Abstract
Aquaculture struggles with sustainability due to the use of fishmeal, and insects are one potential alternative. Insects have low long-chain omega−3 polyunsaturated fatty acid content, and insect-fed fish reflect this in their composition. In total, 500 rainbow trout (Oncorhynchus mykiss, ~46 g) were fed until the fish reached a commercial size (~415 g). Five diets were used: one control based on fishmeal as main source of protein; two with a 50% replacement of fishmeal with yellow mealworm meal (Tenebrio molitor), one with full-fat insect, and another partially defatted; two other diets similar to the one with the full-fat insect, but with the addition of an experimental algal oil rich in omega−3 in two different concentrations (one equivalent to control, the other one to the defatted insect diet). Growth was unaffected, as well as texture and organoleptic profile of the fillets. Lightness, brightness and colour of the fillets were slightly modified by the experimental diets. An increase in omega−3 levels over those of the full-fat insect diet is described. An omega−3 sparing effect was highlighted, causing lipid accumulation in fillets and liver, and a mild increase in oxidative damage. More research is encouraged to evaluate the fatty acid profile of the liver.
Keywords:
aquaculture; fishmeal replacement; insect meal; nutrition; fillet quality; liver intermediary metabolism; liver antioxidant status Key Contribution:
The current manuscript provides further insight on the so called “omega−3 sparing effect” of fish, showing different repercussions on the fatty acid profile of the fillets after trying different dietary combinations. Additionally, some details concerning the repercussions of these same diets and the defatting process of the insect meal over the quality of the final product, liver physiology and fish welfare indicators are given.
1. Introduction
Aquaculture is one of the fastest developing sectors related to food production [1]. Even though the growth ratios of fish make their farming more efficient than most conventional land-based animal species, fish need higher amounts of protein to satisfy their nutritional needs in the short term [2]. The biggest part of these protein requirements has been fulfilled traditionally through the use of fishmeal (FM). Since this ingredient comes mostly from extractive fishing practices its use is considered as not sustainable [3].
The replacement of FM is a complicated task, because it is known that reducing its inclusion below certain thresholds causes a reduction in fish growth [4]. It is also known that even complex diets with different sources of protein can suffer from a similar problem [5], which is why it is still important to search for other protein alternatives. A good counterpart is that this problem has been known and addressed for decades now and the usage of protein alternatives to FM in fish feedings is a reality nowadays. However, this has led to other secondary problems directly related to these alternatives. The most typical case is that of plant-based ingredients like soybean meal, which are known for having antinutrients in their composition [6] and for disrupting a correct and healthy status of the fish gastrointestinal tract [7,8,9]. Even though many of these consequences are now very well known, which allows the use of these ingredients working around their disadvantages [10,11], the search and study of other sources of protein is still a good way to go for aquaculture research in order to offer a higher diversity of ingredients.
Insect meals (IMs) appear as one of the most promising of these alternatives. The production of insects on an industrial scale can be environmentally friendly due to their feed and growth ratios, the high speed of their reproduction cycles, and the high adaptability of many of these animals to different feeding substrates [12,13]. Once they are processed and turned into ingredients, insects count with other advantages such as the good quality of their protein [14,15]. Insects have already proven their efficiency in several rainbow trout (Oncorhynchus mykiss) nutrition trials, either as full-fat [16,17,18] or defatted ingredients [19,20,21]. Additionally, it seems to be possible to use them as functional ingredients [22,23,24]. However, most terrestrial insects have an extremely low proportion of long-chain omega−3 polyunsaturated fatty acids (LC n−3 PUFA) within their composition [14], an important detail that is reflected in the fatty acid (FA) profile of the fillets of insect-fed fish [17,25,26,27,28]. This is why the big fat fraction found in full-fat IMs acts as a limiting factor for aquafeed formulations, making the use of defatted IMs more attractive. Depending on the situation, this might not be such a big concern. It is known that, even after this reduction in LC n−3 PUFA in the fish fillets, their fish lipid quality indices can stay between reasonable, healthy levels [29,30]. Nevertheless, one of the strongest points of fish as a component of a balanced diet is its FA profile, so the possibility of worsening it using novel ingredients should not be left unchecked.
This problem concerning insects as an ingredient for fish feeding has already been addressed through different strategies. The most typical among them is to perform a defatting process on the IM to reduce the amount of insect fat in the feed, which is a widespread practice in current studies [31,32,33]. Another possibility is to make use of the already mentioned ability of insects to use different feeding substrates, as well as the capacity to modify their internal composition according to these substrates. “Enriched” IMs come from insects fed on substrates rich in LC n−3 PUFA, such as fish by-products [34,35,36,37]. Last but not least, there is also the possibility of simply readjusting the feed formula to modify the lipid sources, using ingredients with a higher concentration of essential LC n−3 PUFA such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) [38]. In this sense algal oil obtained from Schizochytrium sp. rich in EPA and DHA has been successful used as source of LC n−3 PUFA in rainbow trout [39].
As for many other changes in the diet, and as for any other novel ingredient, different interactions and changes in physiology should be expected and not underestimated. As previously stated, IMs may act similar to functional ingredients due to their proven capacity to boost the antioxidant and the immunological systems [40,41,42,43,44]. Following the line of previous research [17,18], the present study tried to unify as many of the mentioned ideas as possible. Two different strategies were used to solve the n−3 problem of insect-fed rainbow trout grown up to commercial size: (I) reducing the insect fat proportion; (II) adding an ingredient rich in LC n−3 PUFA. Several variables were measured to follow the possible repercussions of these diets, from pure physiological parameters such as growth, liver physiology and antioxidant analyses, to indirect effects such as the composition of the fillet and the perception of the final product.
2. Materials and Methods
2.1. Experimental Diets
The dried larval stage of yellow mealworm (Tenebrio molitor; TM) was used for the diets of this study in the form of processed IMs (Table 1). Five isoproteic (≈48.9%) and isolipidic (≈18.5%) diets were formulated with the following principles: a control diet (C) with no IM based on FM as the main source of protein; one diet with a 50% FM replacement using full fat TM meal (T; 18% diet inclusion); one diet similar to the previous one, but using defatted TM meal (dT; 18% diet inclusion); two diets similar to T, but with an increasing level of fish oil replacement with an experimental algal oil (EO, proceeding from a culture of Schizochytrium sp.; the supplier decided to remain anonymous) containing 15.7% of EPA and 39.8% of DHA. The inclusion of EO in these diets was 3.09% for TO1 diet and 7.24% for TO2 diet. The concentration of the EO was adjusted to match the theoretical concentration of EPA and DHA, being TO1 equivalent to dT, and TO2 equivalent to C (Table 2a). Diets were enriched with methionine and lysine to satisfy the requirements of the fish [45,46], were manufactured by the Experimental Diets Service of the University of Almería (Almería, Spain) and extruded as pellets of 3 and 4 mm for the different stages of fish. The dough was passed through a two-screw laboratory extruder (Evolum 25, Clextral, France). The extruder barrel had four sections, with a temperature per section of (from inlet to outlet) 100 °C, 95 °C, 90 °C and 85 °C, respectively. Pellets were kept in a drying chamber at 30 °C for 24 h (Airfrio, Pechina, Spain) and stored in sealed plastic bags at −20 °C until they were used.

Table 1.
Proximate and amino acids compositions of fishmeal and insect meals.

Table 2.
(a). Formulation and proximate composition of experimental diets. (b) Amino acid and mineral composition of experimental diets. (c). Fatty acid composition of experimental diets.
2.2. Experimental Animals and Rearing Conditions
The private company Mundova (Albacete, Spain) provided rainbow trout (Oncorhynchus mykiss) eggs to the experimental facilities of the Aquaculture Research Centre of “Instituto Tecnológico Agrario de Castilla y León” (ITACyL), where they were hatched and reared until they were ready for the experiment. Afterwards, a total of 500 female triploid fish were allocated into 20 cylindrical fiber-glass tanks (500 L; four replicates per treatment; 25 fish per tank) in a recirculating system, and stayed in acclimation during three weeks before the beginning of the growth trial (initial body weight of 46.1 ± 0.1 g). The recirculating system was maintained under controlled conditions as follows: water temperature of 14.8 ± 0.7 °C, water dissolved oxygen of 7.8 ± 0.7 mg/L (87% saturation), room photoperiod of 12 h light: 12 h dark, ammonia < 0.1 mg/L and nitrite < 0.1 mg/L (daily analyses).
2.3. Growth Trial and Sample Collection
The growth trial lasted 89 days. Fish were fed daily and by hand (9 a.m.) until apparent satiation was reached (3% tank biomass as maximum feed intake). Feed intake and mortality were monitored daily. A biometry procedure was carried out every 21 days to measure and weigh the fish after being fasted for one day, using a scale (±0.1 g) and a graduated icthyometer (±0.1 mm), and being previously anesthetized with tricaine methanesulfonate (MS-222; 80 mg/L).
After the end of the feeding trial, all fish were weighed and measured to calculate growth. The rest of the sampling procedure was scheduled to take place during the course of a week. Two fish per tank were sampled to take blood for fish welfare indicators, liver for histomorphology, intermediary metabolism, and antioxidant status, and complete fillet samples for proximate and fatty acid composition, and for instrumental analyses of texture and colour. All fish were chosen at random from the tanks and sacrificed by an overdose of MS-222 (300 mg/L). One exception was made for the previously mentioned due to logistic reasons: instead of two fish per tank, four fish per treatment were taken for sensorial analyses of whole raw fish and fillets, which was performed in the following two hours, and other four fish per treatment for cooked fillets analysis. The fish meant for cooked fillet analysis were sacrificed according to AENOR UNE 173300:2016 by beheading, previously stunned by live chilling [47], and their fillets were kept at −20 °C until the analysis was performed. Blood samples (1 mL) were collected with pre-heparinised syringes for centrifugation (6600× g force, 4 °C, 20 min) and plasma extraction. Plasma samples were kept at −80 °C in Eppendorf tubes until their analyses. A thin slice (~0.2 cm width) was cut from each liver sample for histomorphology. They were fixed in 4% buffered formalin for 48 h before dehydration and processing. The rest of the liver samples were kept in liquid nitrogen during the sampling procedure for enzyme determinations, and frozen at −80 °C until their individual analyses.
The Directive of the European Union Council and the Spanish Government [48,49] was followed for the care and handling of the fish. The Bioethical Committee of “ITACyL” approved this experiment (Authorization number: 2017/19/CEEA).
2.4. Final Product Quality
2.4.1. Instrumental Texture and Colour
Samples were thawed overnight at 5 °C. Texture was analysed using a TA-XT2i Texture Analyzer (Stable Micro Systems, Spain) equipped with a 5 kg load cell and two different probes, one cylindrical (2 mm diameter) for a brittleness test, and one spherical (2.5 cm diameter) for a complete texture profile analysis (TPA). Texture parameters were determined as described by Rosenthal [50] in such conditions as at pre-test speed 8 mm/s, test speed 1 mm/s, post-test speed 10 mm/s, distance of 5 mm, trigger type 1 g, time 5 s. A Chroma Meter CR 400 (Minolta, Spain) was used for colour determination, measuring 10 random points per fillet that were expressed as CIELab coordinates: lightness (L*) is expressed on a 0–100 scale of black to white; scale of red (+) or green (−) colour, expressed as redness (a*); scale of yellow (+) or blue (−) colour, expressed as yellowness (b*); chroma value (C*ab = (a*2 + b*2)1/2) as an expression of the intensity and clarity of the colour; hue (H°ab = arctan (b*/a*)) is the name of a colour as it is found in its pure state on the spectrum, and is expressed in degrees°, ranging from 0° (red) to 90° (yellow), 180° (green), 270° (blue) and back to 0° [51].
2.4.2. Direct Sensorial Analyses
The sensorial evaluation of raw rainbow trout was interpreted in two stages.
The first one, for raw fish and fillets, was analysed by thirteen panellists as was previously described by Tomás-Almenar et al. [52], on whole raw fish and on raw fish fillets in four fish per diet (two for whole raw fish, and two for raw fish fillets). Briefly, for the sensorial assessment of raw fish fillets, a quantitative descriptive analysis of four attributes (acceptability, colour, texture and odour) was performed using an equal-interval scale, where the panellists had to place a vertical mark across a 10 cm horizontal line. Whole fish were evaluated for external characteristics using a scoring system similar to the quality index method (QI), including 9 parameters related to 3 main attributes (general appearance, eyes and gills), and the total score for all parameters (QI from 0 to 20) was used as another attribute to evaluate the perception of the panellists. For acceptability, the scale went from 1 (acceptable) to 10 (non-acceptable); for colour, from 1 (white) to 10 (dark brown); for texture, from 1 (elastic) to 10 (flaccid); for odour, from 1 (fresh) to 10 (rotten). For QI (0 to 20), lower scores meant better levels of freshness.
Fillets of four fish per diet were used for cooked fillet analyses. For this procedure, the dorsal fillet was used as a standard. Dorsal fillets were cooked in an oven at 130 °C for 20 min. The most cranial and caudal sections of the fillets were discarded before cutting the fillets in a total of three individual portions. After proper labelling, the different portions were given to eight trained panellists, making sure that all of them could analyse a minimum of two different samples from each treatment. The variables analysed are described in Results and Discussion section. Informed consent was obtained from all panellists involved in the study.
2.5. Analytical Determinations
2.5.1. Chemical Analyses
The moisture and fat content of both IMs, the five diets and fish fillets were analysed following AOAC methods [53], while a modified Dumas method [54] was used to determine their crude protein content, using a nitrogen analyser (FP 528, LECO, St. Joseph, MO, USA). The conversion factors for protein analyses were 6.25 for feeds, and 4.75 for both TM meals [55]. Chitin was isolated from IMs, washed with acetone, dried and weighed as described by the method of Gamage and Shahidi [56]. Samples of TM meal and defatted TM meal were hydrolysed at 110 °C for 22 h, with 6 N HCl [14]. Ion-exchange liquid chromatography and postcolumn continuous reaction with ninhydrin (Biochrom 30; United Kingdom) was used for the amino acid determination. Tryptophan was not determined. For the mineral composition of feeds, the pellets were prepared using a Mignon SS hydraulic press (Nannetti, Italy), and the quantification of elements was carried out with an X-ray fluorescence equipment (Bruker S4 Pioneer, Bruker, Spain), through wavelength dispersion; results were analysed with the Plus EVALUATION program of the Spectra plus package (Bruker, Spain). For the case of fillets, calcium was determined with X-ray fluorescence method of Dispersive Energy, as described by Pessoa et al. [57], and phosphorus determination was carried out by molecular absorption spectrophotometry (UV/Vis UV2, UNICAM, Cambridge, UK) according to ISO standards [58].
2.5.2. Fatty Acid Determination
The FA profile of diets and fish fillets was analysed following the method described in previous studies [59,60]. After a direct derivatization to FA methyl esters (FAMEs), and as described by Guil-Guerrero et al. [61] these FAMEs were analysed in a chromatograph Agilent Technologies 6880 N (Santa Clara, CA, USA) equipped with a flame ionisation detector and an Omegawax capillary column (30 m × 0.25 mm i.d. × 0.25 μm film thickness; Supelco, Bellefonte, PA, USA). The retention times of known FAME standards (PUFAs No. 1, 47,033 from Sigma, St. Louis, MO, USA) were used to identify the peaks of the analysed FAMEs, and methyl nonadecanoate (19:0; 98.0% purity; 74,208 Fluka; Sigma, St. Louis, MO, USA) was used as internal standard to estimate the FA contents. The method described by Cladis [62] was followed to estimate the relative retention factors of each FA. The peroxidability index was calculated as described by García-Márquez et al. [63].
2.5.3. Liver Intermediary Metabolism
Nine volumes of icecold 100 mM Tris-HCl buffer pH 7.8 containing 0.1 mM EDTA and 1 g/kg (v/v) Triton X-100 were used to individually homogenize liver samples, on ice. Samples were then centrifuged for 30 min at 30,000× g (4 °C). Aliquots were separated from the supernatants and kept at −80 °C for the following enzyme assays. The enzymatic activity of fructose 1,6-bisphosphatase (FBPase; EC 3.1.3.11), pyruvate kinase (PK, EC 2.7.1.40), glutamate pyruvate transaminase (GPT; EC 2.6.1.2), glutamate oxaloacetate transaminase (GOT; EC 2.6.1.1), and glutamate dehydrogenase (GDH; EC 1.4.1.2) was measured following the method of Furné et al. [64], at 25 °C, monitoring the changes in absorbance with a PowerWaveX micro-plate scanning spectrophotometer (Bio-Tek Instruments, Winooski, VT, USA). The method described by Bradford [65] was followed to quantify the concentration of soluble protein in tissue homogenates, using as standard bovine serum albumin.
2.5.4. Liver Antioxidant Status and Fish Welfare Indicators
In order to evaluate the antioxidant status of the fish, and following the method of Pérez-Jiménez et al. [66], the activity of superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6), glutathione peroxidase (GPx, EC 1.11.1.9), glutathione reductase (GR, EC 1.6.4.2), and glucose-6-phosphate dehydrogenase (G6PDH; EC 1.1.1.49) were analysed monitoring the absorbance changes with a PowerWaveX microplate scanning spectrophotometer (Bio-Tek Instruments, Winooski, VT, USA). The optimal substrate and protein concentrations for the measurement of maximal activity for each enzyme were established by preliminary assays. For NADH/NADPH, DTNB, and H2O2, the millimolar extinction coefficients were, respectively, 6.22, 13.6 and 0.039/mM·cm. For SOD activity, one unit was defined as the amount of enzyme necessary to inhibit half of the ferricytochrome C reduction rate. For the rest of the enzymes, one unit of activity was defined as the amount of enzyme needed to transform 1 mol of substrate in one minute. All analyses were carried out at 25 °C. To complete the redox status analysis, the level of lipid peroxidation was also measured through the quantification of malondialdehyde (MDA). Thiobarbituric acid reacts in the presence of MDA, producing coloured thiobarbituric acid reacting substances.
Commercial kits were used to analyse glucose and triglyceride levels in plasma, following the manufacturer instructions (Glucose-TR, ref. 41011; Triglycerides-LQ, ref. 41030, Spinreact, Spain), and the absorbance was measured in 96-well microplates with a microplate reader (ELx800TM; Bio-Tek Instruments, Winooski, VT, USA).
2.6. Histomorphology
2.6.1. Samples Processing
Increasing ethanol solutions (25, 50, 75 and 100%) were used to dehydrate the fixed samples, before embedding them in synthetic paraffin. A rotary microtome (FINESSE ME+ Thermo Scientific, Waltham, MA, USA) was used to obtain histological sections (3–4 µm). Hematoxylin and eosin technique was used to process the samples before the light microscopy histomorphology analyses. Graded objective lenses were used to evaluate five random regions per tissue sample, with an Olympus CX31 microscope and an Olympus EP50 microscope camera (Olympus, Barcelona, Spain).
2.6.2. Liver Histomorphology Analysis
The following protocol was the same described in Melenchón et al. [18]. Briefly, hepatocyte nucleus and cytoplasm widths were measured as quantitative variables, while the levels of hepatocyte intranuclear vacuolization and liver inflammation (inflammation and necrosis focuses) were evaluated as qualitative variables. In order to reduce a potential tilting effect of the samples, 5 measures were taken from each sample, leading to a total of 40 measures per treatment.
2.7. Statistical Analyses
Statistical analyses were carried out with the open-source programming tool R [67] making use of Rstudio interface [68]. The analysis of variance (ANOVA) was performed to determine the effect of the diet, and the multiple comparison of the means was performed by Tukey test being statistically different at p-Value < 0.05. Values are shown as mean ± standard error of the mean. Figures were created with Microsoft Excel 2019.
3. Results and Discussion
3.1. Growth Performance
There were no differences in growth performance among the assayed dietary treatments (Table 3).

Table 3.
Growth performance, protein utilization, and biometric indices in rainbow trout fed experimental diets.
In most cases, it is well accepted that IMs do not interfere with fish growth when their inclusion in the diet is not too high [27,69], especially for the case of salmonids, which seem to have good performances with these kind of diets [70]. For rainbow trout (Oncorhynchus mykiss), our own previous studies [17,18], together with what can be found in the bibliography [16,20,71] prove that using TM as an alternative source of protein offers, in general, adequate results when it comes to comparing growth, showing feed conversion ratios that move from 0.75 to 1.2. There are, however, some minor discrepancies to this, such as the case of meagre (Argyrosomus regius), a species whose growth seems to struggle frequently with the use of IMs [72,73,74]. Studies that described better growth performances with the inclusion of IMs for other fish species are also reported [75,76,77]. Due to the high number of variables involved, such as the fish and insect species, the nutritional quality of the IMs, the different strains of the experimental animals, or even abiotic factors such as water temperature and oxygen saturation, it is uneasy to give a definite conclusion of why this happens. However, and taking a broader scope on the topic, the idea that low to mid-levels of FM replacement with IMs do not impair fish growth is quite solid nowadays.
3.2. Final Product Quality
3.2.1. Fillet Proximate and Fatty Acid Composition
The proximate composition of the fillets suffered a significant change in the levels of fat, and a compensatory change in moisture (Table 4). The levels of fat were higher for T and TO1 than for C, while dT and TO2 stayed within intermediate levels. The most probable cause for this is that the presence of insect fat interfered with the lipid deposition in muscle, as well as in liver (discussed further at a later point). The deposition of lipids in the muscle of insect-fed fish generates an intense controversy within the scientific literature, as there have been cases of increases [78,79,80], decreases [35,81,82], and no changes [42,83,84] for this variable. It is known that fish physiology counts with different metabolic “sparing effects”, such as the omega−3 (n−3) sparing effect that happens when there are enough monounsaturated fatty acids (MUFA) and saturated fatty acids (SFA) in the diet [85]. Diets based on TM were rich in oleic and linoleic acid (Table 2c), so it is possible that the fish used these FAs as preferential sources of energy, while other FAs tended to accumulate in different tissues such as muscle and liver. Continuing with this idea, it has been reported that the metabolism of fish is less efficient when processing SFA and MUFA than PUFA [86,87]. It would be reasonable to assume that this slower consumption of FAs would produce a temporary surplus of lipids that could accumulate in tissues such as muscle. This would also make sense with the intermediate result in dT, because the TM meal used for this diet was partially defatted. This could have allowed the fish to still use a small amount of insect fat as a preferential source of energy. Cases similar to this one have already been described, where the addition of alternative and different sources of fat led to its accumulation in organs such as muscle or liver, or an increase in whole body fat [88,89,90].

Table 4.
Effect of dietary treatments on proximate composition of rainbow trout raw fillets.
The FAs profile of the fillets (Table 5) underwent changes that mostly agreed with the composition of the diets (Table 2c). The proportion of SFA (myristic, palmitic and stearic acids) was reduced in most experimental diets. Diets rich on insect fat went up on oleic (T diet) and linoleic (T, TO1 and TO2) acids. Some among the main LC n−3 PUFA, EPA, docosapentaenoic acid (DPA) and DHA suffered decreases in T diet, whereas these decreases did not happen in the cases of dT, TO1 and TO2. It is known that the fat of terrestrial insects has a very different FA composition than that of fish, especially regarding MUFA such as oleic acid, and LC n−3 PUFA such as EPA and DHA [14]. For this reason, it is not surprising that T diet had the highest levels of oleic acid and the lowest levels of LC n−3 PUFA. Actually, this is a point strongly supported by several studies [17,25,26,27,28], and is possibly the first nutritional drawback of using insects as ingredients for fish. Two strategies were followed in the present experiment to solve this problem: lowering the contribution of insects to the overall fat of the diet with a partially defatted IM (dT diet), and supplementing other diets with EO (diets TO1 and TO2). In all three cases, indeed, the problem was solved. However, this problem was not only solved but overcompensated in some cases, such as the case of an increase in EPA and DPA in treatments TO1 and TO2, when comparing with C. Turchini et al. [85] described that the metabolism of Murray cod (Maccullochella peelii) has an n−3 sparing effect mechanism, which tends to prioritise particular FAs for energy production, especially MUFA and, to a lesser extent, SFA. TO1 and TO2 were diets with more diverse sources of fat than the other three, so it is possible that this n−3 sparing effect made the production of energy more efficient, allowing a surplus of LC n−3 PUFA (EPA and DPA in this case) to accumulate in other tissues such as muscle. This same effect was partially highlighted in dT diet as well, showing higher levels of eicosatetraenoic acid and DPA than C diet. The defatting process of the IM was not absolute, so it is possible that the small amount of TM fat within the defatted IM (8.9%, around 1.6% of total feed) allowed again the use of insect-sourced MUFA to promote a partial sparing of the LC n−3 PUFA, making sense with the previous idea. This, however, does not enter in conflict with what is already known about the ability of salmonids to produce a limited amount of LC n−3 PUFA [91,92]. On the contrary, both points should be considered as synergistic actors towards the objective of increasing the final levels of LC n−3 PUFA. Lastly, it should be noted that there was a decrease in the fish oil inclusion of diets T, TO1 and TO2 respect to dT, more marked in TO2. This fish oil reduction would imply more sustainable diets.

Table 5.
Effect of dietary treatments on fatty acids composition of rainbow trout fillets.
Basing ourselves on the current bibliography [93], another analysis was carried out to support the previously mentioned. The proportion of FAs between the different experimental treatments and C was calculated, both for diets and fish fillets, and expressed in the form of graphics (Figure 1). Comparing these proportions and values on a feed-to-fillet basis, the data gives an interesting representation on how the physiology of the fish may be saving and accumulating n−3, whereas the number of other FAs, especially MUFA, becomes proportionally lower.
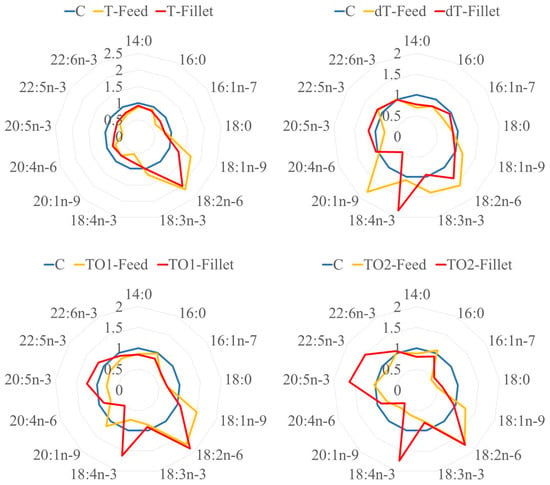
Figure 1.
Comparison between feed and fillet FA profiles, on an experimental treatment vs. control treatment (C) basis, using C diet as a normality baseline with a value of 1 (for both feed and fillet). C: control diet (no fishmeal replacement); T: 50% fishmeal replacement with Tenebrio molitor; dT: 50% fishmeal replacement with partially defatted Tenebrio molitor; TO1: T diet supplemented with 3.09% of EO; TO2: T diet supplemented with 7.24% of EO.
3.2.2. Fillet Perceived Quality
The perceived quality of the final product is another important feature during its evaluation. In this experiment, the texture and colour of fillets were analysed objectively through instrumental methods, but other traits were also subjectively analysed through direct sensorial methods. For the case of direct sensorial analyses, both raw and cooked fillets were evaluated.
Talking about the instrumental analyses, no differences were found among treatments for texture (Table 6). Colour analyses revealed a higher level of L in TO1 than in T, and a lower level of a* in TO2 than in C diet (Table 7). The entire set of direct sensorial analyses only highlighted a higher level of brightness in the general appearance of cooked fillets for C and T diets than the case of TO2 diet (Table 8 and Table 9).

Table 6.
Effect of dietary treatments on the texture of raw rainbow trout fillets (instrumental analysis).

Table 7.
Effect of dietary treatments on the colour of raw rainbow trout fillets (instrumental analysis).

Table 8.
Effect of dietary treatments on whole fish and fillets of raw rainbow trout (sensorial analysis).

Table 9.
Effect of dietary treatments on cooked rainbow trout fillets (sensorial analysis).
Texture, colour, and other traits related to the perceived quality of the fillets can be modified through the diets of the fish, either from an instrumental [94,95] or sensorial perspective [95,96]. Put together, the current results aim to the idea that the tested ingredients did not alter the organoleptic traits of the fish in an important way, which is supported by the bibliography [31,34]. However, the lower level of a* in TO2 of raw fillets suggests that the EO could provoke a more greenish shading colour in these fillets. It is interesting to notice that raw, objective L*, and cooked, subjective brightness, which should be similar traits, offered results very close to opposite. This suggests that the effects of the experimental ingredients (IMs and EO) were different before and after the cooking process. There are not many insect-fed fish studies in which the organoleptic profile was evaluated. However, some of them agree with the present results, since it is not unusual to see changes in flesh [97,98], skin colour [99], or both parameters [82,100]. Differences in other traits are less common [101]. It is possible that these changes are due to the different nature of insect fat, or to other components such as the riboflavin (vitamin B2) that can be found in species such as TM, a yellow-coloured pigment that might contribute to these changes in colour [102].
3.3. Liver Performance and Antioxidant Status
There were no changes from the point of view of liver intermediary metabolism (Table 10), while liver histomorphology only showed minor differences for hepatocyte lipidic accumulation (Figure 2), being higher on dT, TO1 and TO2 treatments (Table 11). Liver antioxidant status showed lower levels of GPx in T, TO1 and TO2, and higher levels of MDA in TO1 and TO2, while no differences were found in the levels of plasmatic glucose and triglycerides (Table 12).

Table 10.
Effect of dietary treatments on liver intermediary metabolism enzymes of rainbow trout.
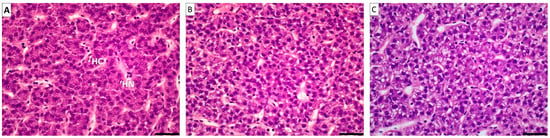
Figure 2.
Visual example of the degree of lipid accumulation in hepatocytes. (A): almost no lipid accumulation (−); (B): minor degree of lipid accumulation (+); (C): medium degree of lipid accumulation (++). Picture A also shows an example of quantitative measures (HC: hepatocyte cytoplasm; HN: hepatocyte nucleus).

Table 11.
Effect of dietary treatments on liver histomorphology of rainbow trout.

Table 12.
Effect of dietary treatments on antioxidant status (liver) and animal welfare indicators (plasma metabolites) of rainbow trout.
Liver function remained mostly unaffected among diets. This is often described in insect-fed studies that measured similar enzymatic and histomorphological variables in salmonids [18,20,24,103]. However, the few changes highlighted in the current study can be explained with a link between liver lipid accumulation and antioxidant status. As previously mentioned about fillet FA composition, it is possible that the higher availability of MUFA and linoleic acid in insect-based diets, together with an n−3 sparing effect [85] allowed insect-fed fish to have a surplus of these n−3. Additionally, it has been described that, in zebrafish (Danio rerio), an increase in the n−6/n−3 ratio (Table 2c and Table 5) can be related to an accumulation of lipids [44]. Following these lines of thought, this was reflected in the higher level of lipid accumulation in three out of four insect-based diets (dT, TO1 and TO2). Furthermore, if livers followed the performance of the fillets, this would have led to the higher level of MDA in liver that was described as well. LC n−3 PUFA are characterised for having higher peroxidability indices than other FAs [63,104], something that was reflected in the results of this work (Table 5). In this way, and even though IMs and/or their chitin are believed to have a positive effect enhancing the antioxidant system [22,23,40,105,106], the levels of GPx went down in T, TO1 and TO2, probably as a consequence of a long-term depletion of this this enzyme [107], resulting in an oxidative damage observed with the increase in MDA levels. Perhaps, the level of antioxidants in the experimental diets was suboptimal, because another experiment with gilthead seabream (Sparus aurata) under similar conditions described different results [38]. As such, it is possible that a special addition of antioxidant would have prevented this effect in TO1 and TO2, which was not carried out due to being a non-expected consequence of these diets. However, growth and other relevant variables related to animal welfare such as plasmatic glucose levels were unaffected. This makes it is easy to assume that even these different levels of MDA and GPx can be considered within sustainable, healthy limits, at least for the length of the experiment (89 days). This should be considered for longer experiments or growth cycles.
4. Conclusions
First and foremost, the diets did not compromise the growth of rainbow trout or the viability of the final product. Additionally, one of the main objectives of this feeding trial, which was proposing two strategies to compensate the reduction in long-chain omega−3 polyunsaturated fatty acids in fillets of insect-fed fish, was successful. Secondarily, this work also supports some of the points that can be found in the bibliography, such as the fact that the nature of the lipids can be decisive due to the existence of an omega−3 sparing effect in the physiology of rainbow trout. This could have provoked the accumulation of lipids in certain organs such as muscle and liver. However, more complex and specialized experiments are required to truly confirm it. As a whole, and particularly due to the unexpected outcome of the antioxidant system, dT was probably the diet with the most interesting performance. TO1 and TO2 also showed a good outcome, and knowing that the algal oil coming from Schizochytrium sp. can replace part of the fish oil, these diets should be the most environmentally sustainable. More research is encouraged to support some points that remain unclear, such as the biochemical mechanisms behind the effects of IMs in general, and chitin in particular, on the antioxidant system of fish.
Author Contributions
Conceptualization, F.G.B., A.E.M., M.C.H. and C.T.-A.; Data curation, F.M. and C.T.-A.; Formal analysis, F.M. and C.T.-A.; Funding acquisition, A.M.L., F.G.B., A.E.M., M.C.H. and C.T.-A.; Investigation, C.T.-A.; Methodology, F.M., A.M.L., M.-Á.S., D.R., D.F., A.G., F.J.A., A.E.M., M.C.H., H.M.L., M.-F.P. and C.T.-A.; Project administration, A.M.L. and C.T.-A.; Supervision, F.M. and A.M.L.; Writing—original draft, F.M. and C.T.-A.; Writing—review and editing, F.M., F.J.A. and C.T.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by INIA (Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria) and co-funded by FEDER funds (Ref. RTA 2015-00021-C03). F. Melenchón was supported by a fellowship funded by AEI (Agencia Estatal de Investigación) awarded through the financial help of reference BES2017-080567 for PhD contracts with FSE funds. A. Galafat was supported by a fellowship funded by AquaTech4Feed (grant # PCI2020-112204) awarded by MCIN/AEI/10.13039/501100011033 and the EU “NextGenerationEU”/PRTR within the ERA-NET BioBlue COFUND.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Instituto Tecnológico Agrario de Castilla y León (protocol code 2017/19/CEEA and date of approval 16 March 2017).
Informed Consent Statement
Informed consent was obtained from all panellists involved in the study.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank the Experimental diets service of the University of Almería (Almería, Spain) for the support in the feed formulation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Oliva-Teles, A.; Couto, A.; Enes, P.; Peres, H. Dietary protein requirements of fish—A meta-analysis. Rev. Aquac. 2020, 12, 1445–1477. [Google Scholar] [CrossRef]
- Cashion, T.; Le Manach, F.; Zeller, D.; Pauly, D. Most fish destined for fishmeal production are food-grade fish. Fish Fish. 2017, 18, 837–844. [Google Scholar] [CrossRef]
- Glencross, B.; Rutherford, N.; Jones, B. Evaluating options for fishmeal replacement in diets for juvenile barramundi (Lates calcarifer). Aquac. Nutr. 2011, 17, e722–e732. [Google Scholar] [CrossRef]
- Glencross, B.; Blyth, D.; Irvin, S.; Bourne, N.; Campet, M.; Boisot, P.; Wade, N.M. An evaluation of the complete replacement of both fishmeal and fish oil in diets for juvenile Asian seabass, Lates calcarifer. Aquaculture 2016, 451, 298–309. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Penn, M.; Thorsen, J.; Refstie, S.; Bakke, A.M. Important antinutrients in plant feedstuffs for aquaculture: An update on recent findings regarding responses in salmonids. Aquac. Res. 2010, 41, 333–344. [Google Scholar] [CrossRef]
- Urán, P.A.; Gonçalves, A.A.; Taverne-Thiele, J.J.; Schrama, J.W.; Verreth, J.A.; Rombout, J.H. Soybean meal induces intestinal inflammation in common carp (Cyprinus carpio L.). Fish Shellfish. Immunol. 2008, 25, 751–760. [Google Scholar] [CrossRef]
- Urán, P.A.; Schrama, J.W.; Rombout, J.H.; Taverne-Thiele, J.J.; Obach, A.; Koppe, W.; Verreth, J.A. Time-related changes of the intestinal morphology of Atlantic salmon, Salmo salar L., at two different soybean meal inclusion levels. J. Fish Dis. 2009, 32, 733–744. [Google Scholar] [CrossRef]
- Djordjevic, B.; Morales-Lange, B.; Øverland, M.; Mercado, L.; Lagos, L. Immune and proteomic responses to the soybean meal diet in skin and intestine mucus of Atlantic salmon (Salmo salar L.). Aquac. Nutr. 2021, 27, 929–940. [Google Scholar] [CrossRef]
- Mundheim, H.; Aksnes, A. Growth, feed efficiency and digestibility in salmon (Salmo salar L.) fed different dietary proportions of vegetable protein sources in combination with two fish meal qualities. Aquaculture 2004, 237, 315–331. [Google Scholar] [CrossRef]
- Albrektsen, S.; Mundheim, H.; Aksnes, A. Growth, feed efficiency, digestibility and nutrient distribution in Atlantic cod (Gadus morhua) fed two different fish meal qualities at three dietary levels of vegetable protein sources. Aquaculture 2006, 261, 626–640. [Google Scholar] [CrossRef]
- Van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Van Huis, A. Insects as food and feed, a new emerging agricultural sector: A review. J. Insects Food Feed 2020, 6, 27–44. [Google Scholar] [CrossRef]
- Barroso, F.G.; de Haro, C.; Sánchez-Muros, M.-J.; Venegas, E.; Martínez-Sánchez, A.; Pérez-Bañón, C. The potential of various insect species for use as food for fish. Aquaculture 2014, 422–423, 193–201. [Google Scholar] [CrossRef]
- Makkar, H.P.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Belforti, M.; Gai, F.; Lussiana, C.; Renna, M.; Malfatto, V.; Rotolo, L.; De Marco, M.; Dabbou, S.; Schiavone, A.; Zoccarato, I.; et al. Tenebrio molitor meal in rainbow trout (Oncorhynchus mykiss) diets: Effects on animal performance, nutrient digestibility and chemical composition of fillets. Ital. J. Anim. Sci. 2015, 14, 4170. [Google Scholar] [CrossRef]
- Melenchón, F.; Larrán, A.M.; de Mercado, E.; Hidalgo, M.C.; Cardenete, G.; Barroso, F.G.; Fabrikov, D.; Lourenço, H.M.; Pessoa, M.F.; Tomás-Almenar, C. Potential use of black soldier fly (Hermetia illucens) and mealworm (Tenebrio molitor) insectmeals in diets for rainbow trout (Oncorhynchus mykiss). Aquac. Nutr. 2021, 27, 491–505. [Google Scholar] [CrossRef]
- Melenchón, F.; de Mercado, E.; Pula, H.J.; Cardenete, G.; Barroso, F.G.; Fabrikov, D.; Lourenço, H.M.; Pessoa, M.-F.; Lagos, L.; Weththasinghe, P.; et al. Fishmeal dietary replacement up to 50%: A comparative study of two insect meals for rainbow trout (Oncorhynchus mykiss). Animals 2022, 12, 179. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, L.; Biasibetti, E.; et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. 2017, 8, 57. [Google Scholar] [CrossRef]
- Chemello, G.; Renna, M.; Caimi, C.; Guerreiro, I.; Oliva-Teles, A.; Enes, P.; Biasato, I.; Schiavone, A.; Gai, F.; Gasco, L. Partially defatted Tenebrio molitor larva meal in diets for grow-out rainbow trout, Oncorhynchus mykiss (Walbaum): Effects on growth performance, diet digestibility and metabolic responses. Animals 2020, 10, 229. [Google Scholar] [CrossRef]
- Cardinaletti, G.; Di Marco, P.; Daniso, E.; Messina, M.; Donadelli, V.; Finoia, M.G.; Petochi, T.; Fava, F.; Faccenda, F.; Contò, M.; et al. Growth and welfare of rainbow trout (Oncorhynchus mykiss) in response to graded levels of insect and poultry by-product meals in fishmeal-free diets. Animals 2022, 12, 1698. [Google Scholar] [CrossRef]
- Henry, M.A.; Gai, F.; Enes, P.; Pérez-Jiménez, A.; Gasco, L. Effect of partial dietary replacement of fishmeal by yellow mealworm (Tenebrio molitor) larvae meal on the innate immune response and intestinal antioxidant enzymes of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2018, 83, 308–313. [Google Scholar] [CrossRef]
- Xu, X.; Ji, H.; Yu, H.; Zhou, J. Influence of dietary black soldier fly (Hermetia illucens Linnaeus) pulp on growth performance, antioxidant capacity and intestinal health of juvenile mirror carp (Cyprinus carpio var. specularis). Aquac. Nutr. 2020, 26, 432–443. [Google Scholar] [CrossRef]
- Kumar, V.; Fawole, F.J.; Romano, N.; Hossain, M.S.; Labh, S.N.; Overturf, K.; Small, B.C. Insect (black soldier fly, Hermetia illucens) meal supplementation prevents the soybean meal-induced intestinal enteritis in rainbow trout and health benefits of using insect oil. Fish Shellfish Immunol. 2021, 109, 116–124. [Google Scholar] [CrossRef] [PubMed]
- St-Hilaire, S.; Sheppard, C.; Tomberlin, J.K.; Irving, S.; Newton, L.; McGuire, M.A.; Mosley, E.E.; Hardy, R.W.; Sealey, W. Fly prepupae as a feedstuff for rainbow trout, Oncorhynchus mykiss. J. World Aquac. Soc. 2007, 38, 59–67. [Google Scholar] [CrossRef]
- Fabrikov, D.; Barroso, F.G.; Sánchez-Muros, M.J.; Hidalgo, M.C.; Cardenete, G.; Tomás-Almenar, C.; Melenchón, F.; Guil-Guerrero, J.L. Effect of feeding with insect meal diet on the fatty acid compositions of sea bream (Sparus aurata), tench (Tinca tinca) and rainbow trout (Oncorhynchus mykiss) fillets. Aquaculture 2021, 545, 737170. [Google Scholar] [CrossRef]
- Shafique, L.; Abdel-Latif, H.; Hassan, F.-U.; Alagawany, M.; Naiel, M.; Dawood, M.; Yilmaz, S.; Liu, Q. The feasibility of using yellow mealworms (Tenebrio molitor): Towards a sustainable aquafeed industry. Animals 2021, 11, 811. [Google Scholar] [CrossRef]
- Jeong, S.M.; Khosravi, S.; Kim, K.-W.; Lee, B.-J.; Hur, S.-W.; You, S.-G.; Lee, S.-M. Potential of mealworm, Tenebrio molitor, meal as a sustainable dietary protein source for juvenile black porgy, Acanthopagrus schlegelii. Aquac. Rep. 2022, 22, 100956. [Google Scholar] [CrossRef]
- Ulbritch, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 8773, 985–992. [Google Scholar] [CrossRef]
- Orkusz, A. Edible insects versus meat—Nutritional comparison: Knowledge of their composition is the key to good health. Nutrients 2021, 13, 1207. [Google Scholar] [CrossRef]
- Belghit, I.; Liland, N.S.; Gjesdal, P.; Biancarosa, I.; Menchetti, E.; Li, Y.; Waagbo, R.; Krogdahl, Å.; Lock, E.-J. Black soldier fly larvae meal can replace fish meal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture 2019, 503, 609–619. [Google Scholar] [CrossRef]
- Basto, A.; Calduch-Giner, J.; Oliveira, B.; Petit, L.; Sá, T.; Maia, M.R.G.; Fonseca, S.C.; Matos, E.; Pérez-Sánchez, J.; Valente, L.M.P. The use of defatted Tenebrio molitor larvae meal as a main protein source is supported in European sea bass (Dicentrarchus labrax) by data on growth performance, lipid metabolism, and flesh quality. Front. Physiol. 2021, 12, 659567. [Google Scholar] [CrossRef]
- Bordignon, F.; Gasco, L.; Birolo, M.; Trocino, A.; Christian, C.; Ballarin, C.; Bortoletti, M.; Nicoletto, C.; Maucieri, C.; Xiccato, G. Performance and fillet traits of rainbow trout (Oncorhynchus mykiss) fed different levels of Hermetia illucens meal in a low-tech aquaponic system. Aquaculture 2022, 546, 737279. [Google Scholar] [CrossRef]
- Sealey, W.M.; Gaylord, T.G.; Barrows, F.T.; Tomberlin, J.K.; McGuire, M.A.; Ross, C.; St-Hilaire, S. Sensory analysis of rainbow trout, Oncorhynchus mykiss, fed enriched black soldier fly prepupae, Hermetia illucens. J. World Aquac. Soc. 2011, 42, 34–45. [Google Scholar] [CrossRef]
- Barroso, F.G.; Sánchez-Muros, M.J.; Rincón, M.A.; Rodríguez-Rodríguez, M.; Fabrikov, D.; Morote, E.; Guil-Guerrero, J.L. Production of n-3-rich insects by bioaccumulation of fishery waste. J. Food Compos. Anal. 2019, 82, 103237. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Zimbelli, A.; Randazzo, B.; Compagni, M.D.; Truzzi, C.; Antonucci, M.; Riolo, P.; Loreto, N.; Osimani, A.; Milanović, V.; et al. Black Soldier Fly (Hermetia illucens) reared on roasted coffee by-product and Schizochytrium sp. as a sustainable terrestrial ingredient for aquafeeds production. Aquaculture 2020, 518, 734659. [Google Scholar] [CrossRef]
- Fabrikov, D.; Morote, E.; Montes, J.; Sánchez-Muros, M.J.; Barroso, F.G.; Rodríguez-Rodríguez, M.; González-Fernández, M.J.; Guil-Guerrero, J.L. Facing the challenge of discarded fish: Improving nutritional quality of two insect species larvae for use as feed and food. J. Insects Food Feed 2021, 7, 345–355. [Google Scholar] [CrossRef]
- Santigosa, E.; Brambilla, F.; Milanese, L. Microalgae oil as an effective alternative source of EPA and DHA for gilthead seabream (Sparus aurata) aquaculture. Animals 2021, 11, 971. [Google Scholar] [CrossRef]
- Santigosa, E.; Constant, D.; Prudence, D.; Wahli, T.; Verlhac-Trichet, V. A novel marine algal oil containing both EPA and DHA is an effective source of omega-3 fatty acids for rainbow trout (Oncorhynchus mykiss). JWAS 2020, 51, 649–665. [Google Scholar] [CrossRef]
- Li, S.; Ji, H.; Zhang, B.; Zhou, J.; Yu, H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture 2017, 477, 62–70. [Google Scholar] [CrossRef]
- Su, J.; Gong, Y.; Cao, S.; Lu, F.; Han, D.; Liu, H.; Jin, J.; Yang, Y.; Zhu, X.; Xie, S. Effects of dietary Tenebrio molitor meal on the growth performance, immune response and disease resistance of yellow catfish (Pelteobagrus fulvidraco). Fish Shellfish Immunol. 2017, 69, 59–66. [Google Scholar] [CrossRef]
- Lu, R.; Chen, Y.; Yu, W.; Lin, M.; Yang, G.; Qin, C.; Meng, X.; Zhang, Y.; Ji, H.; Nie, G. Defatted black soldier fly (Hermetia illucens) larvae meal can replace soybean meal in juvenile grass carp (Ctenopharyngodon idellus) diets. Aquac. Rep. 2020, 18, 100520. [Google Scholar] [CrossRef]
- Hender, A.; Siddik, M.A.B.; Howieson, J.; Fotedar, R. Black soldier fly, Hermetia illucens as an alternative to fishmeal protein and fish oil: Impact on growth, immune response, mucosal barrier status, and flesh quality of juvenile barramundi, Lates calcarifer (Bloch, 1790). Biology 2021, 10, 505. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Randazzo, B.; Secci, G.; Notarstefano, V.; Giorgini, E.; Lock, E.J.; Parisi, G.; Olivotto, I. Application of laboratory methods for understanding fish responses to black soldier fly (Hermetia illucens) based diets. J. Insects Food Feed 2021, 8, 1173–1195. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Fish; National Academies Press: Washington, DC, USA, 1993. [Google Scholar]
- Blanco Cachafeiro, M.C. La Trucha: Cría Industrial, 2nd ed.; Mundi-Prensa: Madrid, Spain, 2005. [Google Scholar]
- AENOR UNE 173300; Pisciculture. Guide to Good Practice for Sacrifice. AENOR: Madrid, Spain, 2016.
- European Parliament. Book Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; European Parliament: Strasbourg, France, 2010; pp. 33–78. [Google Scholar]
- Real Decreto 53/2013, de 1 de Febrero, por el que se Establecen las Normas Básicas Aplicables para la Protección de los Animales Utilizados en Experimentación y Otros Fines Científicos, Incluyendo la Docencia. 2013. Available online: https://www.boe.es/eli/es/rd/2013/02/01/53 (accessed on 27 January 2023).
- Rosenthal, A.J. Relation between instrumental and sensory measures of food texture. In Food Texture: Measurement and Perception; Aspen Publications: Boston, MA, USA, 1999. [Google Scholar]
- Skrede, G.; Storebakken, T.; Næs, T. Color evaluation in raw, baked and smoked flesh of rainbow trout (Onchorhynchus mykiss) fed astaxanthin or canthaxanthin. J. Food Sci. 1990, 55, 1574–1578. [Google Scholar] [CrossRef]
- Tomás-Almenar, C.; Larrán, A.M.; de Mercado, E.; Sanz-Calvo, M.A.; Hernández, D.; Riaño, B.; García-González, M.C. Scenedesmus Almeriensis from an integrated system waste-nutrient, as sustainable protein source for feed to rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 497, 422–430. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Communities International, 18th ed.; AOAC: Rockville, MD, USA, 2005. [Google Scholar]
- Saint-Denis, T.; Goupy, J. Optimization of a nitrogen analyser based on the Dumas method. Anal. Chim. Acta 2004, 515, 191–198. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio Molitor, Alphitobius Diaperinus, and Hermetia Illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Gamage, A.; Shahidi, F. Use of chitosan for the removal of metal ion contaminants and proteins from water. Food Chem. 2007, 104, 989–996. [Google Scholar] [CrossRef]
- Pessoa, M.F.; Scotti-Campos, P.; Pais, I.; Feteiro, A.; Canuto, D.; Simões, M.; Pelica, J.; Pataco, I.; Ribeiro, V.; Reboredo, F.H.; et al. Nutritional profile of the Portuguese cabbage (Brassica Oleracea L. var. costata) and its relationship with the elemental soil analysis. Emir. J. Food Agric. 2016, 28, 381–388. [Google Scholar] [CrossRef]
- ISO 13730; Meat and Meat Products—Determination of Total Phosphorus Content—Spectrometric Method: ISO Technical Committee TC 34/SC 6. ISO: Geneva, Switzerland, 1996.
- Lepage, G.; Roy, C.C. Improved recovery of fatty acid through direct transesterification without prior extraction or purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, J.; Belarbi, E.H.; Sánchez, J.L.G.; Alonso, D.L. Rapid simultaneous lipid extraction and transesterification for fatty acid analyses. Biotechnol. Tech. 1998, 12, 689–691. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Gómez-Mercado, F.; Ramos-Bueno, R.P.; Rincón-Cervera, M.Á.; Venegas-Venegas, E. Restricted-range boraginaceae species constitute potential sources of valuable fatty acids. J. Am. Oil Chem. Soc. 2014, 91, 301–308. [Google Scholar] [CrossRef]
- Cladis, D.P.; Kleiner, A.C.; Freiser, H.H.; Santerre, C.R. Fatty acid profiles of commercially available finfish fillets in the United States. Lipids 2014, 49, 1005–1018. [Google Scholar] [CrossRef]
- García-Márquez, J.; Galafat, A.; Vizcaíno, A.J.; Barany, A.; Martos-Sitcha, J.A.; Mancera, J.M.; Acién, G.; Figueroa, F.L.; Alarcón, F.J.; Arijo, S.; et al. Dietary use of the microalga Chlorella fusca Improves growth, metabolism, and digestive functionality in thick-lipped grey mullet (Chelon labrosus, Risso 1827) juveniles. Front. Mar. Sci. 2022, 9, 902203. [Google Scholar] [CrossRef]
- Furné, M.; Morales, A.E.; Trenzado, C.E.; García-Gallego, M.; Hidalgo, M.C.; Domezain, A.; Rus, A.S. The metabolic effects of prolonged starvation and refeeding in sturgeon and rainbow trout. J. Comp. Physiol. B 2012, 182, 63–76. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.; Hidalgo, M.C.; Morales, A.E.; Arizcun, M.; Abellán, E.; Cardenete, G. Antioxidant enzymatic defenses and oxidative damage in Dentex dentex fed on different dietary macronutrient levels. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 150, 537–545. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for statistical computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 27 April 2023).
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2022; Available online: http://www.rstudio.com/ (accessed on 27 April 2023).
- Liland, N.S.; Araujo, P.; Xu, X.X.; Lock, E.-J.; Radhakrishnan, G.; Prabhu, A.J.P.; Belghit, I. A meta-analysis on the nutritional value of insects in aquafeeds. J. Insects Food Feed 2021, 7, 743–759. [Google Scholar] [CrossRef]
- Weththasinghe, P.; Hansen, J.Ø.; Mydland, L.T.; Øverland, M. A systematic meta-analysis based review on black soldier fly (Hermetia illucens) as a novel protein source for salmonids. Rev. Aquac. 2021, 14, 938–956. [Google Scholar] [CrossRef]
- Rema, P.; Saravanan, S.; Armenjon, B.; Motte, C.; Dias, J. Graded incorporation of defatted yellow mealworm (Tenebrio molitor) in rainbow trout (Oncorhynchus mykiss) diet improves growth performance and nutrient retention. Animals 2019, 9, 187. [Google Scholar] [CrossRef]
- Guerreiro, I.; Castro, C.; Antunes, B.; Coutinho, F.; Rangel, F.; Couto, A.; Serra, C.R.; Peres, H.; Pousão-Ferreira, P.; Matos, E.; et al. Catching black soldier fly for meagre: Growth, whole-body fatty acid profile and metabolic responses. Aquaculture 2020, 516, 734613. [Google Scholar] [CrossRef]
- Coutinho, F.; Castro, C.; Guerreiro, I.; Rangel, F.; Couto, A.; Serra, C.R.; Peres, H.; Pousão-Ferreira, P.; Rawski, M.; Oliva-Teles, A.; et al. Mealworm larvae meal in diets for meagre juveniles: Growth, nutrient digestibility and digestive enzymes activity. Aquaculture 2021, 535, 736362. [Google Scholar] [CrossRef]
- Estévez, A.; Blanco, B.; Fernández, L.; Ferreira, M.; Soula, M. Effects of alternative and sustainable ingredients, insect meal, microalgae and protein and lipid from tuna cooking water, on meagre (Argyrosomus regius) growth, food conversion and muscle and liver composition. Aquaculture 2022, 548, 737549. [Google Scholar] [CrossRef]
- Taufek, N.M.; Aspani, F.; Muin, H.; Raji, A.A.; Razak, S.A.; Alias, Z. The effect of dietary cricket meal (Gryllus bimaculatus) on growth performance, antioxidant enzyme activities, and haematological response of African catfish (Clarias gariepinus). Fish Physiol. Biochem. 2016, 42, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Rapatsa, M.; Moyo, N.A.G. Evaluation of Imbrasia belina meal as a fishmeal substitute in Oreochromis mossambicus diets: Growth performance, histological analysis and enzyme activity. Aquac. Rep. 2017, 5, 18–26. [Google Scholar] [CrossRef]
- Sankian, Z.; Khosravi, S.; Kim, Y.-O.; Lee, S.-M. Effects of dietary inclusion of yellow mealworm (Tenebrio molitor) meal on growth performance, feed utilization, body composition, plasma biochemical indices, selected immune parameters and antioxidant enzyme activities of mandarin fish (Siniperca scherzeri) juveniles. Aquaculture 2018, 496, 79–87. [Google Scholar] [CrossRef]
- Vongvichith, B.; Morioka, S.; Sugita, T.; Phousavanh, N.; Phetsanghanh, N.; Chantasone, P.; Pommachan, P.; Nakamura, S. Evaluation of the efficacy of aquaculture feeds for the climbing perch Anabas testudineus: Replacement of fishmeal by black soldier fly Hermetia illucens prepupae. Fish. Sci. 2020, 86, 145–151. [Google Scholar] [CrossRef]
- Agbohessou, P.S.; Mandiki, S.N.M.; Gougbédji, A.; Megido, R.C.; Hossain, M.S.; de Jaeger, P.; Larondelle, Y.; Francis, F.; Lalèye, P.A.; Kestemont, P. Total replacement of fish meal by enriched-fatty acid Hermetia illucens meal did not substantially affect growth parameters or innate immune status and improved whole body biochemical quality of Nile tilapia juveniles. Aquac. Nutr. 2021, 27, 880–896. [Google Scholar] [CrossRef]
- Gebremichael, A.; Sándor, Z.J.; Kucska, B. Does dietary inclusion of defatted yellow mealworm (Tenebrio molitor) affect growth and body composition of juvenile common carp (Cyprinus carpio)? S. Afr. J. Anim. Sci. 2022, 52, 444–451. [Google Scholar] [CrossRef]
- Mastoraki, M.; Ferrándiz, P.M.; Vardali, S.C.; Kontodimas, D.C.; Kotzamanis, Y.P.; Gasco, L.; Chatzifotis, S.; Antonopoulou, E. A comparative study on the effect of fish meal substitution with three different insect meals on growth, body composition and metabolism of European sea bass (Dicentrarchus labrax L.). Aquaculture 2020, 528, 735511. [Google Scholar] [CrossRef]
- Jeong, S.-M.; Khosravi, S.; Yoon, K.-Y.; Kim, K.-W.; Lee, B.-J.; Hur, S.-W.; Lee, S.-M. Mealworm, Tenebrio molitor, as a feed ingredient for juvenile olive flounder, Paralichthys olivaceus. Aquac. Rep. 2021, 20, 100747. [Google Scholar] [CrossRef]
- Mancini, S.; Medina, I.; Iaconisi, V.; Gai, F.; Basto, A.; Parisi, G. Impact of black soldier fly larvae meal on the chemical and nutritional characteristics of rainbow trout fillets. Animal 2018, 12, 1672–1681. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Fawole, F.J.; Labh, S.N.; Small, B.C.; Overturf, K.; Kumar, V. Insect meal inclusion as a novel feed ingredient in soy-based diets improves performance of rainbow trout (Oncorhynchus mykiss). Aquaculture 2021, 544, 737096. [Google Scholar] [CrossRef]
- Turchini, G.M.; Francis, D.S.; Senadheera, S.P.S.D.; Thanuthong, T.; de Silva, S.S. Fish oil replacement with different vegetable oils in Murray cod: Evidence of an “omega-3 sparing effect” by other dietary fatty acids. Aquaculture 2011, 315, 250–259. [Google Scholar] [CrossRef]
- Emery, J.A.; Norambuena, F.; Trushenski, J.; Turchini, G.M. Uncoupling EPA and DHA in fish nutrition: Dietary demand is limited in Atlantic salmon and effectively met by DHA alone. Lipids 2016, 51, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Turchini, G.; Francis, D.S.; Liang, M.; Mock, T.S.; Rombenso, A.; Ai, Q. Are fish what they eat? A fatty acid’s perspective. Prog. Lipid Res. 2020, 80, 101064. [Google Scholar] [CrossRef]
- Piedecausa, M.A.; Mazón, M.J.; García, B.G.; Hernández, M.D. Effects of total replacement of fish oil by vegetable oils in the diets of sharpsnout seabream (Diplodus puntazzo). Aquaculture 2007, 263, 211–219. [Google Scholar] [CrossRef]
- Karalazos, V.; Bendiksen, E.Å.; Bell, J.G. Interactive effects of dietary protein/lipid level and oil source on growth, feed utilisation and nutrient and fatty acid digestibility of Atlantic salmon. Aquaculture 2011, 311, 193–200. [Google Scholar] [CrossRef]
- Mozanzadeh, M.T.; Agh, N.; Yavari, V.; Marammazi, J.G.; Mohammadian, T.; Gisbert, E. Partial or total replacement of dietary fish oil with alternative lipid sources in silvery-black porgy (Sparidentex hasta). Aquaculture 2016, 451, 232–240. [Google Scholar] [CrossRef]
- Tocher, D.R. Fatty acid requirements in ontogeny of marine and freshwater fish. Aquac. Res. 2010, 41, 717–732. [Google Scholar] [CrossRef]
- Tocher, D.R. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 2015, 449, 94–107. [Google Scholar] [CrossRef]
- Gause, B.R.; Trushenski, J.T. Sparing fish oil with beef tallow in feeds for rainbow trout: Effects of inclusion rates and finishing on production performance and tissue fatty acid composition. N. Am. J. Aquac. 2013, 75, 495–511. [Google Scholar] [CrossRef]
- Asghari, M.; Shabanpour, B.; Pakravan, S. Evaluation of some qualitative variations in frozen fillets of beluga (Huso huso) fed by different carbohydrate to lipid ratios. J. Food Sci. Technol. 2014, 51, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Pavón, Y.; Cian, R.E.; Soldini, M.A.C.; Hernández, D.R.; Sánchez, S.; Drago, S.R. Sensory and instrumental textural changes in fillets from Pacú (Piaractus mesopotamicus) fed different diets. J. Texture Stud. 2018, 49, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Chaiyapechara, S.; Liu, K.K.M.; Barrows, F.T.; Hardy, R.W.; Dong, F.M. Proximate composition, lipid oxidation, and sensory characteristics of fillets from rainbow trout Oncorhynchus mykiss fed diets containing 10% to 30% lipid. J. World Aquac. Soc. 2007, 34, 266–277. [Google Scholar] [CrossRef]
- Stadtlander, T.; Stamer, A.; Buser, A.; Wohlfahrt, J.; Leiber, F.; Sandrock, C. Hermetia illucens meal as fish meal replacement for rainbow trout on farm. J. Insects Food Feed 2017, 3, 165–175. [Google Scholar] [CrossRef]
- Bruni, L.; Randazzo, B.; Cardinaletti, G.; Zarantoniello, M.; Mina, F.; Secci, G.; Tulli, F.; Olivotto, I.; Parisi, G. Dietary inclusion of full-fat Hermetia illucens prepupae meal in practical diets for rainbow trout (Oncorhynchus mykiss): Lipid metabolism and fillet quality investigations. Aquaculture 2020, 529, 735678. [Google Scholar] [CrossRef]
- Iaconisi, V.; Bonelli, A.; Pupino, R.; Gai, F.; Parisi, G. Mealworm as dietary protein source for rainbow trout: Body and fillet quality traits. Aquaculture 2018, 484, 197–204. [Google Scholar] [CrossRef]
- Iaconisi, V.; Marono, S.; Parisi, G.; Gasco, L.; Genovese, L.; Maricchiolo, G.; Bovera, F.; Piccolo, G. Dietary inclusion of Tenebrio molitor larvae meal: Effects on growth performance and final quality treats of blackspot sea bream (Pagellus bogaraveo). Aquaculture 2017, 476, 49–58. [Google Scholar] [CrossRef]
- Borgogno, M.; Dinnella, C.; Iaconisi, V.; Fusi, R.; Scarpaleggia, C.; Schiavone, A.; Monteleone, E.; Gasco, L.; Parisi, G. Inclusion of Hermetia illucens larvae meal on rainbow trout (Oncorhynchus mykiss) feed: Effect on sensory profile according to static and dynamic evaluations. J. Sci. Food Agric. 2017, 97, 3402–3411. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Hoffmann, L.; Rawski, M.; Nogales-Merida, S.; Mazurkiewicz, J. Dietary inclusion of Tenebrio molitor meal in sea trout larvae rearing: Effects on fish growth performance, survival, condition, and GIT and liver enzymatic activity. Ann. Anim. Sci. 2020, 20, 579–598. [Google Scholar] [CrossRef]
- Nogueira, M.S.; Scolaro, B.; Milne, G.L.; Castro, I.A. Oxidation products from omega-3 and omega-6 fatty acids during a simulated shelf life of edible oils. LWT—Food Sci. Technol. 2019, 101, 113–122. [Google Scholar] [CrossRef]
- Secci, G.; Mancini, S.; Iaconisi, V.; Gasco, L.; Basto, A.; Parisi, G. Can the inclusion of black soldier fly (Hermetia illucens) in diet affect the flesh quality/nutritional traits of rainbow trout (Oncorhynchus mykiss) after freezing and cooking? Int. J. Food Sci. Nutr. 2019, 70, 161–171. [Google Scholar] [CrossRef]
- Hidalgo, M.C.; Morales, A.E.; Pula, H.J.; Tomás-Almenar, C.; Sánchez-Muros, M.J.; Melenchón, F.; Fabrikov, D.; Cardenete, G. Oxidative metabolism of gut and innate immune status in skin and blood of tench (Tinca tinca) fed with different insect meals (Hermetia illucens and Tenebrio molitor). Aquaculture 2022, 529, 735731. [Google Scholar] [CrossRef]
- Sreejai, R.; Jaya, D.S. Studies on the changes in lipid peroxidation and antioxidants in fishes exposed to hydrogen sulfide. Toxicol. Int. 2010, 17, 71–77. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).