.

Table 1.
Described actions of insulin-like growth factors (IGF) on oocyte maturational competence (OMC) and oocyte maturation (OM) among species.
2. Materials and Methods
2.1. Fish Husbandry
Fish were from stocks maintained at the USDA National Center for Cool and Cold Water Aquaculture (NCCCWA) in Kearneysville, WV, USA. All fish were reared and maintained in processed continuous-flow groundwater with an ambient temperature of 13 ± 1 °C and supplemental dissolved oxygen during the sampling period. Photoperiod was maintained with artificial lighting that was adjusted weekly to simulate the ambient photoperiod. Fish were fed Zeigler Gold food (Zeigler Bros. Inc., Gardners, PA, USA) at 1% body weight per day.
2.2. Tissue Collection and In Vitro Bioassay
Incubations of ovarian follicles and subsequent evaluation of oocyte maturational stages followed methods previously described for this species [10,34,35]. Ovaries were excised from maturing virgin rainbow trout following anesthetization by immersion in 150 mg per L of tricaine methanesulfonate (Tricaine-S, Western Chemical, Ferndale, WA, USA) and decapitation, and placed into sterile ice-cold trout mineral medium (TMM) [10]. Multiple ovarian fragments containing a total of 20 oocytes in intact follicles were placed in wells of Falcon-well culture plates (Becton Dickinson, Franklin Lakes, NJ, USA) in an incubator at 10 °C, under air, on an orbital oscillator at 80 rpm. Tissues were preincubated for 2 h in control medium, TMM without hormone, which was then replaced with new control medium or medium containing hormones to be tested. An additional 20–25 follicles from each fish were collected and fixed and cleared in Davidson’s solution (2 parts 37% formaldehyde:3 parts 95% EtOH:1 part acetic acid:3 parts H2O) to be staged based on the extent of GVM. An estimate of follicle diameter for each fish used in the experiments was obtained by measuring the diameter of 25 additional fresh follicles collected at the start of incubation to the nearest 100 µm using a dissecting stereomicroscope fitted with an ocular micrometer. The steroid 17,20βP was purchased from Steraloids, Inc. (Wilton, NH, USA). The recombinant human-IGF1 (rhIGF1) and rhIG2 used in Experiment 1 were purchased from R&D Systems (Minneapolis, MN, USA). The rhIGF1 used in Experiment 2 was obtained from the National Hormone and Peptide Program, Harbor-UCLA (Torrance, CA, USA). The 17,20βP was dissolved in absolute ethanol and the rhIGF1 and rhIGF2 used in Experiment 1 were reconstituted in PBS, and the rhIGF1 used in Experiment 2 was dissolved in 10 mM HCl, before dilution in culture medium. Solvents for these chemicals never exceeded 0.1% (v/v) of the culture medium. Following incubation for ~96 h, follicles were fixed and cleared in Davidson’s solution and scored for germinal vesicle breakdown (GVBD) as marker for the resumption of meiosis.
2.3. Experimental Design
Two experiments were conducted. In Experiment 1, the efficacy of rhIGF1 and rhIGF2 to induce GVBD in vitro was examined. Two trials were conducted on two different days with tissues from three females in each trial. In total, tissues from six fish with oocytes at around late GV migration were incubated in each of ten treatment solutions: control medium, 29 nM, 72.5 nM, or 290 nM 17,20βP, or 1 nM, 10 nM, or 100 nM rhIGF1, or 1 nM, 10 nM, or 100 nM rhIGF2. In Experiment 2, the effect of rhIGF1 on 17,20βP-induced GVBD in follicles at different stages of migration was examined. A total of six trials were conducted on six different days with tissues from six females in each trial. Tissues from one of the fish did not survive incubations and therefore the data are not included in the study, and data from another fish were eliminated because 98% of the follicles completed GVBD in response to 17,20βP. Tissues from each fish in Experiment 2 were incubated in each of three treatment solutions: control medium, 290 nM 17,20βP, or 290 nM 17,20βP plus 100 nM rhIGF1. There were 20 follicles incubated in triplicate wells for each treatment for each fish in each experiment.
2.4. Statistical Analysis
Two-way analysis of variance followed by the Holm–Sidak multiple comparisons test was used to test the main effects of MIS concentration, fish, and fish by MIS concentration interactions, and to detect differences among treatment groups for both Experiment 1 and Experiment 2. For Experiment 1, data from all six fish from both trials were included in the analysis, and since GVBD was only induced in treatments receiving 17,20βP, only the treatments receiving 17,20βP were included in the analysis. Only data from the eight competent fish were included in the analysis for Experiment 2, and only treatments receiving 17,20βP or 17,20βP plus rhIGF1, and not controls, were included in the analysis. Statistical analyses were performed using SigmaStat 3.1 (Jandel Scientific, San Rafael, CA, USA) software. For all analyses, a priori level of statistical significance used was p ≤ 0.05, and assumptions of normality and equal variance of the data were confirmed using Shapiro–Wilks and Browne–Forsythe, respectively.
3. Results
3.1. Effects of rhIGF1 and rhIGF2 on Induction of GVBD In Vitro
Follicles from all six fish competent to respond to the MIS, 17,20βP, for induction of GVBD failed to respond to rhIGF1 or rhIGF2 (Figure 1). There was a significant overall effect of 17,20βP concentration on GVBD (p < 0.001) with a graded response among the three concentrations (p < 0.05). Fish also had a significant effect on 17,20βP induction of GVBD (p < 0.001). Competence to respond to MIS treatment ranged from fish with little to no response at concentrations at or below 72.5 nM, such as Fish 1 and 6: to fish in which the lowest concentration of 17,20βP induced GVBD in greater than 45% of follicles such as in Fish 2, 4, and 5 (Figure 1). The extent of GVM or follicle diameter (Table 2) did not appear to predict competence within the ranges tested in the present study. As mentioned, the least competent follicles were from Fish 1, which was the only fish with some follicles at the mid GVM stage before incubation, and Fish 6, which had the highest proportion of follicles with GVs at the oocyte periphery. These two fish also had among the smallest and largest follicles, respectively.
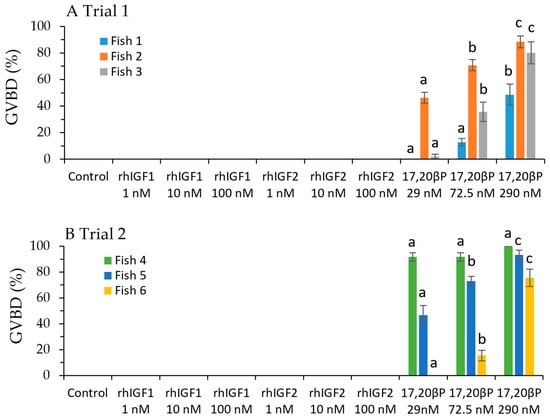
Figure 1.
Effects of rhIGF1, rhIGF2, or 17,20βP on germinal vesicle breakdown (GVBD) in vitro. Values are presented as mean ± SEM (n = 3). Shared letters indicate within fish means, which do not differ among the three treatments receiving 17,20βP (p ≤ 0.05).

Table 2.
Stage and size of follicles in ovarian fragments from fish incubated in vitro with or without rhIGF1, rhIGF2, or 17,20βP in Experiment 1.
3.2. Effects of rhIGF1 on MIH-Induced GVBD In Vitro
Follicles from 8 of 34 fish tested went through GVBD in response to 290 nM 17,20βP (Table 3). Tissues from all 26 fish whose follicles did not respond to 17,20βP alone, also did not respond to a combination of 17,20βP and rhIGF1 indicating IGF1 does not induce OMC in rainbow trout follicles. Although the mean follicle diameter of the fish that responded to the MIH was slightly larger than that of those that did not respond, 4.30 ± 0.14 and 3.86 ± 0.08 mm, respectively, there was overlap in mean follicle diameters among those that were MIS-competent and MIS-incompetent (Table 3). Follicles began to acquire competence between the mid to late GVM. Follicles from fish whose follicles were at mid GVM or earlier failed to respond to the MIS whereas all those that reached the late migration stage were competent. There was a highly significant effect of treatment (p < 0.001) and therefore rhIGF1 on MIS-induced GVBD in MIS-competent follicles suggesting a synergistic action between the hormones, however, there was also a significant effect of fish (p < 0.001) and treatment by fish interactions (p = 0.018) and therefore the effect of rhIGF1 on MIS-induced GVBD was only significant in two of the eight fish tested (Figure 2).

Table 3.
Stage and size of follicles from fish that did (competent) or did not (incompetent) complete germinal vesicle breakdown (GVBD) in vitro in response to treatment with 17,20βP alone or in combination with rhIGF1 in Experiment 2.
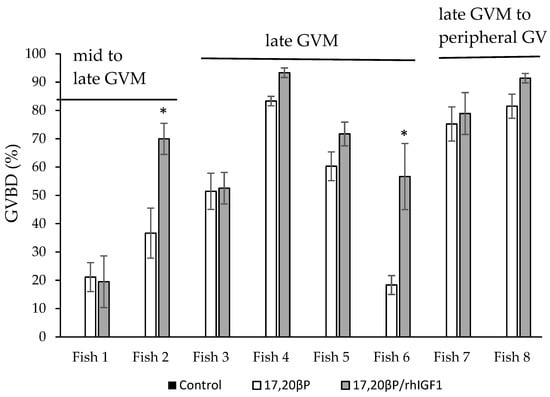
Figure 2.
Effects of no hormone control medium, 17,20βP (290 nM), or 17,20βP (290 nM) plus rhIGF1 (100 nM) on germinal vesicle breakdown (GVBD) in vitro. Values are presented as mean ± SEM (n = 3). All values for controls were 0. An asterisk indicates a significant difference between the 17,20βP treatment and the 17,20βP plus rhIGF1 treatment means for an individual fish (p ≤ 0.05).
4. Discussion
Our study shows IGFs cannot induce meiotic resumption in rainbow trout oocytes. Treatment with IGF1 or IGF2 did not induce GVBD in follicles competent to respond to MIS in Experiment 1. In most fish in which IGFs were found to induce GVBD in vitro, IGFs were able to induce OM in earlier stage follicles than MIS. Insulin-like growth factor-1 was able to induce GVBD in MIS-incompetent follicles in red seabream (Pagrus major), common carp (Cyprinus carpio), southern flounder (Paralichthys lethostigma), and striped bass [19,25,26,31]. The MIS has not induced GVBD at an earlier stage than IGFs in follicles of any fish in which an IGF can induce GVBD. Therefore, finding no effect of IGF1 even in combination with the MIS in MIS-incompetent follicles in Experiment 2 supports there is no stage in which IGFs can induce OM in rainbow trout. The rainbow trout joins the white bass and white perch in being among the few fish in which IGFs cannot induce OM [20,21].
The involvement of IGFs in regulating OMC has received less attention than their roles in inducing the resumption of meiosis. The failure of a combination of MIS and IGF1 to induce GVBD in follicles from any of the 26 MIS-incompetent fish in Experiment 2 not only supports IGFs cannot induce OM, but also indicates IGFs cannot induce OMC. These results included follicles from five fish that were in the mid to late GVM stage, the same stage in which two fish in Experiment 2 and one fish in Experiment 1 were found to be MIS-competent. Insulin-like growth factor induction of OFM in follicles that are not yet competent to undergo GVBD in response to MIS has been investigated in six species other than rainbow trout. As mentioned, IGFs were able to induce OMC but not GVBD in white bass and white perch [20,21]. In red seabream, pretreatment with IGF1 enabled subsequent treatment with 17,20βP to induce GVBD in follicles that did not respond to just 17,20βP [25], and in southern flounder co-incubation of 17,20βP with IGF2, but not with IGF1, was able to induce GVBD when 17,20βP had no effect on its own [31]. Treatment of MIS-incompetent follicles with IGF either as a pretreatment to MIS exposure or a co-treatment with MIS suggests IGF can induce OMC in red seabream and southern flounder. Furthermore, induction of OMC by IGF has been shown to be associated with increased formation of homologous and heterologous gap junctions in red seabream [36] and increased oocyte mPR expression in southern flounder [31] and spotted seatrout (Cynoscion nebulosus) [37]. The rainbow trout joins striped bass [19] and common carp [26] as the only fish in which IGFs were found to be ineffective at inducing OMC in MIS-incompetent follicles, although IGFs can induce GVBD in vitro in both striped bass and common carp.
Even though IGF1 was not able to induce OMC in MIS-incompetent rainbow trout follicles, there was an overall increase in the percent of follicles completing GVBD in response to a combination of 17,20βP plus IGF1 as compared with MIS alone in clutches of follicles from eight fish competent to respond to MIS (Figure 2). The mean increase in GVBD for the eight fish was minor, however, 53.5% for MIS alone compared with 66.7% for the hormone combination, and the potentiation effect of IGF1 was only significant for follicles from two of the eight fish. Furthermore, although the number of follicles completing GVBD nearly doubled for one of these two fish and tripled in the other, these two fish were among the three with the lowest response to MIS alone. Although synergism between IGF1 and the MIS to induce GVBD is apparent, how IGF increases maturation in response to the MIS remains unresolved.
The addition of IGF1 to follicles in which the MIS alone can induce greater than 50% GVBD did not significantly increase GVBD in the present study. It is possible OMC was already highly acquired in these fish and therefore there was not an opportunity for IGF to have an impact. Although a combination of IGF1 and MIS did not induce GVBD in MIS-incompetent follicles demonstrates IGFs cannot induce OMC, the increase in GVBD in two fish with low MIS competence leaves open the possibility IGFs can increase OMC during early stages of the acquisition of OMC in rainbow trout follicles. On the other hand, IGF1 had no synergistic effect on GVBD induction in one fish in the present study in which MIS alone only induced 20% GVBD. Insulin-like growth factors have been shown to induce or increase OMC in fish by promoting changes in the follicle such as an increase in gap junction formation or increased expression of mPR on the oocyte membrane [31,36]. An increase in gene expression of a gap junction protein, connexin 43 (cx43.2), coincides with progression through the acquisition of OMC in rainbow trout [11] and coho salmon [33] suggesting an increase in gap junction formation contributes to OMC in salmonids. However, in coho salmon IGF1 reduces cx43.2 in late vitellogenic stage follicles in vitro and has no effect on gene expression of a second connexin protein, connexin 34 (cx34.3) [33]. Expression of cx34.3 does not change during later stages of oogenesis in coho salmon but was increased in response to LH in the same study. Thus, the role of IGFs in promoting OMC through the regulation of gap junctions is not clearly established in salmonids, and the effects of IGFs on mPR have not been investigated.
Follicles expressing low MIS competence have been used in previous studies to investigate the ability of IGFs to induce OMC and additive effects of the hormones on induction of GVBD in vitro were observed. As mentioned, in red seabream IGF1 pretreatment of MIS-incompetent follicles induced MIS competence [25]. In the same study using MIS-competent follicles, IGF1 exhibited an additive effect with the MIS when lower concentrations of IGF1 were administered that could only induce low levels of GVBD on their own, but not at a higher concentration of IGF1 that induced over 70% GVBD on its own with the same incubation period. Keeping in mind the rate of GVBD induction achieved in a study is in part a function of incubation time, a GVBD induction rate of less than 100% can still be a maximal response. Thus, it may be at the high concentrations of IGF1, OFM was maximally stimulated and therefore there was no opportunity to have an additive effect in the red seabream. In zebrafish (Danio rerio), a fish with asynchronous oocyte development, it is difficult to obtain a clutch of follicles without some follicles that are competent to respond to MIS and some that will undergo spontaneous maturation in vitro. In zebrafish, an additive effect of IGF1 and MIS was evident in the mid-vitellogenic zebrafish follicles but not fully grown follicles. Pretreatment with IGF1 before incubation with 17,20βP increased GVBD in vitro in follicles from mid-vitellogenic zebrafish which had low MIS competence, but not in fully grown follicles in which each of the hormones alone induced about 70% GVBD [22]. Pretreatment with IGF2 showed similar trends but the effects were not statistically significant. It is possible OMC was completed before incubation in the fully grown follicles but not in the mid-vitellogenic follicles. Still at the concentrations used the IGFs were more potent alone than the MIS at inducing GVBD so it is difficult to conclude IGF1 increased OMC as opposed to the increased response being a dosage effect of the combined hormones directly on OM in that study. Nevertheless, a synergism between IGF1 and MIS to overcome the effects of E2 to inhibit GVBD has been demonstrated in zebrafish follicles. The steroid E2 participates in maintaining meiotic arrest [23]. The addition of E2 to incubations of zebrafish follicles can block the effects of MIS or IGF1 at concentrations that could otherwise induce GVBD, however, the blockage by E2 can be overcome if the MIS and IGF1 are added together suggesting a synergism beyond an additive effect of the two hormones or increased OMC by traditional mechanisms [6,23].
The ability of IGFs to act directly on the oocyte to induce the resumption of meiosis and to promote the production of MIS in many fishes [7,9] can complicate the investigation of their actions in OMC. Our study indicates IGFs do not induce OM directly in rainbow trout, eliminating this possibility. However, the increase in GVBD in low MIS-competent follicles receiving both 17,20βP and IGF1 compared with 17,20βP alone may be due to increased 17,20βP production by the follicles receiving IGF1 since IGFs have been shown to stimulate the production of MIS in salmonids [14,18]. Alternatively, IGF1 is commonly added to culture media to increase viability [38] and therefore may enhance cell or tissue development or activities due to general effects on metabolism and not necessarily due to specific action in regulating or altering signaling pathways regulating OMC or OM. General metabolic effects of IGFs on tissue viability are especially a consideration in experiments with long incubation times as in the present study, which was ~96 h. The bioassay used in the present study is a crude evaluation of the capacity to induce OMC. The bioassay combined with additional analyses such as histological examinations of gap junction formation as shown in red seabream [36], or mRNA expression analysis of components associated with OMC such as expression of connexin proteins or mPR as shown in southern flounder [31] and spotted seatrout [37] are needed to provide a more determinative interpretation of IGF actions in regulating or potentiating OMC in rainbow trout. Overall, our results indicate IGFs are not able to induce OMC in rainbow trout follicles.
The different forms of IGFs likely play disparate roles during ovarian development in fish that varies with species [6,8,31]. Expression levels and patterns of expression of multiple IGF ligands have been measured in the rainbow trout follicle during follicle maturation [11] and shown to vary. Most previous studies have investigated the actions of IGF1 or IGF2 in OFM since reproductive actions of IGF3 have only recently been identified and the ligand is not commercially available. Neither IGF1 nor IGF2 was able to induce OM in MIS-competent follicles in the present study. Insulin-like growth factor-1 was selected for examining IGF actions on OMC because IGF1 has usually been found to be the most potent form of IGF for induction of OM or OMC in most species. Insulin-like growth factor-1 was more potent than IGF2 at inducing GVBD in vitro in red seabream, mummichog (Fundulus heteroclitus), striped bass, and common carp [19,25,26,32]; and for inducing OMC in red seabream, spotted seatrout, white bass, and white perch [20,21,25,37]. In each of these studies, IGF1 was effective between 10–100 nM which is within the range used in the present study. Thus far, IGF1 over this range of concentration was able to induce GVBD or OMC in vitro in all fish species in which any form of IGF was effective except southern flounder in which IGF2 but not IGF1 at 100 nM was able to induce competence to respond to the MIS [31]. Furthermore, evidence suggests the IGFs signal through IGF1 receptors (IGF1r) in the ovary [7]. In zebrafish, although differences among the four IGF ligands (IGF1, IGF2a, IGF2b, IGF3) in their ovarian gene expression patterns during the progression through maturation and in response to GtH treatment suggests their having disparate roles, the ability of each to induce GVBD in vitro is abrogated by the addition of an IGF1r inhibitor (NVP-AEW541 or NVP-ADW742) [24] or anti-IGF1r [23] to the culture. In addition, 13 nM IGF1 was effective at regulating steroidogenic enzymes in rainbow trout follicles [39] further supporting the dosage range used in the present study was appropriate for eliciting a physiological response in rainbow trout follicles in vitro. Thus, the lack of a response to IGF1 or IGF2 to induce OM and IGF1 to induce OMC in our studies suggests the inefficacy of other IGF ligands in the bioassays.
The identification of actions or lack thereof of IGFs on OMC and OM in a salmonid helps to clarify a phylogenetic or reproductive strategy context in which to understand the basis for variation in IGF actions among teleosts. However, the number of species representing different orders and reproductive strategies, and the data available for each representative species remains very limited. The rainbow trout is the first fish in which IGFs were found not to induce or at least have a potent effect on either OM or OMC. This is unique among fishes thus far and therefore may be considered rare among fishes, however, there is also the possibility that such negative results have not been reported. As it stands, the rainbow trout joins the white bass and white perch in being among the few fish species in which IGFs cannot induce OM although IGFs can induce OMC in these temperate basses [20,21]. All three of these species display group-synchronous oocyte development, although the rainbow trout is a single-clutch ovulator whereas white perch and white bass are multiple-clutch ovulators. Therefore, the inability of IGFs to induce OM is found in both single-clutch and multiple-clutch ovulators.
The rainbow trout joins the striped bass and common carp as species in which IGFs cannot induce OMC although IGFs can induce OM in striped bass and common carp [19,26]. Interestingly, all three of these species are group-synchronous single-clutch ovulators. To date, the ability of IGFs to induce OMC has been demonstrated in all asynchronous ovulators and all group-synchronous multiple-clutch ovulators, but not in any group-synchronous single-clutch ovulator. Furthermore, striped bass is a temperate bass and therefore even within the same genus, Morone, is found fish in which IGF can only induce OM or only induce OMC. The ability of IGFs to induce both OM and OMC is found in both species investigated with asynchronous oocyte development, red seabream and zebrafish, and in the southern flounder, a fish with group-synchronous oocyte development and ovulates multiple clutches of eggs in a single spawning season [22,23,24,25,31]. The ability of IGFs to induce OM has been reported in several species in which the ability to induce OMC has not been investigated representing a wide phylogenetic distribution and variation in reproductive strategies including the mummichog, Indian carp (Labeo rohita), stinging catfish (Heteropneustes fossilis), grey mullet (Mugil cephalus), and walking catfish (Clarias batrachus (L.)) [27,28,29,30,32]. Overall, the ability of IGFs to induce OM, OMC, or both is widespread among divergent orders and among fishes expressing disparate reproductive strategies. Other than Salmoniformes, there are at least some species of fish from all orders of teleost and reproductive strategies investigated, in which an IGF can induce OM. The ability of IGFs to induce OMC has been investigated in fewer fish and in less detail than OM but also has been found in most fish investigated. Given the high prevalence of IGF actions on OFM among teleosts, and distinct differences in actions among species, the evolution of the IGF system likely contributes to the evolution of reproductive strategies among fishes.
5. Conclusions
Previous studies of patterns of ovarian expression of IGF system genes suggest the IGF system is involved in the regulation of late stages of ovarian development in salmonids, including rainbow trout. Furthermore, steroidogenic pathways leading to the production of MIS have been shown to be affected by IGFs in salmonids. In our studies, IGFs did not induce OM or OMC in rainbow trout follicles. The rainbow trout is the first species of fish in which IGFs were found not able to induce either OM or OMC. Despite the many diverse actions of IGFs in regulating OFM among teleost species, patterns of association with reproductive strategies are limited. The ability of IGFs to induce OMC in asynchronous and group-synchronous multiple-clutch ovulators, but not group-synchronous single-clutch ovulators has to date been consistent.
Funding
This work was supported by the USDA, Agricultural Research Service CRIS Project 8082-31000-013-00D “Integrated Research Approaches for Improving Efficiency in Rainbow Trout”.
Institutional Review Board Statement
The Institutional Animal Care and Use Committee (Leetown, WV, USA) reviewed and approved all animal husbandry practices per standards set forth in the USDA, ARS Policies and Procedures 130.4.v.3 titled “Institutional Animal Care and Use Committee”. First phase: Approval Code: IACUC #072. Approval Date: 30 November 2010. Second phase: Approval Code: IACUC #098. Approval Date: 19 December 2021.
Data Availability Statement
Raw data are available upon reasonable request from the corresponding author.
Acknowledgments
The author acknowledges Jill Birkett for her assistance in collecting the samples, conducting the cultures, and collecting the data. The author would also like to thank the hatchery crew at NCCCWA for their contributions to animal care. The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the USDA or the ARS of any product or service to the exclusion of others that may be suitable. USDA is an equal opportunity provider and employer.
Conflicts of Interest
The author declares no conflict of interest.
References
- Patiño, R.; Yoshizaki, G.; Thomas, P.; Kagawa, H. Gonadotropic control of ovarian follicle maturation: The two-stage concept and its mechanisms. Comp. Biochem. Physiol. Part B 2001, 129, 427–439. [Google Scholar] [CrossRef]
- Nagahama, Y.; Yamashita, M. Regulation of oocyte maturation in fish. Dev. Growth Differ. 2008, 50, S195–S219. [Google Scholar]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdà, J. Oogenesis in teleosts: How fish eggs are formed. Gen. Comp. Endocrinol. 2010, 165, 367–389. [Google Scholar]
- Mohajer, L.E.; Bulteau, R.; Fontaine, P.; Milla, S. Maturation Inducing Hormones in teleosts: Are progestogens always the first to be nominated? Aquaculture 2022, 546, 737315. [Google Scholar]
- Lubzens, E.; Bobe, J.; Young, G.; Sullivan, C.V. Maternal investment in fish oocytes and eggs: The molecular cargo and its contributions to fertility and early development. Aquaculture 2017, 472, 107–143. [Google Scholar]
- Das, D.; Khan, P.P.; Maitra, S. Endocrine and paracrine regulation of meiotic cell cycle progression in teleost oocytes: cAMP at the centre of complex intra-oocyte signalling events. Gen. Comp. Endocrinol. 2017, 241, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, M. Insulin-like growth factors and fish reproduction. Biol. Reprod. 2010, 82, 656–661. [Google Scholar] [PubMed]
- Li, J.; Liu, Z.; Kang, T.; Li, M.; Wang, D.; Cheng, C.H.K. Igf3: A novel player in fish reproduction. Biol. Reprod. 2021, 104, 1194–1204. [Google Scholar]
- Ndandala, C.B.; Dai, M.; Mustapha, U.F.; Li, X.; Liu, J.; Huang, H.; Li, G.; Chen, H. Current research and future perspectives of GH and IGFs family genes in somatic growth and reproduction of teleost fish. Aquac. Rep. 2022, 26, 101289. [Google Scholar] [CrossRef]
- Bobe, J.; Maugars, G.; Nguyen, T.; Rime, H.; Jalabert, B. Rainbow trout follicular maturational competence acquisition is associated with an increased expression of follicle stimulating hormone receptor and insulin-like growth factor 2 messenger RNAs. Mol. Reprod. Dev. 2003, 66, 46–53. [Google Scholar] [CrossRef]
- Bobe, J.; Nguyen, T.; Jalabert, B. Targeted gene expression profiling in the rainbow trout (Oncorhynchus mykiss) ovary during maturational competence acquisition and oocyte maturation. Biol. Reprod. 2004, 71, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Kamangar, B.B.; Gabillard, J.C.; Bobe, J. Insulin-like growth factor-binding protein (IGFBP)-1,-2,-3,-4,-5, and -6 and IGFBP-related protein 1 during rainbow trout postvitellogenesis and oocyte maturation: Molecular characterization, expression profiles, and hormonal regulation. Endocrinology 2006, 147, 2399–2410. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, S.M.; Ge, W. Expression and functional characterization of intrafollicular GH-IGF system in the zebrafish ovary. Gen. Comp. Endocrinol. 2016, 232, 32–42. [Google Scholar] [CrossRef]
- Maestro, M.A.; Planas, J.V.; Moriyama, S.; Gutiérrez, J.; Planas, J.; Swanson, P. Ovarian receptors for insulin and insulin-like growth factor I (IGF-I) and effects of IGF-I on steroid production by isolated follicular layers of the preovulatory coho salmon ovarian follicle. Gen. Comp. Endocrinol. 1997, 106, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Maestro, M.A.; Méndez, E.; Planas, J.V.; Gutiérrez, J.; Swanson, P. Dynamics of insulin and insulin-like growth factor-I (IGF-I) ovarian receptors during maturation in the brown trout (Salmo trutta). Fish Physiol. Biochem. 1999, 20, 341–349. [Google Scholar] [CrossRef]
- Young, G.; Adachi, S.; Nagahama, Y. Role of ovarian theca-interstitial layer and granulosa cells in gonadotropin induced synthesis of a salmonid maturation-inducing substance (17α, 20β-dihydroxy-4-pregnen-3-one). Dev. Biol. 1986, 118, 1–8. [Google Scholar] [CrossRef]
- Nagahama, Y. Gonadotropin action on gametogenesis and steroidogenesis in teleost gonads. Zool. Sci. 1987, 4, 209–222. [Google Scholar]
- Maestro, M.A.; Planas, J.V.; Gutiérrez, J.; Moriyama, S.; Swanson, P. Effects of insulin-like growth-factor-I (Igf-I) on steroid-production by isolated ovarian theca and granulosa layers of preovulatory coho salmon. Neth. J. Zool. 1995, 45, 143–146. [Google Scholar]
- Weber, G.M.; Sullivan, C.V. Effects of insulin-like growth factor-I on in vitro final oocyte maturation and ovarian steroidogenesis in striped bass, Morone saxatilis. Biol. Reprod. 2000, 63, 1049–1057. [Google Scholar] [CrossRef]
- Weber, G.M.; Sullivan, C.V. Insulin-like growth factor-I induces oocyte maturational competence but not meiotic resumption in white bass (Morone chrysops) follicles in vitro: Evidence for rapid evolution of Insulin-like growth factor action. Biol. Reprod. 2005, 72, 1177–1186. [Google Scholar]
- Weber, G.M.; Moore, A.B.; Sullivan, C.V. In vitro actions of insulin-like growth factor-I on ovarian follicle maturation in white perch (Morone americana). Gen. Comp. Endocrinol. 2007, 151, 180–187. [Google Scholar] [CrossRef]
- Nelson, S.N.; Van Der Kraak, G. The role of the insulin-like growth factor (IGF) system in zebrafish (Danio rerio) ovarian development. Gen. Comp. Endocrinol. 2010, 168, 103–110. [Google Scholar] [CrossRef]
- Das, D.; Pal, S.; Maitra, S. Releasing prophase arrest in zebrafish oocyte: Synergism between maturational steroid and Igf1. Reproduction 2016, 151, 59–72. [Google Scholar] [CrossRef]
- Li, J.; Chu, L.; Sun, X.; Liu, Y.; Cheng, C.H.K. IGFs mediate the action of LH on oocyte maturation in zebrafish. Mol. Endocrinol. 2015, 29, 373–383. [Google Scholar] [CrossRef]
- Kagawa, H.; Kobayashi, M.; Hasegawa, Y.; Aida, K. Insulin and insulin-like growth factors I and II induce final maturation of oocytes of red seabream, Pagrus major, in vitro. Gen. Comp. Endocrinol. 1994, 95, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Mukherjee, D.; Sen, U.; Paul, S.; Bhattacharyya, S.P. In vitro effects of insulin-like growth factors and insulin on oocyte maturation and maturation-inducing steroid production in ovarian follicles of common carp, Cyprinus carpio. Comp. Biochem. Physiol. Part A 2006, 144, 63–77. [Google Scholar] [CrossRef]
- Dasgupta, S.; Basu, D.; Ravi Kumar, L.; Bhattacharya, S. Insulin alone can lead to a withdrawal of meiotic arrest in the carp oocyte. J. Biosci. 2001, 26, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, T.K.; Joy, K.P. Estrogen-2/4-hydroxylase activity is stimulated during germinal vesicle breakdown induced by hCG, IGF-1, GH and insulin in the catfish Heteropneustes fossilis. Gen. Comp. Endocrinol. 2008, 155, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Hajra, S.; Das, D.; Ghosh, P.; Pal, S.; Nath, P.; Maitra, S. Regulation of recombinant human insulin-induced maturational events in Clarias batrachus (L.) oocytes in vitro. Zygote 2016, 24, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Pramanick, K.; Mukherjee, D.; Maiti, B.R. In vitro induction of oocyte maturation and steroidogenesis by gonadotropins, insulin, calcitonin and growth factor in an estuarine flat head grey mullet, Mugil cephalus L. Fish Physiol. Biochem. 2014, 40, 105–116. [Google Scholar] [CrossRef]
- Picha, M.E.; Shi, B.; Thomas, P. Dual role of IGF-II in oocyte maturation in southern flounder Paralichthys lethostigma: Up-regulation of mPRα and resumption of meiosis. Gen. Comp. Endocrinol. 2012, 177, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Negatu, Z.; Hsiao, S.M.; Wallace, R.A. Effects of insulin-like growth factor-I on final oocyte maturation and steroid production in Fundulus heteroclitus. Fish Physiol. Biochem. 1998, 19, 13–21. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Luckenbach, A.; Middleton, M.M.; Swanson, P. The spatiotemporal expression of multiple coho salmon ovarian connexin genes and their hormonal regulation in vitro during oogenesis. Reprod. Biol. Endocrinol. 2011, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Jalabert, B.; Fostier, A. The modulatory effect in vitro of oestradiol-17β, testosterone or cortisol on the output of 17α-hydroxy-20β-dihydroprogesterone by rainbow trout (Salmo gairdneri) ovarian follicles stimulated by the maturational gonadotropin s-GtH. Reprod. Nutr. Dev. 1984, 24, 127–136. [Google Scholar] [CrossRef]
- Lankford, S.E.; Weber, G.M. The maturation-inducing hormone 17α,20β-dihydroxy-4-pregnen-3-one regulates gene expression of inhibin βA and bambi (bone morphogenetic protein and activin-membrane-bound inhibitor) in the rainbow trout ovary. Gen. Comp. Endocrinol. 2010, 168, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Patiño, R.; Kagawa, H. Regulation of gap junctions and oocyte maturational competence by gonadotropin and insulin-like growth factor-I in ovarian follicles of red seabream. Gen. Comp. Endocrinol. 1999, 115, 454–462. [Google Scholar] [CrossRef]
- Thomas, P.; Pinter, J.; Das, S. Upregulation of the maturation-inducing steroid membrane receptor in spotted seatrout ovaries by gonadotropin during oocyte maturation and its physiological significance. Biol. Reprod. 2001, 64, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Miki, H.; Takagi, M. Design of serum-free medium for suspension culture of CHO cells on the basis of general commercial media. Cytotechnology 2015, 67, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Kusakabe, M.; Swanson, P.; Young, G. Regulation of sex steroid production and mRNAs encoding gonadotropin receptors and steroidogenic proteins by gonadotropins, cyclic AMP and insulin-like growth factor-I in ovarian follicles of rainbow trout (Oncorhynchus mykiss) at two stages of vitellogenesis. Comp. Biochem. Physiol. Part A 2016, 201, 132–140. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).