1. Introduction
Organic aquaculture is a rapidly growing sector that focuses on sustainability, animal welfare, and reducing environmental impact. Organic production involves a combination of traditional methods, modern technology, and scientific knowledge to protect the environment, support fair and equitable relationships, and improve quality of life. Organic fish farming aims to reduce antibiotics, pesticides, and synthetic fertilizers, among other things, and promote natural inputs [
1]. In the 27 European Union (EU) countries, the organic production of European sea bass and gilthead seabream in 2020 was 2750 tons, representing only 1.5% of the total production of these species [
2]. The European Market Observatory for Fisheries and Aquaculture (EUMOFA) states that the primary obstacles to organic aquaculture include the general lack of growth, insufficient innovation, and increased costs, particularly regarding organic feed [
2]. To be considered organic certified production, the feed must be produced from: by-products of other organic aquaculture species; organic plant production; trimmings and by-products; fishmeal; and fish oil from certified fisheries, according to EU Regulation 1380/2013.
The demand for certified organic feed ingredients for aquaculture is much higher than the supply worldwide, leading to elevated prices and high production costs [
3]. Although revised EU regulations now allow fishmeal and fish oil derived from sustainable fisheries, other alternative feed ingredients with high levels of essential amino acids (EAA) and lipids are still required. According to Gambelli et al. [
4], it is suggested that enhanced research in aquafeed is necessary to improve the competitiveness of organic fish farming and foster the future growth of organic aquaculture. As such, replacing fishmeal with alternative protein sources in organic aquaculture feeds is particularly interesting. Alternative protein sources can totally or partially replace fishmeal (FM) in aquaculture diets, reducing feed costs and improving sustainability [
5].
With various degrees of success, the replacement of FM in aquaculture diets with various soy products, such as soybean meal, soy protein concentrate, and soy protein isolate, has been described [
6,
7]. Animal protein by-products, such as poultry by-product meal (PBM), have been studied as a partial or total replacement for FM in aquaculture diets due to the increasing cost and limited availability of FM [
8]. Aquaculture protein by-products are a cost-effective and sustainable alternative to traditional fishmeal-based feeds. In addition to reducing the environmental impact of fish processing, these by-products can also help reduce the pressure on wild fish stocks harvested for fishmeal production. A few studies have explored aquaculture protein by-products in aquaculture feed [
9,
10,
11,
12]. Li et al. [
12] evaluated using a mixture of shrimp hydrolysate and plant proteins in diets for largemouth bass. The results showed that the combination could replace up to 30% of the FM in the diet without negatively impacting growth performance [
12]. Similarly, a study by Gunathilaka et al. [
11] investigated the use of shrimp protein hydrolysate and krill meal in the diets of red seabream (
Pagrus major). The results showed that incorporating shrimp protein hydrolysate into the red seabream diet can decrease FM usage by up to 20% [
11]. Previous studies evaluated the use of fish protein hydrolysate (FPH) in the diets of Nile tilapia. The results showed that the use of 10% FPH gave the highest growth performance, feed utilization, and protein utilization [
10].
Gilthead seabream (
Sparus aurata) is a notable species of farmed fish in the Mediterranean countries, with an annual global production of approximately 282.1 thousand metric tons in European countries [
13]. Despite implementing new nutritional strategies that have reduced the use of fishmeal in seabream diets, it is still necessary to minimize fishmeal levels to improve aquaculture sustainability, and certified organic production continues to be a testimonial. Research on organic farming of gilthead seabream is scarce, and most studies focus on growth performance, welfare, and quality aspects compared to conventional farming [
14,
15,
16]. A study on an industrial scale found differences in growth performance, metabolic status, and fillet composition between organically produced fish and conventional ones, indicating the need for more research to improve organic feed formulation [
17]. On the other hand, it was reported by Estevez et al. [
3] that to evaluate new organic ingredients, such as green pea protein and brown seaweed, to replace fish meals in feeds suitable for organic production. No adverse effects were observed on fish growth, their muscles’ composition, health, quality, or nutritional value [
3].
Improving the sustainability of aquaculture through the organic substitution of fishmeal is an important goal that must be reached as soon as possible. Success in achieving this goal must ensure that such substitution does not harm the performance or efficiency of the growth of gilthead seabream.
2. Materials and Methods
2.1. Ethics Approval
Following Royal Decree 53/2013 and the European Directive 2010/63/EU on the protection of animals used for scientific research, the experimental protocol was reviewed and approved by the Ethics and Animal Welfare Committee of the Universitat Politècnica de València (Official Bulletin No. 80 of 06/2014) to minimize the suffering of animals.
2.2. Experimental Conditions
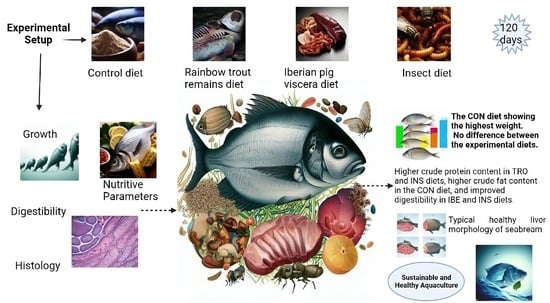
Four different diets were fed to the seabreams for 120 days. In total, 300 fish with an average mean weight of ~93 ± 3.82 g and an average mean length of 16 ± 1.6 were used and were distributed in 12 concrete tanks of 4000 L each with octagonal-shaped tanks.
The experiment was carried out on the Animal Science Department of the Polytechnic University of Valencia (UPV) farm from August to November 2021, after ten days of adaptation for the seabream, which weighed an average of ~93 ± 3.82 g. The trial was within a saltwater recirculating system of 75 m3 of capacity with a rotary mechanical filter and a 6 m3 capacity gravity biofilter, with 25 animals in each tank.
Feeding was done manually until apparent satiety, twice daily, at 09:00 and 16:00, six days a week, from Monday to Saturday. Additionally, throughout the experiment, the physical and chemical parameters of the tanks were monitored: dissolved oxygen 8.7 ± 1.6 mg L−1, temperature 21 ± 1 °C, ammonium level 0.22 mg L−1, nitrates 104.2 mg L−1, nitrites 0.38 mg L−1, salinity 33.3 ± 2.4 g L−1, and pH 7.49. The photoperiod was natural (~12.5 h) (August to November 2021), and the lighting in each tank was the same.
2.3. Experimental Diets
The diets were manufactured at the Universitat Politècnica de València (UPV) facilities using a Clextral BC45 semi-industrial extruder using the cooking-extrusion process (CLEXTRAL BC-45, St. Etienne, France). Four diets were tested in triplicate: the CON diet, with FM as a protein source; the TRO diet, with FM coming from organic trout remains after processing; the INS diet, which uses organic insect meal instead of FM; and the IBE diet, which contains organic Iberian pig viscera to replace FM (
Table 1). The replacement of fishmeal was 100% in all experimental diets.
The following processing parameters were used: a screw speed of 100 rpm, a pressure range of 40–50 atm, and a temperature of 110 °C. Organic raw materials formulated the four extruded diets (
Table 1). All the organic ingredients come from organically certified producers with the EU organic label. Trout was self-processed to extract the meat slices, and just the remaining parts were carefully cut, oven-dried, and ground into a suitable form for use in the feed. The remaining parts used for the Iberian pig meal were the liver, intestines, and heart, which were cut into small pieces. After oven drying, they were ground into a suitable form for incorporation into the feed. The insect component, larval insects, was utilized. They were already dried and then ground for inclusion in the diets. The level of inclusion of the TRO, IBE, and INS was sufficient to replace fishmeal and maintain the total protein constant. The diets were supplemented with sustainable supplies of vegetable methionine and calcium phosphate. Dietary formulation and processing using organic raw components labeled and approved following Regulation (EU) 2018/848 [
18]. Once completed, they were packaged and stored in a commercial refrigerator at 4 °C.
2.4. Growth Control
The fish were monitored daily in tanks and weighed every 30 days while anesthetized with clove oil containing 87% eugenol (Guinama®, Valencia, Spain) in 150 mg L−1 of water. This was done to assess fish growth throughout the experiment, establish growth parameters, and determine the health of the fish. The fish were not fed the day before being weighed. Five fish were sampled at the beginning of the experiment and kept at −30 °C for further analysis of total body composition. At the end of the study, ten animals in each tank were sampled to evaluate biometric parameters. Three fish from each tank (to ensure we have representative samples for analysis) were randomly selected for sampling and pooling to determine the approximate composition and amino acids.
2.5. Analysis of Nutritional Composition and Amino Acids
The whole body of the fish and the composition of the diets were examined using the procedures outlined in AOAC (Association of Official Analytical Chemists) [
19]: dry matter (105 °C to constant weight), ash (incinerated at 550 °C for five hours), crude protein (determined using the direct combustion method DUMAS using LECO CN628, Geleen, The Netherlands), and crude lipid. Diets are extracted with methyl ether using the ANKOMXT10 extractor (Macedon, NY, USA). Each analysis was carried out three times. The procedure previously described by Bosch et al. [
20] was used to analyze the AA of diets and body fish. A Waters HPLC system (Waters 474, Waters, Milford, MA, USA) consisting of two pumps (Model 515, Waters), an autosampler (Model 717, Waters), a fluorescence detector (Model 474, Waters), and a temperature control module was used.
Aminobutyric acid was first introduced as a standard internal pattern prior to hydrolysis. The AA was derivatized using AQC (6-aminoquinolyl-N-hydroxysuccinimidyl carbamate). Methionine and cysteine were recognized as methionine sulphone and cystic acid, respectively, following oxidation with performic acid. After being separated with a reverse-phase C-18 column by Waters Acc, the tag AA was changed to methionine and cystine (150 mm, 3.9 mm). The EAA content of the experimental diets is indicated in
Table 2. Amino acid tests were performed in duplicate on every sample.
2.6. Indices of Growth and Biometric Parameters
With the sampling carried out, it was possible to obtain data on the weight of the individuals, the number of fish in each tank, and their total biomass. Growth and nutrient efficiency indices, such as final weight (FW), specific growth rate (SGR), survival (S), feed intake (FI), and feed conversion ratio (FCR), were calculated at the end of the study, considering the tank as an experimental unit.
Ten fish were randomly selected from each tank to obtain the biometric parameters. The fish were anesthetized with clove oil containing 87% eugenol (Guinama®, Valencia, Spain) in 150 mg/L−1 of water. Total length (cm), total weight (g), liver weight (g), carcass weight (g), and visceral fat weight (g) were measured to obtain the visceromatic index (VSI), hepatosomatic index (HSI), visceral fat index (VFI), and condition factor (CF).
2.7. Digestibility Assay
After the growth experiment, another experiment was conducted in another system prepared to estimate the digestibility of different diets for gilthead seabream. The study was conducted at the Animal Science Department of the Polytechnic University of Valencia (UPV) farm using a semi-closed marine recirculating system with 190-L fiberglass tanks; each trial lasted 30 days. Faecal material was collected in a settling column, dried in an oven, and analyzed for its nutritional content and inert markers. Chromic oxide (5 g kg
−1) was used as an indigestible marker to assess the apparent digestibility of the diets. Dry matter, crude protein, energy, calcium, and phosphorus were also analyzed using the same methods. The apparent digestibility coefficients (ADCs) were calculated for each diet, and the results were compared to determine the most digestible diet [
21]. The following formulas were used to estimate the ADC of diets for dry matter (ADC
dm, %):
The following equations determined the percentage ADCs of every dietary nutrient, including calcium, phosphorus, protein, and energy:
In this equation, the variables “nutrient diet (g/kg)” and “nutrient feces (g/kg)” represent the relevant nutritional components of the diet and feces, such as protein or energy. Marker levels of the diet and feces are indicated by the measurements “marker diet” (g/kg) and “marker feces” (g/kg), respectively.
2.8. In Vitro Hydrolysis Assay
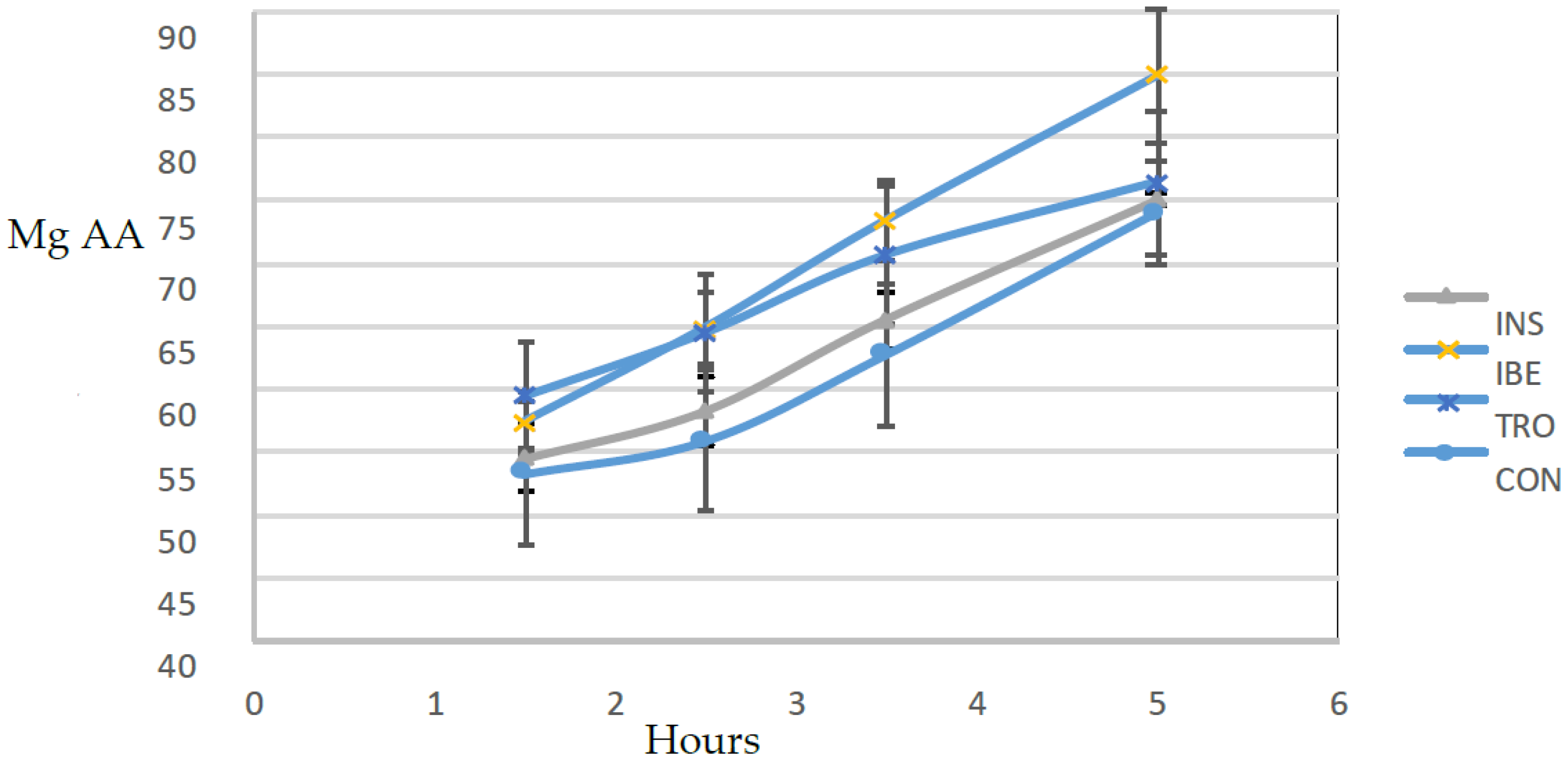
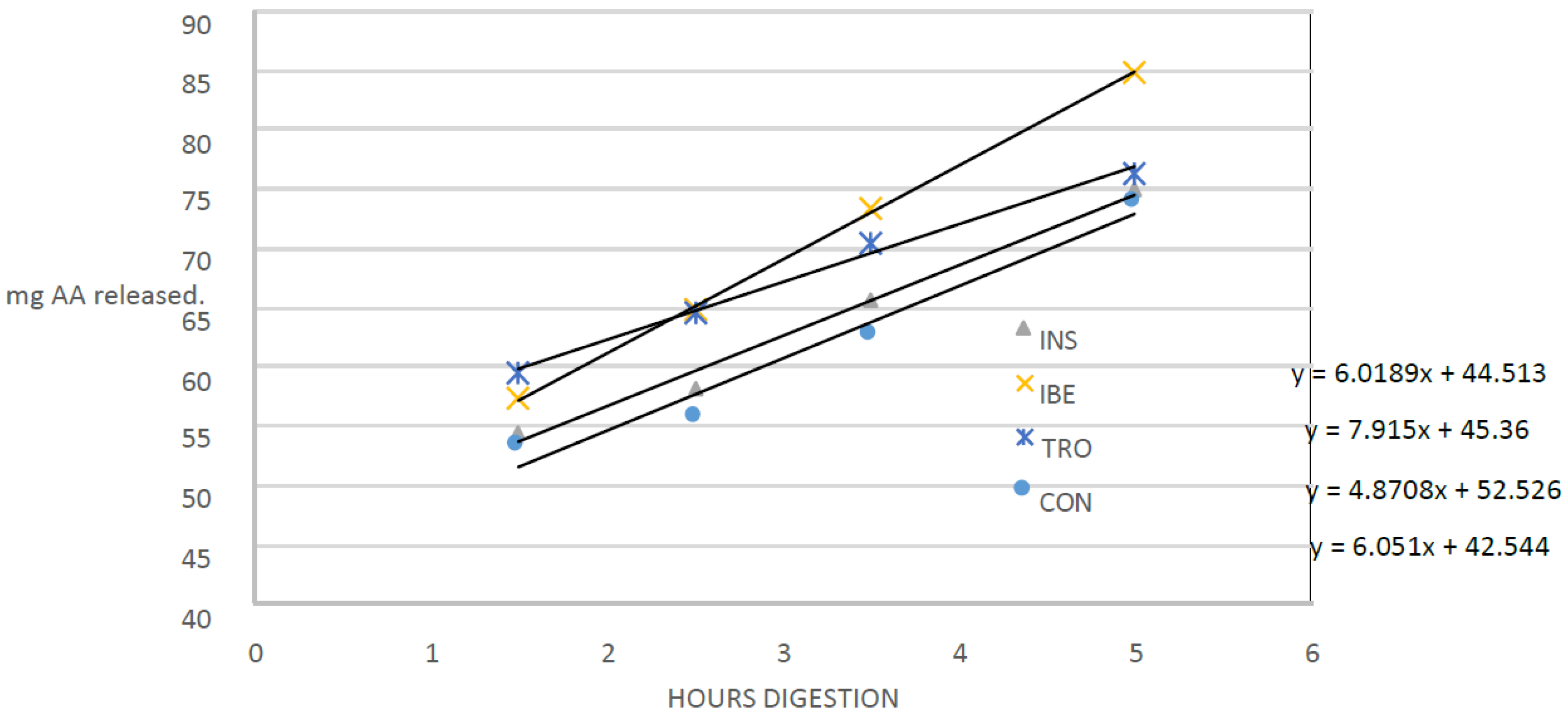
Conditions that simulated the digestive tract of juvenile gilthead seabream were used to conduct an in vitro hydrolysis test [
22]. Six hours after feeding, samples of seven juvenile gilthead seabream were taken, each weighing an average of 256 g, to confirm the presence of digestive and intestinal-related enzymes. The fish were euthanized by immersing them in ice-cold water with a small amount of clove oil as an anesthetic. They were then promptly dissected to obtain the digestive tract. The digestive tract was divided into two sections: 1—the proximal intestine, which included the diffuse pancreas and the pyloric caecum; and 2—the stomach. The tissues were used to produce the extracts to measure proteases and amylase activities following the conditions shown in
Table 3.
An assay was carried out for each diet in triplicate plus a blank. The blank was constructed by inactivating the enzymatic extracts with heat before their inclusion in the bioreactors. It allowed quantifying the amount of amino acids in the extracts and diet. That is released by solubilization and not by enzymatic hydrolysis.
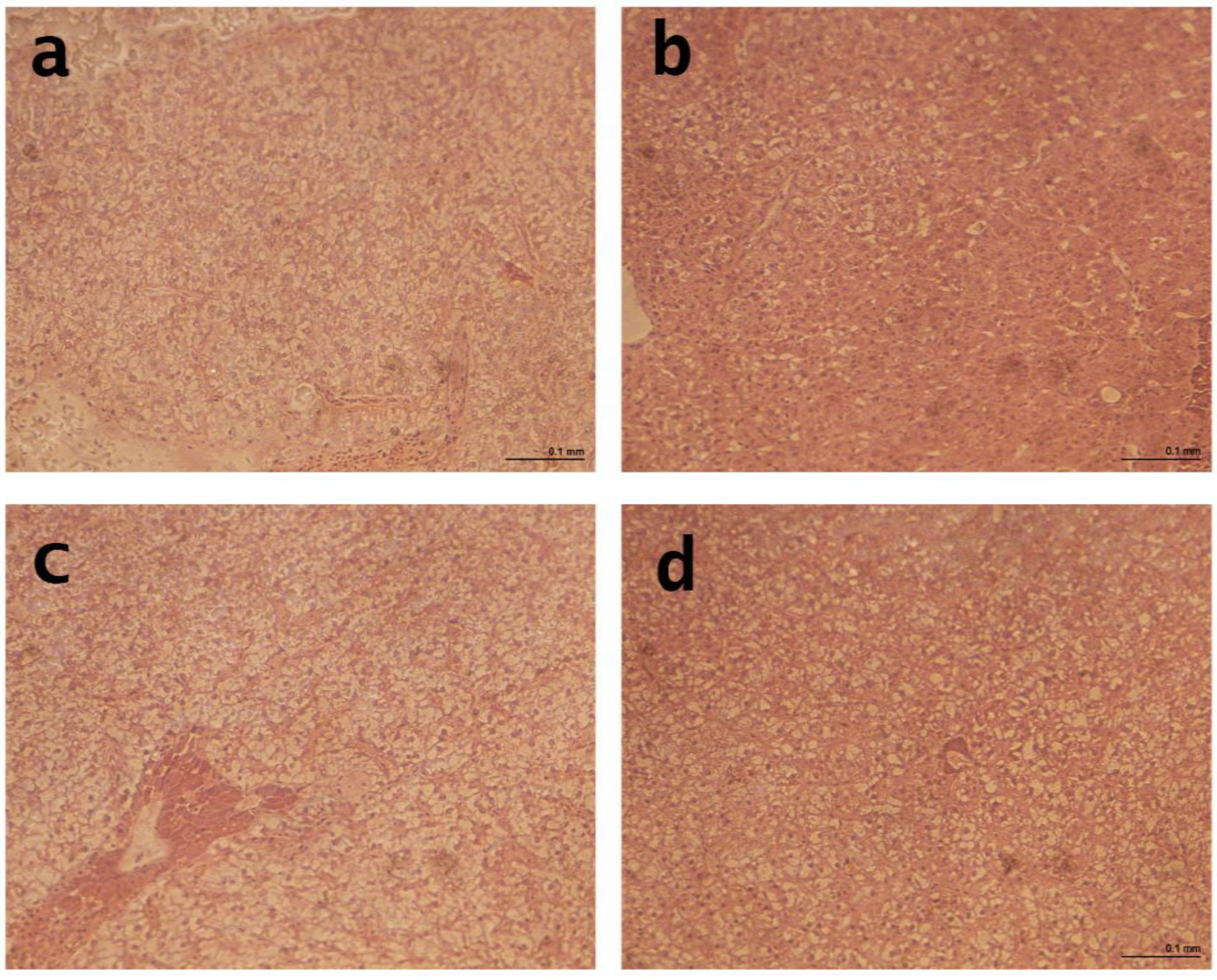
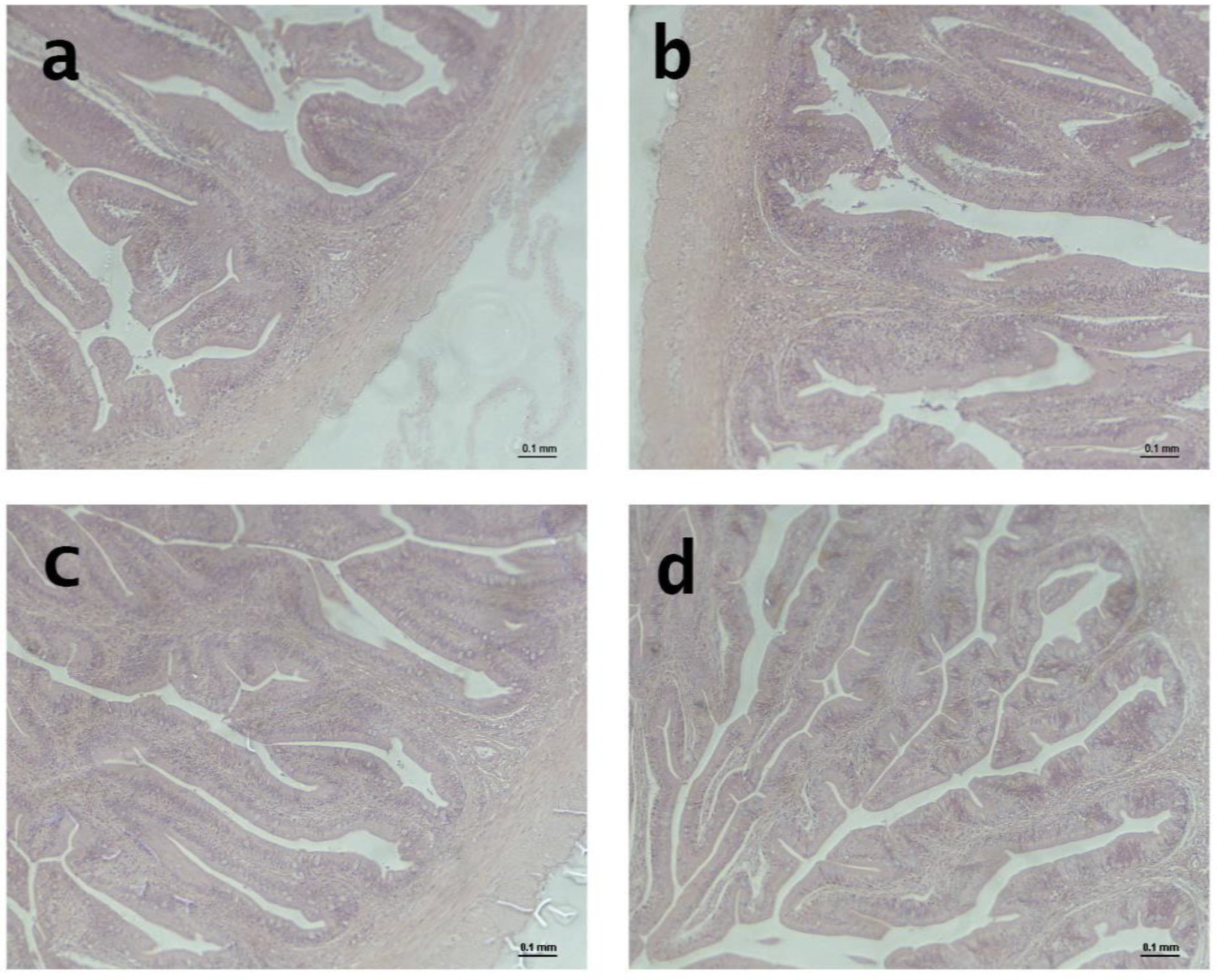
2.9. Liver and Intestinal Histology
After the growth experiment, the liver and distal intestine (DI) were dissected from the guts of three fish; each tank was fed experimental diets. Samples were kept in formalin buffered with phosphate (4%, pH 7.4).
Following standard histological practices, all formalin-fixed tissues were systematically dehydrated in ethanol, equilibrated under ultra-clean conditions, and embedded in paraffin. Transverse sections of each paraffin block were cut using a Shandon hyper-cut microtome and then stained with hematoxylin and eosin for analysis. An Eclipse E400 Nikon light microscope from Izasa S.A. in Barcelona, Spain, was used to analyze 100 sections of the distal intestine and 400 sections of the liver. Hepatocytes and their nuclei were examined and their diameters measured to evaluate the effect of diet on the liver. We evaluated villus length (VL), villus thickness (VT), lamina propria (LP), muscle layer (ML), submucous layer (SML), and serous layer (SL).
2.10. Statistical Analysis
Using the Statgraphics® Plus 5.1 statistical program (Statistical Graphics Corp., Rockville, MO, USA), the results of various growth and nutrient indices, biometric indices, retention of AA, ADC, in vitro hydrolysis, and histological measurements were analyzed using an analysis of variance with a Newman-Keuls test for the comparison of means. Initial covariate weights were used for the study of growth indices. The findings are represented as the mean ± standard error (SEM). The significance level was established at p < 0.05.
4. Discussion
Improving organic fish’s growth and value is a critical research area, particularly in developing suitable feeds for organic farming. However, the need for appropriate raw materials ideal for creating well-balanced diets and EU regulations have been significant obstacles to producing organic carnivorous fish feeds, which have limited the growth of organic aquaculture production [
23]. The focus on feed formulation for organic production has shifted to the search for alternative raw materials that can serve as good protein sources due to the scarcity of fishmeal. This has become crucial in meeting the growing demand for protein sources [
24]. However, the optimal ratio of marine and plant-origin proteins [
25], marine and animal by-products, and insects in organic aquaculture feed has yet to be established.
Furthermore, while organic marine and animal by-products and insects can be incorporated into feed to some extent, the effect on growth, feed utilization, digestibility, retention efficiency, and histology must also be considered.
Organic diets can substitute fishmeal without affecting the growth performance of gilthead seabream. However, the highest final weight was observed on the CON diet.
These results are consistent with previous studies that show better growth performance in organically reared fish than in conventionally reared fish [
17]. However, the survival rates of the organic groups in this study were not significantly different from those of the conventional group, which differs from some previous studies that reported higher survival rates in organic fish.
It should be noted that the specific ingredients (remains of trout, visceral Iberian pig, and insects) used in the organic diets can significantly impact the growth and composition of fish, as demonstrated by the differences observed between the different organic and control diets in this study.
According to Di Marco’s studies [
17] on the organic farming of European sea bass and gilthead seabream, researchers found that organic fish exhibited better growth performance, as indicated by their lower feed conversion ratio and higher metabolic status, supported by their protein and energy profiles and higher hepatosomatic index (HSI), but a lower mesenteric fat index and higher lipid content in organic European sea bass fillets. These differences were attributed to the composition of the feeds provided to the different groups, as shown in a similar study by Trocino et al. [
16] on European sea bass and Mente et al. [
14] on gilthead seabream.
Only a few studies have examined the substitution of fishmeal in organic diets, and most of them have focused on freshwater species. Lunger et al. [
26,
27] used organic yeast (NuPro) to replace up to 25% of FM in the cobia and tilapia feeds, respectively, and found that it did not affect the growth or feed conversion rate.
The FCR values obtained in this study indicate that fish could probably be fed slightly less while maintaining the same growth at the trial temperature. However, FCR is consistent with the temperature, growth, and feed intake shown.
Aquaculture and animal by-product protein-based ingredients in organic aquaculture have yet to be well studied. More research is needed to determine their effects on product quality and other aspects, such as histology and digestibility, in organic aquaculture. Further research is required to optimize the formulation of organic feed and evaluate the long-term effects of organic farming on fish health and growth.
Experimental organic diets positively affected the digestibility of dry matter, calcium, gross protein, and gross energy in gilthead seabream (
Table 7). Some organic diets had higher apparent digestibility coefficients (ADC) for these nutrients than the CON diet, indicating that fish could better digest and utilize them. Previous studies have had different results on the effect of organic feeds on digestibility. For example, the study conducted by Amirkolaie et al. [
28] revealed that substituting fishmeal with poultry by-product meal (PBM) reduced the digestibility of dry matter, fat, and protein in rainbow trout. Other studies, instead, reported that aquafeeds formulated with alternative protein sources had higher ADCs for protein and energy in various fish species, such as tilapia and catfish [
8]. In general, these studies suggest that organic raw materials have the potential to improve the digestibility and utilization of nutrients in aquafeeds, which can lead to improved growth and health in farmed fish. However, digestibility is influenced by the raw ingredients and must be studied separately.
Studying the dynamics of protein hydrolysis in experimental diets for gilthead seabream is essential to aquaculture nutrition. The results of this study show that the rate of protein hydrolysis varied between the experimental diets, which indicates that different organic raw materials may have other effects on protein hydrolysis and subsequent nutrient availability. The current study provides new information on the impact of different organic raw materials on protein hydrolysis in gilthead seabream diets. The study found that the diet with the highest hydrolysis rate was IBE, which contained visceral Iberian pig meal as a protein source. This finding is consistent with previous studies showing variations in protein hydrolysis rates among raw materials [
29].
As is known, EAAs cannot be synthesized and must be obtained through the diet, making them essential for optimal growth and health. Retention efficiency can vary depending on the specific EAA and the composition of the diet.
The results did not indicate significant differences in the retention efficiency of EAA in the various diets except for methionine (Met). Met retention efficiency was significantly higher on the INS diet. Mente et al. [
25] reported differences in the retention efficiency of essential amino acids in European sea bass fed with different organic diets. These findings differ from previous studies that have reported differences in the retention efficiency of EAA in fish fed a blend of animal and plant protein diets. Monge-Ortiz et al. [
30] reported that the retention ratio of seven EAA in the whole-body profile of fish varied significantly between the diets of
Seriola dumerili. Overall, the findings from the present study suggest that the retention efficiency of EAA in fish-fed organic diets can vary depending on the type and composition of the organic raw materials used. Methionine is a limited amino acid in fish, and its higher retention in CON and INS diets (
Table 8) could affect the PPV value of
Table 5.
The gilthead seabream in the study did not show significant lipid accumulation or nucleus displacement caused by vacuoles, indicating a typical healthy morphology. The results indicated that the experimental organic diets significantly affected the histological measures of the liver in gilthead seabream. The diameter of the hepatocyte was significantly reduced in fish-fed organic diets compared to the CON diet. The reduced hepatocyte diameter in fish-fed organic diets may indicate decreased lipid accumulation and improved liver health. Furthermore, the diameter of the nuclei was significantly reduced in fish-fed organic diets compared to the control diet, which may indicate improved liver function. This finding is consistent with a study by Mourente et al. [
31], which investigated the effects of plant-based diets on the liver morphology of sea bass and found that the diameter of the hepatocyte was significantly reduced in fish fed plant-based diets. These findings suggest that organic diets can positively affect gilthead seabreams’ liver health and morphology. To date, no studies have investigated the histological effects of organic diets on the fish liver.
The study results showed that the different organic diets significantly affected the distal measurements of the gut in gilthead seabream. The diets affected the SL, ML, SML, VL, and VT. Specifically, gilthead seabream fed the CON and IBE diets had significantly longer SL and VL than those fed the TRO and INS diets. Regarding ML, SML, and VT, gilthead seabream fed the IBE diet had significantly longer measurements than those fed the TRO and INS diets. Finally, LP was considerably longer in gilthead seabream fed the CON and IBE diets than in those fed the TRO and INS diets.
Limited studies have investigated the effects of different organic diets on intestinal measurements in gilthead seabream. However, Fronte et al. [
32] examined the impact of different nitrogen-rich ingredients, such as hydrolyzed fish protein and autolyzed yeast, on the histological intestinal morphology of gilthead seabream (
Sparus aurata) and found that villi branching and thickening were significantly affected by diets.
Furthermore, according to Torrecillas et al. [
33], who investigated the effects of a vegetable-based diet on the gut health of sea bass and found that villi height and epithelial thickness of the gut were significantly reduced in fish fed the vegetable-based diet compared to those fed a fishmeal-based diet. These findings suggest that different organic diets can dramatically affect the health and morphology of the intestinal tract of gilthead seabream.
Based on the results presented in the study, it can be reported that gilthead seabream fed the INS, TRO, and IBE diets had lower final weights than those fed the CON diet. However, survival rates were similar among all diets. Gilthead seabream fed the TRO, INS, and IBE diets had a lower protein content than those fed the CON diet. On the contrary, the fat content was higher in the TRO and IBE diets compared to the CON diet. The retention efficiencies of fat were also lower in the TRO and IBE diets than in the CON and INS diets. The apparent digestibility coefficients (ADC) of dry matter, gross protein, and gross energy were higher in the INS and IBE diets compared to the CON and TRO diets. The retention efficiency of EAA is mostly the same among diets except for Met. The IBE and TRO diets had lower retention efficiency for Met than the CON and INS diets. Gilthead seabream fed the TRO and INS diets had smaller nuclei and hepatocyte diameters in their livers than those fed the CON and IBE diets. Gilthead seabream fed the TRO and INS diets had shorter distal intestinal measurements than those fed the CON and IBE diets.