Abstract
Using vegetable protein sources as a replacement for fish meal (FM) in the diet of Pacific white shrimp (PWS) has a negative impact on their health. Acute hepatopancreatic necrosis disease (AHPND), caused by Vibrio parahaemolyticus, affects PWS and causes financial losses. Nucleotides modulate the immune response and could contribute to counteracting these issues. Our objective was to evaluate the effects of nucleotide supplementation on performance, immune response, and survival when challenged with V. parahaemolyticus, in PWS receiving a diet with FM partially replaced with vegetable protein sources. A feeding trial (1000 PWS; 56 days) and a challenge trial (600 PWS; 10 days) were performed using diets with different FM inclusion levels (26%, 23.4%, 22.1%, and 20.8%), with or without 0.1% nucleotides. A non-challenged, non-supplemented group was also used in the challenge trial. Adding nucleotides to diets with reduced FM allowed significantly better results in growth performance parameters and total hemocyte count (THC). In the challenge trial, compared to control, nucleotide supplementation led to significantly higher THC and survival rate 15 h post-challenge. In conclusion, adding nucleotides to PWS diets improves their immune response and resistance to aquatic pathogens, allowing FM to be replaced by vegetable protein sources without negatively affecting performance.
1. Introduction
Aquaculture production continues to grow globally, particularly in Asia, and the sector is expected to perform a key role in providing food and nutrition in the future. The global consumption of aquatic foods will continue to rise, but growth must be sustainable [1]. Therefore, there is a need for sustainable and efficacious solutions that can be used in the production of aquatic species and can ensure the health of the animals while enhancing their performance and resistance to diseases.
Vietnam is an example of a country where aquaculture development has been successful [1]. More specifically, the production of Pacific white shrimp (PWS), Litopenaeus vannamei, in Vietnam was 743,500 metric tons in 2022, which represents an 11.6% increase compared to the production volume in 2021. Intensive and super-intensive farming with plenty of innovations and technologies have been applied efficiently in the past few years in this country [2].
Fish meal (FM) is the most commonly used protein source in aquaculture diets, as it provides high protein levels, an excellent amino acid profile, low carbohydrate levels, digestibility, and few antinutritional factors. It is, however, also one of the most expensive ingredients. For this reason, vegetable protein sources, such as soybean meal (SBM), are increasingly being used as replacement for FM in the diet for PWS in Vietnam and other countries of the Southeast Asia region. This has a negative impact on the health of PWS due to their high content in anti-nutritional factors [3].
Acute hepatopancreatic necrosis disease (AHPND) is a bacterial disease mainly caused by Vibrio parahaemolyticus, which represents a major threat to the PWS industry in many Asian countries, including Vietnam and Indonesia, as it leads to substantial economic losses. Treatment is complex, and it comes with some limitations, which is why novel options are needed [4,5,6]. AHPND causes huge financial losses in countries where shrimp are produced in large amounts, such as Vietnam and Indonesia. Bacterial diseases are normally treated with antibiotics, but using them in shrimp aquaculture poses a risk of promoting the development of drug resistance, which could eventually have a negative impact on shrimp and people. For this reason, alternatives to antibiotics would be highly desired in order to avoid such problems [7]. Nucleotides could become an option to consider when facing challenging situations caused by AHPND.
Nucleotides are low molecular weight bioactive compounds which perform key roles in many physiological processes in living organisms. Dietary nucleotide supplementation becomes essential when there is increased nucleotide demand, and the body is not able to produce sufficient amounts. This occurs in situations of physiological stress, immunosuppression, infection, and disease [8,9,10,11]. Prior publications demonstrate the benefits of Nucleoforce®, a proprietary brand of a highly sustainable nucleotide-rich yeast extract from Saccharomyces cerevisiae developed by Bioiberica S.A.U. (Palafolls, Spain), in several animal species, including aquatic ones [8,12,13,14,15,16,17,18,19,20,21,22,23]. In fact, yeast derivatives have been described as promising immunostimulants to control diseases in aquaculture [24,25]. The efficacy of Nucleoforce® nucleotides in PWS has recently been further supported by two studies performed in Indonesia, showing a positive impact of nucleotide supplementation on performance, immune response, profitability and disease resistance against Vibrio harveyi [16,23]. Our research group hypothesized that they could also be beneficial for PWS in a production system based in Vietnam and supporting the immune health of shrimp against similar pathogens. Therefore, our objective was to evaluate the effects of dietary nucleotide supplementation on performance, immune response, and survival when challenged with V. parahaemolyticus in PWS-receiving diets where FM has been partially replaced by vegetable protein sources.
2. Materials and Methods
A feeding trial was performed at ShrimpVet Rand D Demonstration Farm (Cần Giờ, Ho Chi Minh City, Vietnam); meanwhile, a challenge trial was conducted at ShrimpVet Laboratory (Thủ Đức City, Ho Chi Minh City, Vietnam) in order to evaluate a potential immunoregulatory effect of dietary nucleotide supplementation in PWS and a possible positive impact on performance and resistance to aquatic pathogens.
Diets were prepared with different FM inclusion levels, which were partially replaced by vegetable sources (Table 1). The control diet (260FM, containing 26% FM) was designed to serve as a representative for commonly used diets for PWS production in Vietnam. Nucleotides (N, Nucleoforce®, Bioiberica, S.A.U., Palafolls, Spain) were supplemented at 0.1% in the other diets used in this study, in which a reduction in FM content of 0% (260FMN) 10% (234FMN), 15% (221FMN) and 20% (208FMN) was applied.

Table 1.
Composition of diets used in the trials. Diets were formulated using various levels (26%, 23.4%, 22.1%, and 20.8%) of fish meal (FM), and with or without adding dietary nucleotides (N).
2.1. Feeding Trial
A total of 1000 specific pathogen free (SPF) Litopenaeus vannamei PWS were acclimated for 2 days and thereafter classified into five study groups according to the study diet (5 replicate tanks per group; 500 L/tank; 40 juvenile shrimp/tank; maximum density of 160 shrimp/m3) under a recirculating aquaculture system (RAS). Shrimp were fed 6 times daily. Table 2 depicts the nutrient composition after analyzing the different diets used in these studies. Water temperature was maintained around 28–32 °C.

Table 2.
Nutrient composition of the diets administered to Pacific white shrimp in these studies.
At the end of the trial, hemolymph samples were obtained from 5 PWS/tank and used for quantification of total hemocyte count (THC) and phenoloxidase (PO) activity. Hemolymph was withdrawn from the pleopod base of the first abdominal segment near the genital pore using a syringe (26G needle) containing 0.1 mL of anticoagulant solution precooled (4 °C) (30 mM trisodium citrate, 340 mM sodium chloride, 115 mM glucose, and 10 mM EDTA at pH 7.55). 0.1 mL of hemolymph were collected from each shrimp. The total volume of the hemolymph-anticoagulant (1/1 v/v) mixture after pooled from 10 shrimp hemolymph samples was 2 mL. Thereafter, 0.1 mL of the hemolymph-anticoagulant mixture were put in a tube containing 0.9 mL of Natt-Herricks’s stain solution and, after that, on a Neubauer hemocytometer. TCH was then counted under the microscope (cells/mL). The PO activity was determined through the level of dopachrome at 490 nm wavelength. The PO reaction was performed on a 96-well plate. Briefly, hemolymph plasma was extracted by centrifuging 0.6 mL of the hemolymph-anticoagulant mixture at 6000 g/4 °C for 5 min. The supernatant was then collected and stored at −80 °C. 0.1 mL of plasma was added in wells of the 96-well. After that, 0.1 mL of L-DOPA solution were added in 1 mL of 100 mM sodium cacodylate buffer solution containing 100 mM CaCl2. The absorbance was measured at 490 nm at an interval of 110 s 15 times altogether. One unit of PO activity was defined by the increase of 0.001 in OD minute/mL of plasma.
Growth performance parameters were evaluated during the study, and profitability (return on investment; ROI) was also assessed by considering the revenues and production costs in each treatment group. Survival rate was also quantified at trial termination (day 56).
2.2. Challenge Trial
A total of 600 PWS (0.72 ± 0.15 g) were used in this trial. After acclimatation for 2 days, PWS were classified into 6 groups (4 replicate tanks per group; 120 L/tank; 25 PWS/tank; maximum density of 250 shrimp/m3) and received different diets for 28 days. Compared to the feeding trial, an additional study group (no challenge; 260FMNoCh—Negative Control) was added and shrimps received the same diet as in the 260FM group. The rest of the groups were challenged with the EMS/AHPND-causing V. parahaemolyticus strain and received the same diets than those in the feeding trial: 260FM (Positive Control group; 26% FM; 0% N), 260FMN (26% FM; 0.1% N), 234FMN (23.4% FM; 0.1% N), 221FMN (22.1% FM; 0.1% N) and 208FMN (20.8% FM; 0.1% N). PWS were followed-up for 10 days post-challenge.
The challenge was carried out as follows: V. parahaemolyticus isolate was collected from AHPND-affected L. vannamei from a shrimp farm in Vietnam. The isolate was confirmed by PCR and bioassay challenge on L. vannamei. PWS from the study were subjected to an immersion challenge. Bacterial suspensions were added to the tanks to achieve the desired density, measured by optical density absorbance (OD600 nm), to achieve a lethal dose of 90% mortality within 10 days of challenge. Shrimp in non-challenged tanks were treated with sterile tryptone soya broth (TSB) added directly into the tanks. THC was also quantified at the end of the trial.
Histology of the hepatopancreas was performed after the 10-day follow-up post-challenge. Live shrimps were soaked for 12 to 24 h (for small shrimp) and 24 to 72 h (for adult shrimp) in Davidson’s alcohol, formalin, acetic acid (AFA) fixative, then transferred to 50% ethanol for storage. Shrimp tissues were immersed in ethanol, from low to high concentrations (70%, 80%, 95%, and 99.5%) and dipped in xylene as the last step. After dehydration, the tissue samples were embedded in paraffin. Paraffin blocks containing tissues were then cut up to 4 µm thick under the microtome for the target tissue sample. After that, the slices were stained with Hematoxylin and Eosin. Then, slides were covered in Permount Mounting Medium and observed under the microscope. Groups were compared based on the following criteria and staging [26,27,28]: Grade 0 (−): no signs of infection by pathogen, parasite or epicommensal present; Grade 1–2 (+): pathogen, parasite, or epicommensal present but in low numbers. Light lesions characteristic of syndrome present, but “disease” not significant. Prognosis is for insignificant effect, except in developing infections by highly virulent pathogens; Grade 3 (++): Moderate numbers of pathogen, parasite, or epicommensal present. Moderate to severe lesions characteristic of syndrome present. Potentially lethal prognosis if no treatment (if treatable) is applied; Grade 4 (+++): High numbers of pathogen, parasite, or epicommensal present. Severe lesions characteristic of syndrome present. Lethal prognosis.
2.3. Statistical Analysis
Laboratory and performance parameters were analyzed by one-way analysis of variance (ANOVA), followed by a Duncan test to determine differences between treatments using SPSS v.25 statistic calculation software. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Feeding Trial
After 56 days, THC levels were significantly higher in all N-supplemented groups, compared to Control. Group 260FMN showed THC levels significantly higher than any other group. THC in the 234FMN, 221FMN, and 208FMN groups (with a 10%, 15%, and 20% FM reduction, respectively) were also significantly improved compared to the control group (regular 26% FM inclusion level) (Figure 1). No significant differences were found between groups in PO activity.

Figure 1.
THC in shrimp from the different study groups after 56-day feeding trial. Different superscript letters indicate significantly different values (p < 0.05).
Nucleotide supplementation using regular FM levels (260FMN) led to improved performance, achieving better final mean body weight, final biomass, mean weight gain, average daily gain, and feed consumption, compared to control (260FM), although this effect did not reach the level of statistical significance. On the other hand, several performance parameters including final mean weight, final biomass, mean weight gain, average daily gain, specific growth ratio, and feed consumption were significantly better in the 260FMN group compared to 234FMN, 221FMN, or 208FMN (Table 3).

Table 3.
Growth performance data. Results (mean ± SD) in the same rows with different superscript letters are significantly different (p < 0.05).
Survival rate after 56 days was very high in all study groups (91.5%, 91%, 93.5%, 92.5%, and 93% in groups 260FM, 260FMN, 234FMN, 221FMN, and 208FMN, respectively), with no statistically significant differences between them.
It was also noticed during the trial that PWS fed with nucleotides featured higher locomotion activity and shorter time to consume the feed, compared to the non-supplemented groups.
Economic analyses revealed improved profitability after adding nucleotides to the diet with regular FM inclusion levels (260FMN vs. 260FM) (Table 4).

Table 4.
Return on investment (ROI) obtained in each of the study groups in the feeding trial receiving various levels (26%, 23.4%, 22.1%, and 20.8%) of fish meal (FM), with or without adding dietary nucleotides (N).
3.2. Challenge Trial
Challenge by immersion achieved the desired AHPND infection in all corresponding tanks (Figure 2).
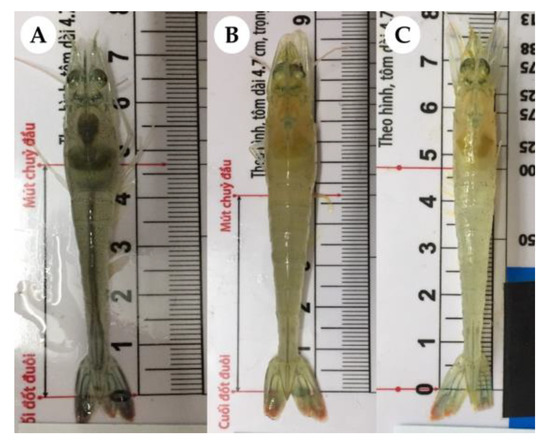
Figure 2.
Pacific white shrimp from the study 24 h after challenge with Vibrio parahaemolyticus belonging to groups 260FMNoCh (A), 260FM (B) and 260FMN (C). Infected shrimp show pale hepatopancreas and empty gut tract, while non-infected shrimp feature brownish hepatopancreas, and full stomach and gut tract.
THC levels at the end of the trial were significantly higher (p < 0.05) in groups 234FMN (17.44 ± 5.32 × 106 cells/mL) and 221FMN (18.15 ± 4.16 × 106 cells/mL), compared to 260FM (11.04 ± 3.384 × 106 cells/mL).
At 10 days post-challenge, survival rate was significantly lower in the positive control group (260FM), compared to the negative control (260FMNoCh). All groups in which the diets incorporated nucleotides showed higher survival rates than the positive control group during the post-challenge period (Figure 3). Fifteen hours post-challenge, a significantly (p < 0.05) higher survival rate compared to the positive control group (69.72 ± 18.08%) was achieved with 221FMN (95.00 ± 10.00%) and 208FMN (91.98 ± 9.52%), while survival rate in these groups at that time was not statistically inferior to the negative control group (86.76 ± 5.27%).
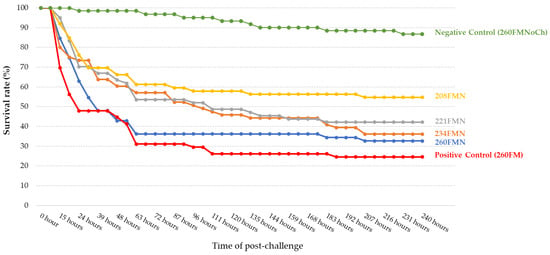
Figure 3.
Survival rate of shrimp in each study group after challenge.
Histological staging showed differences between groups after challenge. More specifically, groups were graded as follows: 260FMNoCh −; 260FM +++; 260FMN ++; 234FMN ++; 221FMN ++; 208FMN +++ (Figure 4).
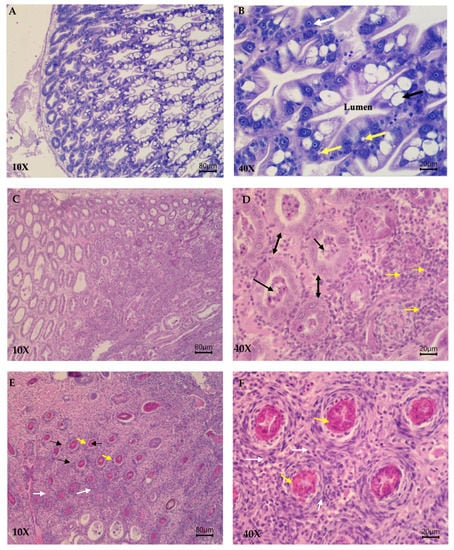
Figure 4.
Representative histopathological images of hematoxylin and eosin-stained sections of the hepatopancreas from Pacific white shrimp (PWS) belonging to different study groups. Histological images from a non-challenged shrimp from the negative control group at 10× (A) and 40× (B) showing normal hepatopancreas structures. Hepatopancreas cells and tubules are in good shape. White arrow: F cells; black arrow: B cells; yellow arrows: R cells. Histological images from shrimps belonging to group 221FMN at 10× (C) and 40× (D) showing G3 infection (early terminal phase). Severe tubules atrophy (double headed arrows) and detachment due to the degenerate and necrotic process can be observed. Severe sloughing of hepatopancreas cells and sloughing of hepatopancreas epithelium (black arrows) and hemocyte infiltration (yellow arrows) can also be seen. Histological images from shrimps belonging to the positive control group at 10× (E) and 40× (F) showing G4 infection (terminal phase). hepatopancreas tubules appear totally damaged (black narrows). Collapsed epithelia invaded by bacteria and severe hemocyte infiltration are seen. Hemocytic capsule formation (white narrows) and melanization of bacteria or necrotic material inside the tubules (yellow narrows) are also observed.
4. Discussion
Shrimp production continues to grow globally and faces several challenges, at the same time aiming to align with blue transformation, whose target is to promote sustainable aquaculture ensuring food security and environmental sustainability [1]. According to what was observed in this trial and in prior research studies [16,23], nucleotides could contribute to that goal.
In the research presented herein, the incorporation of dietary nucleotides led to several beneficial effects in the feeding trial. Adding nucleotides to a diet with regular FM levels led to significantly better immune response, seen as higher THC levels. Using nucleotides in a diet with regular FM levels also led to significantly better growth parameters, compared to groups with lower FM levels, while the performance results in the control diet (without nucleotides) did not reach such significant effect, compared to the rest of the diets. On the other hand, when regular FM levels were used, adding nucleotides allowed a numerical improvement, although this effect did not reach the level of statistical significance.
Profitability is key for shrimp producers. To gain more profits and competitive advantages, shrimp producers must consider minimizing production cost and tackling sustainable development. Nowadays, many novel ingredients, feed additives, and functional products have been explored, tested, and partially applied in shrimp farming in order to farm more efficiently, to increase productivity, and to boost shrimp health and disease tolerance. In Vietnam, although over 743,500 tons of L. vannamei shrimp (2022) are produced per year [2], the production cost is still much higher than in Ecuador and India. The investment in infrastructures becomes high and costly, and these do not last for long due to depreciation. The profit margin for shrimp producers is unprecedentedly slim and Vietnam shrimp products would lose their competitive advantage on the market if they cannot find appropriate solutions. In near future, the Vietnamese government is aiming to achieve 10 billion USD in shrimp exports by 2025. To achieve this goal, the shrimp industry in Vietnam is currently focusing on hi-tech farming with more intensification and sustainability. Alternative ingredients have been used to replace fish meal and animal protein ingredients; functional feeds have also been generated by many reputable feed millers in the last few years; more and more potential feed additives have been demonstrated to enhance growth performance, feed utilization, and disease tolerance [29,30]. In this study, we demonstrated that adding nucleotides to shrimp diets improved profitability. This might be explained by the improvements in performance which, despite some not being statistically significant, taken together with the minimal impact of nucleotides on diet costs and their contribution to sustainability, eventually result in economic advantages for the producers.
The pathogenesis of AHPND is not yet fully understood. However, there seems to be a dysregulation of the immune response and alterations in the epithelial cells in the shrimp stomach [4]. Nucleotides, being compounds that contribute to maintaining intestinal growth and improving cellular function, along with their immunomodulatory effects [9,10], could explain the observed benefits against this particular aquatic pathogen. Immune response was evaluated in both trials presented herein and, in both cases, a significant improvement in THC was observed. This is in line with a recent trial performed with nucleotides in PWS in Indonesia, in which significantly higher THC and lysozyme activity were observed after a Vibrio harveyi challenge [16].
Survival of shrimp in the challenge trial was clearly affected by the infection with V. parahaemolyticus, but adding nucleotides counteracted this negative impact by improving survival of PWS. This further supports the benefits of Nucleoforce® against shrimp pathogens, as it was also seen with a V. harveyi study [16].
The hepatopancreas is a very important organ of shrimp. It is structured by many tubules with various hepatopancreas cells present from the proximal region to distal region in every tubule. In the hepatopancreas structure, B (blister-like or Blastozellen) cells, F (fibrillar) cells, and R (resorptive/absorptive or Restzellen) cells perform vital roles in the digestion process, and in nutrient absorption, and excretion. More specifically, F-cells are responsible for the production of digestive enzymes [31]. B-cells are responsible for intracellular digestion, while R cells are liable for the storage of nutrients by forming lipid and/or glycogen droplets [32]. In histopathology, although all challenged shrimp showed the severity of AHPND infection (G3 and G4), the tissues of shrimp fed with nucleotide diets (234FMN and 221FMN) look better than the shrimp fed with the control diet and 260FMN diet as still in the early terminal phase of the infection. Meanwhile, shrimp in the negative control group did not show any abnormalities in their hepatopancreas tissues. The histology examination indicates the integrity of shrimp hepatopancreas structure and no necroses with the abundance of B cells, R cells, and F cells.
Nucleotides have been implicated as feed attractants which are supplemented in feed to improve the attractability and palatability of fish and shrimp [33,34]. This was also observed in this study. Although there was no significant difference in terms of feed consumption among the groups, shrimp fed with nucleotide dietary treatments look better in terms of appetite and palatability as their feed consumption was higher than the control, they consumed the feed faster and exhibited a higher locomotion activity.
On the other hand, shrimp fed with nucleotides diets appear to have a higher probability of survival than those fed without nucleotides, and a delay in mortality of about 1–2 days compared to the control. Thus, nucleotide supplementation has efficacy in modulating shrimp immune responses and improving shrimp disease tolerance. Therefore, it should provide cultivators with more time to apply interventions and make decisions accordingly, in order to protect profit margins or avoid detrimental effects at harvest.
The main limitation of the study was the lack of a study group, or more than one, in which there was a partial replacement of FM by SBM and without nucleotide supplementation. This would have allowed comparisons between diets with reduced FM utilization in which nucleotides are either added or not. With the current design, it is possible to evaluate the effect of supplementing diets with regular FM levels with nucleotides and the impact of reducing FM in diets also containing nucleotides, but further studies would be warranted in order to confirm a clear effect of adding nucleotides to diets with reduced fish meal levels, compared to non-supplemented diets.
5. Conclusions
Supplementation with 0.1% nucleotides for 56 days in Pacific white shrimp under recirculating aquaculture system conditions in Vietnam leads to improved immune function, profitability and survival upon challenge with an EMS/AHPND-causing V. parahaemolyticus strain, allowing FM replacement by vegetable protein sources without having a negative impact on performance. Therefore, nucleotides could be used as sustainable and effective functional ingredient in PWS production.
Author Contributions
Conceptualization, T.C., P.H., L.T. and S.S.; methodology, T.C., P.H., L.T. and S.S.; formal analysis, T.C., P.H. and L.T.; writing—original draft preparation, S.S.; writing—review and editing, T.C., P.H., L.T. and S.S.; supervision, L.T.; project administration, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This project was sponsored by Bioiberica S.A.U., Spain (PJ-00103).
Institutional Review Board Statement
This study was approved by the ethical committee of the Law on Animal Health (No. 79/2015/QH13) and the Law on Fisheries (No. 18/2017/QH14) in scientific testing, research, animal welfare, and the use of animals in research.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
S.S. is employed by Bioiberica S.A.U. The rest of the authors state no conflict of interest.
References
- FAO. The State of World Fisheries and Aquaculture 2022; Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Vietnam Directorate of Fisheries. Report No. 331/BC-TCTK; General Statistics Office of Vietnam: Hanoi, Vietnam, 2022.
- Yue, Y.R.; Liu, Y.J.; Tian, L.X.; Gan, L.; Yang, H.J.; Liang, G.Y. Effects of Replacing Fish Meal with Soybean Meal and Peanut Meal on Growth, Feed Utilization and Haemolymph Indexes for Juvenile White Shrimp Litopenaeus Vannamei, Boone. Aquac. Res. 2012, 43, 1687–1696. [Google Scholar] [CrossRef]
- Kumar, R.; Ng, T.H.; Wang, H.C. Acute Hepatopancreatic Necrosis Disease in Penaeid Shrimp. Rev. Aquac. 2020, 12, 1867–1880. [Google Scholar] [CrossRef]
- Tran, L.; Nunan, L.; Redman, R.M.; Mohney, L.L.; Pantoja, C.R.; Fitzsimmons, K.; Lightner, D.V. Determination of the Infectious Nature of the Agent of Acute Hepatopancreatic Necrosis Syndrome Affecting Penaeid Shrimp. Dis. Aquat. Organ. 2013, 105, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Shinn, A.P.; Pratoomyot, J.; Griffiths, D.; Trong, T.Q.; Vu, N.T.; Jiravanichpaisal, P.; Briggs, M. Asian Shrimp Production and the Economic Costs of Disease. Asian Fish. Sci. 2018, 661, 29–58. [Google Scholar] [CrossRef]
- Kumar, R.; Chauhan, S.B.; Ng, S.S.; Sundar, S.; Engwerda, C.R. Immune Checkpoint Targets for Host-Directed Therapy to Prevent and Treat Leishmaniasis. Front. Immunol. 2017, 8, 1492. [Google Scholar] [CrossRef]
- Segarra, S. Nutritional Modulation of the Immune Response Mediated by Nucleotides in Canine Leishmaniosis. Microorganisms 2021, 9, 2601. [Google Scholar] [CrossRef]
- Fontana, L.; Martínez-Augustin, O.; Gil, Á. Role of Dietary Nucleotides in Immunity. Funct. Food Rev. 2010, 2, 91–100. [Google Scholar] [CrossRef]
- Gil, A. Modulation of the Immune Response Mediated by Dietary Nucleotides. Eur. J. Clin. Nutr. 2002, 56, S1–S4. [Google Scholar] [CrossRef]
- Hess, J.R.; Greenberg, N.A. The Role of Nucleotides in the Immune and Gastrointestinal Systems: Potential Clinical Applications. Nutr. Clin. Pract. 2012, 27, 281–294. [Google Scholar] [CrossRef]
- Reda, R.M.; Selim, K.M.; Mahmoud, R.; El-Araby, I.E. Effect of Dietary Yeast Nucleotide on Antioxidant Activity, Non-Specific Immunity, Intestinal Cytokines, and Disease Resistance in Nile Tilapia. Fish Shellfish Immunol. 2018, 80, 281–290. [Google Scholar] [CrossRef]
- Selim, K.M.; Reda, R.M.; Mahmoud, R.; El-Araby, I.E. Effects of Nucleotides Supplemented Diets on Growth Performance and Expressions of Ghrelin and Insulin-like Growth Factor Genes in Nile Tilapia, Oreochromis Niloticus. J. Appl. Aquac. 2020, 32, 157–174. [Google Scholar] [CrossRef]
- Yaseen, M.S.; Abdelaziz, M.; Abdel-Moneam, D.A.; Abd-Elhay, E.; Wassif, I.M.; Moustafa, M. Efficacy of Dietary Nucleotides (NucleoforceTM) on Growth, Haemato-Immunological Response and Disease Resistance in Pangasianodon Hypophthalmus Fish (Sauvage, 1878) in Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 405–424. [Google Scholar] [CrossRef]
- El-Nokrashy, A.M.; El-Banna, R.A.; Edrise, B.M.; Abdel-Rahim, M.M.; Jover-Cerdá, M.; Tomás-Vidal, A.; Prince, A.; Davies, S.J.; El-Haroun, E.R.; Goda, A.M.A.S. Impact of Nucleotide Enriched Diets on the Production of Gilthead Seabream, Sparus Aurata Fingerlings by Modulation of Liver Mitochondrial Enzyme Activitity, Antioxidant Status, Immune Gene Expression, and Gut Microbial Ecology. Aquaculture 2021, 535, 736398. [Google Scholar] [CrossRef]
- Novriadi, R.; Ilham, I.; Roigé, O.; Segarra, S. Effects of Dietary Nucleotides Supplementation on Growth, Total Haemocyte Count, Lysozyme Activity and Survival upon Challenge with Vibrio Harveyi in Pacific White Shrimp, Litopenaeus Vannamei. Aquac. Rep. 2021, 21, 100840. [Google Scholar] [CrossRef]
- Borda, E.; Estévez, A.; Tort, L. Effect of the Free Nucleotides on the Immune Response during First Stage of Fattening in Gilthhead Bream (Sparus Aurata). In Proceedings of the Aquaculture Europe, Trondheim, Norway, 5–9 August 2005; pp. 5–9. [Google Scholar]
- Estruch, G.; Tortosa, I.; Monge, R.; Godoy, S. Inclusión de Aditivos En Dietas Vegetales Para Doradas. Efectos Sobre El Crecimiento, Parámetros Biométricos y Nutritivos, Digestibilidad e Histología. In Proceedings of the Actas del XV Congreso Nacional y I Congreso Ibérico de Acuicultura, Guayaquil, Ecuador, 19–22 October 2015. [Google Scholar]
- Magouz, F.I.; Abdel-Rahim, M.M.; Lotfy, A.M.; Mosbah, A.; Alkafafy, M.; Sewilam, H.; Dawood, M.A.O. Dietary Nucleotides Enhanced Growth Performance, Carcass Composition, Blood Biochemical, and Histology Features of European Sea Bass, Dicentrarchus labrax L. Aquac. Rep. 2021, 20, 100738. [Google Scholar] [CrossRef]
- Borda, E.; de la Fuente, E. Efficacy of Dietary Nucleotides Supplementation on the Immune Response in Atlantic Salmon (Salmo salar): Effects on Salt Water Transfer and Vaccination. In Proceedings of the Aquaculture Europe, Krakow, Poland, 15–18 September 2008. [Google Scholar]
- Sáenz de Rodrigáñez, M.A.; Barata, M.; Dias, J.; Pousão-Ferreira, P.; Morales, G.; Márquez, L.; Moyano, F.J.; Ribeiro, L. Evaluation of the Effect of Nucleotides on Intestinal Function in Juveniles of Meagre (Argyrosomus regius) Fed on High Plant Protein Diets. In Proceedings of the Aquaculture Europe, Prague, Czech Republic, 1–5 September 2012. [Google Scholar]
- Romano, N.; Fischer, H.; Rossi, W.; Quintero, H.; Limbaugh, N. Effects of Bioprocessed Soybean Meal and Nucleotide Supplementation on Growth, Physiology and Histomorphology in Largemouth Bass, Micropterus Salmoides, Juveniles. Comp. Biochem. Physiol. 2021, 260, 111038. [Google Scholar] [CrossRef]
- Novriadi, R.; Roigé, O.; Segarra, S. Effects of Dietary Nucleotide Supplementation on Performance, Profitability, and Disease Resistance of Litopenaeus Vannamei Cultured in Indonesia under Intensive Outdoor Pond Conditions. Animals 2022, 12, 2036. [Google Scholar] [CrossRef]
- Mastan, S.A. Use of Immunostimulants in Aquaculture Disease Management. Int. J. Fish. Aquat. Stud. 2015, 2, 277–280. [Google Scholar]
- del Valle, J.C.; Bonadero, M.C.; Ferández-Gimenez, A.V. Saccharomyces Cerevisiae as Probiotic, Prebiotic, Synbiotic, Postbiotics and Parabiotics in Aquaculture: An Overview. Aquaculture 2023, 1, 739342. [Google Scholar] [CrossRef]
- Bell, T.A.; Lightner, D.V. A Handbook of Normal Penaeid Shrimp Histology; World Aquaculture Society: Baton Rouge, LA, USA, 1988. [Google Scholar]
- Lightner, D.V. A Handbook of Shrimp Pathology and Diagnostic Procedures for Diseases of Cultured Penaeid Shrimp; World Aquaculture Society: Baton Rouge, LA, USA, 1996. [Google Scholar]
- Tang, K.F.J.; Bondad-Reantaso, M.G.; Arthur, J.R.; MacKinnon, B.; Hao, B.; Alday-Sanz, V.; Liang, Y.; Dong, X. Shrimp Acute Hepatopancreatic Necrosis Disease Strategy Manual; FAO Fisheries and Aquaculture Circular No. 1190; FAO: Rome, Italy, 2020. [Google Scholar]
- Hoe, T.D. Finding the Reason for the High Production Cost of Vietnamese Shrimp. Vietnamagriculture 2023. Available online: https://vietnamagriculture.nongnghiep.vn/finding-the-reason-for-the-high-production-cost-of-vietnamese-shrimp-d348607.html (accessed on 31 May 2023).
- Luc, H.Q. Vietnamese Shrimp’s Global Integration. Vietnam Fisheries Magazine, 28 June 2021; 10–13. [Google Scholar]
- Vicentini, I.B.F.; Ribeiro, K.; Papa, L.P.; Junior, J.M.; Vicentini, C.A.; Moraes-Valenti, P.M.C. Histoarchitectural features of the hepatopancreas of the Amazon river prawn Macrobrachium amazonicum. Int. J. Morphol. 2009, 27, 121–128. [Google Scholar]
- Wang, X.; Li, E.; Xu, C.; Qin, J.G.; Wang, S.; Chen, X.; Cai, Y.; Chen, K.; Gan, L.; Yu, N.; et al. Growth, body composition, ammonia tolerance and hepatopancreas histology of white shrimp Litopenaeus vannamei fed diets containing diferente carbohydrate sources at low salinity. Aquac. Res. 2014, 47, 1932–1943. [Google Scholar] [CrossRef]
- Lee, P.G.; Meyers, S.P. Chemoattraction and Feeding Stimulation. In Crustacean Nutrition, Advances in World Aquaculture; The World Aquaculture Society: Baton Rouge, LA, USA, 1997; pp. 292–352. [Google Scholar]
- Suresh, A.V.; Kumaraguru Vasagam, K.P.; Nates, S. Attractability and Palatability of Protein Ingredients of Aquatic and Terrestrial Animal Origin, and Their Practical Value for Blue Shrimp, Litopenaeus Stylirostris Fed Diets Formulated with High Levels of Poultry Byproduct Meal. Aquaculture 2011, 319, 132–140. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).