Abstract
Environmental challenges related to open sea cage production of Atlantic salmon have sparked interest in developing commercial-scale semi-closed sea systems for post-smolt Atlantic salmon (100–1000 g). Determining the mass-specific water flow required by post-smolts will largely influence the design and dimensioning of such systems. In this experiment, post-smolts were exposed to four levels of specific water flow: 0.2, 0.3, 0.4, and 0.5 L kg fish−1 min−1. All treatments involved flow-through seawater with full oxygenation, a salinity of 34‰, and a mean temperature of 9.3 °C. The stocking density was kept stable at 75 kg m−3. Water pH decreased with reduced flow, while partial pressure of carbon dioxide (pCO2) and total ammonia nitrogen (TAN) in the water increased. The increase in water CO2 was reflected in the blood with increased pCO2, HCO3−, and decreased Cl− in the lowest water flow treatment (0.2 L kg fish−1 min−1), indicating a typical regulatory response to increased water CO2 over the eight-week experimental period. No negative effects on osmoregulation, external macroscopic welfare, or performance indicators were observed, suggesting that within the time period of this experiment, post-smolts can compensate for reductions in water flow down to 0.2 L kg fish−1 min−1. However, to avoid activating and exhausting potentially energy-costly physiological regulatory mechanisms, it is suggested to keep specific water flow above 0.3 L kg fish−1 min−1 in large-scale operations with semi-closed sea systems at intermediate temperatures.
Key Contribution:
It is suggested that post-smolt Atlantic salmon should be reared at a water flow of 0.3 L kg fish−1 min−1 in large-scale operations with semi-closed sea systems at intermediate temperatures. This will ensure no negative effects on osmoregulation, external macroscopic welfare, or performance indicators.
1. Introduction
Currently, global aquaculture production of Atlantic salmon post-smolts occurs mainly in sea cages. This part of the production cycle has the potential for the largest environmental impact and is when the industry faces the highest losses [1,2]. Thus, developing new production methods that increase the overall sustainability of salmon aquaculture is of fundamental importance. Extending the time fish spend in closed-containment systems (CCS), where the rearing environment can be controlled and optimized, prior to stocking in open sea cages is expected to produce larger and more resilient post-smolts. This strategy could lower losses, and by shortening the time that fish spend in open sea cages, it will contribute to an overall reduction in environmental impact. Both land-based systems and semi-closed systems in the sea (S-CCS) are currently of interest. However, post-smolt production in CCS and S-CCS is predicted to have higher initial costs compared to open sea cages [3,4]. Investment costs for S-CCS can potentially be offset by increasing stocking density and/or reducing biomass-specific water flow, hereafter referred to as specific water flow (SWF). Nevertheless, economic profitability will depend on whether the resulting rearing conditions have a negative impact on fish physiology, performance, and overall welfare.
Most of the knowledge on water quality requirements for salmon in flow-through systems is based on freshwater studies on earlier life stages, from eggs/fry to smolts [5,6,7,8]. Accordingly, it is of great relevance to establish safe limits and guidelines regarding SWF rates for the seawater post-smolt life stage. These water quality requirements will to a large extent influence the design of closed and semi-closed flow-through systems by determining the necessary water inflow volume, retention time, accumulation of feces, carbon dioxide (CO2), ammonia (NH3-N), and oxygen supply.
Reduced access to water at any given stocking density will reduce pO2, pH, and increase pCO2, total ammonia nitrogen (TAN), in the ambient water and the blood of the fish. Ammonia (NH3-N) is the toxic metabolic waste product from the catabolism of amino acids and some nucleotides and is mainly excreted through the gills [9,10]. In water, TAN is present in two forms: ionized NH4+ and unionized NH3, and the equilibrium of the two species depends mainly on pH but also on temperature and salinity. NH3 is considered more toxic since it can easily cross gill membranes [9]. Depending on the energy substrate, fish excrete approximately 10 times more CO2 than TAN [11,12]. CO2, produced through the aerobic metabolism of the fish, will accumulate and decrease the water pH, which shifts the TAN equilibrium towards the ionized, less toxic form (NH4+). Thus, in intensive flow-through systems without aeration and where oxygen is added to the inflow water, CO2 has been suggested to be the first limiting production factor [13,14].
Due to the close contact between the gills and the ambient water, ventilation will quickly equilibrate any differences in CO2 tension between the water and the blood of the fish. The acid-base response to increased ambient CO2 is a decrease in blood pH, which reduces the oxygen-transporting ability of the blood [15]. However, to compensate for the increased pCO2 level and reduced pH, fish excrete H+ and take up HCO3− from the surrounding water via the HCO3/Cl exchanger in the gill epithelium [16], and in general, within 2–7 days, the blood pH is restored [17]. A decreased water exchange rate will not only increase the metabolite load but also the amount of total suspended solids (TSS) and the bacterial load in the system [18]. As decreasing the water flow creates complex water quality issues, it is important to establish safe levels for reduced SWF instead of relying on individual water quality parameters.
From a functional perspective, good fish welfare entails the ability of an animal to adapt to its environment and maintain normal biological function [19,20]. Stressors, like reduced water quality [21], may require energy-demanding physiological adjustments, known as allostasis, that enable the animal to adapt to a changing environment [19]. Increased blood glucose, osmoregulatory, and hematological changes are examples of physiological alterations that allow the fish to react and compensate for the stressful stimuli [22,23,24]. However, long-term or repeated stress can lead to an allostatic overload of these adaptive mechanisms with chronic adverse effects on welfare like reduced growth, immune function, and reproductive capacity [25,26,27]. The rearing environment may not only induce stress responses but is also suggested as a cause of fin and bodily damage in farmed fish, representing a clear welfare issue that must be addressed [28,29,30].
An earlier study focusing on determining the optimal fish density for post-smolt Atlantic salmon suggested 75 kg m−3 as a viable option [30]. However, in that study, a high SWF rate was used in all groups, thus eliminating water quality as a factor for poor performance. Therefore, the aim of this study was to document the potential effects of reduced specific water flow on performance and welfare at the proposed stocking density of 75 kg m−3. Furthermore, open flow respirometry, i.e., the difference in O2 content between the tank inlet and outlet, flow rate, and biomass were used to estimate oxygen uptake and indirectly the metabolic effect of reduced SWF. It is hypothesized that below the SWF threshold level, higher water CO2 may result in increased oxygen demand, osmoregulatory and hematological changes, and potentially chronic effects such as poorer growth.
2. Materials and Methods
2.1. Fish Stock and Rearing Conditions
The fish used in this study were out-of-season smolts of the AquaGen strain produced by Lerøy Vest, Flateråker, in Western Norway. Smolts were produced according to standard rearing protocols; for more details, see [30]. Before the acclimation phase of the experiment, all fish showed normal morphological and physiological signs of smolting, including silvery scales, dark fin margins, a low condition factor, and high gill Na+ and K+-ATPase (NKA) activity [31,32].
2.2. Experimental Design
All experimental procedures were approved by the Norwegian Animal Research Authority (reference no. 4692). This study was carried out at the Department of Biological Sciences at the University of Bergen, Norway, between October and December 2012. This was part of a combined experiment, and other aspects of the experiment have previously been published [30]. On 10 October, 2500 smolts (mean ± SEM; fork length = 22.0 cm ± 0.1, weight = 113.6 g ± 1.1) were randomly distributed among eight 1 m3 square fiberglass tanks (500 L, stocking density 75.0 kg fish m−3) with freshwater (pH adjusted and treated with SiO2) in a flow-through mode (0.6 L kg fish−1 min−1). From the 16th to the 18th of October, the freshwater in each tank was gradually and stepwise replaced with deep flow-through seawater (−105 m; 9.3 °C ± 0.01); i.e., increasing salinity from 0 to 17‰ on 16 October (D1), from 17 to 25‰ on 17 October (D2), and from 25‰ to full strength seawater (34‰) on October 18 (D3).
In seawater, the fish were exposed to a simulated natural photoperiod (60°25′ N). The experimental treatments were established on the 24th of October and included four different levels of specific water flow (SWF): 0.2, 0.3, 0.4, and 0.5 L kg fish−1 min−1. Each treatment was conducted in two identical tanks. The chosen SWFs tested are in the range of those previously investigated in other life stages [5,6,7,8] and slightly lower to identify a possible threshold level. The fish in all treatments were fed a commercial freshwater dry diet (Optiline 3 mm, Skretting AS, Stavanger, Norway) in 10% excess versus tables (Skretting AS, Stavanger, Norway) with an automatic feeder daily between 09:00–10:00 and 15:00–16.00 throughout the study. A freshwater feed was used to reduce the sinking rate of the pellets, thereby increasing the time they were available to the fish. All tanks were checked twice daily, and dead fish were removed immediately and weighed. Bulk weight measurements of the total biomass in each tank were recorded at the start of the experiment, in the middle (4 weeks), and at the end (8 weeks). At week 4, the actual biomass gain was recorded and removed to maintain the original treatment density of 75 kg m−3 (range 71.2–89.1 kg m−3). At weeks 2 and 6, biomass gain was estimated from sampled fish and removed. The study lasted for 8 weeks and was terminated on December 20.
2.3. Blood Chemistry Sampling and Analysis
All individuals fasted for 24 h prior to sampling. Fish were quickly netted and anesthetized in 200 mg L−1 buffered tricaine methanesulphonate (MS222, Sigma-Aldrich, St. Louis, MO, USA). Blood samples from one fish per tank (n = 12 fish) were collected during the gradual acclimation to full-strength seawater (D1–D4) from October 16th to 19th and at the start of the experiment (24 October). Samples from six fish per tank (n = 12 fish) were also collected after 2, 4, 6, and 8 weeks of exposure to different SWF treatments. Blood was sampled with heparinized syringes from the caudal vessels. A sub-sample of blood was immediately analyzed by an ISTAT analyzer (Abbot Norge AS, Billingstad, Norway).
Analytical cassettes (EC8+) were used with the ISTAT analyzer to measure blood levels of glucose, sodium (Na+), chloride (Cl−), bicarbonate (HCO3−), blood pH, and partial pressure of carbon dioxide (pCO2). Both blood pCO2 [33] and plasma pH [17] values were adjusted according to the temperature difference between 37 °C and the temperature of the fish (pHblood equals pHmeasured + 0.013·ΔT) [17]. Values for HCO3− were calculated according to the Henderson-Hasselbach equation [34], in which the solubility of CO2 and the apparent pK were adjusted according to temperature. When used for diagnostics in fish, deviations between the ISTAT and conventional laboratory values have been found [35,36,37,38]. However, it is also the only tool that allows for a large number of parameters of interest to be analyzed simultaneously. This is particularly useful when a more extensive overview of blood chemical changes is needed, and the main objective is to compare relative differences between treatments and not to obtain absolute values for both blood ions and gases. Therefore, identical handling and sampling of fish were prioritized in this study to allow for comparison between treatments.
2.4. Gill Activity Sampling and Analysis
Gill tissue from 12 fish was collected for determination of Na+, K+-ATPase (NKA) activity, and NKA α-subunit isoforms during the gradual acclimation to full-strength seawater (D1–D4) and at the start of the experiment (0). Samples from six fish per tank were also collected after 4 and 8 weeks of exposure to different SWF treatments. For NKA activity determination, gill tissue was collected from the second gill arch, immediately immersed in ice-cold SEI buffer, and frozen at −80 °C until subsequent analysis according to the procedure of [31]. Readings were taken at 340 nm for 10 min at 25 °C. Protein in the homogenate was determined by a bicinchoninic acid method [39], and NKA activity is expressed as µmol ADP mg−1 protein h−1.
For determination of NKA α-subunit isoforms, gill filaments were preserved in RNA later stabilization solution (Ambion, Foster City, CA, USA) for real-time quantitative PCR analysis. Total RNA was isolated from approximately 50 mg of gill tissue by phenol-chloroform extraction using TRI Reagent® (Sigma, St. Louis, MO, USA) as outlined by [40]. Total RNA concentration and purity were determined by the NanoDrop® ND-1000 UV-Vis Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA), and RNA integrity was evaluated with the Agilent 2100 Bioanalyzer using the RNA 6000 Nano LabChip® kit (Agilent Technologies, Palo Alto, CA, USA). cDNA was reversely transcribed using 2000 ng of total RNA and an oligo (dT) 20 primer in conjunction with the SuperScript III kit (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s instructions. Gill NKA α-subunit isoforms (α1a, α1b) and elongation factor 1A (EF α1a) mRNA levels were measured using the CFX-96 Real-Time PCR detection system platform (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Melt-curve analysis verified that the primer sets for each Q-PCR assay generated one single product and no primer-dimer artifacts. Primers and PCR conditions have been published by [41,42]. For each assay, a triplicate, two-fold cDNA dilution series made from total RNA from different exposure groups was used to determine amplification efficiencies (E). These were calculated as the slope from the plot of log cDNA concentration versus threshold cycle (Ct) values using the following formula: E = 10(−1/slope). This efficiency was used to correct for differences in amplification efficiency when calculating gene expression, according to [43]. Expression is presented as relative to the endogenous reference gene EF α1a [44]. The EF α1a did not change over time or differ between treatments in the present study.
2.5. External Welfare Analysis
External welfare indicators were scored as described in [45]. In short, external welfare indicators were recorded for 10 fish per tank at the final sampling point. Fin condition was assessed according to [46], where each fin was examined and given a score from 0 (no damage/fin erosion) to 5 (severe damage/fin erosion). In addition, the left and right pectoral and pelvic fins were measured for length (mm). Each fish was also examined for the presence of cataracts, skin lesions, and operculum shortening and scored as described in [47].
2.6. Water Quality and Specific Oxygen Consumption
The tank water flow rates were adjusted by measuring the time it took to fill a 10-liter container with outlet water from each tank and were continually monitored and adjusted weekly throughout the experiment. The water velocity in each tank was kept stable and equal by adjusting the angle of the inlet water pipe. The water temperature was measured daily in the outlet of each tank with a handheld YSI 550A (Xylem Inc., Yellow Springs, OH, USA) and was 9.3 °C ± 0.3 throughout the experiment.
To achieve a good basis for accurate MO2 estimations in the present trial, an automatic oxygen level regulation and monitoring system was used throughout the whole experiment, and various water quality parameters were closely followed (Table 1). The oxygen level in the header tank and in the outlet water was continuously monitored and logged (every 10 min) from each tank by an Oxyguard Commander System (Oxyguard, Farum, Denmark). Based on actual oxygen level information, the same system controlled the injection of oxygen into the inlet water via the corresponding header tank to always maintain oxygen saturation above 80% in the outlet water. The overall specific oxygen consumption (MO2; mgO2 kg−1 min−1) was based on the difference in O2 between the header tank and tank outlet logged every 10 min throughout the experiment. The specific oxygen consumption () was calculated from the 1st measurement every hour, every second day in the period October 27–December 19 (n = 28), based on the formula:
MO2 = (Cinlet − Coutlet) × Q/B
C is the oxygen concentration (mg L−1), Q is the flow (L min−1), and B is the fish biomass (kg).

Table 1.
Water quality at 4 different levels of specific water flow in full strength sea water (34‰) over the 8-week experimental period (n = 2 tanks). O2 (Oxygen), TAN (total ammonia nitrogen) and CO2 (Carbon dioxide) levels were measured in outlet of each tank. Un-ionized ammonia (NH3-N; µg L−1) is calculated from TAN, pH, salinity and temperature. Significant differences between SWF treatments are indicated by different letters (one-way ANOVA, p < 0.05). Data given are means ± SEM.
Table 1.
Water quality at 4 different levels of specific water flow in full strength sea water (34‰) over the 8-week experimental period (n = 2 tanks). O2 (Oxygen), TAN (total ammonia nitrogen) and CO2 (Carbon dioxide) levels were measured in outlet of each tank. Un-ionized ammonia (NH3-N; µg L−1) is calculated from TAN, pH, salinity and temperature. Significant differences between SWF treatments are indicated by different letters (one-way ANOVA, p < 0.05). Data given are means ± SEM.
| Parameter | 0.2 L kg−1 min−1 | 0.3 L kg−1 min−1 | 0.4 L kg−1 min−1 | 0.5 L kg−1 min−1 |
|---|---|---|---|---|
| Water flow (L min−1) | 7.5 | 11.25 | 15 | 18.75 |
| Tank exchange time (min) | 66.6 | 44.4 | 33.3 | 26.6 |
| Temperature (°C) | 9.3 ± 0.01 | 9.3 ± 0.01 | 9.3 ± 0.01 | 9.3 ± 0.01 |
| O2 (mg L−1) | 7.97 ± 0.01 a | 8.10 ± 0.01b | 9.82 ± 0.01 d | 8.63 ± 0.01 c |
| pH | 6.94 ± 0.05 a | 7.20 ± 0.05 b | 7.37 ± 0.04 c | 7.46 ± 0.05 c |
| CO2 (mg L−1) | 15.74 ± 1.83 a | 8.6 ± 0.88 b | 5.60 ± 0.48 bc | 4.79 ± 0.62 c |
| TAN (mg N L−1) NH3-N (µg L−1) | 0.76 ± 0.11 a 1.0 a | 0.48 ± 0.07 b 1.2 ab | 0.35 ± 0.05b 1.3 b | 0.36 ± 0.05 b 1.6 c |
Every second week, pH was measured directly in the outlet of each tank (Seven Easy pH meter, Mettler-Toledo AG, Schwerzenbach, Germany; Table 1), and water samples were collected in sealable airtight glass bottles to monitor CO2 levels [48] and in acid-washed tubes for TAN measurements. The carbon dioxide concentrations were calculated based on the percentage of carbon dioxide in the total carbonate concentration [49]. Before TAN was analyzed, pH was reduced to below 2 in each sample using sulfuric acid (H2SO4). TAN concentrations were analyzed according to ‘Norwegian Standard 2005, NS-EN ISO 11732’ using a Seal autoanalyzer (Omni Process AB, Solna, Sweden).
2.7. Growth and Condition Factor
To assess the effects of reduced SWF on growth and condition factors, a sub-group of 15 randomly selected fish from each tank were individually tagged (11 October, PIT tags, Trovan Ltd., Melton, North Ferriby, UK). Fork length (L) was measured to the nearest 0.1 cm and weight (W) to the nearest 0.1 g and was recorded during tagging and at the end of the experiment. The growth was calculated as a specific growth rate (SGR), where W1 and W2 are weights at days T1 (start of experiment) and T2 (after 8 weeks), according to the equation:
SGR = (lnW2 − lnW1) × 100/(T2 − T1).
Fulton’s condition factor (K) was calculated based on the formula:
K = 100W × L−3
2.8. Statistics
Statistical analyses and graphics were made using STATISTICA (version 12). All data sets were tested for normality using the Kolmogorov-Smirnov test. The Hartley F-max test was used to test for homogeneity of variances. A two-way nested ANOVA [50] with tanks nested within specific water flow (SWF) and sample time points was used to compare the effect of treatment time on physiological parameters (n = 12). A two-way nested ANOVA with the tank nested in SWF was also used to assess the effect of SWF on performance parameters (SGR, weight, fork length, and condition factor) of individually tagged fish (n = 27–30). The overall MO2 (n = 28) after 8 weeks of treatment was tested with a one-way ANOVA, and a two-way ANOVA [50] was used to compare the effect of SWF and time of day on MO2 (n = 28). The change in MO2 after feeding was tested with a linear regression, and the parallelism of regression lines was tested with an analysis of covariance (ANCOVA [50]). All welfare score data (n = 20), except fin length, were arcsine square root transformed. Subsequently, the effect of SWF on external welfare was analyzed with a two-way nested ANOVA, with tank nested in SWF. Differences in water quality were also tested with a one-way ANOVA (n = 2 tanks). Significant ANOVAs were followed by a Student-Newman-Keuls multiple comparison post hoc test (SNK). All data given are means ± SEM. A significance level (α) of 0.05 was used unless stated otherwise.
3. Results
3.1. Blood Chemistry
Measurements conducted prior to the establishment of the experimental SWF treatments showed that seawater transfer and acclimation did not affect blood pCO2 and HCO3− levels (p < 0.05; results not shown). Both blood pCO2 (Figure 1A) and HCO3− (Figure 1B) levels were significantly influenced by SWF and the duration of treatment (p < 0.001). The most prominent increase in blood pCO2 level was in the lowest SWF treatment (0.2 L kg fish−1 min−1) which had higher levels compared to other treatments from week 2 till the end of the experiment (p < 0.01). Blood HCO3− level followed a similar development as blood pCO2, with an initial rise in blood HCO3− in all treatments in the first two weeks of the experiment (p < 0.001; Figure 1B), followed by a period of stabilization. Similarly to the blood pCO2, the HCO3− level was also significantly higher in the 0.2 L kg fish−1⋅min−1 treatment from week two and on (p < 0.01). Furthermore, the 0.3 L kg fish−1 min−1 treatment had elevated HCO3− levels compared to the two higher SWF treatments (0.4 and 0.5 L kg fish−1 min−1; p < 0.05).
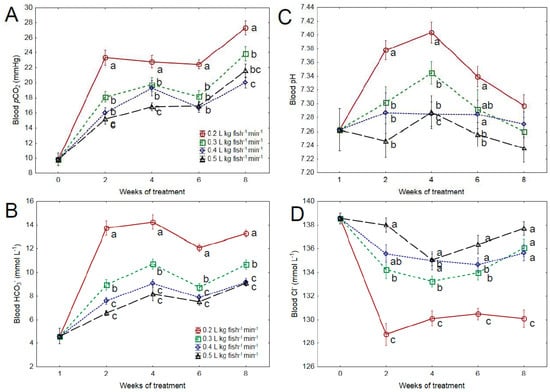
Figure 1.
(A) Blood pCO2 (mmHg), (B) blood bicarbonate (HCO3−, mmol L−1), (C) blood pH levels, and (D) blood chloride (Cl−, mmol L−1) in Atlantic salmon post-smolts reared at four different levels of specific water flow (SWF: 0.2, 0.3, 0.4, and 0.5 L kg fish−1 min−1). Significant differences between SWF treatments at each time point are indicated by different letters (two-way nested ANOVA, p < 0.05). n = 12, and all data given are means ± SEM.
Blood pH was significantly influenced by time and reduced SWF (Figure 1C, p < 0.001). In the lowest SWF treatment (0.2 L kg fish−1 min−1), blood pH was significantly elevated compared to the highest SWF (0.5 L kg fish−1 min−1) for the first 6 weeks of the experiment (p < 0.001).
Prior to the establishment of SWF treatments, a significant increase in blood chloride levels (Cl−) was observed when increasing the salinity from 25 to 34 ppt (D3; p < 0.05). Cl− levels remained at the increased level until the start of the experiment (results not shown). Plasma Cl− levels were inversely related to the observed changes in blood pCO2 and HCO3− in all treatments and decreased in a dose-response manner to reduced specific water flow (Figure 1D, p < 0.001). In the 0.2 L kg fish−1 min−1 treatment, Cl− levels were significantly lower than in other treatments throughout the experiment (p < 0.01). There was also a reduction in blood Cl− concentration in the 0.3 L kg fish−1 min−1 treatment compared to the 0.5 L kg fish−1 min−1 treatment at weeks 2 and 6.
Prior to the establishment of SWF treatments, there was a significant increase in blood sodium (Na+) when increasing the salinity from 0 ppt to 17 ppt on D1. Na+ levels stabilized at this level until the start of the experiment (results not shown). Neither exposure time nor SWF influenced blood sodium levels (Na+, p > 0.05, Figure 2).
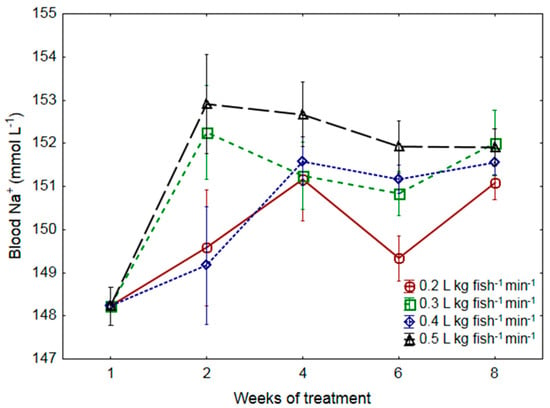
Figure 2.
Blood sodium (Na+; mmol L−1) levels in Atlantic salmon post-smolts raised at four different levels of specific water flow (SWF: 0.2, 0.3, 0.4, and 0.5 L kg fish−1 min−1). Significant differences between different levels of SWF at each time point are indicated by different letters (two-way nested ANOVA, p < 0.05). n = 12, and all data given are means ± SEM.
No significant differences in blood glucose between treatments were observed at any of the sampling points (results not shown).
3.2. Gill NKA α1a and α1b mRNA Levels and Gill NKA
A gradual increase in gill NKA was observed during the seawater acclimation period, reaching peak levels of 11.2, 12.2, 12.3, and 15.1 µmol ADP mg protein−1 h−1 in the 0.2, 0.3, 0.4, and 0.5 L kg fish−1 min−1 treatments, respectively, at week 2 (Figure 3). During the gradual 4-day transition to seawater, D1 to D4, a significant decline in gill NKA α1a (freshwater isoform) mRNA levels was recorded, which reached transcript levels close to 0 after 2 weeks in seawater (Figure 4, p < 0.001). There were no significant differences among groups during this period. In the same period, the seawater isoform NKA α1b mRNA showed the opposite pattern, with levels increasing to reach peak levels at the start of the experiment. Following the peak, gill NKA α1b mRNA levels declined to significantly lower levels by the end of the experiment (Figure 4, p < 0.05). No differences among treatments were registered.
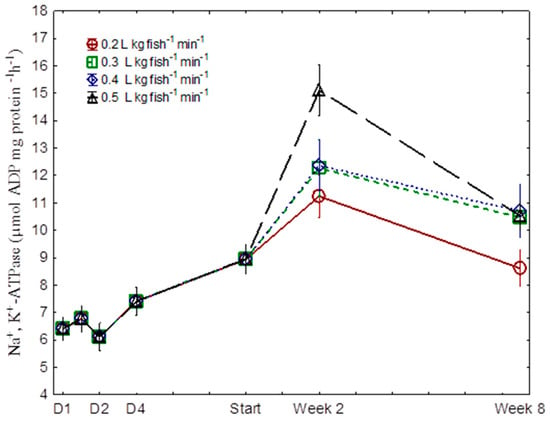
Figure 3.
Gill Na+, K+-ATPase (NKA) activity in Atlantic salmon post-smolts reared at four different levels of specific water flow (SWF: 0.2, 0.3, 0.4, and 0.5 L kg fish−1 min−1). D1–D4 refers to the four days of gradual acclimation to full-strength sea water. Significant differences between different levels of SWF at each time point are indicated by different letters (two-way nested ANOVA, p < 0.05). n = 12, and the data given are means ± SEM.
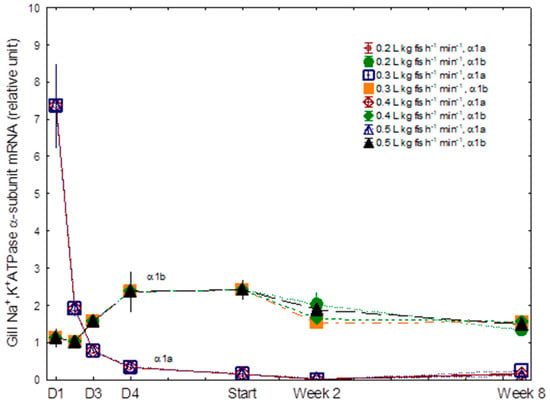
Figure 4.
Gill NKA α1a and α1b relative mRNA expression in Atlantic salmon post-smolts reared at four different levels of specific water flow (0.2, 0.3, 0.4, and 0.5 L kg fish−1 min−1). See Figure 5 for other details.
3.3. External Welfare Indicators
Fin damage such as erosion, splitting, malformations, and fin ray damage were the most commonly observed pathologies. However, these were not affected by SWF, nor were the lengths of the pelvic and pectoral fins (p > 0.05, lengths not shown). Overall, reduced SWF did not affect any of the external welfare indicators (fin, skin, cataracts, and operculum shortening) studied (p > 0.05, Table 2).

Table 2.
External welfare analysis of post-smolt Atlantic salmon after 8 weeks exposure to four different levels of specific water flow (SFW: 0.2, 0.3, 0.4 and 0.5 L kg fish−1 min−1). Each indicator was given a score, from 0 (no damage) to 2 (severe damage) for operculum, cataract, and skin lesions. Fins are scored from 0 (no damage) to 5 (severe damage). No Significant differences between SWF treatments were detected (two-way nested ANOVA, p > 0.05). n = 20 and data given are means ± SEM.
3.4. Oxygen Consumption
The overall highest mean oxygen consumption was observed in the two treatments with the lowest SWF, with 2.6 mg O2 kg−1 min−1 being consumed both in the 0.2 and 0.3 L kg fish−1 min−1 treatments (Table 3, p < 0.05). The overall MO2 was 2.4 mg O2 kg−1 min−1 in the 0.5 L kg fish−1 min−1 treatment, a significant reduction compared to the 0.2 and 0.3 L kg fish−1 min−1 treatments (p < 0.05). In the 0.4 L kg fish−1 min−1 treatment, the MO2 was 1.8 mg O2 kg−1 min−1, which was significantly lower than all other treatments (p < 0.001). A significant increase in oxygen consumption was observed during the period 08:00–13:00 in the 0.3, 0.4, and 0.5 L kg fish−1 min−1 treatments (Figure 5, p < 0.05). During 13:00–15:00, the oxygen consumption in the same treatments started to decline, and 10–12 h later (approximately 02:00), values returned to the baseline MO2 for the treatment (p < 0.05). Such a pattern was not observed in the lowest SWF treatment (0.2 L kg fish−1 min−1); instead, here the MO2 significantly decreased following the commencement of feeding (09:00–10:00) (p < 0.05), resulting in non-parallel regression lines (p < 0.05, ANCOVA) for this feeding time.

Table 3.
Specific growth rate (SGR; % bodyweight day−1), fork length (L), Weight (W), condition factor (K) and specific oxygen consumption (MO2; mg kg−1 min−1) of Atlantic salmon post-smolts reared at four different levels of specific water flow (SWF: 0.2, 0.3, 0.4 and 0.5 L kg fish−1 min−1). All data were collected after an experimental period of 8 weeks (20 December). Data given are means ± SEM. Significant differences between SWF treatments are indicated by different letters (p < 0.05, two-way nested ANOVA).
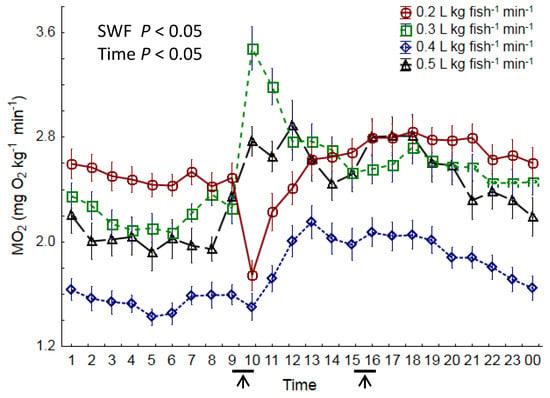
Figure 5.
Diurnal specific oxygen consumption (MO2) in Atlantic salmon post-smolts reared at four different levels of specific water flow (0.2, 0.3, 0.4, and 0.5 L kg fish−1 min−1) for 8 weeks. ↑ indicates feeding time. n = 28 days, and all data are given as means ± SEM.
3.5. Growth and Condition Factors
The fish density range was similar in all treatments (Table 3). The mortality was low in the experiment and not related to the experimental treatment (p > 0.25). Fish in the 0.2 L kg fish−1 min−1 had a significantly higher average start weight of 121.4 g compared to 113.8, 110.7, and 108.7 g in the 0.3, 0.4, and 0.5 L kg fish−1 min−1, respectively (p < 0.05, Table 3). Fish in the 0.2 L kg fish−1 min−1 also had a higher average fork length than other treatments and a higher condition factor than fish in the 0.3 and 0.5 L kg fish−1 min−1 at the start of the experiment (p < 0.05, results not shown). At the end of the experiment, there was no longer a significant difference in weights between treatments (p > 0.05, Table 3). Furthermore, no difference in average length or condition factor was registered at the end of the experiment. Furthermore, reduced SWF did not affect the specific growth rate during the experimental period (p > 0.45, Table 3).
4. Discussion
4.1. Water Quality and Blood Chemistry
As SWF is reduced, less new water dilutes the metabolic waste from the fish in the culture tank. The effect of this increased intensity was clear on the water quality in this study. Both TAN and CO2 increased as conditions intensified, and with a rise in CO2 levels, the pH declined accordingly. The mean CO2 level in the water was three times higher in the lowest SWF treatment compared to the highest SWF. In this experiment, the first blood samples were taken after 2 weeks, and blood pH had been restored in the 0.3, 0.4, and 0.5 L kg fish−1 min−1 treatments but was significantly increased in the lowest SWF treatment (0.2 L kg fish−1 min−1), likely due to the elevated pCO2 and increased level of blood bicarbonate. Furthermore, to maintain electroneutrality in the blood, the branchial chloride influx rate is reduced, subsequently decreasing plasma chloride levels [51]. This compensatory response was sustained in the 0.2 L kg fish−1 min−1 treatment throughout the whole experiment. Long-term reductions in Cl− in response to increased water CO2 have earlier been observed in post-smolts exposed to 26 mg L−1 of CO2 for 43 days [52] and in parr exposed to 17–18 mg L−1 of CO2 for 42 days in freshwater [53]. As no effects on growth were observed in this study, which is considered a chronic stress response, the scope for the physiological compensatory responses (described above) was likely not exceeded in the lowest SWF. However, these responses are energy-intensive, as shown by increased oxygen consumption. It can be speculated that if the experiment had lasted longer, a redistribution of energy from maintenance functions such as growth and immune functions may have been observed in the lowest SWF (0.2 L kg fish−1 min−1) treatment.
4.2. Osmoregulation
The sharp decrease in NKA α1a isoform expression and the increase in NKA α1b with increased salinity support earlier findings demonstrating that α1b is the seawater adaptive isoform and α1a is the freshwater isoform [32,41,54,55]. There was no difference in gene expression of either of the NKA isoforms or differences in enzyme activity between treatments, suggesting that within the time span of this study, reducing SWF down to 0.2 L kg fish−1 min−1 does not impact osmoregulation in post-smolts in seawater.
4.3. External Welfare Indicators
Cataracts, fin, skin, and opercular damage represent injuries to live tissue and have been associated with common rearing practices in farmed salmonids [20,29,45]. Damaged epithelia can lead to osmotic disturbances, represent invasion routes for pathogens, and therefore increase the risk for disease [56,57]. In the present study, reduced flow did not have a detectable effect on any of the macroscopic external welfare indicators studied; to our knowledge, this has not earlier been addressed in relation to reduced specific water flow. The present results are in line with [30], which showed that post-smolts in stocking densities up to 75 kg m−3 had no negative effect on the same external welfare indicators, and [47], which concluded that sub-optimal water quality conditions did not affect external welfare in Atlantic salmon. In a skin health analysis that was performed as part of the present study, it was found that a SWF of 0.3 L kg fish−1 min−1 and below activated transcription of genes associated with immune responses and mucus production in the skin [58]. Hence, a skin health analysis is suggested as a useful tool for detecting early responses to environmental changes.
4.4. Oxygen Consumption
In any aquaculture facility, the balance between oxygen consumption (MO2) and oxygen supply is critical; however, it has been difficult to estimate MO2 rates in commercial operations due to the large number of effectors and their unpredictability (reviewed in [3]). The overall MO2 was lowest in the 0.4 L kg fish−1 min−1 treatment and not in the highest SWF treatment, 0.5 L kg fish−1 min−1 (control), as might be expected; this is likely due to the large number of factors affecting MO2. Although measures were taken to maintain equal water velocities in each tank, it cannot be excluded that a slightly higher water velocity in the 0.5 L kg fish−1 min−1, due to a higher SWF rate, caused the increased MO2 in this treatment.
The diurnal variation in MO2 with an increase following feeding is known as specific dynamic action (SDA) and accounts for all metabolic expenses associated with digestion, absorption, and storage of nutrients [59]. In the present experiment, the SDA effect was most apparent after the first meal in the 0.3–0.5 L kg fish−1 min−1, whereas no clear SDA effect was observed in the lowest SWF treatment over a 24-h period. Instead, at the lowest SWF (0.2 L kg fish−1 min−1), the overall oxygen demand over the 24-h period was higher, indicating that energy was allocated to physiological compensatory processes instead. Similarly, [60] found a reduced SDA effect in sea bass, Dicentrarchus labrax, and turbot, Scophthalmus maximus, exposed to hypoxic conditions; for sea bass, this effect was explained by a reduced feed intake. Reduced feed intake was not observed in the turbot, indicating that behavioral adaptations like reduced swimming activity may be important in reallocating energy during unfavorable water quality conditions. In line with this, the decreased MO2 observed after feeding in the 0.2 L kg fish−1 min−1 treatment in the present trial may indicate decreased activity. In support of this, Ref. [61] found that chronic but mild CO2 exposure increased feed intake and decreased swimming activity in sea bass; similarly, no effects on growth were observed in that study. Overall, the diurnal fluctuations and the effect of SWF on oxygen demand need to be taken into consideration when designing the oxygen supply system for large-scale semi-closed sea systems.
4.5. Growth
No effects on any of the growth indicators measured were detected in the present study. This is consistent with [62], who did not detect any effects on growth or FCR after 145 days of exposure to 0.2 L kg fish−1 min−1 in 8 °C seawater. Assuming that CO2 is the first limiting factor in our experiment, our results are also in agreement with [52], which saw only slight effects on growth in post-smolts after 43 days of CO2 exposure up to 26 mg L−1 in 15–16 °C seawater.
4.6. Application at the Industrial Scale
This experiment investigated four rearing intensities at an intermediate (9.3 °C) temperature. A higher experimental temperature would have increased the fish metabolism and intensified the rearing conditions accordingly, and vice versa for lower temperatures. Thus, the lower SWF limit suggested in this study could be too low for higher temperatures and too conservative for temperatures lower than 9.3 °C [63]. However, according to available reports from commercial S-CCS production [64,65,66], a temperature of 9.3 ± 2 °C appears to be highly relevant for most of the production period in the reported S-CCS cases. The SWF limit will also depend on post-smolt size since smaller fish have a higher mass excretion rate [10]. According to recent post-smolt growth models [67], it takes approximately 4–7 months to produce post-smolts from 100 g up to 1 kg. Therefore, results from the present study need to be verified in longer-term, large-scale studies covering this size range for post-smolt Atlantic salmon. Although the SWF guidelines in this paper should be applied with consideration to the prevailing environmental and biological factors, this paper reveals information that is highly requested by salmon farmers considering or producing post-smolts in S-CCS.
5. Conclusions
The present study shows that post-smolt Atlantic salmon kept at an intermediate temperature (9.3 °C) in flow-through systems can elicit physiological responses to compensate for reduced specific water flow down to 0.2 L kg fish−1 min−1. However, the responses observed have an energetic cost, as revealed by increased oxygen consumption. Hence, based on the present results, it is suggested that without any in-tank water treatment, specific water flow should be maintained above 0.3 L kg fish−1 min−1 at a post-smolt size of 100–200 g because physiological regulatory responses are energy-costly. Future studies should concentrate on the longer-term effects and the allostatic costs of physiological regulatory responses to decreased water flow/increased CO2.
Author Contributions
S.O.H., T.O.N., B.F.T., L.O.E.E., H.S., H.T., F.M., S.O.S. and A.K.D.I. established the project, gathered the funding, and designed the experiment. S.C., T.O.N., J.K., C.D.H., S.F., C.P. and S.O.H. carried out the samplings, performed water quality, NKA, and blood chemistry analyses. S.C., A.K.D.I. and S.O.H. carried out the data analysis. S.C. drafted and wrote the manuscript. A.K.D.I., T.O.N., C.D.H. and S.O.H. provided editorial assistance and helped write the document. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Fishery and Aquaculture Industry Research Fund FHF (project Postsmolt, 900816) and the Research Council of Norway, project Optimized Postsmolt Production OPP (217502/E40).
Institutional Review Board Statement
The present field trials were approved by the local responsible laboratory animal science specialist under the surveillance of the Norwegian Animal Research Authority (NARA) and registered by the Authority.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pillay, T.V.R. Aquaculture and the Environment, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; p. 208. [Google Scholar]
- Gullestad, P.; Bjørgo, S.; Eithun, I.; Ervik, A.; Gudding, R.; Hansen, H.; Johansen, R.; Osland, A.; Rødseth, M.; Røsvik, I.; et al. Effektiv og Bærekraftig Arealbruk i Havbruksnæringen—Areal Til Begjær; Kystdepartementet: Oslo, Norway, 2011; p. 190. [Google Scholar]
- Thorarensen, H.; Farrell, A.P. The biological requirements for post-smolt Atlantic salmon in closed-containment systems. Aquaculture 2011, 312, 1–14. [Google Scholar] [CrossRef]
- Iversen, A.; Andreassen, O.; Hermansen, Ø.; Andre, T.; Terjesen, B.F. Aquaculture technology and competitiveness. Nofima. Rapp. 2013, 1–32. [Google Scholar]
- Fivelstad, S.; Binde, M. Effects of reduced waterflow (increased loading) in soft water on Atlantic salmon smolts (Salmo salar L.) while maintaining oxygen at constant level by oxygenation of the inlet water. Aquac. Eng. 1994, 13, 211–238. [Google Scholar] [CrossRef]
- Fivelstad, S.; Olsen, A.B.; Kloften, H.; Ski, H.; Stefansson, S. Effects of carbon dioxide on Atlantic salmon (Salmo salar L.) smolts at constant pH in bicarbonate rich freshwater. Aquaculture 1999, 78, 171–187. [Google Scholar] [CrossRef]
- Fivelstad, S.; Bergheim, A.; Holland, P.M.; Fjermedal, A.B. Water flow requirements in the intensive production of Atlantic salmon (Salmo salar L.) parr-smolt at two salinity levels. Aquaculture 2004, 231, 263–277. [Google Scholar] [CrossRef]
- Stefansson, S.O.; Nilsen, T.O.; Ebbesson, L.O.E.; Wargelius, A.; Madsen, S.S.; Bjornsson, B.T.; McCormick, S. Molecular mechanisms of continuous light inhibition of Atlantic salmon parr-smolt transformation. Aquaculture 2007, 273, 235–245. [Google Scholar] [CrossRef]
- Randall, D.J.; Tsui, T.K.N. Ammonia toxicity in fish. Mar. Poll. Bull. 2002, 45, 17–23. [Google Scholar] [CrossRef]
- Terjesen, B.F. Nitrogen excretion. In Fish Larval Physiology; Finn, R., Ed.; Science Publishers: New York, NY, USA, 2008; pp. 263–302. [Google Scholar]
- Randall, D.; Wright, P. The interaction between carbon dioxide and ammonia excretion and water pH in fish. Can. J. Zool. 1989, 67, 2936–2942. [Google Scholar] [CrossRef]
- Forsberg, O.I. The impact of varying feeding regimes on oxygen consumption and excretion of carbon dioxide and nitrogen in post-smolt Atlantic salmon Salmo salar L. Aquac. Res. 1997, 28, 29–41. [Google Scholar] [CrossRef]
- Bergheim, A.; Seymour, E.A.; Sanni, S.; Tyvold, T.; Fivelstad, S. Measurements of oxygen consumption and ammonia excretion of Atlantic salmon (Salmo salar L.) in commercial-scale, single-pass freshwater and seawater landbased culture systems. Aquac. Eng. 1991, 10, 251–267. [Google Scholar] [CrossRef]
- Sanni, S.; Forsberg, O.I. Modelling pH and carbon dioxide in single-pass sea-water aquaculture systems. Aquac. Eng. 1996, 15, 91–110. [Google Scholar] [CrossRef]
- Eddy, F.; Lomholt, J.; Weber, R.; Johansen, K. Blood respiratory properties of rainbow trout (Salmo gairdneri) kept in water of high CO2 tension. J. Exp. Biol. 1997, 67, 37–47. [Google Scholar] [CrossRef]
- Claiborne, J.B.; Edwards, S.L.; Morrison-Shetlar, A.I. Acid–base regulation in fishes: Cellular and molecular mechanisms. J. Exp. Zool. 2002, 293, 302–319. [Google Scholar] [CrossRef]
- Heisler, N. Role of ion transfer processes in acid-base regulation with temperature changes in fish. Am. J. Physiol. 1984, 246, R441–R451. [Google Scholar] [CrossRef]
- Cripps, S.J.; Bergheim, A. Solids management and removal for intensive land-based aquaculture production systems. Aquac. Eng. 2000, 22, 33–56. [Google Scholar] [CrossRef]
- Segner, H.; Sundh, H.; Buchmann, K.; Douxfils, J.; Sundell, S.K.; Mathieu, C.; Ruane, N.; Jutfelt, F.; Toften, H.; Vaughan, L. Health of farmed fish: Its relation to fish welfare and its utility as welfare indicator. Fish Physiol. Biochem. 2012, 38, 85–105. [Google Scholar] [CrossRef]
- Turnbull, J.; Kadri, S. Safeguarding the many guises of farmed fish welfare. Dis. Aquat. Org. 2007, 75, 173–182. [Google Scholar] [CrossRef]
- Kristensen, T.; Åtland, Å.; Rosten, T.; Urke, H.A.; Rosseland, B.O. Important influent-water quality parameters at freshwater production sites in two salmon producing countries. Aquac. Eng. 2009, 41, 53–59. [Google Scholar] [CrossRef]
- Wright, P.A.; Perry, S.F.; Moon, T.W. Regulation of hepatic gluconeogenesis and glycogenolysis by catecholamines in rainbow trout during environmental hypoxia. J. Exp. Biol. 1989, 147, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Ann. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef]
- Korte, S.M.; Olivier, B.; Koolhaas, J.M. A new animal welfare concept based on allostasis. Physiol. Behav. 2007, 92, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.B. Stress and fish reproduction: The roles of allostasis and hormesis. Gen. Comp. Endocrinol. 2010, 165, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Sterling, P. Allostasis: A model of predictive regulation. Physiol. Behav. 2012, 106, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Broom, D.M. Animal welfare: Concepts and measurement. J. Anim. Sci. 1991, 69, 4167–4175. [Google Scholar] [CrossRef] [PubMed]
- Ellis, T.; North, B.; Scott, A.; Bromage, N.; Porter, M.; Gadd, D. The relationships between stocking density and welfare in farmed rainbow trout. J. Fish Biol. 2002, 61, 493–531. [Google Scholar] [CrossRef]
- Calabrese, S.; Nilsen, T.O.; Kolarevic, J.; Ebbesson, L.O.E.; Pedrosa, C.; Fivelstad, S.; Hosfeld, C.; Stefansson, S.; Terjesen, B.; Takle, H. Stocking density limits for post-smolt Atlantic salmon (Salmo salar L.) emphasis on production performance and welfare. Aquaculture 2007, 468, 363–370. [Google Scholar] [CrossRef]
- McCormick, S.D. Methods for Nonlethal Gill Biopsy and Measurement of Na+, K+-ATPase Activity. Can. J. Fish. Aquat. Sci. 1993, 50, 656–658. [Google Scholar] [CrossRef]
- Stefansson, S.O.; Björnsson, B.T.; Sundell, K.; Nyhammer, G.; McCormick, S.D. Physiological characteristics of wild Atlantic salmon post-smolts during estuarine and coastal migration. J. Fish Biol. 2003, 63, 942–955. [Google Scholar] [CrossRef]
- Boutilier, R.G.; Heming, T.A.; Iwama, G.K. Appendix: Physicochemical parameters for use in fish respiratory physiology. Fish Physiol. 1984, 10, 403–430. [Google Scholar]
- Boutilier, R.G.; West, T.G.; Webber, D.M.; Pogson, G.H.; Mesa, K.A.; Wells, J.; Wells, M.J. The protective effects of hypoxia-induced hypometabolism in the Nautilus. J. Comp. Physiol. B. 2000, 170, 261–268. [Google Scholar] [CrossRef]
- Harrenstien, L.A.; Tornquist, S.J.; Miller-Morgan, T.J.; Fodness, B.G.; Clifford, K.E. Evaluation of a point-of-care blood analyzer and determination of reference ranges for blood parameters in rockfish. J. Am. Vet. Med. Ass. 2005, 226, 255–265. [Google Scholar] [CrossRef]
- Cooke, S.J.; Suski, C.D.; Danylchuk, S.E.; Danylchuk, A.J.; Donaldson, M.F.; Pullen, C.; Bulte, G.; O’Toole, A.; Murchie, K.J.; Goldberg, T.J. Effects of different capture techniques on the physiological condition of bonefish Albula vulpes evaluated using field diagnostic tools. J. Fish Biol. 2008, 73, 1351–1375. [Google Scholar] [CrossRef]
- DiMaggio, M.A.; Ohs, C.L.; Petty, B.D. Evaluation of a Point-of-Care Blood Analyzer for Use in Determination of Select Hematological Indices in the Seminole Killifish. North Am. J. Aquac. 2010, 72, 261–268. [Google Scholar] [CrossRef]
- Harter, T.S.; Shartau, R.B.; Brauner, C.J.; Farrell, A.P. Validation of the i-STAT system for the analysis of blood parameters in fish. Cons. Physiol. 2014, 2, cou037. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Chomczynski, P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Bio. Tech. 1993, 15, 532–535. [Google Scholar]
- Nilsen, T.O.; Ebbesson, L.O.; Madsen, S.S.; McCormick, S.D.; Anderson, E.; Bjornsson, B.T.; Prunet, P.; Stefansson, S.O. Differential expression of gill Na+, K+-ATPaseα-and β-subunits, Na+, K+, 2Cl-cotransporter and CFTR anion channel in juvenile anadromous and landlocked Atlantic salmon Salmo salar. J. Exp. Biol. 2007, 210, 2885–2896. [Google Scholar] [CrossRef]
- Nilsen, T.O.; Ebbesson, L.O.E.; Kverneland, O.G.; Kroglund, F.; Finstad, B.; Stefansson, S.O. Effects of acidic water and aluminum exposure on gill Na+, K+-ATPase α-subunit isoforms, enzyme activity, physiology and return rates in Atlantic salmon (Salmo salar L.). Aquat. Toxiol. 2010, 97, 250–259. [Google Scholar] [CrossRef]
- Pfaffl, M.W. Quantification strategies in real-time PCR. In AZ of Quantitative PCR; Bustin, S.A., Ed.; International University Line: La Jolla, CA, USA, 2004; pp. 89–113. [Google Scholar]
- Olsvik, P.A.; Lie, K.K.; Jordal, A.-E.O.; Nilsen, T.O.; Hordvik, I. Evaluation of potential reference genes in real-time RT-PCR studies of Atlantic salmon. BMC Molecul. Biol. 2005, 6, 21. [Google Scholar] [CrossRef]
- Kolarevic, J.; Baeverfjord, G.; Takle, H.; Ytteborg, E.; Reiten, B.K.M.; Nergård, S.; Terjesen, B.F. Performance and welfare of Atlantic salmon smolt reared in recirculating or flow through aquaculture systems. Aquaculture 2014, 432, 15–25. [Google Scholar] [CrossRef]
- Hoyle, I.; Oidtmann, B.; Ellis, T.; Turnbull, J.; North, B.; Nikolaidis, J.; Knowles, T.G. A validated macroscopic key to assess fin damage in farmed rainbow trout (Oncorhynchus mykiss). Aquaculture 2007, 270, 142–148. [Google Scholar] [CrossRef]
- Kolarevic, J.; Selset, R.; Felip, O.; Good, C.; Christopher, M.; Snekvik, K.; Takle, H.R.; Ytteborg, E.; Bæverfjord, G.; Åsgård, T.E.; et al. Influence of long term ammonia exposure on Atlantic salmon (Salmo salar L.) parr growth and welfare. Aquac. Res. 2013, 44, 1649–1664. [Google Scholar] [CrossRef]
- Fivelstad, S.; Olsen, A.B.; Åsgård, T.; Bæverfjord, G.; Rasmussen, T.; Vindheim, T.; Stefansson, S. Long-term sublethal effects of carbon dioxide on Atlantic salmon smolts (Salmo salar L.): Ion regulation, haematology, element composition, nephrocalcinosis and growth parameters. Aquaculture 2003, 215, 301–319. [Google Scholar] [CrossRef]
- Gebauer, R.; Eggen, G.; Hansen, E.; Eikebrokk, B. Oppdrettsteknologi; Tapir: Oslo, Norway, 1992; p. 576. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1984; p. 718. [Google Scholar]
- Lloyd, R.; White, W.R. Effect of High Concentration of Carbon Dioxide on the Ionic Composition of Rainbow Trout Blood. Nature 1967, 216, 1341–1342. [Google Scholar] [CrossRef]
- Fivelstad, S.; Haavik, H.; Lovik, G.; Olsen, A.B. Sublethal effects and safe levels of carbon dioxide in seawater for Atlantic salmon postsmolts (Salmo salar L.): Ion regulation and growth. Aquaculture 1998, 160, 305–316. [Google Scholar] [CrossRef]
- Hosfeld, C.D.; Engevik, A.; Mollan, T.; Lunde, T.M.; Waagbø, R.; Olsen, A.B.; Breck, O.; Stefansson, S.; Fivelstad, S. Long-term separate and combined effects of environmental hypercapnia and hyperoxia in Atlantic salmon (Salmo salar L.) smolts. Aquaculture 2008, 280, 146–153. [Google Scholar] [CrossRef]
- McCormick, S.D.; Regish, A.; Christensen, A. Distinct freshwater and seawater isoforms of Na+/K+-ATPase in gill chloride cells of Atlantic salmon. J. Exp. Biol. 2009, 212, 3994–4001. [Google Scholar] [CrossRef]
- Handeland, S.O.; Imsland, A.K.; Nilsen, T.O.; Ebbesson, L.O.E.; Hosfeld, C.D.; Pedrosa, C.; Toften, H.; Stefansson, S.O. Osmoregulation in Atlantic salmon Salmo salar smolts transferred to seawater at different temperatures. J. Fish Biol. 2014, 85, 1163–1176. [Google Scholar] [CrossRef]
- Sundh, H.; Olsen, R.E.; Fridell, F.; Gadan, K.; Evensen, Ø.; Glette, J.; Taranger, G.L.; Myklebust, R.; Sundell, K. The effect of hyperoxygenation and reduced flow in fresh water and subsequent infectious pancreatic necrosis virus challenge in sea water, on the intestinal barrier integrity in Atlantic salmon, Salmo salar L. J. Fish Dis. 2009, 32, 687–698. [Google Scholar] [CrossRef]
- Stien, L.H.; Bracke, M.B.M.; Folkedal, O.; Nilsson, J.; Oppedal, F.; Torgersen, T.; Kittilsen, S.; Midtlyng, P.J.; Vindas, M.A.; Øverli, Ø.; et al. Salmon Welfare Index Model (SWIM 1.0): A semantic model for overall welfare assessment of caged Atlantic salmon: Review of the selected welfare indicators and model presentation. Rev. Aquac. 2013, 5, 33–57. [Google Scholar] [CrossRef]
- Sveen, L.R.; Timmerhaus, G.; Torgersen, J.S.; Ytteborg, E.; Jørgensen, S.M.; Handeland, S.; Stefansson, S.O.; Nilsen, T.O.; Calabrese, S.; Ebbesson, L.; et al. Impact of fish density and specific water flow on skin properties in Atlantic salmon (Salmo salar L.) post-smolts. Aquaculture 2016, 464, 629–637. [Google Scholar] [CrossRef]
- Jobling, M. The influences of feeding on the metabolic rate of fishes: A short review. J. Fish Biol. 1981, 18, 385–400. [Google Scholar] [CrossRef]
- Pichavant, K.; Person-Le-Ruyet, J.; Bayon, N.L.; Severe, A.; Roux, A.L.; Boeuf, G. Comparative effects of long-term hypoxia on growth, feeding and oxygen consumption in juvenile turbot and European sea bass. J. Fish Biol. 2001, 59, 875–883. [Google Scholar] [CrossRef]
- Santos, G.A.; Schrama, J.W.; Capelle, J.; Rombout, J.H.W.M.; Verreth, J.A.J. Effects of dissolved carbon dioxide on energy metabolism and stress responses in European seabass (Dicentrarchus labrax). Aquac. Res. 2013, 44, 1370–1382. [Google Scholar] [CrossRef]
- Forsberg, O.I.; Bergheim, A. The impact of constant and fluctuating oxygen concentrations and two water consumption rates on post-smolt Atlantic salmon production parameters. Aquac. Eng. 1996, 15, 327–347. [Google Scholar] [CrossRef]
- Mathisen, F. Vannforbruksfaktor-ny Parameter for Vurdering av Intensitet og Vannbehov i Settefiskanlegg. Available online: https://www.kyst.no/vannforbruksfaktor-ny-parameter-for-vurdering-av-intensitet-og-vannbehov-i-settefiskanlegg/712880 (accessed on 15 March 2023). (In Norwegian).
- Storsul, T.; Arnfinn, A.; Dalum, A.S.; Romstad, S.; Urke, H.; Calabrese, S.; Larsson, T.; Marte, F. Aquatraz. Report to the Norwegian Directorate of Fisheries. Directorate of Fisheries: Bergen, Norway, 2021. [Google Scholar]
- Øvrebø, T.K.; Balseiro, P.; Imsland, A.K.D.; Stefansson, S.O.; Tveterås, R.; Sveier, H.; Handeland, S. Investigation of growth performance of post-smolt Atlantic salmon (Salmo salar L.) in semi closed containment system: A big-scale benchmark study. Aquac. Res. 2022, 53, 4178–4189. [Google Scholar] [CrossRef]
- Nilsen, A.; Nielsen, K.V.; Bergheim, A. A closer look at closed cages: Growth and mortality rates during production of post-smolt Atlantic salmon in marine closed confinement systems. Aquac. Eng. 2020, 91, 102124. [Google Scholar] [CrossRef]
- Føre, M.; Alver, M.; Alfredsen, J.A.; Marafioti, G.; Senneset, G.; Birkevold, J.; Willumsen, F.V.; Lange, G.; Espmark, Å.; Terjesen, B.F. Modelling growth performance and feeding behaviour of Atlantic salmon (Salmo salar L.) in commercial-size aquaculture net pens: Model details and validation through full-scale experiments. Aquaculture 2016, 464, 268–278. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).