Abstract
The level of carbon dioxide, which resembles water acidification, is critical to the success of juvenile fish production. The growth, skeletal deformities, and blood parameters of the economically important freshwater fish mahseer, Tor tambroides, were assessed in different concentrations of pCO2 (400, 700, and 1000 µatm pCO2). The highest growth properties (survival rate, body weight gain (BWG), specific growth rate (SGR)) in mahseer were observed at 400 µatm, whereas the lowest growth indices were found in the treatment of 1000 µatm pCO2. The lowest pCO2 concentration was found to be within the optimum healthy blood parameter range. The fish exposed to acidic conditions (700 and 1000 µatm) exhibited considerably higher levels of haemoglobin and haematocrit compared to the control fish. Glucose levels were significantly lower in the acidic conditions, while total cholesterol levels in mahseer fish exposed to acidic conditions were higher. The fish displayed skeleton malformations as the concentration of pCO2 was elevated. The findings from this research could be set as a standard technique in the juvenile rearing of mahseer fish under acidified conditions.
1. Introduction
In recent years, there has been huge interest in freshwater acidification research due to acid rain caused by anthropogenic activities [1]. Most freshwater rivers and streams are acidic, with pH values between 6 and 8. This is mostly due to the release of protons (H+) when water breaks down bicarbonate (HCO3−). Recent research indicates that certain freshwater environments are at risk of acidification as a result of elevated CO2 [2]. Acidification caused by increased CO2 concentrations arises as a result of multiple factors, such as increased atmospheric CO2, invasive species, and soaring land-based, terrestrial primary productivity due to global warming [3,4]. Some freshwater bodies could be in danger from high levels of dissolved CO2, which is alarming given how acidic the ocean is becoming right now.
In reference to acidification, a decline in growth performance has been observed in freshwater fishes. This decline in the rate of growth was most probably triggered by the acid–base regulatory mechanisms which counter fluid and tissue acidification [5]. Additionally, many freshwater fish experience a physiological stress response in acidified water, which also requires energy. Therefore, the energetic costs of maintaining homeostasis in the presence of severe acidification can modify the energy of freshwater fish, resulting in a decrease in rates of growth and body mass [6].
Fish in their juvenile stage may be more vulnerable to changes in CO2 since their physiological functions have not yet developed. When fish are exposed to a high level of CO2, the frequency of skeleton abnormalities increases [7]. It has been demonstrated that acidification influences the incidence of branchiostegal ray deformation and vertebral column malformations, including lordosis [8]. As fry grow into the juvenile stage, their tissues and organs undergo significant changes, along with the calcification of skeletal components, which are necessary for swimming skills and otolith development and vital for equilibrium [9]. The impact of acidification on otolith morphometric characteristics and skeletal abnormalities has been extensively studied [8,10,11]. These studies yielded inconsistent findings, ranging from no implications to beneficial outcomes in terms of increased density and decreased malformations. Furthermore, blood haematological and biochemical parameters have been used as indicators of fish responses to stressors such as temperature and CO2 [12,13]. As a result, using these factors in the monitoring of fish reactions to progressively acidic pH levels is critical. One of the most critical parameters determining water quality and fish health is pH. Long-term exposure to an aquatic environment with an acidic pH of less than 6.5 or an alkaline pH of more than 9.0 causes stress in fish [14].
Mahseer are commercially important freshwater fish. Mahseer are well-known, migratory, large-bodied Asian river freshwater fishes. These fish have long been regarded as a food supply and are used for other social functions, but they are now gravely threatened by a variety of interrelated and growing human activities, including dam construction, water pollution, overfishing, and habitat degradation. Mahseer fish are advantageous to people in the rivers they inhabit because they serve as both direct providers of ecosystem services and indicators of the health of the environment. Mahseer fish are in high demand in Southeast Asian countries as an edible and attractive fish [1]. Mahseer have a high nutritional content and can provide food security in areas with high levels of poverty; hence, there have been accounts of extensive exploitation of this resource dating back to the eighteenth century [6]. Mahseer are currently experiencing tremendous population pressures in several Asian countries as a result of a number of anthropogenic factors, including invasive species, diminishing water quality, and massive river engineering projects [1]. Despite the significant economic and cultural value of the Tor genus, levels of population-level data are low, and basic knowledge of the biology and ecology of the majority of species is lacking [6]. While the genus has seen an increase in research efforts recently, these efforts have been largely geared toward aquaculture [8]. The regions of the developing globe where mahseer are found are experiencing escalating resource demands from a rapidly industrialising and expanding population. Walton et al. identified four main risks to freshwater fishes while analysing trends in large-scale hydrological changes throughout Asia: flow alteration and management (such as dam construction and abstraction), pollution, drainage basin alteration (such as deforestation), and over-harvesting [4]. Threats affecting the population status of the Tor species fall into each of these categories in a significant way. There is an urgent need to give practitioners, regulators, and policymakers standardised points of reference to benchmark the current state of knowledge and conservation status of the genus Tor. This is due to a growing interest in conserving this group of iconic fishes among a variety of stakeholder groups (e.g., scientists, conservationists, recreational anglers, land and water resource managers).
Due to the likely persistence of acidification in some freshwater environments as a result of climatic change and other human activity affecting these environments, researchers must try to advance their knowledge of acidification’s impacts on freshwater fish. Aquatic ecosystems offer vast biodiversity, food, and water security and are vital components of the climate and the health of our ecosystems [15]. Together, the diversity in freshwater system responses to elevated CO2 and the high risk of human disturbances and environmental degradation [16] are reasons why forecasting the impacts of climatic change on freshwater systems is especially difficult. As some freshwater ecosystems have already reached century-end CO2 concentrations, there is a sense of urgency to identifying the implications of acidification for freshwater organisms [17]. Thus, the purpose of this research was to emphasise possible physiologic impacts during acidification on the growth, blood parameters, and vertebral column of mahseer fish juveniles.
2. Materials and Methods
2.1. Fish Sampling and Experimental Design
Mahseer fish (n = 270; initial wet weight expressed as mean ± standard deviation = 2.4 ± 0.01 g) were obtained from the Aquaculture Development Centre in Perak, Malaysia (3.8428° N, 102.2081° E), and transferred to the Universiti Kebangsaan Malaysia (UKM) wet laboratory in Bangi, Malaysia. Mahseer were then randomly allocated into two aquaria (2.5 m × 1.2 m × 0.7 m, size and capacity of 1800 L; 135 fish in each tank). Each aquarium was provided with running fresh water and kept at a temperature of approximately 28 °C using the previous feed given in the hatchery. Mahseer were assigned to nine experimental aquariums (30 fish per aquarium) during a four-month period after they began feeding and defecating. The experimental design and fish-rearing protocols described in De et al. [18] were used as guidance. Juveniles were fed commercial fish food on a daily basis (JB Kelah, Daya Aquatic Sdn Bhd, crude protein: 45%, crude fat: 5%, crude ash: 1%, crude fibre: 3%). Fish were hand-fed twice a day to apparent visual satiety in order to avoid any uneaten pellets, and the feed distributed was recorded. The photoperiod was adapted each week to simulate the natural environment, with 12 h of light and 12 h of darkness [19]. Every day, leftover feed and faeces were emptied from the aquaria to make sure that the feed and excretion did not affect the pH value [1,19]. The rate of water flow ensured that the dissolved oxygen (DO) remained above 5.5.
Based on the assessment by the IPCC [20], three different acidification treatments were used to culture the juveniles. Anthropogenic CO2 does indeed affect freshwater carbon hydrogeochemistry. The pCO2-dependent acidification rate is expected to decrease by 3–0.5 pH units until 2100, at the same rate as observed in the oceans [21]. Investigated reservoirs appear to be climate change sentinels, acidifying at a faster rate of 0.01 pH units per year. As demonstrated by Phillips et al. [21], the effect of increased pCO2 on freshwater pH is consistent with current IPCC projections for the growth in atmospheric pCO2 from the present to 2100. Three distinct CO2 partial pressures were used in the acidification process (pCO2), derived from the IPCC projections. Control was set at 400 μatm, while the simulated scenarios used 700 μatm and 1000 μatm. Climate forecasts show that the water will attain approximately 1000 µatm pCO2 within the next century, whereas 700 µatm was based on the IPCC’s report for the upcoming 50 years [20]. Water quality during the experimental period is tabulated in Table 1. Continuous pH measurements were taken, and the system was programmed to control a solenoid valve connected to a CO2 cylinder. This valve was used to regulate the CO2 being infused into the fish aquaria. pH was controlled in each aquarium to within ±0.05 pH units using a continuous pH-stat system (IKS) that controlled the addition of CO2 into the beakers, which were continuously aerated with CO2-free air. A multi-probe HI98194 was used to measure pH and temperature (Hanna Instruments, Woonsocket, Rhode Island USA). Total alkalinity was determined weekly by mixing 15 mL of HCl (0.01 M) with a 50 mL sample of filtered water (200 μm nylon mesh).

Table 1.
Water quality (mean ± standard deviation, SD) of mahseer during experimental period. Different superscript letters indicated significance difference among treatments.
A macro CO2 system was run in Microsoft Excel with the constants from Kortazar et al. to compute the water carbonate chemistry [22]. After reaching the desired pCO2 concentration, mahseer were cultured for 4 months in flow-through freshwater systems with a water exchange rate of 2 L per minute in 500 L aquaria (1.0 m × 0.6 m × 0.3 m) with different pCO2 levels (Figure 1). Mahseer fish were collected at the end of the experimental period for growth and vertebral column formation analyses, which are described in detail in the following sections.
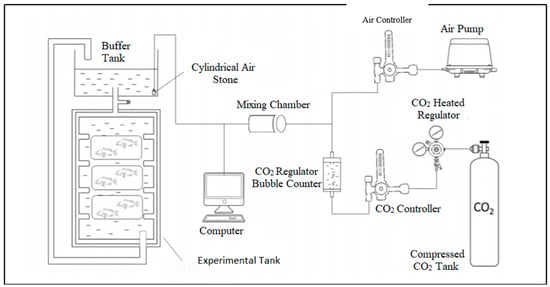
Figure 1.
Experimental setup during experimental period for mahseer.
2.2. Growth Properties
The body weight of the fish was determined via an electronic balance (XPR204S, Mettler Toledo, Columbus, OH, USA), and they were anaesthetised for 10 min with MS222 solution (20 mg per 1 L of water, Sigma Aldrich, St. Louis, MO, USA). The survival rate was calculated and converted to a percentage for each aquarium based on the number of mahseer that survived at the beginning and end of the experiment. The following equations were used to determine the growth features [18]:
where W1 is the initial weight of the fish, W2 is the final weight of the fish, and T is the length of the duration of the study.
Body weight gain, BWG (g) = (W2 − W1) × n
Specific growth rate, SGR = [(ln W2 − ln W1)/T] × 100,
2.3. Vertebral Column Formation
On the final experimental day, three mahseer were sacrificed and fixed for 24 h in a 10% buffered formalin solution [8]. Afterwards, the fixed samples were kept in 70% ethanol. The cartilaginous portion of the skeletal system was stained for 72 h in ethanol and 0.02% alcian blue. Samples were subsequently immersed in muscle digestion buffer containing borate until stained cartilage became visible. The sample was transferred to a 0.5% aqueous potassium hydroxide solution. Alizarin Red S was applied to the mixture until the solution was dark purple. Samples were left for 36 h and finally stored in 100% glycerol for permanent storage. Samples were then detected under stereomicroscope (Leica EZ4, Wetzlar, Germany). In accordance with reports by Boglione [8], skeletal abnormalities were divided into four categories (fusion, lordosis, kyphosis, and scoliosis), and the percentage for every type of deformity in each treated group was determined using the total number of fish.
2.4. Blood Haematology and Biochemical Parameters
Following the experimental period, three mahseer were obtained from replicates of each pCO2 condition and anaesthetised for ten minutes within MS222 solution (20 mg per 1 L of water, Sigma Aldrich). Blood was collected from caudal vein puncture with a syringe (5 mL) rinsed with 10% EDTA, which acts as an anticoagulant to prevent blood clotting. Blood samples were taken from caudal blood vessels and separated into two parts, one of which was mixed with anticoagulant, and the other was centrifuged without anticoagulant to separate the serum. The Baker and Silverton standard cyanmethemoglobin method was used to measure haemoglobin [23]. The value of haematocrit was calculated using the Wintrobe technique and was calculated as a percentage. Blood samples were loaded into Wintrobe tubes and spun at 3000 rpm for 5 min before being measured [23]. Biochemical analysis of total protein (TP) was performed by assay kits (Thermo Fischer Scientific, Waltham, MA, USA). A glucose and cholesterol analyser (Vitros DTEII, Johnson & Johnson, New Brunswick, NJ, USA) was used to determine the levels of glucose and cholesterol according to quality control protocols established by Johnson & Johnson.
2.5. Statistical Analysis
All statistical analyses of growth, vertebral formation, and blood parameters in this study were conducted by Minitab version 17 software (Minitab Inc., State College, PA, USA). The results of this study were derived in terms of mean ± standard deviation. The values were checked for variance and normality equality prior to one-way ANNOVA. A Tukey’s HSD post hoc analysis was run to compare whether the specific group means (in comparison to one another) were statistically different at p < 0.05.
3. Results
3.1. Growth
The survival rate of mahseer at 1000 µatm pCO2 was quarterly reduced compared to 400 µatm (Figure 2). A significant difference was observed between the pCO2 groups. Overall, the growth rate of mahseer periodically declined when the culture conditions reached a high concentration of pCO2 (Figure 3). Based on Figure 3, BWG and SGR had the same correlation because an increase or decrease in one of the parameters directly affected other values of growth. The SGR for mahseer was significantly influenced by pCO2 when the pCO2 rose to 1000 µatm, which caused the SGR value to fall.
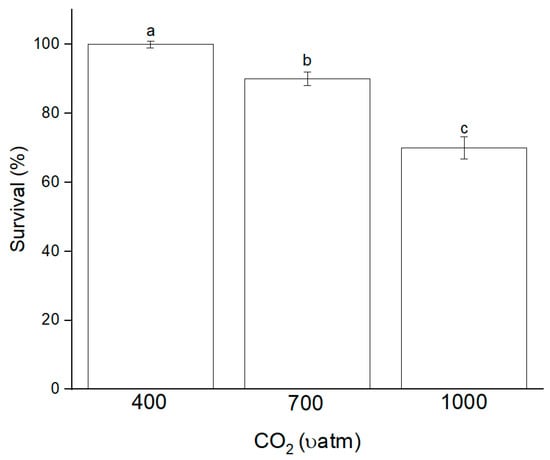
Figure 2.
Survival percentage of mahseer in different pCO2 conditions. Values are means from three groups of fish (n = 30). Superscript letter indicates significant difference (p < 0.05) among treatments.
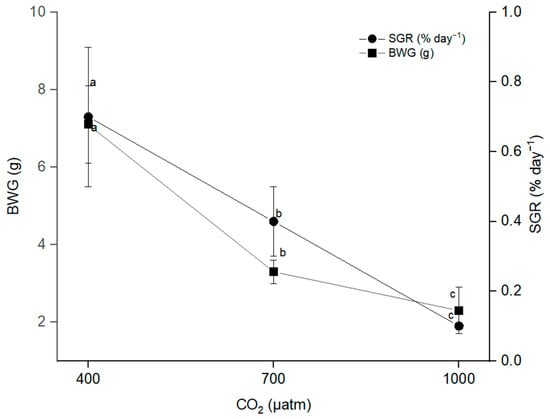
Figure 3.
Body weight gain (BWG) and specific growth rate (SGR) of mahseer in different CO2 conditions. Values are means from three groups of fish (n = 3). Superscript letter indicates significant differences in BWG and SGR in different pCO2 conditions. µ−1−1.
3.2. Vertebral Formation
The vertebral column was compared to the red, straight line in Figure 4 to illustrate the increasing angle of lordosis from 400 (Figure 4b) to 1000 µatm (Figure 4f). Lordosis is a term referring to the spine’s normal inward curvatures in the cervical and lumbar regions. At 400 µatm, a regular vertebral column was visible, as illustrated in Figure 4a,b. The percentages of skeletal abnormalities for each treated group are presented in Table 2. After 4 months of experimentation under 400 µatm, no skeletal deformities were identified in the fish. However, fish in 1000 µatm had the highest incidence of lordosis (13.33%), which was significantly higher than in the other groups. Lordosis followed by spinal fusion damaged the 11 to 20 vertebrae (Figure 4e,f). Those fish with lordosis demonstrated generally lethargic behaviour in the aquarium. Several of the fish merely laid on the bottom side and consumed food whenever it fell directly in front of their mouths. The results indicated that, as pCO2 concentrations rose, a few deformities became visible in the mahseer vertebral columns.
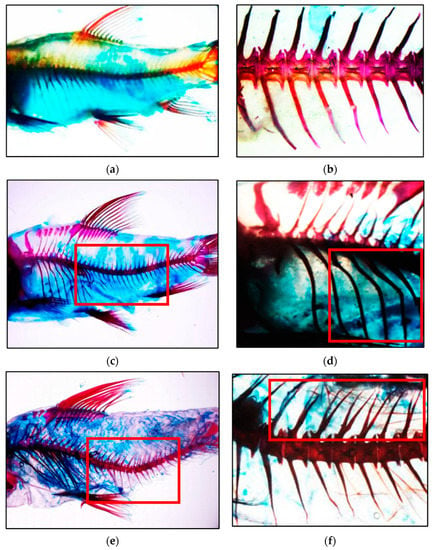
Figure 4.
Observation of vertebral column in mahseer: (a) normal spine in 400 µatm, (b) normal vertebral column in 400 µatm, (c) mild lordosis in 700 µatm, (d) deformed spine in 700 µatm, (e) severe lordosis and mild fusion in 1000 µatm, and (f) split spine in 1000 µatm.

Table 2.
Vertebral deformities occurrence in mahseer cultured at different levels of pCO2.
3.3. Blood Haematological and Biochemical Parameters
Table 3 shows the results of an experiment on the haematology and biochemistry of the mahseer fish exposed to different pCO2 levels. In comparison to the control fish, the fish exposed to acidic conditions (700 and 1000 µatm) had significantly higher haemoglobin levels. Haematocrit levels were also higher in the acidic conditions. Glucose levels were significantly lower in the acid-condition fishes than in the control group. The total cholesterol levels in mahseer fish exposed to acidic conditions were reported to be lower.

Table 3.
Blood haematological and biochemical parameters (mean ± standard deviation, SD) of mahseer during experimental period. Different superscript letters indicate significant difference between treatments.
4. Discussion
We demonstrated in this study that mahseer fish are susceptible to acidification conditions, as the survival rates were significantly reduced. Previous findings may imply that the mahseer fish’s internal pH is poorly controlled and maintained, resulting in a decrease in protein biosynthesis [25,26], for example, growth [27], as some studies have shown that silver carp Hypophthalmichthys molitrix, Nile Tilapia Oreochromis niloticus, rainbow trout Oncorhynchus mykiss, and pink salmon Oncorhynchus gorbuscha grow less well in elevated-pCO2 environments [28,29,30,31]. Others appear to suggest that fish, such as smallmouth bass Micropterus dolomieu, Arctic charr Salvelinus alpinus, and also rainbow trout Oncorhynchus mykiss [32,33,34], grow well in high-pCO2 environments. The decrease in growth rate under elevated pCO2 was most probably triggered by acid–base regulatory mechanisms which mitigate fluid and tissue acidification [35]. These acidosis buffering regulatory mechanisms typically involve the acquisition and retention of HCO3− from the surrounding water as accumulated H+ is excreted [36]. Along with buffering acidosis, most freshwater fish exhibit a physiological stress reaction in response to acidified water, which needs energy [33]. Thus, the energetic cost of supporting homeostasis in the presence of acidification can modify the amount of energy in freshwater fish, resulting in poor growth rates and body weight gain.
As blood is a particularly sensitive tissue to environmental changes, it is frequently included as an indicator for physiological health responses to endogenous and exogenous modifications in fish [37]. Mahseer’s haematological parameters revealed that they experience a significant effect in various pCO2 conditions. The present study’s findings indicate that a high pCO2 concentration decreases the values of haematological parameters such as haemoglobin and haematocrit, which is in agreement with Kaya et al. [38], who investigated the haematological characteristics of Mozambique tilapia Oreochromis mossambicus in high pCO2 (>400 µatm) concentrations. Sopinka et al. [39] suggested that Hct may be one of the most straightforward cardiovascular variables to investigate in fish. Hct was the most important indicator of Hb levels in their studies due to its high percent correlation coefficient. HCT values decrease as pCO2 concentration rises and Hb levels fall. Additionally, Hct values are an excellent indicator of fish health. Blood glucose levels have been widely used to determine the degree of stress in fish. According to Hoseini et al. [40], this variable may be notably essential for evaluating the level of stress in fish exposed to extreme conditions. The findings achieved here support the use of glucose as a marker of stress in an acidic environment. Vargas-Chacoff et al. [41] previously observed an increase in glucose values in Atlantic salmon Salmo salar exposed to moderately acidic water (>700 µatm). Blood total protein illustrates the organism’s state and the modifications that occur as a result of internal and external factors [34]. Thus, the impact of surrounding parameters on the total protein concentration has to be taken into account when assessing their response to extreme conditions and, as a result, their increasing energy demand [42]. The other biochemical parameters, such as cholesterol, were reported to be significantly higher than in the control group. It is well established that cholesterol contributes to higher total lipids. The surge in these energy stores in response to environmental stressors can be accounted for by the opinion that organisms require excess energy reserves (glucose and cholesterol) to mitigate extreme conditions. This is most likely because increasing the pCO2 causes stressful conditions which inhibit fish growth. Similar trends were observed as pCO2 had a significant negative effect on the Sagor catfish blood analysis of Hb, Hct, and glucose since fish weight gain fell correspondingly with the increase in pCO2 [43].
Acidification has a significant impact on the occurrence of vertebral skeletal malformations. The effect of acidification results in significantly higher levels of malformations. Vertebral column malformations increase significantly as the environment became more acidic. Additionally, other research has revealed a higher incidence of abnormalities in vertebral development in juvenile fish exposed to acidified water [37,44]. It has been suggested that axial deviations can occur during early development as a result of abnormal development of the notochord and perinotochordal connective sheet [45], which leads to abnormalities in the vertebral column such as lordosis. These deformities may impair the fish’s ability to balance their position in the surrounding water. Earlier published research indicated a decrease in the skeletal deformities of brook charr Salvelinus fontinalis [46], an increment in the skeletal deformities of gilthead seabream Sparus aurata [45], and no change in the skeletal deformities of little skate Leucoraja erinacea [44] when exposed to acidified conditions. One hypothesis that could account for the increased vertebral column deformation associated with increased pCO2 is the continuation of a trade-off between the skeletal structure and bone stiffness. While acidification accelerates the development of the skeleton, bone density reduces, allowing the skeletal structure to be more susceptible to developing deformities [7].
5. Conclusions
The rate of vertebral column malformations was accelerated by elevated pCO2, implying stress in mahseer fish blood haematological and biochemical parameters under elevated pCO2. Thus, the effect of acidification may result in significant physical impairment and, as a result, a decline in the fitness of mahseer juveniles. The response of mahseer juvenile fish described in the present investigation includes the perspective that future acidification may affect the progress of mahseer fish. Future research should take into account the investigation conducted at various stages of development, with additional analysis of the muscle and other skeletal segments (the jaw and operculum). Researchers should also consider key genes of growth with details on the influence of these environmental factors at higher organisational levels (e.g., population level) on commercially important fish species. These data can assist managers and policymakers in carrying out effective actions. With a variety of potential factors, including climatic change and human practices, increasing dissolved pCO2 in the freshwater environment, it is essential for scientists to initially realise the implications of freshwater acidification.
Author Contributions
Conceptualization, N.S.I. and S.K.D.; methodology, N.S.I., Z.C.C. and S.K.D.; software, N.S.I. and S.K.D.; validation, N.S.I., Z.C.C. and S.K.D.; formal analysis, N.S.I., N.M.N. and S.K.D.; investigation, N.S.I., Z.C.C. and S.K.D.; resources N.S.I. and S.K.D.; data curation, N.S.I., N.M.N., Z.C.C. and S.K.D.; writing—original draft preparation N.S.I., N.M.N., Z.C.C. and S.K.D.; writing—review and editing, N.S.I., N.M.N., Z.C.C. and S.K.D.; visualization, S.K.D.; supervision, Z.C.C. and S.K.D.; project administration, S.K.D.; funding acquisition, Z.C.C., N.M.N. and S.K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by UKM-Sime Darby Foundation Chair in Climate Change grant number ZF-2019-003 and GGPM-2022-073.
Institutional Review Board Statement
This research was approved by the Universiti Kebangsaan Malaysia on 27 July 2016. Approval code: FST/2016/SIMON/27-JULY/763-JULY-2016-MAY-2017.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to express our gratitude to the staff of UKM Wet Laboratory and Institute of Climate Change for their continuous assistance throughout the experimental period. This research was supported by UKM-Sime Darby Foundation Chair in Climate Change grant (project no.: ZF-2019-003), which was granted to the corresponding author.
Conflicts of Interest
The authors have declared that no conflict of interests exists.
References
- Parra, J.E.G.; Baldisserotto, B. Effect of water pH and hardness on survival and growth of freshwater teleosts. In Fish Osmoregulation; CRC Press: Boca Raton, FL, USA, 2019; pp. 135–150. [Google Scholar]
- Messina, S.; Costantini, D.; Eens, M. Impacts of rising temperatures and water acidification on the oxidative status and immune system of aquatic ectothermic vertebrates: A meta-analysis. Sci. Total Environ. 2023, 868, 161580. [Google Scholar] [CrossRef] [PubMed]
- Hasler, C.T.; Butman, D.; Jeffrey, J.D.; Suski, C.D. Freshwater biota and rising pCO2? Ecol. Lett. 2016, 19, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Walton, S.E.; Gan, H.M.; Raghavan, R.; Pinder, A.C.; Ahmad, A. Disentangling the taxonomy of the mahseers (Tor spp.) of Malaysia: An integrated approach using morphology, genetics and historical records. Rev. Fish. Sci. Aquac. 2017, 25, 171–183. [Google Scholar] [CrossRef]
- Brauner, C.J.; Shartau, R.B.; Damsgaard, C.; Esbaugh, A.J.; Wilson, R.W.; Grosell, M. Acid-base physiology and CO2 homeostasis: Regulation and compensation in response to elevated environmental CO2. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2019; Volume 37, pp. 69–132. [Google Scholar]
- Jaafar, F.; Na-Nakorn, U.; Srisapoome, P.; Amornsakun, T.; Duong, T.Y.; Gonzales-Plasus, M.M.; Parhar, I.S. A current update on the distribution, morphological features, and genetic identity of the Southeast Asian mahseers, Tor species. Biology 2021, 10, 286. [Google Scholar] [CrossRef]
- Lopes, I.G.; Araújo-Dairiki, T.B.; Kojima, J.T.; Val, A.L.; Portella, M.C. Predicted 2100 climate scenarios affects growth and skeletal development of tambaqui (Colossoma macropomum) larvae. Ecol. Evol. 2018, 8, 10039–10048. [Google Scholar] [CrossRef]
- Dhakal, A.; Pandey, M.; Kayastha, P.; Suwal, G.; Suwal, B. An Overview of Status and Development Trend of Aquaculture and Fisheries in Nepal. Adv. Agric. 2022, 2022, 4206401. [Google Scholar] [CrossRef]
- Stiasny, M.H.; Sswat, M.; Mittermayer, F.H.; Falk-Petersen, I.B.; Schnell, N.K.; Puvanendran, V.; Clemmesen, C. Divergent responses of Atlantic cod to ocean acidification and food limitation. Glob. Chang. Biol. 2019, 25, 839–849. [Google Scholar] [CrossRef]
- Jesus, T.F.; Rosa, I.C.; Repolho, T.; Lopes, A.R.; Pimentel, M.S.; Almeida-Val, V.M.; Rosa, R. Different ecophysiological responses of freshwater fish to warming and acidification. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 216, 34–41. [Google Scholar] [CrossRef]
- Majeed, Z.; Ajab, Z.; Zuberi, A.; Akthar, S.; Muhammad, A. Meristic variations and skeletal deformities in natural population of mahseer fish, Tor putitora (Hamilton, 1822). Iran. J. Fish. Sci. 2018, 17, 208–216. [Google Scholar]
- Mazumder, S.K.; Das, S.K.; Bakar, Y.; Ghaffar, M.A. Effects of temperature and diet on length-weight relationship and condition factor of the juvenile Malabar blood snapper (Lutjanus malabaricus Bloch & Schneider, 1801). J. Zhejiang Univ. Sci. B 2016, 17, 580–590. [Google Scholar]
- Noor, N.M.; De, M.; Cob, Z.C.; Das, S.K. Welfare of scaleless fish, Sagor catfish (Hexanematichthys sagor) juveniles under different carbon dioxide concentrations. Aquac. Res. 2021, 52, 2980–2987. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.R.; Kim, S.K.; Kang, H.W. Effects of pH changes on blood physiology, antioxidant responses and Ig M of juvenile olive flounder, Paralichthys olivaceus. Aquac. Rep. 2021, 21, 100790. [Google Scholar] [CrossRef]
- Cantonati, M.; Poikane, S.; Pringle, C.M.; Stevens, L.E.; Turak, E.; Heino, J.; Znachor, P. Characteristics, main impacts, and stewardship of natural and artificial freshwater environments: Consequences for biodiversity conservation. Water 2020, 12, 260. [Google Scholar] [CrossRef]
- Greaver, T.L.; Clark, C.M.; Compton, J.E.; Vallano, D.; Talhelm, A.F.; Weaver, C.P.; Haeuber, R.A. Key ecological responses to nitrogen are altered by climate change. Nat. Clim. Chang. 2016, 6, 836–843. [Google Scholar] [CrossRef]
- Weiss, L.C.; Pötter, L.; Steiger, A.; Kruppert, S.; Frost, U.; Tollrian, R. Rising pCO2 in freshwater ecosystems has the potential to negatively affect predator-induced defenses in Daphnia. Curr. Biol. 2018, 28, 327–332. [Google Scholar] [CrossRef]
- De, M.; Ghaffar, M.A.; Bakar, Y.; Das, S.K. Effect of temperature and diet on growth and gastric emptying time of the hybrid, Epinephelus fuscoguttatus × E. lanceolatus. Aquac. Rep. 2016, 4, 118–124. [Google Scholar] [CrossRef]
- Das, S.K.; Noor, N.M.; Kai, K.S.; Juan, Q.Z.; Iskandar, N.S.M.; De, M. Effects of temperature on the growth, gastric emptying time, and oxygen consumption rate of mahseer (Tor tambroides) under laboratory conditions. Aquac. Rep. 2018, 12, 20–24. [Google Scholar] [CrossRef]
- IPCC. AR6 Climate Change 2021: Impacts, Adaptation and Vulnerability—IPCC. 2021. Available online: https://www.ipcc.ch/report/sixth-assessment-report-working-group-ii/ (accessed on 28 December 2022).
- Phillips, J.C.; McKinley, G.A.; Bennington, V.; Bootsma, H.A.; Pilcher, D.J.; Sterner, R.W.; Urban, N.R. The potential for CO2-induced acidification in freshwater: A Great Lakes case study. Oceanography 2015, 28, 136–145. [Google Scholar] [CrossRef]
- Kortazar, L.; Duval, B.; Liñero, O.; Olamendi, O.; Angulo, A.; Amouroux, D.; Fernandez, L.A. Accurate determination of the total alkalinity and the CO2 system parameters in high-altitude lakes from the Western Pyrenees (France–Spain). Microchem. J. 2020, 152, 104345. [Google Scholar] [CrossRef]
- Gupta, N.; Nigar, S. Detection of Blood Parasites and Estimation of Hematological Indices in Fish. In Experimental Protocols in Biotechnology; Humana: New York, NY, USA, 2020; pp. 43–73. [Google Scholar]
- Hoseini, S.M.; Mirghaed, A.T.; Iri, Y.; Ghelichpour, M. Effects of dietary cineole administration on growth performance, hematological and biochemical parameters of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 495, 766–772. [Google Scholar] [CrossRef]
- Kunwar, P.S.; Sinha, A.K.; De Boeck, G.; Sapkota, K. Modulations of blood biochemical parameters of golden mahseer, Tor putitora following exposures to single and mixed organophosphate. Comparat. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 251, 109207. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.; Howes, E.L.; Lacoue-Labarthe, T.; Forêt, S.; Hanna, B.; Medina, M.; Gattuso, J.P. Near-future pH conditions severely impact calcification, metabolism and the nervous system in the pteropod Heliconoides inflatus. Glob. Chang. Biol. 2016, 22, 3888–3900. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, J.D.; Hannan, K.D.; Hasler, C.T.; Suski, C.D. Responses to elevated CO2 exposure in a freshwater mussel, Fusconaia flava. J. Comparat. Physiol. B 2017, 187, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Tix, J.A.; Hasler, C.T.; Sullivan, C.; Jeffrey, J.D.; Suski, C.D. Elevated carbon dioxide has the potential to impact alarm cue responses in some freshwater fishes. Aquat. Ecol. 2017, 51, 59–72. [Google Scholar] [CrossRef]
- Mustapha, M.K.; Atolagbe, S.D. Tolerance level of different life stages of Nile tilapia Oreochromis niloticus (Linnaeus, 1758) to low pH and acidified waters. J. Basic Appl. Zool. 2018, 79, 46–54. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, Y.; Hu, X.; Yang, X.; Xu, S. Energy metabolism responses in muscle tissue of rainbow trout Oncorhynchus mykiss fry to CO2-induced aquatic acidification based on metabolomics. Aquatic Toxicol. 2020, 220, 105400. [Google Scholar] [CrossRef]
- Ou, M.; Hamilton, T.J.; Eom, J.; Lyall, E.M.; Gallup, J.; Jiang, A. Responses of pink salmon to CO2-induced aquatic acidification. Nat. Clim. Chang. 2015, 5, 950–955. [Google Scholar] [CrossRef]
- Heino, J.; Virkkala, R.; Toivonen, H. Climate change and freshwater biodiversity: Detected patterns, future trends and adaptations in northern regions. Biol. Rev. 2009, 84, 39–54. [Google Scholar] [CrossRef]
- Wrona, F.J.; Prowse, T.D.; Reist, J.D.; Hobbie, J.E.; Lévesque, L.M.; Vincent, W.F. Climate change effects on aquatic biota, ecosystem structure and function. AMBIO 2006, 35, 359–369. [Google Scholar] [CrossRef]
- McMillan, J.R.; Katz, S.L.; Pess, G.R. Observational evidence of spatial and temporal structure in a sympatric anadromous (winter steelhead) and resident rainbow trout mating system on the Olympic Peninsula, Washington. Trans. Am. Fish. Soc. 2007, 136, 736–748. [Google Scholar] [CrossRef]
- Munday, P.L.; Watson, S.A.; Parsons, D.M.; King, A.; Barr, N.G.; Mcleod, I.M.; Pether, S.M. Effects of elevated CO2 on early life history development of the yellowtail kingfish, Seriola lalandi, a large pelagic fish. ICES J. Mar. Sci. 2016, 73, 641–649. [Google Scholar] [CrossRef]
- Noor, N.M.; De, M.; Iskandar, A.; Keng, W.L.; Cob, Z.C.; Ghaffar, M.A.; Das, S.K. Effects of elevated carbon dioxide on the growth and welfare of Juvenile tiger grouper (Epinephelus fuscoguttatus) × giant grouper (E. lanceolatus) hybrid. Aquaculture 2019, 513, 734448. [Google Scholar] [CrossRef]
- Noor, N.M.; Das, S.K. Effects of elevated carbon dioxide on marine ecosystem and associated fishes. Thalassas 2019, 35, 421–429. [Google Scholar] [CrossRef]
- Wood, C.M. Internal spatial and temporal CO2 dynamics: Fasting, feeding, drinking, and the alkaline tide. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2019; Volume 37, pp. 245–286. [Google Scholar]
- Mazumder, S.K.; Das, S.K.; Rahim, S.M.; Abd Ghaffar, M. Temperature and diet effect on the pepsin enzyme activities, digestive somatic index and relative gut length of Malabar blood snapper (Lutjanus malabaricus Bloch & Schneider, 1801). Aquac. Rep. 2018, 9, 1–9. [Google Scholar]
- Kaya, H.; Hisar, O.; Yılmaz, S.; Gürkan, M.; Hisar, Ş.A. The effects of elevated carbon dioxide and temperature levels on tilapia (Oreochromis mossambicus): Respiratory enzymes, blood pH and hematological parameters. Environ. Toxicol. Pharmacol. 2016, 44, 114–119. [Google Scholar] [CrossRef]
- Sopinka, N.M.; Donaldson, M.R.; O’Connor, C.M.; Suski, C.D.; Cooke, S.J. Stress indicators in fish. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 405–462. [Google Scholar]
- Hoseini, S.M.; Mirghaed, A.T.; Mazandarani, M.; Zoheiri, F. Serum cortisol, glucose, thyroid hormones’ and non-specific immune responses of Persian sturgeon, Acipenser persicus to exogenous tryptophan and acute stress. Aquaculture 2016, 462, 17–23. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Regish, A.M.; Weinstock, A.; McCormick, S.D. Effects of elevated temperature on osmoregulation and stress responses in Atlantic salmon Salmo salar smolts in fresh water and seawater. J. Fish Biol. 2018, 93, 550–559. [Google Scholar] [CrossRef]
- Martens, L.G.; Witten, P.E.; Fivelstad, S.; Huysseune, A.; Sævareid, B.; Vikeså, V.; Obach, A. Impact of high water carbon dioxide levels on Atlantic salmon smolts (Salmo salar L.): Effects on fish performance, vertebrae composition and structure. Aquaculture 2006, 261, 80–88. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Yu, G.; Yang, C.; Shan, B.; Liu, S.; Sun, D. Osteological development of the vertebral column and caudal complex in larval and juvenile blackhead seabream, Acanthopagrus schlegelii (Perciformes, Sparidae) (Bleeker, 1854). Pak. J. Zool. 2019, 51, 1859. [Google Scholar] [CrossRef]
- Crespel, A.; Dupont-Prinet, A.; Bernatchez, L.; Claireaux, G.; Tremblay, R.; Audet, C. Divergence in physiological factors affecting swimming performance between anadromous and resident populations of brook charr Salvelinus fontinalis. J. Fish Biol. 2017, 90, 2170–2193. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).